Searching for Metabolic Markers of Stroke in Human Plasma via NMR Analysis
Abstract
:1. Introduction
2. Results
2.1. Personal, Sociodemographic and Clinical Data
2.2. NMR Spectra Peaks Integration and Identification
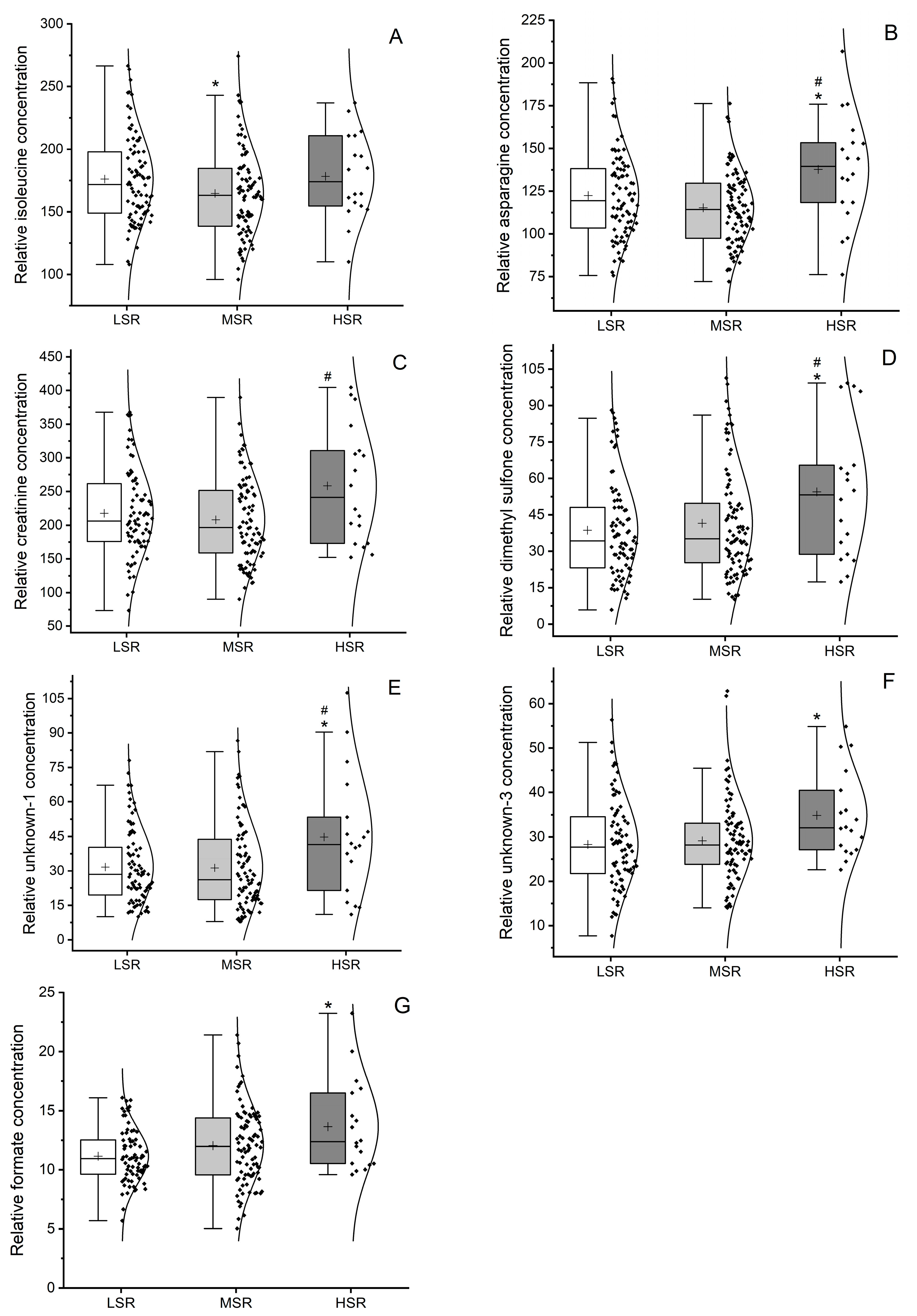
2.3. Univariate Metabolomic Analysis of Metabolites Levels
2.4. Comorbidities and Drugs Effect on Plasma Metabolome
3. Discussion
4. Materials and Methods
4.1. Study Groups’ Constitution
4.2. Sample Preparation
4.3. NMR Data Acquisition and Processing
4.4. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Feigin, V.L.; Brainin, M.; Norrving, B.; Martins, S.; Sacco, R.L.; Hacke, W.; Fisher, M.; Pandian, J.; Lindsay, P. World Stroke Organization (WSO): Global Stroke Fact Sheet 2022. Int. J. Stroke 2022, 17, 18–29. [Google Scholar] [CrossRef] [PubMed]
- Benjamin, E.J.; Blaha, M.J.; Chiuve, S.E.; Cushman, M.; Das, S.R.; Deo, R.; de Ferranti, J.F.; Fornage, M.; Gillespie, C.; Isasi, C.R.; et al. Heart Disease and Stroke Statistics-2017 Update: A Report From the American Heart Association. Circulation 2017, 135, e146–e603. [Google Scholar] [CrossRef] [PubMed]
- Ingall, T. Stroke-incidence, mortality, morbidity and risk. J. Insur. Med. 2004, 36, 143–152. [Google Scholar]
- World Health Organization. World Health Statistics 2023: Monitoring Health for the SDGs, Sustainable Development Goals; World Health Organization: Geneva, Switzerland, 2023. [Google Scholar]
- Meschia, J.F.; Brott, T. Ischaemic stroke. Eur. J. Neurol. 2018, 25, 35–40. [Google Scholar] [CrossRef] [PubMed]
- Montaño, A.; Hanley, D.F.; Hemphill, J.C., 3rd. Hemorrhagic stroke. Handb. Clin. Neurol. 2021, 176, 229–248. [Google Scholar] [CrossRef] [PubMed]
- Smith, E.E.; Saposnik, G.; Biessels, G.J.; Doubal, F.N.; Fornage, M.; Gorelick, P.B.; Greenberg, S.M.; Higashida, R.T.; Kasner, S.E.; Seshadri, S.; et al. Prevention of Stroke in Patients with Silent Cerebrovascular Disease: A Scientific Statement for Healthcare Professionals from the American Heart Association/American Stroke Association. Stroke 2017, 48, e44–e71. [Google Scholar] [CrossRef]
- Guzik, A.; Bushnell, C. Stroke Epidemiology and Risk Factor Management. Contin. Lifelong Learn. Neurol. 2017, 23, 15–39. [Google Scholar] [CrossRef]
- Pletcher, M.J.; Moran, A.E. Cardiovascular Risk Assessment. Med. Clin. N. Am. 2017, 101, 673–688. [Google Scholar] [CrossRef]
