Adult-Onset Transcriptomic Effects of Developmental Exposure to Benzene in Zebrafish (Danio rerio): Evaluating a Volatile Organic Compound of Concern
Abstract
:1. Introduction
2. Results
2.1. Benzene Exposure
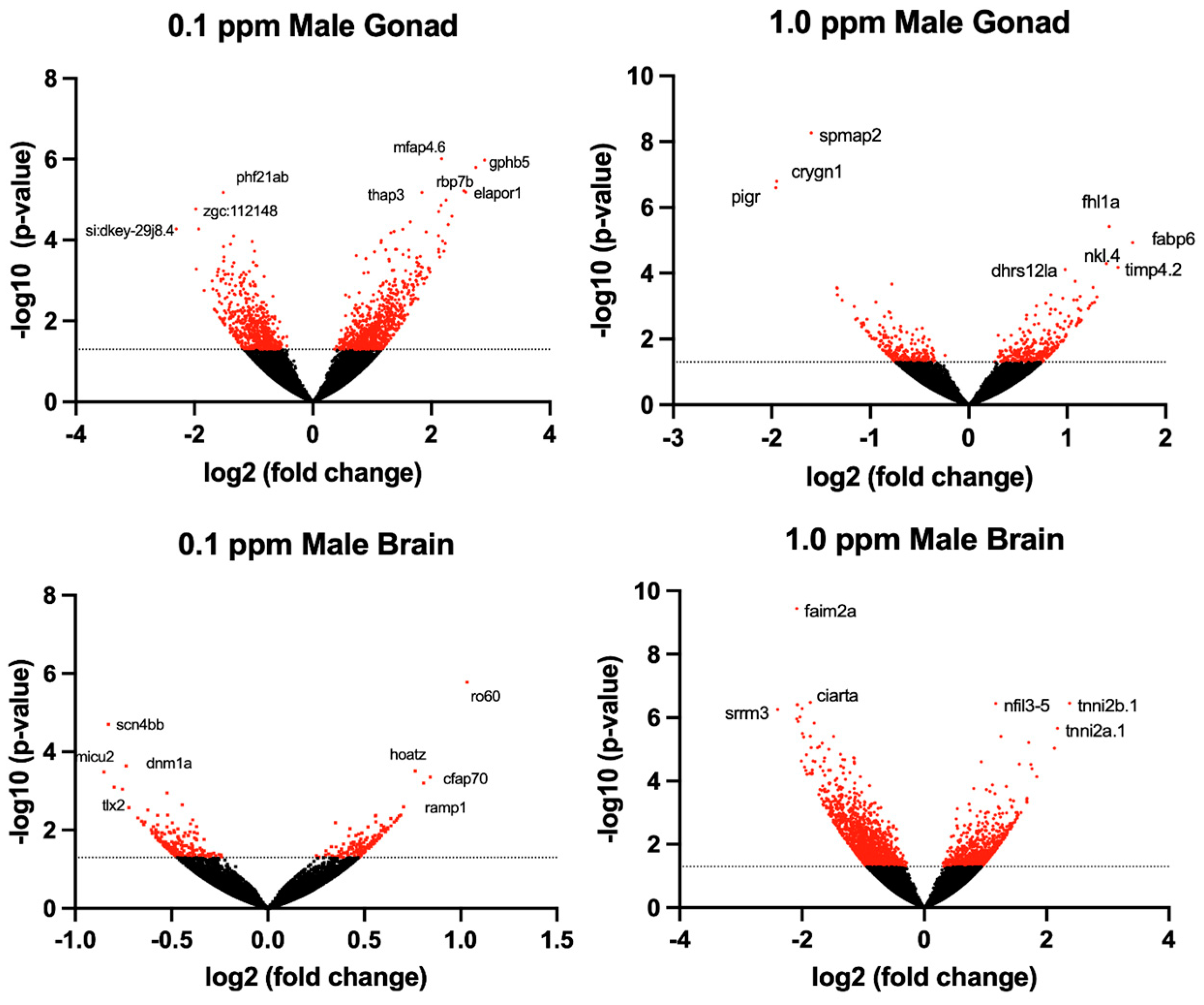
2.2. Differential Gene Expression
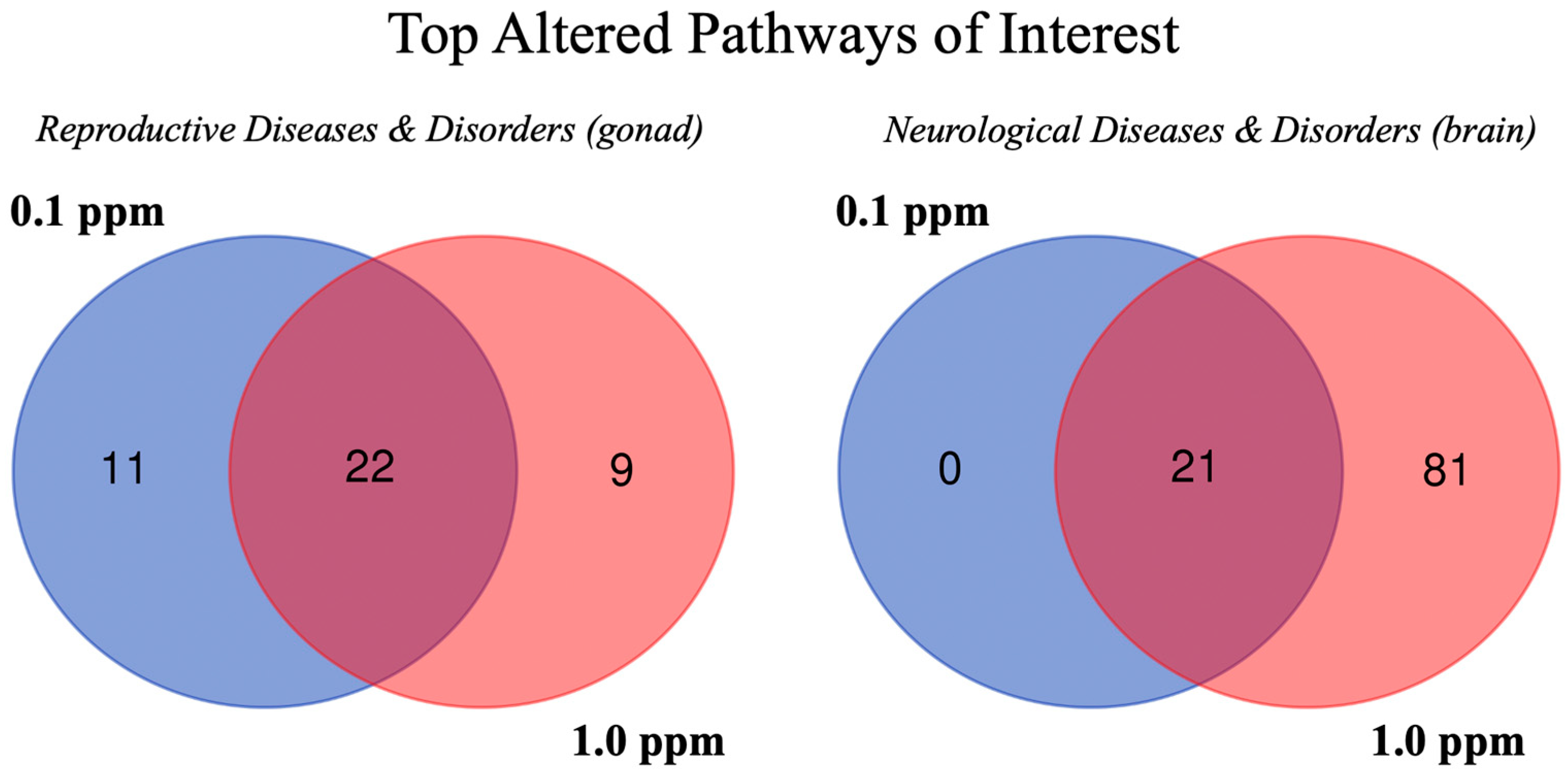
2.3. Pathway Analysis
3. Discussion
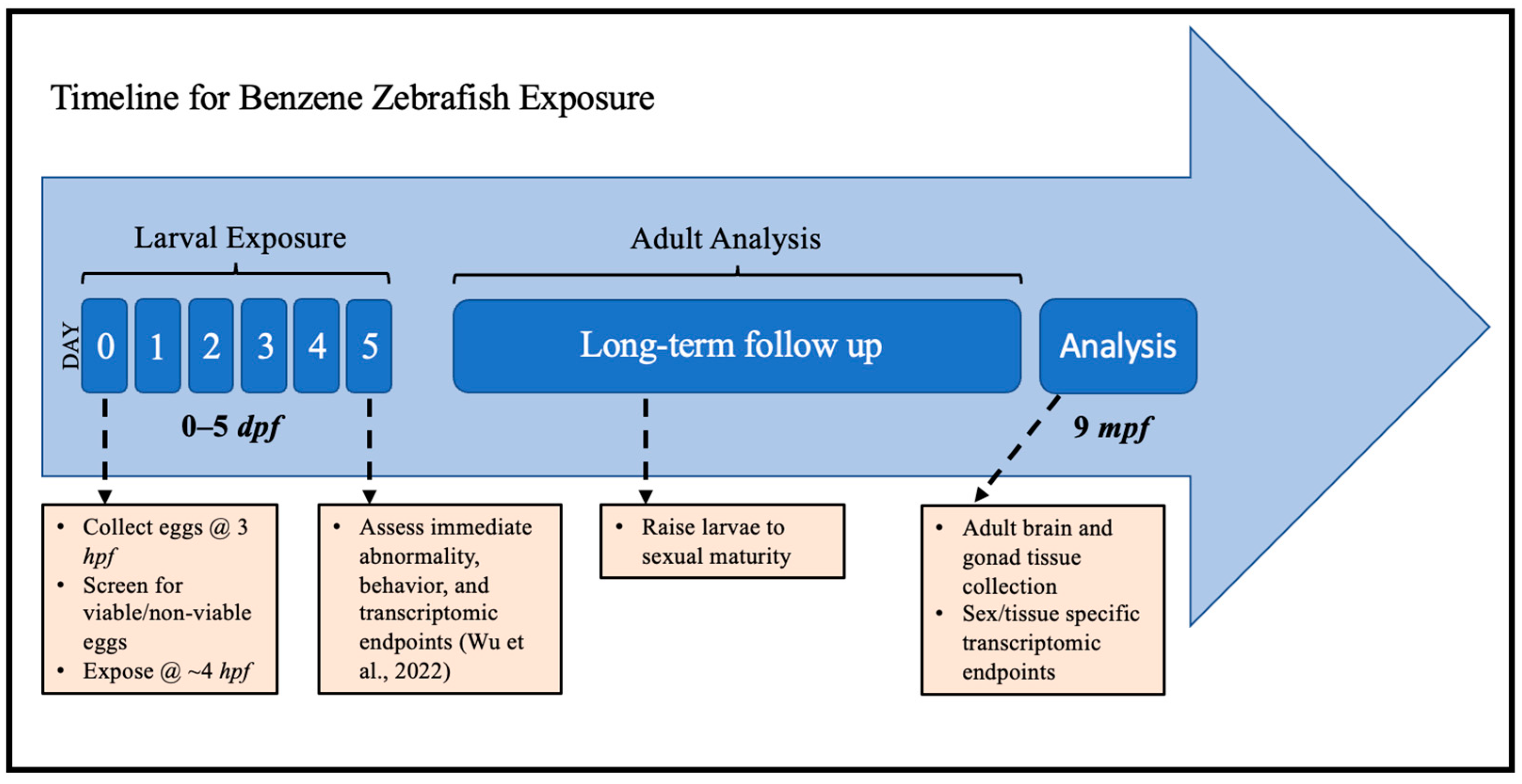
4. Materials and Methods
4.1. Animal Husbandry
4.2. Spawning
4.3. Benzene Exposures
4.4. Tissue Collection
4.5. RNA Collection and Isolation
4.6. RNA-Seq QuantSeq
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Du, L.; Batterman, S.; Godwin, C.; Rowe, Z.; Chin, J.-Y. Air Exchange Rates and Migration of VOCs in Basements and Residences. Indoor Air 2015, 25, 598–609. [Google Scholar] [CrossRef]
- McDonald, B.C.; de Gouw, J.A.; Gilman, J.B.; Jathar, S.H.; Akherati, A.; Cappa, C.D.; Jimenez, J.L.; Lee-Taylor, J.; Hayes, P.L.; McKeen, S.A.; et al. Volatile Chemical Products Emerging as Largest Petrochemical Source of Urban Organic Emissions. Science 2018, 359, 760–764. [Google Scholar] [CrossRef] [PubMed]
