Prenatal Metal Exposure Alters the Placental Proteome in a Sex-Dependent Manner in Extremely Low Gestational Age Newborns: Links to Gestational Age
Abstract
:1. Introduction
2. Results
2.1. Demographic Characteristics of Study Participants
2.2. Trace Elements in Umbilical Cord Tissue
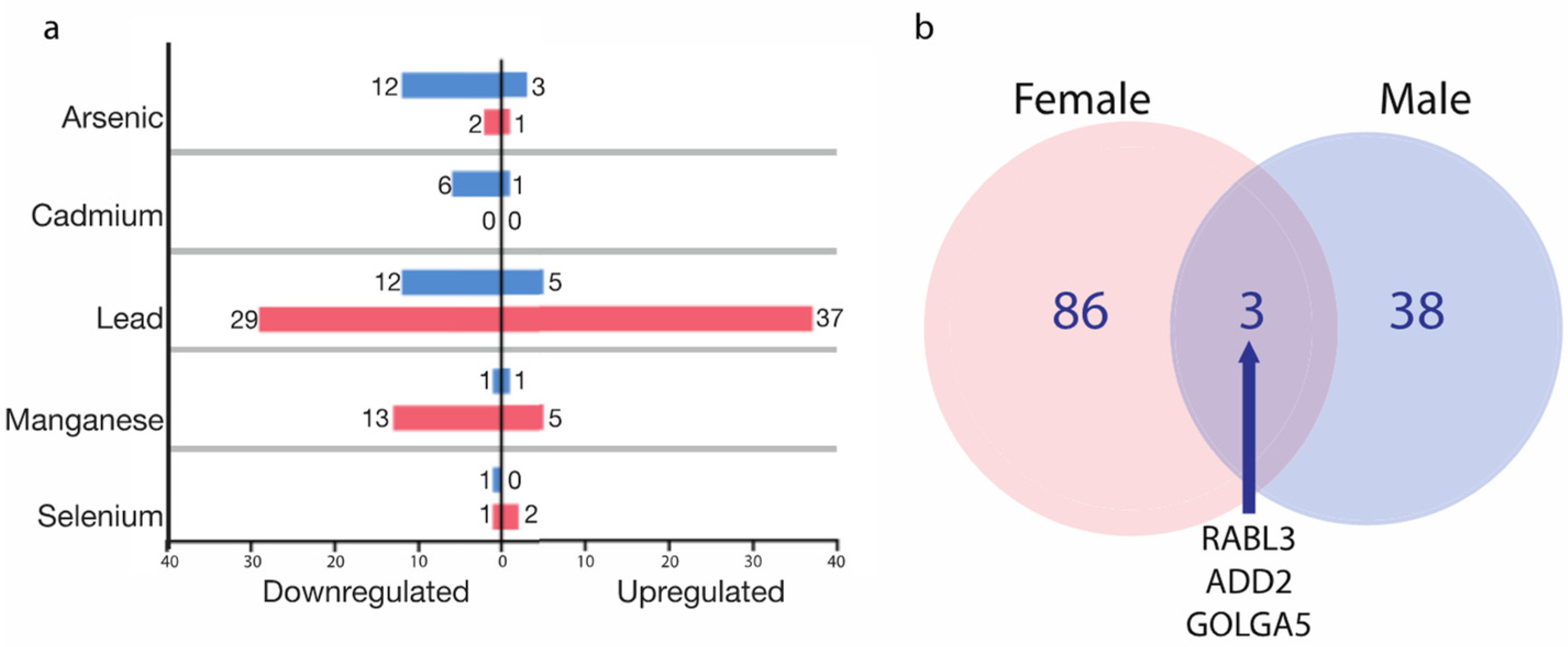
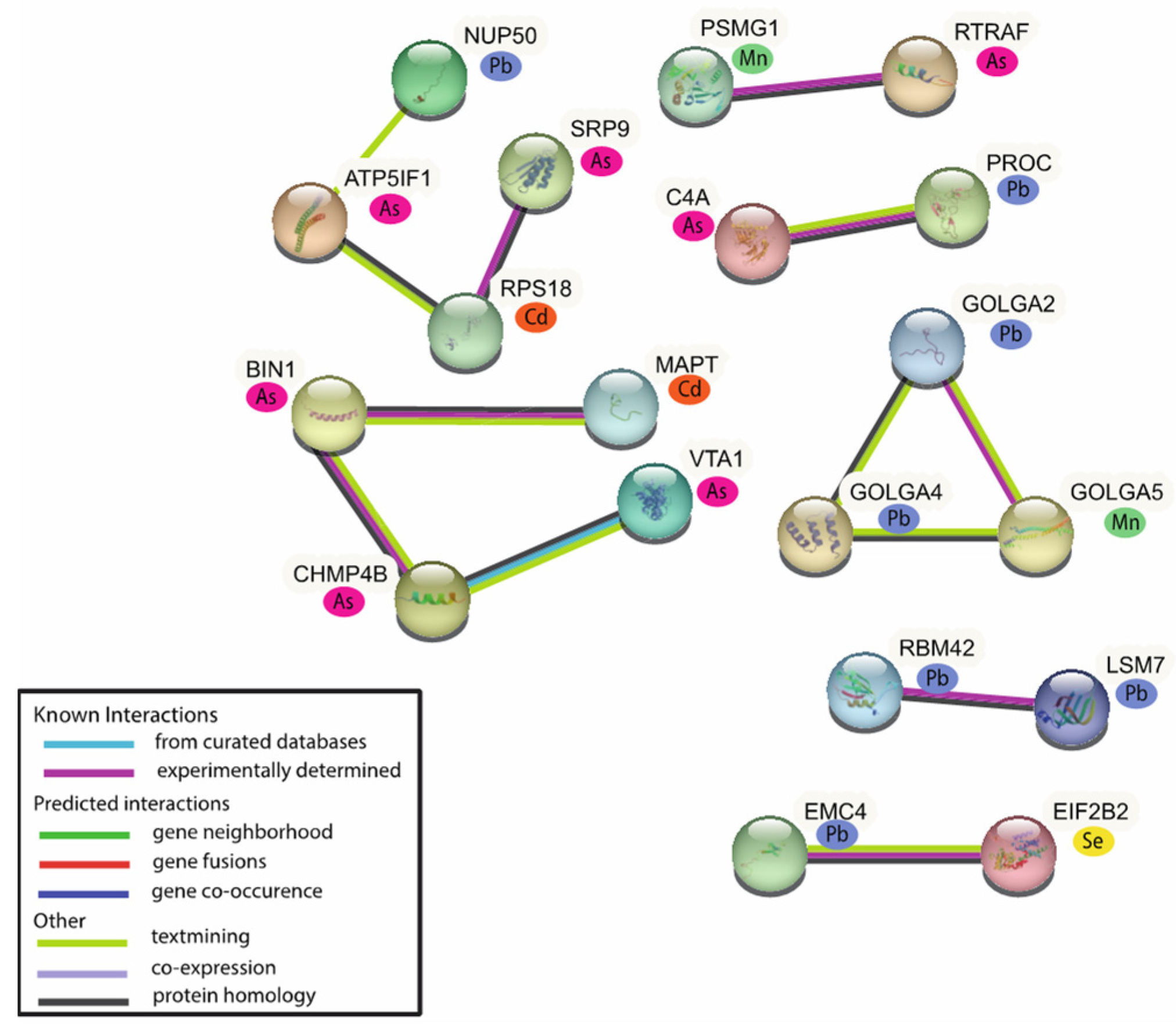
2.3. The Identification of Metal-Associated Proteins
2.4. Biological Functional Analysis of the MAPs
2.5. Upstream Regulator Analysis of the MAPs
2.6. Associations of MAPs with Placental Weight and Gestational Age
3. Discussion
4. Materials and Methods
4.1. The ELGAN Cohort
4.2. Placental Tissue Collection and Quantification of Protein Expression
4.3. Umbilical Cord Tissue Collection and Trace Element Measurements
4.4. Covariate Selection
4.5. Significance Tests across Demographic Characteristics
4.6. Evaluation of the Relationship between Trace Elements and Placental Protein Expression
4.7. Assessment of MAP Associations with Pregnancy or Neonatal Outcomes
4.8. Network Interaction Analysis
4.9. Biological Functional Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tchounwou, P.B.; Yedjou, C.G.; Patlolla, A.K.; Sutton, D.J. Heavy metal toxicity and the environment. Exp. Suppl. 2012, 101, 133–164. [Google Scholar] [PubMed]