- Bernini, P.; Bertini, I.; Luchinat, C.; Tenori, L.; Tognaccini, A. The cardiovascular risk of healthy individuals studied by NMR metabonomics of plasma samples. J. Proteome Res. 2011, 10, 4983–4992. [Google Scholar] [CrossRef]
- Sun, D.; Tiedt, S.; Yu, B.; Jian, X.; Gottesman, R.F.; Mosley, T.H.; Boerwinkle, E.; Dichgans, M.; Fornage, M. A prospective study of serum metabolites and risk of ischemic stroke. Neurology 2019, 92, e1890–e1898. [Google Scholar] [CrossRef]
- Ke, C.; Pan, C.W.; Zhang, Y.; Zhu, X.; Zhang, Y. Metabolomics facilitates the discovery of metabolic biomarkers and pathways for ischemic stroke: A systematic review. Metabolomics 2019, 15, 152. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.; Shin, M.S.; Jee, S.H.; Park, Y.H. Global metabolomics analysis of serum from humans at risk of thrombotic stroke. Analyst 2020, 145, 1695–1705. [Google Scholar] [CrossRef] [PubMed]
- Au, A. Metabolomics and Lipidomics of Ischemic Stroke. Adv. Clin. Chem. 2018, 85, 31–69. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.H.; Shim, H.S.; Kim, K.H.; Lee, J.; Chung, B.C.; Kowall, N.W.; Ryu, H.; Lee, J. Metabolomic Analysis Identifies Alterations of Amino Acid Metabolome Signatures in the Postmortem Brain of Alzheimer’s Disease. Exp. Neurobiol. 2019, 28, 376–389. [Google Scholar] [CrossRef]
- Laborde, C.M.; Mourino-Alvarez, L.; Akerstrom, F.; Padial, L.R.; Vivanco, F.; Gil-Dones, F.; Barderas, M.G. Potential blood biomarkers for stroke. Expert. Rev. Proteom. 2012, 9, 437–449. [Google Scholar] [CrossRef]
- Rankin, N.J.; Preiss, D.; Welsh, P.; Burgess, K.E.; Nelson, S.M.; Lawlor, D.A.; Sattar, N. The emergence of proton nuclear magnetic resonance metabolomics in the cardiovascular arena as viewed from a clinical perspective. Atherosclerosis 2014, 237, 287–300. [Google Scholar] [CrossRef]
- Smolinska, A.; Blanchet, L.; Buydens, L.M.; Wijmenga, S.S. NMR and pattern recognition methods in metabolomics: From data acquisition to biomarker discovery: A review. Anal. Chim. Acta 2012, 750, 82–97. [Google Scholar] [CrossRef]
- Goulart, V.A.M.; Sena, M.M.; Mendes, T.O.; Menezes, H.C.; Cardeal, Z.L.; Paiva, M.J.N.; Sandrim, V.C.; Pinto, M.C.X.; Resende, R.R. Amino Acid Biosignature in Plasma among Ischemic Stroke Subtypes. Biomed. Res. Int. 2019, 2019, 8480468. [Google Scholar] [CrossRef]