- Porada, E.; Szyszkowicz, M. UNMIX Methods Applied to Characterize Sources of Volatile Organic Compounds in Toronto, Ontario. Toxics 2016, 4, 11. [Google Scholar] [CrossRef]
- Hsu, C.-Y.; Chiang, H.-C.; Shie, R.-H.; Ku, C.-H.; Lin, T.-Y.; Chen, M.-J.; Chen, N.-T.; Chen, Y.-C. Ambient VOCs in Residential Areas near a Large-Scale Petrochemical Complex: Spatiotemporal Variation, Source Apportionment and Health Risk. Environ. Pollut. Barking Essex 1987 2018, 240, 95–104. [Google Scholar] [CrossRef]
- Saravanakumar, K.; Sivasantosh, S.; Sathiyaseelan, A.; Sankaranarayanan, A.; Naveen, K.V.; Zhang, X.; Jamla, M.; Vijayasarathy, S.; Vishnu Priya, V.; MubarakAli, D.; et al. Impact of Benzo[a]Pyrene with Other Pollutants Induce the Molecular Alternation in the Biological System: Existence, Detection, and Remediation Methods. Environ. Pollut. 2022, 304, 119207. [Google Scholar] [CrossRef] [PubMed]
- Benzene|Toxzine|ATSDR. Available online: https://www.atsdr.cdc.gov/sites/toxzine/benzene_toxzine.html (accessed on 16 June 2023).