- WHO. Arsenic. 2018. Available online: https://www.who.int/news-room/fact-sheets/detail/arsenic (accessed on 23 January 2023).
- Bulka, C.M.; Scannell, B.M.; Lombard, M.A.; Bartell, S.M.; Jones, D.K.; Bradley, P.M.; Vieira, V.M.; Silverman, D.T.; Focazio, M.; Toccalino, P.M.; et al. Arsenic in private well water and birth outcomes in the United States. Environ. Int. 2022, 163, 107176. [Google Scholar] [CrossRef] [PubMed]
- Eaves, L.A.; Keil, A.P.; Rager, J.E.; George, A.; Fry, R.C. Analysis of the novel NCWELL database highlights two decades of co-occurrence of toxic metals in North Carolina private well water: Public health and environmental justice implications. Sci. Total Environ. 2022, 812, 151479. [Google Scholar] [CrossRef] [PubMed]
- Balachandran, R.C.; Mukhopadhyay, S.; McBride, D.; Veevers, J.; Harrison, F.E.; Aschner, M.; Haynes, E.N.; Bowman, A.B. Brain manganese and the balance between essential roles and neurotoxicity. J. Biol. Chem. 2020, 295, 6312–6329. [Google Scholar] [CrossRef]
- ATSDR. Toxicological Profile for Lead; U.S. Department of Health and Human Services, Public Health Service: Atlanta, GA, USA, 2020.