- Sidorov, E.; Bejar, C.; Xu, C.; Ray, B.; Reddivari, L.; Chainakul, J.; Vanamala, J.K.; Sanghera, D.K. Potential Metabolite Biomarkers for Acute Versus Chronic Stage of Ischemic Stroke: A Pilot Study. J. Stroke Cerebrovasc. Dis. 2020, 29, 104618. [Google Scholar] [CrossRef]
- Sidorov, E.; Sanghera, D.K.; Vanamala, J.K.P. Biomarker for Ischemic Stroke Using Metabolome: A Clinician Perspective. J. Stroke 2019, 21, 31–41. [Google Scholar] [CrossRef]
- Tao, S.; Xiao, X.; Li, X.; Na, F.; Na, G.; Wang, S.; Zhang, P.; Hao, F.; Zhao, P.; Guo, D.; et al. Targeted metabolomics reveals serum changes of amino acids in mild to moderate ischemic stroke and stroke mimics. Front. Neurol. 2023, 14, 1153193. [Google Scholar] [CrossRef]
- Jung, J.Y.; Lee, H.S.; Kang, D.G.; Kim, N.S.; Cha, M.H.; Bang, O.S.; Ryu, D.H.; Hwang, G.-S. 1H-NMR-based metabolomics study of cerebral infarction. Stroke 2011, 42, 1282–1288. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, E.H. The neurology of folic acid deficiency. Handb. Clin. Neurol. 2014, 120, 927–943. [Google Scholar] [CrossRef] [PubMed]
- Takemoto, Y. Amino acids that centrally influence blood pressure and regional blood flow in conscious rats. J. Amino Acids 2012, 2012, 831759. [Google Scholar] [CrossRef]
- Wang, D.; Kong, J.; Wu, J.; Wang, X.; Lai, M. GC-MS-based metabolomics identifies an amino acid signature of acute ischemic stroke. Neurosci. Lett. 2017, 642, 7–13. [Google Scholar] [CrossRef] [PubMed]
- Kimberly, W.T.; Wang, Y.; Pham, L.; Furie, K.L.; Gerszten, R.E. Metabolite profiling identifies a branched chain amino acid signature in acute cardioembolic stroke. Stroke 2013, 44, 1389–1395. [Google Scholar] [CrossRef]
- Huang, Y.; Zhou, M.; Sun, H.; Wang, Y. Branched-chain amino acid metabolism in heart disease: An epiphenomenon or a real culprit? Cardiovasc. Res. 2011, 90, 220–223. [Google Scholar] [CrossRef]
- Zhang, X.; Hu, W.; Wang, Y.; Wang, W.; Liao, H.; Zhang, X.; Kiburg, K.V.; Shang, X.; Bulloch, G.; Huang, Y.; et al. Plasma metabolomic profiles of dementia: A prospective study of 110,655 participants in the UK Biobank. BMC Med. 2022, 20, 252. [Google Scholar] [CrossRef]
- Peng, R.; Liu, K.; Li, W.; Yuan, Y.; Niu, R.; Zhou, L.; Xiao, Y.; Gao, H.; Yang, H.; Zhang, C.; et al. Blood urea nitrogen, blood urea nitrogen to creatinine ratio and incident stroke: The Dongfeng-Tongji cohort. Atherosclerosis 2021, 333, 1–8. [Google Scholar] [CrossRef]