- Duarte-Davidson, R. Benzene in the Environment: An Assessment of the Potential Risks to the Health of the Population. Occup. Environ. Med. 2001, 58, 2–13. [Google Scholar] [CrossRef]
- Rowe, B.L.; Toccalino, P.L.; Moran, M.J.; Zogorski, J.S.; Price, C.V. Occurrence and Potential Human-Health Relevance of Volatile Organic Compounds in Drinking Water from Domestic Wells in the United States. Environ. Health Perspect. 2007, 115, 1539–1546. [Google Scholar] [CrossRef]
- Zhong, L.; Batterman, S.; Milando, C.W. VOC Sources and Exposures in Nail Salons: A Pilot Study in Michigan, USA. Int. Arch. Occup. Environ. Health 2019, 92, 141–153. [Google Scholar] [CrossRef]
- Lamplugh, A.; Harries, M.; Xiang, F.; Trinh, J.; Hecobian, A.; Montoya, L.D. Occupational Exposure to Volatile Organic Compounds and Health Risks in Colorado Nail Salons. Environ. Pollut. Barking Essex 1987 2019, 249, 518–526. [Google Scholar] [CrossRef]
- Chong, N.S.; Abdulramoni, S.; Patterson, D.; Brown, H. Releases of Fire-Derived Contaminants from Polymer Pipes Made of Polyvinyl Chloride. Toxics 2019, 7, 57. [Google Scholar] [CrossRef]
- U.S. Food&Drug Administration. FDA Alerts Drug Manufacturers to the Risk of Benzene Contamination in Certain Drugs; FDA: Silver Spring, MD, USA, 2022. [Google Scholar]
- Pal, V.K.; Lee, S.; Naidu, M.; Lee, C.; Kannan, K. Occurrence of and Dermal Exposure to Benzene, Toluene and Styrene Found in Hand Sanitizers from the United States. Environ. Int. 2022, 167, 107449. [Google Scholar] [CrossRef]
- Pal, V.K.; Lee, S.; Kannan, K. Occurrence of and Dermal Exposure to Benzene, Toluene and Styrene in Sunscreen Products Marketed in the United States. Sci. Total Environ. 2023, 888, 164196. [Google Scholar] [CrossRef]
- Nakai, J.S.; Chu, I.; Li-Muller, A.; Aucoin, R. Effect of Environmental Conditions on the Penetration of Benzene through Human Skin. J. Toxicol. Environ. Health 1997, 51, 447–462. [Google Scholar] [CrossRef]
- U.S. Environmental Protection Agency. Benzene CASRN 71-43-2|DTXSID3039242|IRIS|US EPA, ORD. Available online: https://cfpub.epa.gov/ncea/iris2/chemicallanding.cfm?substance_nmbr=276#:~:text=Benzene%20is%20classified%20as%20a,Risk%20Assessment%20Guidelines%20of%201986 (accessed on 21 July 2023).
- Wen, Q.; Boshier, P.; Myridakis, A.; Belluomo, I.; Hanna, G.B. Urinary Volatile Organic Compound Analysis for the Diagnosis of Cancer: A Systematic Literature Review and Quality Assessment. Metabolites 2020, 11, 17. [Google Scholar] [CrossRef] [PubMed]
- Hanna, G.B.; Boshier, P.R.; Markar, S.R.; Romano, A. Accuracy and Methodologic Challenges of Volatile Organic Compound-Based Exhaled Breath Tests for Cancer Diagnosis: A Systematic Review and Meta-Analysis. JAMA Oncol. 2019, 5, e182815. [Google Scholar] [CrossRef]
- Rana, I.; Dahlberg, S.; Steinmaus, C.; Zhang, L. Benzene Exposure and Non-Hodgkin Lymphoma: A Systematic Review and Meta-Analysis of Human Studies. Lancet Planet. Health 2021, 5, e633–e643. [Google Scholar] [CrossRef]
- Odutola, M.K.; Benke, G.; Fritschi, L.; Giles, G.G.; van Leeuwen, M.T.; Vajdic, C.M. A Systematic Review and Meta-Analysis of Occupational Exposures and Risk of Follicular Lymphoma. Environ. Res. 2021, 197, 110887. [Google Scholar] [CrossRef] [PubMed]
- D’Andrea, M.A.; Reddy, G.K. Health Risks Associated With Benzene Exposure in Children: A Systematic Review. Glob. Pediatr. Health 2018, 5, 2333794X18789275. [Google Scholar] [CrossRef]
- Smargiassi, A.; Goldberg, M.S.; Wheeler, A.J.; Plante, C.; Valois, M.-F.; Mallach, G.; Kauri, L.M.; Shutt, R.; Bartlett, S.; Raphoz, M.; et al. Associations between Personal Exposure to Air Pollutants and Lung Function Tests and Cardiovascular Indices among Children with Asthma Living near an Industrial Complex and Petroleum Refineries. Environ. Res. 2014, 132, 38–45. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.-H.; Kim, J.H.; Lee, B.-E.; Hong, Y.-C. Urinary Benzene Metabolite and Insulin Resistance in Elderly Adults. Sci. Total Environ. 2014, 482–483, 260–268. [Google Scholar] [CrossRef] [PubMed]
- Cordiano, R.; Papa, V.; Cicero, N.; Spatari, G.; Allegra, A.; Gangemi, S. Effects of Benzene: Hematological and Hypersensitivity Manifestations in Resident Living in Oil Refinery Areas. Toxics 2022, 10, 678. [Google Scholar] [CrossRef] [PubMed]
- Slama, R.; Thiebaugeorges, O.; Goua, V.; Aussel, L.; Sacco, P.; Bohet, A.; Forhan, A.; Ducot, B.; Annesi-Maesano, I.; Heinrich, J.; et al. Maternal Personal Exposure to Airborne Benzene and Intrauterine Growth. Environ. Health Perspect. 2009, 117, 1313–1321. [Google Scholar] [CrossRef] [PubMed]