- Thornton, I. Sources and pathways of cadmium in the environment. IARC Sci. Publ. 1992, 118, 149–162. [Google Scholar]
- Massanyi, P.; Massanyi, M.; Madeddu, R.; Stawarz, R.; Lukac, N. Effects of Cadmium, Lead, and Mercury on the Structure and Function of Reproductive Organs. Toxics 2020, 8, 94. [Google Scholar] [CrossRef]
- The Agency for Toxic Substances and Disease Registry (ATSDR). Toxicological Profile for Selenium; U.S. Department of Health and Human Services, Public Health Service: Atlanta, GA, USA, 2003.
- Michael, T.; Kohn, E.; Daniel, S.; Hazan, A.; Berkovitch, M.; Brik, A.; Hochwald, O.; Borenstein-Levin, L.; Moskovich, M. Prenatal exposure to heavy metal mixtures and anthropometric birth outcomes: A cross-sectional study. Environ. Health 2022, 21, 139. [Google Scholar] [CrossRef]
- Dettwiler, M.; Flynn, A.C.; Rigutto-Farebrother, J. Effects of Non-Essential “Toxic” Trace Elements on Pregnancy Outcomes: A Narrative Overview of Recent Literature Syntheses. Int. J. Environ. Res. Public Health 2023, 20, 5536. [Google Scholar] [CrossRef]
- Rahman, A.; Kumarathasan, P.; Gomes, J. Infant and mother related outcomes from exposure to metals with endocrine disrupting properties during pregnancy. Sci. Total Environ. 2016, 569, 1022–1031. [Google Scholar] [CrossRef]
- Barker, D.J. The origins of the developmental origins theory. J. Intern. Med. 2007, 261, 412–417. [Google Scholar] [CrossRef]
- Gluckman, P.D.; Hanson, M.A.; Cooper, C.; Thornburg, K.L. Effect of in utero and early-life conditions on adult health and disease. N. Engl. J. Med. 2008, 359, 61–73. [Google Scholar] [CrossRef] [PubMed]
- Rich-Edwards, J.W.; Stampfer, M.J.; Manson, J.E.; Rosner, B.; Hankinson, S.E.; Colditz, G.A.; Willett, W.C.; Hennekens, C.H. Birth weight and risk of cardiovascular disease in a cohort of women followed up since 1976. BMJ 1997, 315, 396–400. [Google Scholar] [CrossRef] [PubMed]
- Cooper, C.; Fall, C.; Egger, P.; Hobbs, R.; Eastell, R.; Barker, D. Growth in infancy and bone mass in later life. Ann. Rheum. Dis. 1997, 56, 17–21. [Google Scholar] [CrossRef] [PubMed]
- Bulka, C.M.; Eaves, L.A.; Gardner, A.J.; Parsons, P.J.; Galusha, A.L.; Roell, K.R.; Smeester, L.; O’Shea, T.M.; Fry, R.C. Prenatal exposure to multiple metallic and metalloid trace elements and the risk of bacterial sepsis in extremely low gestational age newborns: A prospective cohort study. Front. Epidemiol. 2022, 2, 27. [Google Scholar] [CrossRef] [PubMed]
- Golub, M.S.; Macintosh, M.S.; Baumrind, N. Developmental and reproductive toxicity of inorganic arsenic: Animal studies and human concerns. J. Toxicol. Environ. Health B Crit. Rev. 1998, 1, 199–241. [Google Scholar] [CrossRef] [PubMed]
- Chandravanshi, L.; Shiv, K.; Kumar, S. Developmental toxicity of cadmium in infants and children: A review. Environ. Anal. Health Toxicol. 2021, 36, e2021003. [Google Scholar] [CrossRef]
- Gidlow, D.A. Lead toxicity. Occup. Med. 2015, 65, 348–356. [Google Scholar] [CrossRef]
- Pieczynska, J.; Grajeta, H. The role of selenium in human conception and pregnancy. J. Trace Elem. Med. Biol. 2015, 29, 31–38. [Google Scholar] [CrossRef]
- Wood, R.J. Manganese and birth outcome. Nutr. Rev. 2009, 67, 416–420. [Google Scholar] [CrossRef]
- Vigeh, M.; Yokoyama, K.; Ramezanzadeh, F.; Dahaghin, M.; Fakhriazad, E.; Seyedaghamiri, Z.; Araki, S. Blood manganese concentrations and intrauterine growth restriction. Reprod. Toxicol. 2008, 25, 219–223. [Google Scholar] [CrossRef]
- Sun, H.; Chen, W.; Wang, D.; Jin, Y.; Chen, X.; Xu, Y. The effects of prenatal exposure to low-level cadmium, lead and selenium on birth outcomes. Chemosphere 2014, 108, 33–39. [Google Scholar] [CrossRef] [PubMed]
- Foster, W.G.; Cheung, A.P.; Davis, K.; Graves, G.; Jarrell, J.; Leblanc, A.; Liang, C.L.; Leech, T.; Walker, M.; Weber, J.P.; et al. Circulating metals and persistent organic pollutant concentrations in Canadian and non-Canadian born primiparous women from five Canadian centres: Results of a pilot biomonitoring study. Sci. Total Environ. 2012, 435, 326–336. [Google Scholar] [CrossRef] [PubMed]