- Koppe, L.; Nyam, E.; Vivot, K.; Manning Fox, J.E.; Dai, X.Q.; Nguyen, B.N.; Trudel, D.; Attané, C.; Moullé, V.S.; MacDonald, P.E.; et al. Urea impairs β cell glycolysis and insulin secretion in chronic kidney disease. J. Clin. Investig. 2016, 126, 3598–3612. [Google Scholar] [CrossRef]
- D’Apolito, M.; Du, X.; Zong, H.; Catucci, A.; Maiuri, L.; Trivisano, T.; Pettoello-Mantovani, M.; Campanozzi, A.; Raia, V.; Pessin, J.E.; et al. Urea-induced ROS generation causes insulin resistance in mice with chronic renal failure. J. Clin. Investig. 2010, 120, 203–213. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Li, J.; Yu, K.; Yang, H.; Min, X.; Chen, H.; Wu, T. Associations of estimated glomerular filtration rate and blood urea nitrogen with incident coronary heart disease: The Dongfeng-Tongji Cohort Study. Sci. Rep. 2017, 7, 9987. [Google Scholar] [CrossRef] [PubMed]
- Arnan, M.K.; Hsieh, T.C.; Yeboah, J.; Bertoni, A.G.; Burke, G.L.; Bahrainwala, Z.; Grega, M.A.; Baumgartner, W.A.; Gottesman, R.F. Postoperative blood urea nitrogen is associated with stroke in cardiac surgical patients. Ann. Thorac. Surg. 2015, 99, 1314–1320. [Google Scholar] [CrossRef]
- Fernstrom, J.D. Branched-chain amino acids and brain function. J. Nutr. 2005, 135 (Suppl. S6), 1539S–1546S. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Canela, M.; Toledo, E.; Clish, C.B.; Hruby, A.; Liang, L.; Salas-Salvadó, J.; Razquin, C.; Corella, D.; Estruch, R.; Ros, E.; et al. Plasma Branched-Chain Amino Acids and Incident Cardiovascular Disease in the PREDIMED Trial. Clin. Chem. 2016, 62, 582–592. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Duan, Y.; Jiang, M.; He, X.; Xu, S.; Guo, J.; Li, M.; Zhou, C.; Wu, D.; Liu, G.; et al. Branched-chain amino acids and risk of stroke: A Mendelian randomization study. Front. Neurosci. 2023, 17, 1143718. [Google Scholar] [CrossRef]
- Liu, M.; Zhou, K.; Li, H.; Dong, X.; Tan, G.; Chai, Y.; Wang, W.; Bi, X. Potential of serum metabolites for diagnosing post-stroke cognitive impairment. Mol. Biosyst. 2015, 11, 3287–3296. [Google Scholar] [CrossRef]
- Tobias, D.K.; Lawler, P.R.; Harada, P.H.; Demler, O.V.; Ridker, P.M.; Manson, J.E.; Cheng, S.; Mora, S. Circulating Branched-Chain Amino Acids and Incident Cardiovascular Disease in a Prospective Cohort of US Women. Circ. Genom. Precis. Med. 2018, 11, e002157. [Google Scholar] [CrossRef]
- Lamarre, S.G.; Morrow, G.; Macmillan, L.; Brosnan, M.E.; Brosnan, J.T. Formate: An essential metabolite, a biomarker, or more? Clin. Chem. Lab. Med. 2013, 51, 571–578. [Google Scholar] [CrossRef]