- Cassidy-Bushrow, A.E.; Burmeister, C.; Lamerato, L.; Lemke, L.D.; Mathieu, M.; O’Leary, B.F.; Sperone, F.G.; Straughen, J.K.; Reiners, J.J. Prenatal Airshed Pollutants and Preterm Birth in an Observational Birth Cohort Study in Detroit, Michigan, USA. Environ. Res. 2020, 189, 109845. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.; Singh, A.B.; Arora, T.; Singh, S.; Singh, R. Critical Review on Emerging Health Effects Associated with the Indoor Air Quality and Its Sustainable Management. Sci. Total Environ. 2023, 872, 162163. [Google Scholar] [CrossRef]
- Klepeis, N.E.; Nelson, W.C.; Ott, W.R.; Robinson, J.P.; Tsang, A.M.; Switzer, P.; Behar, J.V.; Hern, S.C.; Engelmann, W.H. The National Human Activity Pattern Survey (NHAPS): A Resource for Assessing Exposure to Environmental Pollutants. J. Expo. Anal. Environ. Epidemiol. 2001, 11, 231–252. [Google Scholar] [CrossRef]
- Chatterjee, N.; Kim, C.; Im, J.; Kim, S.; Choi, J. Mixture and Individual Effects of Benzene, Toluene, and Formaldehyde in Zebrafish (Danio Rerio) Development: Metabolomics, Epigenetics, and Behavioral Approaches. Environ. Toxicol. Pharmacol. 2023, 97, 104031. [Google Scholar] [CrossRef]
- Marchini, S.; Tosato, M.L.; Norberg-King, T.J.; Hammermeister, D.E.; Hoglund, M.D. Lethal and Sublethal Toxicity of Benzene Derivatives to the Fathead Minnow, Using a Short-Term Test. Environ. Toxicol. Chem. 1992, 11, 187–195. [Google Scholar] [CrossRef]
- Philibert, D.A.; Philibert, C.P.; Lewis, C.; Tierney, K.B. Comparison of Diluted Bitumen (Dilbit) and Conventional Crude Oil Toxicity to Developing Zebrafish. Environ. Sci. Technol. 2016, 50, 6091–6098. [Google Scholar] [CrossRef]
- Bérubé, R.; Gauthier, C.; Bourdin, T.; Bouffard, M.; Triffault-Bouchet, G.; Langlois, V.S.; Couture, P. Lethal and Sublethal Effects of Diluted Bitumen and Conventional Oil on Fathead Minnow (Pimephales promelas) Larvae Exposed during Their Early Development. Aquat. Toxicol. 2021, 237, 105884. [Google Scholar] [CrossRef]
- Chatterjee, S.; Behnam Azad, B.; Nimmagadda, S. The Intricate Role of CXCR4 in Cancer. Adv. Cancer Res. 2014, 124, 31–82. [Google Scholar] [CrossRef]
- He, J.; Zang, S.; Liu, N.; Ji, M.; Ma, D.; Ji, C. Epimedium Polysaccharides Attenuates Hematotoxicity by Reducing Oxidative Stress and Enhancing Immune Function in Mice Model of Benzene-Induced Bone Marrow Failure. Biomed. Pharmacother. 2020, 125, 109908. [Google Scholar] [CrossRef]
- Qiao, Y.; Hu, H.; Zhao, Y.; Jin, M.; Yang, D.; Yin, J.; Wu, P.; Liu, W.; Li, J. Benzene Induces Spleen Injury through the B Cell Receptor Signaling Pathway. Ecotoxicol. Environ. Saf. 2023, 257, 114924. [Google Scholar] [CrossRef]
- Zhao, J.; Sui, P.; Wu, B.; Chen, A.; Lu, Y.; Hou, F.; Cheng, X.; Cui, S.; Song, J.; Huang, G.; et al. Benzene Induces Rapid Leukemic Transformation after Prolonged Hematotoxicity in a Murine Model. Leukemia 2021, 35, 595–600. [Google Scholar] [CrossRef]
- Badham, H.J.; Winn, L.M. In Utero Exposure to Benzene Disrupts Fetal Hematopoietic Progenitor Cell Growth via Reactive Oxygen Species. Toxicol. Sci. 2010, 113, 207–215. [Google Scholar] [CrossRef]
- Varona, A.; Echevarria, E.; Irazusta, J.; Serrano, R.; Gil, J.; Casis, L. Effects of Acute Benzene Exposure on Brain Enkephalin Immunostaining and Degradation. Neurotoxicol. Teratol. 1998, 20, 611–616. [Google Scholar] [CrossRef]
- Koshko, L.; Scofield, S.; Debarba, L.; Stilgenbauer, L.; Sacla, M.; Fakhoury, P.; Jayarathne, H.; Perez-Mojica, J.E.; Griggs, E.; Lempradl, A.; et al. Prenatal Benzene Exposure Alters Offspring Hypothalamic Development Predisposing to Metabolic Disease in Later Life. bioRxiv 2023. [Google Scholar] [CrossRef] [PubMed]
- Sun, R.; Liu, M.; Xu, K.; Pu, Y.; Huang, J.; Liu, J.; Zhang, J.; Yin, L.; Pu, Y. Ferroptosis Is Involved in the Benzene-Induced Hematotoxicity in Mice via Iron Metabolism, Oxidative Stress and NRF2 Signaling Pathway. Chem. Biol. Interact. 2022, 362, 110004. [Google Scholar] [CrossRef] [PubMed]
- Badham, H.J.; Renaud, S.J.; Wan, J.; Winn, L.M. Benzene-Initiated Oxidative Stress: Effects on Embryonic Signaling Pathways. Chem. Biol. Interact. 2010, 184, 218–221. [Google Scholar] [CrossRef]
- Weisel, C.P. Benzene Exposure: An Overview of Monitoring Methods and Their Findings. Chem. Biol. Interact. 2010, 184, 58–66. [Google Scholar] [CrossRef]