- Caserta, D.; Graziano, A.; Lo Monte, G.; Bordi, G.; Moscarini, M. Heavy metals and placental fetal-maternal barrier: A mini-review on the major concerns. Eur. Rev. Med. Pharmacol. Sci. 2013, 17, 2198–2206. [Google Scholar]
- Burton, G.J.; Fowden, A.L. The placenta: A multifaceted, transient organ. Philos. Trans. R Soc. Lond. B Biol. Sci. 2015, 370, 20140066. [Google Scholar] [CrossRef]
- Konkel, L. Lasting Impact of an Ephemeral Organ: The Role of the Placenta in Fetal Programming. Environ. Health Perspect. 2016, 124, A124–A129. [Google Scholar] [CrossRef] [PubMed]
- Punshon, T.; Li, Z.; Marsit, C.J.; Jackson, B.P.; Baker, E.R.; Karagas, M.R. Placental Metal Concentrations in Relation to Maternal and Infant Toenails in a U.S. Cohort. Environ. Sci. Technol. 2016, 50, 1587–1594. [Google Scholar] [CrossRef] [PubMed]
- Needham, L.L.; Grandjean, P.; Heinzow, B.; Jorgensen, P.J.; Neilsen, F.; Patterson, D.G., Jr.; Sjodin, A.; Turner, W.E.; Weihe, P. Partition of environmental chemicals between maternal and fetal blood and tissues. Environ. Sci. Technol. 2011, 45, 1121–1126. [Google Scholar] [CrossRef]
- Concha, G.; Volger, G.; Lezcano, D.; Nermell, B.; Vahter, M. Exposure to inorganic arsenic metabolites during early human development. Toxicol. Sci. 1998, 44, 185–190. [Google Scholar] [CrossRef]
- Romero, R.; Kusanovic, J.P.; Chaiworapongsa, T.; Hassan, S.S. Placental bed disorders in preterm labor, preterm PROM, spontaneous abortion and abruptio placentae. Best Pract. Res. Clin. Obstet. Gynaecol. 2011, 25, 313–327. [Google Scholar] [CrossRef]
- Roberts, J.M.; Cooper, D.W. Pathogenesis and genetics of pre-eclampsia. Lancet 2001, 357, 53–56. [Google Scholar] [CrossRef]
- Cowell, W.J.; Wright, R.J. Sex-Specific Effects of Combined Exposure to Chemical and Non-chemical Stressors on Neuroendocrine Development: A Review of Recent Findings and Putative Mechanisms. Curr. Environ. Health Rep. 2017, 4, 415–425. [Google Scholar] [CrossRef] [PubMed]
- Elsmen, E.; Kallen, K.; Marsal, K.; Hellstrom-Westas, L. Fetal gender and gestational-age-related incidence of pre-eclampsia. Acta Obstet. Gynecol. Scand. 2006, 85, 1285–1291. [Google Scholar] [CrossRef] [PubMed]
- Baron-Cohen, S.; Lombardo, M.V.; Auyeung, B.; Ashwin, E.; Chakrabarti, B.; Knickmeyer, R. Why are autism spectrum conditions more prevalent in males? PLoS Biol. 2011, 9, e1001081. [Google Scholar] [CrossRef] [PubMed]
- Martin, E.; Smeester, L.; Bommarito, P.A.; Grace, M.R.; Boggess, K.; Kuban, K.; Karagas, M.R.; Marsit, C.J.; O’Shea, T.M.; Fry, R.C. Sexual epigenetic dimorphism in the human placenta: Implications for susceptibility during the prenatal period. Epigenomics 2017, 9, 267–278. [Google Scholar] [CrossRef] [PubMed]
- Eaves, L.A.; Phookphan, P.; Rager, J.E.; Bangma, J.; Santos, H.P., Jr.; Smeester, L.; O’Shea, T.M.; Fry, R.C. A role for microRNAs in the epigenetic control of sexually dimorphic gene expression in the human placenta. Epigenomics 2020, 12, 1543–1558. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Roig, M.D.; Pascal, R.; Cahuana, M.J.; Garcia-Algar, O.; Sebastiani, G.; Andreu-Fernandez, V.; Martinez, L.; Rodriguez, G.; Iglesia, I.; Ortiz-Arrabal, O.; et al. Environmental Exposure during Pregnancy: Influence on Prenatal Development and Early Life: A Comprehensive Review. Fetal. Diagn. Ther. 2021, 48, 245–257. [Google Scholar] [CrossRef]
- Dheilly, E.; Battistello, E.; Katanayeva, N.; Sungalee, S.; Michaux, J.; Duns, G.; Wehrle, S.; Sordet-Dessimoz, J.; Mina, M.; Racle, J.; et al. Cathepsin S Regulates Antigen Processing and T Cell Activity in Non-Hodgkin Lymphoma. Cancer Cell 2020, 37, 674–689. [Google Scholar] [CrossRef]
- Xiong, T.C.; Wei, M.C.; Li, F.X.; Shi, M.; Gan, H.; Tang, Z.; Dong, H.P.; Liuyu, T.; Gao, P.; Zhong, B.; et al. The E3 ubiquitin ligase ARIH1 promotes antiviral immunity and autoimmunity by inducing mono-ISGylation and oligomerization of cGAS. Nat. Commun. 2022, 13, 5973. [Google Scholar] [CrossRef]