- Pietzke, M.; Meiser, J.; Vazquez, A. Formate metabolism in health and disease. Mol. Metab. 2020, 33, 23–37. [Google Scholar] [CrossRef]
- Lee, J.; Shin, J.A.; Lee, E.M.; Nam, M.; Park, E.M. Noggin-mediated effects on metabolite profiles of microglia and oligodendrocytes after ischemic insult. J. Pharm. Biomed. Anal. 2023, 224, 115196. [Google Scholar] [CrossRef] [PubMed]
- Clarke, R.; Grimley Evans, J.; Schneede, J.; Nexo, E.; Bates, C.; Fletcher, A.; Prentice, A.; Johnston, C.; Ueland, P.M.; Refsum, H.; et al. Vitamin B12 and folate deficiency in later life. Age Ageing. 2004, 33, 34–41. [Google Scholar] [CrossRef] [PubMed]
- Coull, B.M.; Beamer, N.; de Garmo, P.; Sexton, G.; Nordt, F.; Knox, R.; Seaman, G.V. Chronic blood hyperviscosity in subjects with acute stroke, transient ischemic attack, and risk factors for stroke. Stroke 1991, 22, 162–168. [Google Scholar] [CrossRef]
- Jimoh, O.F.; Brown, T.J.; Bunn, D.; Hooper, L. Beverage Intake and Drinking Patterns-Clues to Support Older People Living in Long-Term Care to Drink Well: DRIE and FISE Studies. Nutrients 2019, 11, 447. [Google Scholar] [CrossRef] [PubMed]
- Engelke, U.F.; Tangerman, A.; Willemsen, M.A.; Moskau, D.; Loss, S.; Mudd, S.H.; Wevers, R.A. Dimethyl sulfone in human cerebrospinal fluid and blood plasma confirmed by one-dimensional (1)H and two-dimensional (1)H-(13)C NMR. NMR Biomed. 2005, 18, 331–336. [Google Scholar] [CrossRef]
- Nakhostin-Roohi, B.; Barmaki, S.; Khoshkhahesh, F.; Bohlooli, S. Effect of chronic supplementation with methylsulfonylmethane on oxidative stress following acute exercise in untrained healthy men. J. Pharm. Pharmacol. 2011, 63, 1290–1294. [Google Scholar] [CrossRef]
- Butawan, M.; Benjamin, R.L.; Bloomer, R.J. Methylsulfonylmethane: Applications and Safety of a Novel Dietary Supplement. Nutrients 2017, 9, 290. [Google Scholar] [CrossRef]
- Miller, L.E. Methylsulfonylmethane decreases inflammatory response to tumor necrosis factor-α in cardiac cells. Am. J. Cardiovasc. Dis. 2018, 8, 31–38. [Google Scholar]
- Miller, L.; Thompson, K.; Pavlenco, C.; Mettu, V.S.; Haverkamp, H.; Skaufel, S.; Basit, A.; Prasad, B.; Larsen, J. The Effect of Daily Methylsulfonylmethane (MSM) Consumption on High-Density Lipoprotein Cholesterol in Healthy Overweight and Obese Adults: A Randomized Controlled Trial. Nutrients 2021, 13, 3620. [Google Scholar] [CrossRef]