- Howe, K.; Clark, M.D.; Torroja, C.F.; Torrance, J.; Berthelot, C.; Muffato, M.; Collins, J.E.; Humphray, S.; McLaren, K.; Matthews, L.; et al. The Zebrafish Reference Genome Sequence and Its Relationship to the Human Genome. Nature 2013, 496, 498–503. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.-C.; Blount, J.R.; Haimbaugh, A.; Heldman, S.; Shields, J.N.; Baker, T.R. Evaluating Phenotypic and Transcriptomic Responses Induced by Low-Level VOCs in Zebrafish: Benzene as an Example. Toxics 2022, 10, 351. [Google Scholar] [CrossRef] [PubMed]
- Lex, A.; Gehlenborg, N.; Strobelt, H.; Vuillemot, R.; Pfister, H. UpSet: Visualization of Intersecting Sets. IEEE Trans. Vis. Comput. Graph. 2014, 20, 1983–1992. [Google Scholar] [CrossRef] [PubMed]
- Conway, J.R.; Lex, A.; Gehlenborg, N. UpSetR: An R Package for the Visualization of Intersecting Sets and Their Properties. Bioinformatics 2017, 33, 2938–2940. [Google Scholar] [CrossRef] [PubMed]
- Baron, J.A.; Nichols, H.B.; Anderson, C.; Safe, S. Cigarette Smoking and Estrogen-Related Cancer. Cancer Epidemiol. Biomark. Prev. Publ. Am. Assoc. Cancer Res. Cosponsored Am. Soc. Prev. Oncol. 2021, 30, 1462–1471. [Google Scholar] [CrossRef]
- Wolff, M.S.; Collman, G.W.; Barrett, J.C.; Huff, J. Breast Cancer and Environmental Risk Factors: Epidemiological and Experimental Findings. Annu. Rev. Pharmacol. Toxicol. 1996, 36, 573–596. [Google Scholar] [CrossRef]
- Costantini, A.S.; Gorini, G.; Consonni, D.; Miligi, L.; Giovannetti, L.; Quinn, M. Exposure to Benzene and Risk of Breast Cancer among Shoe Factory Workers in Italy. Tumori 2009, 95, 8–12. [Google Scholar] [CrossRef]
- Fenga, C.; Gangemi, S.; Costa, C. Benzene Exposure Is Associated with Epigenetic Changes (Review). Mol. Med. Rep. 2016, 13, 3401–3405. [Google Scholar] [CrossRef]
- Maronpot, R.R. Ovarian Toxicity and Carcinogenicity in Eight Recent National Toxicology Program Studies. Environ. Health Perspect. 1987, 73, 125–130. [Google Scholar] [CrossRef]
- Bahadar, H.; Mostafalou, S.; Abdollahi, M. Current Understandings and Perspectives on Non-Cancer Health Effects of Benzene: A Global Concern. Toxicol. Appl. Pharmacol. 2014, 276, 83–94. [Google Scholar] [CrossRef]
- Danysh, H.E.; Mitchell, L.E.; Zhang, K.; Scheurer, M.E.; Lupo, P.J. Traffic-Related Air Pollution and the Incidence of Childhood Central Nervous System Tumors: Texas, 2001–2009. Pediatr. Blood Cancer 2015, 62, 1572–1578. [Google Scholar] [CrossRef]
- Mazzei, A.; Konstantinoudis, G.; Kreis, C.; Diezi, M.; Ammann, R.A.; Zwahlen, M.; Kühni, C.; Spycher, B.D. Childhood Cancer and Residential Proximity to Petrol Stations: A Nationwide Registry-Based Case-Control Study in Switzerland and an Updated Meta-Analysis. Int. Arch. Occup. Environ. Health 2022, 95, 927–938. [Google Scholar] [CrossRef]
- Geist, C.R.; Drew, K.L.; Schoenheit, C.M.; Praed, J.E. Learning Impairments Following Postnatal Exposure to Benzene. Percept. Mot. Skills 1983, 57, 1083–1086. [Google Scholar] [CrossRef]
- Lo Pumo, R.; Bellia, M.; Nicosia, A.; Micale, V.; Drago, F. Long-Lasting Neurotoxicity of Prenatal Benzene Acute Exposure in Rats. Toxicology 2006, 223, 227–234. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Casanova, I.; Stein, A.D.; Barraza-Villarreal, A.; Feregrino, R.G.; DiGirolamo, A.; Hernandez-Cadena, L.; Rivera, J.A.; Romieu, I.; Ramakrishnan, U. Prenatal Exposure to Environmental Pollutants and Child Development Trajectories through 7 Years. Int. J. Hyg. Environ. Health 2018, 221, 616–622. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Hsiao, J.-H.T.; Paxinos, G.; Halliday, G.M.; Kim, W.S. ABCA5 Regulates Amyloid-β Peptide Production and Is Associated with Alzheimer’s Disease Neuropathology. J. Alzheimer’s Dis. JAD 2015, 43, 857–869. [Google Scholar] [CrossRef] [PubMed]
- Bu, P.; Xiao, Y.; Hu, S.; Jiang, X.; Tan, C.; Qiu, M.; Huang, W.; Li, M.; Li, Q.; Qin, C. Identification of ABCA5 among ATP-Binding Cassette Transporter Family as a New Biomarker for Colorectal Cancer. J. Oncol. 2022, 2022, 3399311. [Google Scholar] [CrossRef]
- Talibov, M.; Sormunen, J.; Hansen, J.; Kjaerheim, K.; Martinsen, J.-I.; Sparen, P.; Tryggvadottir, L.; Weiderpass, E.; Pukkala, E. Benzene Exposure at Workplace and Risk of Colorectal Cancer in Four Nordic Countries. Cancer Epidemiol. 2018, 55, 156–161. [Google Scholar] [CrossRef]
- Papuc, S.M.; Abela, L.; Steindl, K.; Begemann, A.; Simmons, T.L.; Schmitt, B.; Zweier, M.; Oneda, B.; Socher, E.; Crowther, L.M.; et al. The Role of Recessive Inheritance in Early-Onset Epileptic Encephalopathies: A Combined Whole-Exome Sequencing and Copy Number Study. Eur. J. Hum. Genet. EJHG 2019, 27, 408–421. [Google Scholar] [CrossRef]