- Fang, X.; Chen, C.; Xia, F.; Yu, Z.; Zhang, Y.; Zhang, F.; Gu, H.; Wan, J.; Zhang, X.; Weng, W.; et al. CD274 promotes cell cycle entry of leukemia-initiating cells through JNK/Cyclin D2 signaling. J. Hematol. Oncol. 2016, 9, 124. [Google Scholar] [CrossRef]
- Sia, D.; Jiao, Y.; Martinez-Quetglas, I.; Kuchuk, O.; Villacorta-Martin, C.; Castro d Moura, M.; Putra, J.; Camprecios, G.; Bassaganyas, L.; Akers, N.; et al. Identification of an Immune-specific Class of Hepatocellular Carcinoma, Based on Molecular Features. Gastroenterology 2017, 153, 812–826. [Google Scholar] [CrossRef]
- Goenka, S.; Kaplan, M.H. Transcriptional regulation by STAT6. Immunol. Res. 2011, 50, 87–96. [Google Scholar] [CrossRef]
- Nissim, S.; Leshchiner, I.; Mancias, J.D.; Greenblat, M.B.; Maertens, O.; Cassa, C.A.; Rosenfeld, J.A.; Cox, A.G.; Hedgepeth, J.; Wucherpfennig, J.I.; et al. Mutations in RABL3 alter KRAS prenylation and are associated with hereditary pancreatic cancer. Nat. Genet. 2019, 51, 1308–1314. [Google Scholar] [CrossRef]
- Khan, A.A.; Hanada, T.; Mohseni, M.; Jeong, J.J.; Zeng, L.; Gaetani, M.; Li, D.; Reed, B.C.; Speicher, D.W.; Chishti, A.H. Dematin and adducin provide a novel link between the spectrin cytoskeleton and human erythrocyte membrane by directly interacting with glucose transporter-1. J. Biol. Chem. 2008, 283, 14600–14609. [Google Scholar] [CrossRef]
- Diao, A.; Rahman, D.; Pappin, D.J.; Lucocq, J.; Lowe, M. The coiled-coil membrane protein golgin-84 is a novel rab effector required for Golgi ribbon formation. J. Cell. Biol. 2003, 160, 201–212. [Google Scholar] [CrossRef] [PubMed]
- Eaves, L.A.; Bulka, C.M.; Rager, J.E.; Gardner, A.J.; Galusha, A.L.; Parsons, P.J.; O’Shea, T.M.; Fry, R.C. Metal mixtures modeling identifies birth weight-associated gene networks in the placentas of children born extremely preterm. Chemosphere 2023, 313, 137469. [Google Scholar] [CrossRef] [PubMed]
- Signes-Pastor, A.J.; Doherty, B.T.; Romani, M.E.; Gleason, K.M.; Gui, J.; Baker, E.; Karagas, M.R. Prenatal exposure to metal mixture and sex-specific birth outcomes in the New Hampshire Birth Cohort Study. Environ. Epidemiol. 2019, 3, e068. [Google Scholar] [CrossRef]
- Bunn, T.L.; Parson, P.J.; Kao, E.; Dietert, R.R. Exposure to lead during critical windows of embryonic development: Differential immunotoxic outcome based on stage of exposure and gender. Toxicol. Sci. 2001, 64, 57–66. [Google Scholar] [CrossRef] [PubMed]
- Chiba, S.; Takeshita, K.; Imai, Y.; Kumano, K.; Kurokawa, M.; Masuka, S.; Shimizu, K.; Nakamura, S.; Ruddle, F.H.; Hirai, H. Homeoprotein DLX-1 interacts with Smad4 and blocks a signaling pathway from activin A in hematopoietic cells. Proc. Natl. Acad. Sci. USA 2003, 100, 15577–15582. [Google Scholar] [CrossRef]
- Lo, H.W.; Zhu, H.; Cao, X.; Aldrich, A.; Ali-Osman, F. A novel splice variant of GLI1 that promotes glioblastoma cell migration and invasion. Cancer Res. 2009, 69, 6790–6798. [Google Scholar] [CrossRef]
- Mastrangelo, E.; Milani, M. Role and inhibition of GLI1 protein in cancer. Lung Cancer 2018, 9, 35–43. [Google Scholar] [CrossRef]
- Vana, A.C.; Lucchinetti, C.F.; Le, T.Q.; Armstrong, R.C. Myelin transcription factor 1 (Myt1) expression in demyelinated lesions of rodent and human CNS. Glia 2007, 55, 687–697. [Google Scholar] [CrossRef] [PubMed]
- Craciunas, L.; Pickering, O.; Chi, J.; Choudhary, M.; Zurauskiene, J.; Coomarasamy, A. The transcriptomic profile of endometrial receptivity in recurrent miscarriage. Eur. J. Obstet. Gynecol. Reprod. Biol. 2021, 261, 211–216. [Google Scholar] [CrossRef]
- Lefranc, M.P. Immunoglobulin and T Cell Receptor Genes: IMGT((R)) and the Birth and Rise of Immunoinformatics. Front. Immunol. 2014, 5, 22. [Google Scholar] [CrossRef] [PubMed]