- Dufouil, C.; Beiser, A.; McLure, L.A.; Wolf, P.A.; Tzourio, C.; Howard, V.J.; Westwood, A.J.; Himali, J.J.; Sullivan, L.M.; Aparicio, H.J.; et al. Revised Framingham Stroke Risk Profile to Reflect Temporal Trends. Circulation 2017, 135, 1145–1159. [Google Scholar] [CrossRef]
- Wolf, P.A.; D’Agostino, R.B.; Belanger, A.J.; Kannel, W.B. Probability of stroke: A risk profile from the Framingham Study. Stroke 1991, 22, 312–318. [Google Scholar] [CrossRef] [PubMed]


| Conditions | LSR | MSR | HSR | p-Value | Statistical Test |
|---|---|---|---|---|---|
| Number (n) | 85 | 94 | 18 | ||
| Age (years) | 79.6 ± 0.88 | 88.2 ± 0.57 | 90.7 ± 1.28 | <0.001 * | KWT |
| Gender, Female, (%, (n)) | 52.9 (45) | 79.8 (75) | 77.8 (14) | <0.001 * | FET |
| Systolic blood pressure (mmHg) | 127.6 ± 2.27 | 122.4 ± 2.04 | 127.4 ± 4.26 | 0.199 | OWAT |
| Diastolic blood pressure (mmHg) | 67.2 ± 1.32 | 69.9 ± 1.13 | 71.8 ± 2.47 | 0.153 | OWAT |
| Heart rate (bpm) | 72.7 ± 1.18 | 72.7 ± 1.05 | 71.7 ± 3.22 | 0.932 | OWAT |
| Body mass index (kg/m2) | 26.1 ± 0.61 | 27.1 ± 0.55 | 28.5 ± 1.32 | 0.174 | OWAT |
| Serum glucose (mg/dL) | 98.3 ± 2.69 | 99.4 ± 3.13 | 104.4 ± 8.14 | 0.740 | OWAT |
| Serum total cholesterol (mg/dL) | 165.3 ± 5.06 | 168.4 ± 4.72 | 159.2 ± 9.73 | 0.738 | OWAT |
| Serum HDL-cholesterol (mg/dL) | 54.4 ± 1.49 | 56.3 ± 1.33 | 59.3 ± 5.01 | 0.399 | OWAT |
| Serum LDL-cholesterol (mg/dL) | 89.6 ± 4.35 | 89.8 ± 4.34 | 79.7 ± 7.79 | 0.651 | OWAT |
| Serum triglyceride (mg/dL) | 106.4 ± 4.58 | 111.6 ± 6.13 | 100.9 ± 13.66 | 0.399 | OWAT |
| Diseases | LSR | MSR | HSR | p-Value | Test |
|---|---|---|---|---|---|
| Cardiovascular diseases | |||||
| Hypertension | 62.4 (53) | 79.8 (75) | 83.3 (15) | 0.048 * | FET |
| Atrial fibrillation | 2.4 (2) | 11.7 (11) | 55.6 (10) | <0.001 * | FET |
| Other Arrythmias | 14.1 (12) | 19.1 (18) | 61.1(11) | <0.001 * | FET |
| Heart Failure | 15.3 (13) | 26.6 (25) | (44.4 (8) | 0.021 * | FET |
| Angina pectoris | 7.1 (6) | 10.6 (10) | (5.6) (1) | 0.123 | FET |
| Atherosclerosis | 3.5(3) | 3.2 (3) | 5.6 (1) | 0.232 | FET |
| Valvulopathies | 1.2 (1) | 4.3 (4) | 11.1(2) | 0.059 | FET |
| Peripheral vascular disease | 7.7 (6) | 7.8 (7) | 5.6 (1) | 1.000 | FET |
| Acute myocardial infarction | 2 (2.4) | 2 (2.1) | 4 (22.2) | 0.004 * | FET |
| Cardiovascular diseases (n, ± s.e.m) | 0.94 ± 0.09 | 1.32 ± 0.09 | 2.06 ± 0.24 | <0.001 * | OWAT |