- Reuter, M.S.; Tawamie, H.; Buchert, R.; Hosny Gebril, O.; Froukh, T.; Thiel, C.; Uebe, S.; Ekici, A.B.; Krumbiegel, M.; Zweier, C.; et al. Diagnostic Yield and Novel Candidate Genes by Exome Sequencing in 152 Consanguineous Families With Neurodevelopmental Disorders. JAMA Psychiatry 2017, 74, 293–299. [Google Scholar] [CrossRef]
- Yang, S.H.; Shrivastav, A.; Kosinski, C.; Sharma, R.K.; Chen, M.-H.; Berthiaume, L.G.; Peters, L.L.; Chuang, P.-T.; Young, S.G.; Bergo, M.O. N-Myristoyltransferase 1 Is Essential in Early Mouse Development. J. Biol. Chem. 2005, 280, 18990–18995. [Google Scholar] [CrossRef]
- Quintero-Rivera, F.; Leach, N.T.; de la Chapelle, A.; Gusella, J.F.; Morton, C.C.; Harris, D.J. Is the Disruption of an N-Myristoyltransferase (NMT2) Associated with Hypoplastic Testes? Am. J. Med. Genet. Part A 2007, 143A, 1796–1798. [Google Scholar] [CrossRef]
- Williams, P.A.; Krug, M.S.; McMillan, E.A.; Peake, J.D.; Davis, T.L.; Cocklin, S.; Strochlic, T.I. Phosphorylation of the RNA-Binding Protein Dazl by MAPKAP Kinase 2 Regulates Spermatogenesis. Mol. Biol. Cell 2016, 27, 2341–2350. [Google Scholar] [CrossRef] [PubMed]
- Huo, M.; Han, H.; Sun, Z.; Lu, Z.; Yao, X.; Wang, S.; Wang, J. Role of IL-17 Pathways in Immune Privilege: A RNA Deep Sequencing Analysis of the Mice Testis Exposure to Fluoride. Sci. Rep. 2016, 6, 32173. [Google Scholar] [CrossRef]
- Farrugia, A.J.; Rodríguez, J.; Orgaz, J.L.; Lucas, M.; Sanz-Moreno, V.; Calvo, F. CDC42EP5/BORG3 Modulates SEPT9 to Promote Actomyosin Function, Migration, and Invasion. J. Cell Biol. 2020, 219, e201912159. [Google Scholar] [CrossRef]
- Furusato, B.; Mohamed, A.; Uhlén, M.; Rhim, J.S. CXCR4 and Cancer. Pathol. Int. 2010, 60, 497–505. [Google Scholar] [CrossRef] [PubMed]
- Yun, J.W.; Lee, S.; Ryu, D.; Park, S.; Park, W.-Y.; Joung, J.-G.; Jeong, J. Biomarkers Associated with Tumor Heterogeneity in Prostate Cancer. Transl. Oncol. 2019, 12, 43–48. [Google Scholar] [CrossRef] [PubMed]
- Diotel, N.; Vaillant, C.; Gueguen, M.-M.; Mironov, S.; Anglade, I.; Servili, A.; Pellegrini, E.; Kah, O. Cxcr4 and Cxcl12 Expression in Radial Glial Cells of the Brain of Adult Zebrafish. J. Comp. Neurol. 2010, 518, 4855–4876. [Google Scholar] [CrossRef]
- Chong, S.W.; Emelyanov, A.; Gong, Z.; Korzh, V. Expression Pattern of Two Zebrafish Genes, Cxcr4a and Cxcr4b. Mech. Dev. 2001, 109, 347–354. [Google Scholar] [CrossRef]
- Lim, Y.S.; Tang, B.L. The Evi5 Family in Cellular Physiology and Pathology. FEBS Lett. 2013, 587, 1703–1710. [Google Scholar] [CrossRef]
- Hoppenbrouwers, I.A.; Aulchenko, Y.S.; Ebers, G.C.; Ramagopalan, S.V.; Oostra, B.A.; van Duijn, C.M.; Hintzen, R.Q. EVI5 Is a Risk Gene for Multiple Sclerosis. Genes Immun. 2008, 9, 334–337. [Google Scholar] [CrossRef]
- Jacob, B.; Osato, M.; Yamashita, N.; Wang, C.Q.; Taniuchi, I.; Littman, D.R.; Asou, N.; Ito, Y. Stem Cell Exhaustion Due to Runx1 Deficiency Is Prevented by Evi5 Activation in Leukemogenesis. Blood 2010, 115, 1610–1620. [Google Scholar] [CrossRef]
- Tang, J.; Ou, J.; Xu, C.; Yi, C.; Xue, F.; Xu, L.; Lai, F.; Tang, J.; Li, S.; Kang, T.; et al. EVI5 Is a Novel Independent Prognostic Predictor in Hepatocellular Carcinoma after Radical Hepatectomy. Oncol. Rep. 2017, 38, 2251–2258. [Google Scholar] [CrossRef] [PubMed]
- Blaker-Lee, A.; Gupta, S.; McCammon, J.M.; De Rienzo, G.; Sive, H. Zebrafish Homologs of Genes within 16p11.2, a Genomic Region Associated with Brain Disorders, Are Active during Brain Development, and Include Two Deletion Dosage Sensor Genes. Dis. Model. Mech. 2012, 5, 834–851. [Google Scholar] [CrossRef] [PubMed]
- Bertrand, R.E.; Wang, J.; Xiong, K.H.; Thangavel, C.; Qian, X.; Ba-Abbad, R.; Liang, Q.; Simões, R.T.; Sampaio, S.A.M.; Carss, K.J.; et al. Ceramide Synthase TLCD3B Is a Novel Gene Associated with Human Recessive Retinal Dystrophy. Genet. Med. Off. J. Am. Coll. Med. Genet. 2021, 23, 488–497. [Google Scholar] [CrossRef]
- Gistelinck, C.; Kwon, R.Y.; Malfait, F.; Symoens, S.; Harris, M.P.; Henke, K.; Hawkins, M.B.; Fisher, S.; Sips, P.; Guillemyn, B.; et al. Zebrafish Type I Collagen Mutants Faithfully Recapitulate Human Type I Collagenopathies. Proc. Natl. Acad. Sci. USA 2018, 115, E8037–E8046. [Google Scholar] [CrossRef]
- Cole, W.G.; Dalgleish, R. Perinatal Lethal Osteogenesis Imperfecta. J. Med. Genet. 1995, 32, 284–289. [Google Scholar] [CrossRef]
- Oestreich, A.K.; DeCata, J.A.; Akers, J.D.; Phillips, C.L.; Schulz, L.C. Fecundity Is Impaired in a Mouse Model of Osteogenesis Imperfecta. Mol. Reprod. Dev. 2020, 87, 927–929. [Google Scholar] [CrossRef] [PubMed]