- Langnaese, K.; Kloos, D.U.; Wehnert, M.; Seidel, B.; Wieacher, P. Expression pattern and further characterization of human MAGED2 and identification of rodent orthologues. Cytogenet. Cell Genet. 2001, 94, 233–240. [Google Scholar] [CrossRef]
- Lefranc, M.P.; Lefranc, G. Human Gm, Km, and Am allotypes and their molecular characterization: A remarkable demonstration of polymorphism. Methods Mol. Biol. 2012, 882, 635–680. [Google Scholar]
- Sanders, A.P.; Flood, K.; Chiang, S.; Herring, A.H.; Wolf, L.; Fry, R.C. Towards prenatal biomonitoring in North Carolina: Assessing arsenic, cadmium, mercury, and lead levels in pregnant women. PLoS ONE 2012, 7, e31354. [Google Scholar] [CrossRef] [PubMed]
- Kozikowska, I.; Binkowski, L.J.; Szczepanska, K.; Slawska, H.; Miszczuk, K.; Sliwinska, M.; Laciak, T.; Stawarz, R. Mercury concentrations in human placenta, umbilical cord, cord blood and amniotic fluid and their relations with body parameters of newborns. Environ. Pollut. 2013, 182, 256–262. [Google Scholar] [CrossRef] [PubMed]
- Sakamoto, M.; Yasutake, A.L.; Domingo, J.L.; Chan, H.M.; Kubota, M.; Murata, K. Relationships between trace element concentrations in chorionic tissue of placenta and umbilical cord tissue: Potential use as indicators for prenatal exposure. Environ. Int. 2013, 60, 106–111. [Google Scholar] [CrossRef]
- Ni, W.; Yang, W.; Yu, J.; Li, Z.; Jin, L.; Liu, J.; Zhang, Y.; Wang, L.; Ren, A. Umbilical Cord Concentrations of Selected Heavy Metals and Risk for Orofacial Clefts. Environ. Sci. Technol. 2018, 52, 10787–10795. [Google Scholar] [CrossRef]
- Mehri, A. Trace Elements in Human Nutrition (II)—An Update. Int. J. Prev. Med. 2020, 11, 2. [Google Scholar]
- Fisher, R.M.; Gupta, V. Heavy Metals. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- O’Shea, T.M.; Allred, E.N.; Dammann, O.; Hirtz, D.; Kuban, K.C.; Peneth, N.; Leviton, A.; ELGAN Study Investigators. The ELGAN study of the brain and related disorders in extremely low gestational age newborns. Early Hum. Dev. 2009, 85, 719–725. [Google Scholar] [CrossRef]
- Addo, K.A.; Bulka, C.; Dhingra, R.; Santos, H.P., Jr.; Smeester, L.O.; Shea, T.M.; Fry, R.C. Acetaminophen use during pregnancy and DNA methylation in the placenta of the extremely low gestational age newborn (ELGAN) cohort. Environ. Epigenet. 2019, 5, dvz010. [Google Scholar] [CrossRef]
- Onderdonk, A.B.; Delaney, M.L.; DuBois, A.M.; Allred, E.N.; Leviton, A.; ELGAN Study Investigators. Detection of bacteria in placental tissues obtained from extremely low gestational age neonates. Am. J. Obstet. Gynecol. 2008, 198, 110.e1–110.e7. [Google Scholar] [CrossRef] [PubMed]
- Huber, W.; von Heydebreck, A.; Sultmann, H.; Poustka, A.; Vingron, M. Variance stabilization applied to microarray data calibration and to the quantification of differential expression. Bioinformatics 2002, 18, S96–S104. [Google Scholar] [CrossRef] [PubMed]
- Stekhoven, D.J.; Buhlmann, P. MissForest—Non-parametric missing value imputation for mixed-type data. Bioinformatics 2012, 28, 112–118. [Google Scholar] [CrossRef] [PubMed]
- Leek, J.T.; Johson, W.E.; Parker, H.S.; Fertig, E.J.; Jaffe, A.E.; Zhang, Y.; Storey, J.D.; Torres, L.C. Sva: Surrogate Variable Analysis, version 3.46.0; R package, 2022. [Google Scholar]
- Rager, J.E.; Clark, J.; Eaves, L.E.; Avula, V.; Niehoff, N.M.; Kim, Y.H.; Jaspers, I.; Gilmour, M.I. Mixtures modeling identifies chemical inducers versus repressors of toxicity associated with wildfire smoke. Sci. Total Environ. 2021, 775, 145759. [Google Scholar] [CrossRef] [PubMed]
- Rager, J.E.; Bailey, K.A.L.; Seester, L.; Miller, S.K.; Parker, J.S.; Laine, J.E.; Drobna, Z.; Currier, J.; Douillet, C.; Olshan, A.F.; et al. Prenatal arsenic exposure and the epigenome: Altered microRNAs associated with innate and adaptive immune signaling in newborn cord blood. Environ. Mol. Mutagen 2014, 55, 196–208. [Google Scholar] [CrossRef]
- Team, R.C. A Language and Environment for Statistical Computing. R Foundation for Statistical Computing. 2021. Available online: https://www.R-project.org/ (accessed on 1 March 2023).