| Metabolic diseases | |||||
| Diabetes | 25.9 (22) | 27.7 (26) | 33.3(6) | 0.361 | FET |
| Dyslipidaemia | 38.8 (33) | 46.8 (44) | 50.0 (9) | 0.081 | FET |
| Respiratory diseases | |||||
| COPD | 5.9 (5) | 9.6 (9) | 5.6 (1) | 0.729 | FET |
| Asthma | 1.2 (1) | 3.2 (3) | 5.6 (1) | 0.380 | FET |
| Central nervous system diseases | |||||
| Depression | 16.5 (14) | 16.0 (15) | 5.6 (1) | 0.587 | FET |
| Treatments | LSR | MSR | HSR | p-Value | Test |
|---|---|---|---|---|---|
| Treatments for cardiovascular diseases | |||||
| Antiarrhythmics | 2.4 (2) | 4.3 (4) | 0 (0) | 0.114 | FET |
| Anti-anginal | 9.4 (8) | 14.9 (14) | 16.7 (3) | 0.087 | FET |
| ACEi | 9.4 (8) | 20.2 (19) | 33.3 (6) | 0.006 * | FET |
| Angiotensin receptor antagonists | 34.1 (29) | 40.4 (38) | 55.6 (10) | 0.060 | FET |
| Alpha and Beta blockers | 18.88 (16) | 16.0 (15) | 38.9 (7) | 0.019 * | FET |
| Calcium channel blockers | 23.5 (20) | 14.9 (14) | 33.3 (6) | 0.020 * | FET |
| Potassium sparing diuretics | 2.4 (2) | 7.4 (7) | 11.1(2) | 0.029 * | FET |
| Loop diuretics | 29.4 (25) | 40.4 (38) | 72.2 (13) | 0.001 * | FET |
| Thiazide diuretics | 20.0 (17) | 20.2 (19) | 16.7 (3) | 0.159 | FET |
| Venotropics | 10.6 (9) | 12.8 (12) | 11.1 (2) | 0.152 | FET |
| Anticoagulants | 41.2 (35) | 53.2 (50) | 77.8 (14) | 0.006 * | FET |
| Treatments for metabolic diseases | |||||
| Sulfonylureas | 4.7 (4) | 3.2 (3) | 11.1 (2) | 0.049 * | FET |
| Biguanides | 14.1 (12) | 18.1 (17) | 11.1 (2) | 0.136 | FET |
| DPP-4 inhibitors | 16.5 (14) | 14.9 (14) | 22.2 (4) | 0.117 | FET |
| Insulin | 5.9 (5) | 4.3 (4) | 5.6 (1) | 0.120 | FET |
| Statins | 35.3 (30) | 39.4 (37) | 50.0 (9) | 0.107 | FET |
| Treatments for respiratory diseases | |||||
| Bronchodilators | 10.6 (9) | 17.0 (16) | 16.4 (3) | 0.087 | FET |
| Treatments for CNS diseases | |||||
| Acetylcholinesterase inhibitors | 10.6 (9) | 10.6 (10) | 5.6 (1) | 0.142 | FET |
| Monoamine oxidase inhibitors | 0.5 (1) | 0 (0) | 0 (0) | 0.052 | FET |
| NMDA antagonist | 7.1 (6) | 12.8 (12) | 5.6 (1) | 0.080 | FET |
| Antiepileptics | 12.9 (11) | 6.4 (6) | 0 (0) | 0.022 * | FET |
| Antipsychotics | 32.9 (28) | 26.6 (25) | 27.8 (5) | 0.099 | FET |
| Antidepressants | 60.0 (51) | 69.1 (65) | 88.9 (16) | 0.028 * | FET |
| Group | Asn | Unk1 | ||||
| Comparison (metabolites) with and without hypertension | LSR | ns | ▲ ** | |||
| MSR | ▲ *** | ns | ||||
| HSR | ns | ns | ||||