- Underhill, L.A.; Barbarita, C.; Collis, S.; Tucker, R.; Lechner, B.E. Association of Maternal Versus Fetal Ehlers-Danlos Syndrome Status with Poor Pregnancy Outcomes. Reprod. Sci. 2022, 29, 3459–3464. [Google Scholar] [CrossRef]
- Lind, J.; Wallenburg, H.C.S. Pregnancy and the Ehlers-Danlos Syndrome: A Retrospective Study in a Dutch Population. Acta Obstet. Gynecol. Scand. 2002, 81, 293–300. [Google Scholar] [CrossRef]
- Sorokin, Y.; Johnson, M.P.; Rogowski, N.; Richardson, D.A.; Evans, M.I. Obstetric and Gynecologic Dysfunction in the Ehlers-Danlos Syndrome. J. Reprod. Med. 1994, 39, 281–284. [Google Scholar]
- Castori, M.; Morlino, S.; Dordoni, C.; Celletti, C.; Camerota, F.; Ritelli, M.; Morrone, A.; Venturini, M.; Grammatico, P.; Colombi, M. Gynecologic and Obstetric Implications of the Joint Hypermobility Syndrome (a.k.a. Ehlers–Danlos Syndrome Hypermobility Type) in 82 Italian Patients. Am. J. Med. Genet. Part A 2012, 158A, 2176–2182. [Google Scholar] [CrossRef] [PubMed]
- Gan, Q.; Liu, Q.; Hu, X.; You, C. Collagen Type I Alpha 2 (COL1A2) Polymorphism Contributes to Intracranial Aneurysm Susceptibility: A Meta-Analysis. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 2017, 23, 3240–3246. [Google Scholar] [CrossRef]
- Seger, R.; Krebs, E.G. The MAPK Signaling Cascade. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 1995, 9, 726–735. [Google Scholar] [CrossRef]
- Paton, E.L.; Turner, J.A.; Schlaepfer, I.R. Overcoming Resistance to Therapies Targeting the MAPK Pathway in BRAF-Mutated Tumours. J. Oncol. 2020, 2020, 1079827. [Google Scholar] [CrossRef]
- Koedoot, E.; Fokkelman, M.; Rogkoti, V.-M.; Smid, M.; van de Sandt, I.; de Bont, H.; Pont, C.; Klip, J.E.; Wink, S.; Timmermans, M.A.; et al. Uncovering the Signaling Landscape Controlling Breast Cancer Cell Migration Identifies Novel Metastasis Driver Genes. Nat. Commun. 2019, 10, 2983. [Google Scholar] [CrossRef] [PubMed]
- Koshko, L.; Debarba, L.K.; Sacla, M.; de Lima, J.B.M.; Didyuk, O.; Fakhoury, P.; Sadagurski, M. In Utero Maternal Benzene Exposure Predisposes to the Metabolic Imbalance in the Offspring. Toxicol. Sci. Off. J. Soc. Toxicol. 2021, 180, 252–261. [Google Scholar] [CrossRef]
- Debarba, L.K.; Mulka, A.; Lima, J.B.M.; Didyuk, O.; Fakhoury, P.; Koshko, L.; Awada, A.A.; Zhang, K.; Klueh, U.; Sadagurski, M. Acarbose Protects from Central and Peripheral Metabolic Imbalance Induced by Benzene Exposure. Brain. Behav. Immun. 2020, 89, 87–99. [Google Scholar] [CrossRef]
- Poli, D.; Mozzoni, P.; Pinelli, S.; Cavallo, D.; Papaleo, B.; Caporossi, L. Sex Difference and Benzene Exposure: Does It Matter? Int. J. Environ. Res. Public. Health 2022, 19, 2339. [Google Scholar] [CrossRef]
- Goizet, C.; Depienne, C.; Benard, G.; Boukhris, A.; Mundwiller, E.; Solé, G.; Coupry, I.; Pilliod, J.; Martin-Négrier, M.-L.; Fedirko, E.; et al. REEP1 Mutations in SPG31: Frequency, Mutational Spectrum, and Potential Association with Mitochondrial Morpho-Functional Dysfunction. Hum. Mutat. 2011, 32, 1118–1127. [Google Scholar] [CrossRef]
- Kolachana, P.; Subrahmanyam, V.V.; Meyer, K.B.; Zhang, L.; Smith, M.T. Benzene and Its Phenolic Metabolites Produce Oxidative DNA Damage in HL60 Cells in Vitro and in the Bone Marrow in Vivo. Cancer Res. 1993, 53, 1023–1026. [Google Scholar]
- Murugesan, K.; Baumann, S.; Wissenbach, D.K.; Kliemt, S.; Kalkhof, S.; Otto, W.; Mögel, I.; Kohajda, T.; von Bergen, M.; Tomm, J.M. Subtoxic and Toxic Concentrations of Benzene and Toluene Induce Nrf2-Mediated Antioxidative Stress Response and Affect the Central Carbon Metabolism in Lung Epithelial Cells A549. Proteomics 2013, 13, 3211–3221. [Google Scholar] [CrossRef] [PubMed]
- Nair, R.R.; Kerätär, J.M.; Autio, K.J.; Masud, A.J.; Finnilä, M.A.J.; Autio-Harmainen, H.I.; Miinalainen, I.J.; Nieminen, P.A.; Hiltunen, J.K.; Kastaniotis, A.J. Genetic Modifications of Mecr Reveal a Role for Mitochondrial 2-Enoyl-CoA/ACP Reductase in Placental Development in Mice. Hum. Mol. Genet. 2017, 26, 2104–2117. [Google Scholar] [CrossRef]
- Chen, Z.; Leskinen, H.; Liimatta, E.; Sormunen, R.T.; Miinalainen, I.J.; Hassinen, I.E.; Hiltunen, J.K. Myocardial Overexpression of Mecr, a Gene of Mitochondrial FAS II Leads to Cardiac Dysfunction in Mouse. PLoS ONE 2009, 4, e5589. [Google Scholar] [CrossRef] [PubMed]
- Hu, R.; Xu, Y.; Han, B.; Chen, Y.; Li, W.; Guan, G.; Hu, P.; Zhou, Y.; Xu, Q.; Chen, L. MiR-202-3p Determines Embryo Viability during Mid-Blastula Transition. Front. Cell Dev. Biol. 2022, 10, 897826. [Google Scholar] [CrossRef] [PubMed]
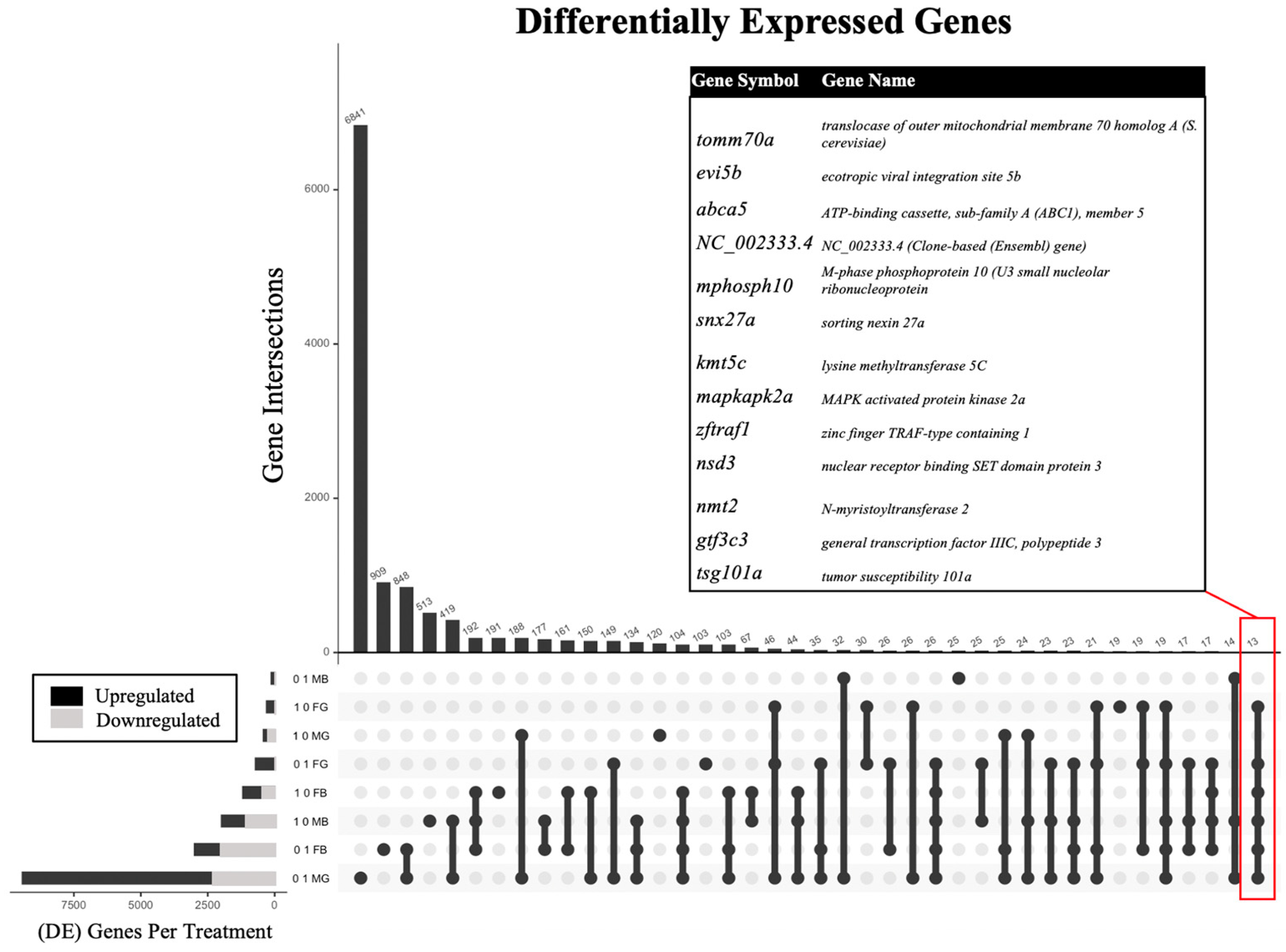