- Textor, J.; van der Zander, B.; Gilthorpe, M.S.; Liskiewicz, M.L.; Ellison, G.T. Robust causal inference using directed acyclic graphs: The R package ‘dagitty’. Int. J. Epidemiol. 2016, 45, 1887–1894. [Google Scholar] [CrossRef]
- Jones, D.H.; Yu, X.; Guo, Q.; Duan, X.; Jia, C. Racial Disparities in the Heavy Metal Contamination of Urban Soil in the Southeastern United States. Int. J. Environ. Res. Public Health 2022, 19, 1105. [Google Scholar] [CrossRef]
- Gee, G.C.; Payne-Sturges, D.C. Environmental health disparities: A framework integrating psychosocial and environmental concepts. Environ. Health Perspect. 2004, 112, 1645–1653. [Google Scholar] [CrossRef]
- Santos, H.P., Jr.; Bhattacharya, A.; Martin, E.M.L.; Addo, K.; Psioda, M.; Smeester, L.; Joseph, R.M.; Hooper, S.R.; Frazier, J.A.; Kuban, K.C.; et al. Epigenome-wide DNA methylation in placentas from preterm infants: Association with maternal socioeconomic status. Epigenetics 2019, 14, 751–765. [Google Scholar] [CrossRef] [PubMed]
- Stekhoven, D.J. Missforest: Nonparametric Missing Value Imputation Using Random Forest; R package, 2013. [Google Scholar]
- Ritchie, M.E.; Phipson, B.; Wu, D.; Hu, Y.; Law, C.W.; Shi, W.; Smyth, G.K. limma powers differential expression analyses for RNA-sequencing and microarray studies. Nucleic Acids Res. 2015, 43, e47. [Google Scholar] [CrossRef] [PubMed]
- Benjamini, Y.; Hochberg, Y. Controlling the False Discovery Rate: A Practical and Powerful Approach to Multiple Testing. J. R. Stat. Society Ser. B (Methodol.) 1995, 57, 289–300. [Google Scholar] [CrossRef]
- Szklarczyk, D.; Gable, A.L.; Nastou, K.C.; Lyon, D.; Kirsch, R.; Pyysalo, S.L.; Doncheva, N.T.L.; Legeay, M.; Feng, T.; Bork, P.; et al. The STRING database in 2021: Customizable protein-protein networks, and functional characterization of user-uploaded gene/measurement sets. Nucleic Acids Res. 2021, 49, D605–D612. [Google Scholar] [CrossRef] [PubMed]
- Gillespie, M.; Jassal, B.; Stephan, R.; Milacic, M.; Rothfels, K.; Senff-Ribeiro, A.; Griss, J.; Sevilla, C.; Matthews, L.; Gong, C. The reactome pathway knowledgebase 2022. Nucleic Acids Res. 2022, 50, D687–D692. [Google Scholar] [CrossRef]



| All Participants (n = 230) | Females (n = 99) | Males (n = 131) | p-Value | |
|---|---|---|---|---|
| Maternal age (average) | 29.6 [6.70] | 28.9 [6.59] | 30.1 [6.75] | 0.159 |
| Gestational age in weeks (average) | 26.0 [1.26] | 26.1 [1.22] | 25.9 [1.28] | 0.230 |
| Birthweight in grams | 825 [183] | 810 [188] | 839 [179] | 0.282 |
| Birthweight z-score | −0.22 [1.03] | −0.37 [1.15] | −0.10 [0.91] | 0.057 |
| Placental weight in grams | 246 [121] | 260 [122] | 236 [119] | 0.139 |
| C-section delivery | ||||
| Yes | 162 (70.4%) | 70 (70.7%) | 92 (70.2%) | 1.000 |
| No | 68 (29.6%) | 29 (29.3%) | 39 (29.8%) | |
| Duration of labor | ||||
| 0 h | 64 (27.8%) | 28 (28.3%) | 36 (27.5%) | 0.851 |
| ≤12 h | 50 (21.7%) | 23 (23.2%) | 27 (20.6%) | |
| >12 h | 116 (50.4%) | 48 (48.5%) | 68 (51.9%) | |
| Public insurance | ||||
| No | 150 (65.2%) | 60 (60.6%) | 90 (68.7%) | 0.262 |
| Yes | 78 (33.9%) | 38 (38.4%) | 40 (30.5%) | |
| “Missing” | 2 (0.87%) | 1 (1.01%) | 1 (0.76%) | |
| Mother’s education (years) | ||||
| ≤12 | 93 (40.9%) | 42 (42.4%) | 51 (38.9%) | 0.260 |
| 3–15 | 49 (21.3%) | 24 (24.2%) | 25 (19.1%) | |
| 16+ | 84 (36.5%) | 30 (30.3%) | 54 (41.2%) | |
| “Missing” | 4 (1.74%) | 3 (3.03%) | 1 (0.76%) | |
| Mother’s race | ||||