| All | ▲ * | ▲ *** | ||||
| Comparison (metabolites) with and without arrythmias | LSR | ns | ns | |||
| MSR | ns | ns | ||||
| HSR | ns | ns | ||||
| All | ▲ * | ▲ * | ||||
| Comparison (metabolites) with and without atrial fibrillation | LSR | ns | ||||
| MSR | ▲ * | |||||
| HSR | ns | |||||
| All | ▲ * | |||||
| Group | Ile | |||||
| Comparison (metabolites) with and without acute myocardial infarction | LSR | ns | ||||
| MSR | ns | |||||
| HSR | ns | |||||
| All | ▲ * | |||||
| Group | Ile | Unk1 | ||||
| Comparison (metabolites) with and without calcium channel blockers treatment | LSR | ns | ▲ * | |||
| MSR | ns | ns | ||||
| HSR | ns | ns | ||||
| All | ▲ * | ▲ * | ||||
| Group | Asn | Cre | Unk1 | Unk3 | Formate | |
| Comparison (metabolites) with and without loop diuretics treatment | LSR | ns | ▲ ** | ▲ ** | ns | ns |
| MSR | ns | ns | ns | ns | ▲ ** | |
| HSR | ns | ns | ns | ns | ns | |
| All | ▲ * | ▲ * | ▲ *** | ▲ * | ▲ * | |
| Group | Ile | Unk1 | Unk3 | Formate | ||
| Comparison (metabolites) with and without anticoagulants treatment | LSR | ns | ns | ns | ns | |
| MSR | ns | ns | ns | ▲ * | ||
| HSR | ns | ns | ns | ns | ||
| All | ▲ * | ▲ * | ▲ * | ▲ * | ||
| Group | Ile | |||||
| Comparison (metabolites) with and without sulfonylureas treatment | LSR | ns | ||||
| MSR | ns | |||||
| HSR | ns | |||||
| All | ▲ *** | |||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oliveira, N.; Sousa, A.; Amaral, A.P.; Graça, G.; Verde, I. Searching for Metabolic Markers of Stroke in Human Plasma via NMR Analysis. Int. J. Mol. Sci. 2023, 24, 16173. https://doi.org/10.3390/ijms242216173
Oliveira N, Sousa A, Amaral AP, Graça G, Verde I. Searching for Metabolic Markers of Stroke in Human Plasma via NMR Analysis. International Journal of Molecular Sciences. 2023; 24(22):16173. https://doi.org/10.3390/ijms242216173
Chicago/Turabian StyleOliveira, Nádia, Adriana Sousa, Ana Paula Amaral, Gonçalo Graça, and Ignacio Verde. 2023. "Searching for Metabolic Markers of Stroke in Human Plasma via NMR Analysis" International Journal of Molecular Sciences 24, no. 22: 16173. https://doi.org/10.3390/ijms242216173
APA StyleOliveira, N., Sousa, A., Amaral, A. P., Graça, G., & Verde, I. (2023). Searching for Metabolic Markers of Stroke in Human Plasma via NMR Analysis. International Journal of Molecular Sciences, 24(22), 16173. https://doi.org/10.3390/ijms242216173