| Exposure Concentration (ppm) | Sex | Tissue | # DEGs |
|---|---|---|---|
| 0.1 | Female | Brain | 2992 |
| 0.1 | Male | Brain | 124 |
| 1.0 | Female | Brain | 1187 |
| 1.0 | Male | Brain | 1981 |
| 0.1 | Female | Gonad | 713 |
| 0.1 | Male | Gonad | 9421 |
| 1.0 | Female | Gonad | 296 |
| 1.0 | Male | Gonad | 415 |
| Gene Symbol | 0.1 ppm | 1.0 ppm | ||||||
|---|---|---|---|---|---|---|---|---|
| FB | MB | FG | MG | FB | MB | FG | MG | |
| col1a2 | −0.57 | 1.29 | 0.91 | 0.61 | ||||
| mapkapk | 0.66 | 1.22 | 0.52 | −0.51 | 0.84 | |||
| larp7 | 0.78 | 1.34 | 0.59 | 1.23 | ||||
| gtf3c3 | 0.53 | 1.39 | 0.58 | 1.28 | ||||
| evi5b | 0.87 | 1.85 | 0.84 | 0.88 | −0.82 | 1.74 | ||
| prpf4bb | 0.76 | 0.87 | 0.57 | −0.59 | 0.74 | |||
| cyhr1 | 0.64 | 1.37 | 0.62 | −0.76 | 1.01 | |||
| tlk1a | 0.98 | 1.43 | 0.69 | 0.80 | ||||
| zbtb16a | 0.93 | 0.71 | 0.76 | 1.08 | ||||
| rev31 | 1.03 | 1.35 | 0.76 | −0.68 | 0.79 | |||
| garre1 | 0.90 | 1.56 | 0.94 | −0.76 | 0.79 | |||
| nsd3 | 0.83 | 1.66 | 0.75 | −0.90 | 0.90 | |||
| nmt2 | 0.71 | 0.95 | 0.62 | −0.96 | 0.90 | |||
| phrf1 | 0.89 | 1.40 | 0.59 | −0.63 | 1.08 | |||
| snx27 | 1.32 | 1.66 | 1.08 | −0.88 | 1.06 | |||
| abca5 | 0.86 | 0.89 | 0.94 | −0.83 | 0.80 | |||
| ankrd12 | 0.74 | 1.50 | 0.58 | 1.05 | ||||
| rsf1b.1 | 0.80 | 1.36 | 0.62 | −0.56 | 1.21 | |||
| Pathway | 0.1 Female | 1.0 Female | 0.1 Male | 1.0 Male |
|---|---|---|---|---|
| Reproductive Diseases and Disorders (Gonad) | ||||
| Genital tumor | −0.439 * | 1.154 * | 1.079 * | 2.59 * |
| Pelvic tumor | −0.595 * | 1.154 * | 0.819 * | 2.581 * |
| Endometrial cancer | * | * | * | * |
| Female genital tract cancer | * | * | * | * |
| Breast or ovarian carcinoma | −1.067 * | * | 1.066 * | * |
| Breast or gynecological cancer | −1.387 * | * | 1.189 * | * |
| Mammary tumor | 1.006 * | 0.254* | 1.049 * | 1.034 * |
| Breast cancer | −1.067 * | * | 0.817 * | * |
| Morphology of testis | ns | ns | * | ns |
| Atrophy of testis | ns | ns | 0.711* | ns |
| Neurological Diseases and Disorders (Brain) | ||||
| Brain tumor | 0.644 * | 0.152 * | * | 0.378 * |
| Congenital neurological disorder | −2.456 * | −1.538 * | * | 3.619 * |
| Nervous system neoplasm | 0.689 * | 0.566 * | −0.283 * | 0.304 * |
| Central nervous system cancer | 0.875 * | 0.069 * | * | 2 * |
| Central nervous system solid tumor | 0.229 * | 0.497 * | * | 0.218 * |
| Locomotion | ns | −0.954 * | ns | 0.398 * |
| Learning | ns | 2.878 * | ns | −3.582 * |
| Proliferation of neuronal cells | ns | 1.739 * | ns | −2.969 * |
| Cognition | ns | 2.939 * | ns | −3.828 * |
| Quantity of neurons | ns | 0.443 * | ns | −1.984 * |
| Development of neurons | ns | 1.693 * | ns | −3.528 * |
| Development central nervous system | ns | 1.815 * | ns | −3.282 * |
| Memory | ns | 1.544 * | ns | −1.571 * |
| Endocrine System Diseases and Disorders (Gonad) | ||||
| Thyroid carcinoma | * | * | * | * |
| Nonpituitary endocrine tumor | 0.404 * | * | * | * |
| Endocrine gland tumor | 0.017 * | * | −0.401 * | ns |
| Endocrine carcinoma | * | * | * | * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Connell, M.L.; Wu, C.-C.; Blount, J.R.; Haimbaugh, A.; Kintzele, E.K.; Banerjee, D.; Baker, B.B.; Baker, T.R. Adult-Onset Transcriptomic Effects of Developmental Exposure to Benzene in Zebrafish (Danio rerio): Evaluating a Volatile Organic Compound of Concern. Int. J. Mol. Sci. 2023, 24, 16212. https://doi.org/10.3390/ijms242216212
Connell ML, Wu C-C, Blount JR, Haimbaugh A, Kintzele EK, Banerjee D, Baker BB, Baker TR. Adult-Onset Transcriptomic Effects of Developmental Exposure to Benzene in Zebrafish (Danio rerio): Evaluating a Volatile Organic Compound of Concern. International Journal of Molecular Sciences. 2023; 24(22):16212. https://doi.org/10.3390/ijms242216212
Chicago/Turabian StyleConnell, Mackenzie L., Chia-Chen Wu, Jessica R. Blount, Alex Haimbaugh, Emily K. Kintzele, Dayita Banerjee, Bridget B. Baker, and Tracie R. Baker. 2023. "Adult-Onset Transcriptomic Effects of Developmental Exposure to Benzene in Zebrafish (Danio rerio): Evaluating a Volatile Organic Compound of Concern" International Journal of Molecular Sciences 24, no. 22: 16212. https://doi.org/10.3390/ijms242216212
APA StyleConnell, M. L., Wu, C.-C., Blount, J. R., Haimbaugh, A., Kintzele, E. K., Banerjee, D., Baker, B. B., & Baker, T. R. (2023). Adult-Onset Transcriptomic Effects of Developmental Exposure to Benzene in Zebrafish (Danio rerio): Evaluating a Volatile Organic Compound of Concern. International Journal of Molecular Sciences, 24(22), 16212. https://doi.org/10.3390/ijms242216212