| White | 136 (59.1%) | 57 (57.6%) | 79 (60.3%) | 0.684 |
| Black | 70 (30.4%) | 33 (33.3%) | 37 (28.2%) | |
| Other | 21 (9.13%) | 8 (8.08%) | 13 (9.92%) | |
| “Missing” | 3 (1.30%) | 1 (1.01%) | 2 (1.53%) | |
| Marital status (married) | ||||
| No | 104 (45.2%) | 49 (49.5%) | 55 (42.0%) | 0.318 |
| Yes | 126 (54.8%) | 50 (50.5%) | 76 (58.0%) | |
| Maternal pre-pregnancy BMI | ||||
| Underweight | 18 (7.83%) | 6 (6.06%) | 12 (9.16%) | 0.573 |
| Normal | 115 (50.0%) | 46 (46.5%) | 69 (52.7%) | |
| Overweight | 39 (17.0%) | 19 (19.2%) | 20 (15.3%) | |
| Obese | 53 (23.0%) | 25 (25.3%) | 28 (21.4%) | |
| “Missing” | 5 (2.17%) | 3 (3.03%) | 2 (1.53%) | |
| Maternal smoking while pregnant | ||||
| No | 204 (88.7%) | 86 (86.9%) | 118 (90.1%) | 0.765 |
| Yes | 23 (10.0%) | 11 (11.1%) | 12 (9.16%) | |
| “Missing” | 3 (1.30%) | 2 (2.02%) | 1 (0.76%) | |
| Maternal SES score | ||||
| 0 | 109 (47.4%) | 43 (43.4%) | 66 (50.4%) | 0.149 |
| 1 | 36 (15.7%) | 13 (13.1%) | 23 (17.6%) | |
| 2 | 55 (23.9%) | 26 (26.3%) | 29 (22.1%) | |
| 3 | 23 (10.0%) | 15 (15.2%) | 8 (6.11%) | |
| 4 | 7 (3.04%) | 2 (2.02%) | 5 (3.82%) | |
| Metal | Sex | Median | Mean | Minimum | Maximum | p-Value |
|---|---|---|---|---|---|---|
| Selenium (Se) | Female | 0.880 | 0.877 | 0.436 | 1.610 | 0.493 |
| Male | 0.851 | 0.892 | 0.600 | 1.980 | ||
| Manganese (Mn) | Female | 0.342 | 0.432 | 0.101 | 5.577 | 0.310 |
| Male | 0.344 | 0.373 | 0.194 | 1.699 | ||
| Lead (Pb) | Female | 0.014 | 0.029 | 0.003 | 0.350 | 0.463 |
| Male | 0.017 | 0.036 | 0.003 | 0.893 | ||
| Arsenic (As) | Female | 0.004 | 0.006 | 0.001 | 0.078 | 0.600 |
| Male | 0.005 | 0.007 | 0.001 | 0.067 | ||
| Cadmium (Cd) | Female | 0.001 | 0.005 | 0.0002 | 0.075 | 0.204 |
| Male | 0.001 | 0.045 | 0.0002 | 4.06 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Freedman, A.N.; Roell, K.; Engwall, E.; Bulka, C.; Kuban, K.C.K.; Herring, L.; Mills, C.A.; Parsons, P.J.; Galusha, A.; O’Shea, T.M.; et al. Prenatal Metal Exposure Alters the Placental Proteome in a Sex-Dependent Manner in Extremely Low Gestational Age Newborns: Links to Gestational Age. Int. J. Mol. Sci. 2023, 24, 14977. https://doi.org/10.3390/ijms241914977
Freedman AN, Roell K, Engwall E, Bulka C, Kuban KCK, Herring L, Mills CA, Parsons PJ, Galusha A, O’Shea TM, et al. Prenatal Metal Exposure Alters the Placental Proteome in a Sex-Dependent Manner in Extremely Low Gestational Age Newborns: Links to Gestational Age. International Journal of Molecular Sciences. 2023; 24(19):14977. https://doi.org/10.3390/ijms241914977
Chicago/Turabian StyleFreedman, Anastasia N., Kyle Roell, Eiona Engwall, Catherine Bulka, Karl C. K. Kuban, Laura Herring, Christina A. Mills, Patrick J. Parsons, Aubrey Galusha, Thomas Michael O’Shea, and et al. 2023. "Prenatal Metal Exposure Alters the Placental Proteome in a Sex-Dependent Manner in Extremely Low Gestational Age Newborns: Links to Gestational Age" International Journal of Molecular Sciences 24, no. 19: 14977. https://doi.org/10.3390/ijms241914977
APA StyleFreedman, A. N., Roell, K., Engwall, E., Bulka, C., Kuban, K. C. K., Herring, L., Mills, C. A., Parsons, P. J., Galusha, A., O’Shea, T. M., & Fry, R. C. (2023). Prenatal Metal Exposure Alters the Placental Proteome in a Sex-Dependent Manner in Extremely Low Gestational Age Newborns: Links to Gestational Age. International Journal of Molecular Sciences, 24(19), 14977. https://doi.org/10.3390/ijms241914977





