Albumin of People with Diabetes Mellitus Is More Reduced at Low HbA1c
Abstract
:1. Introduction
2. Results
2.1. Albumin Redox State in Relation to Demographic and Laboratory Data of Diabetes Mellitus Patients
2.2. Albumin Redox State in Relation to Diabetes Duration
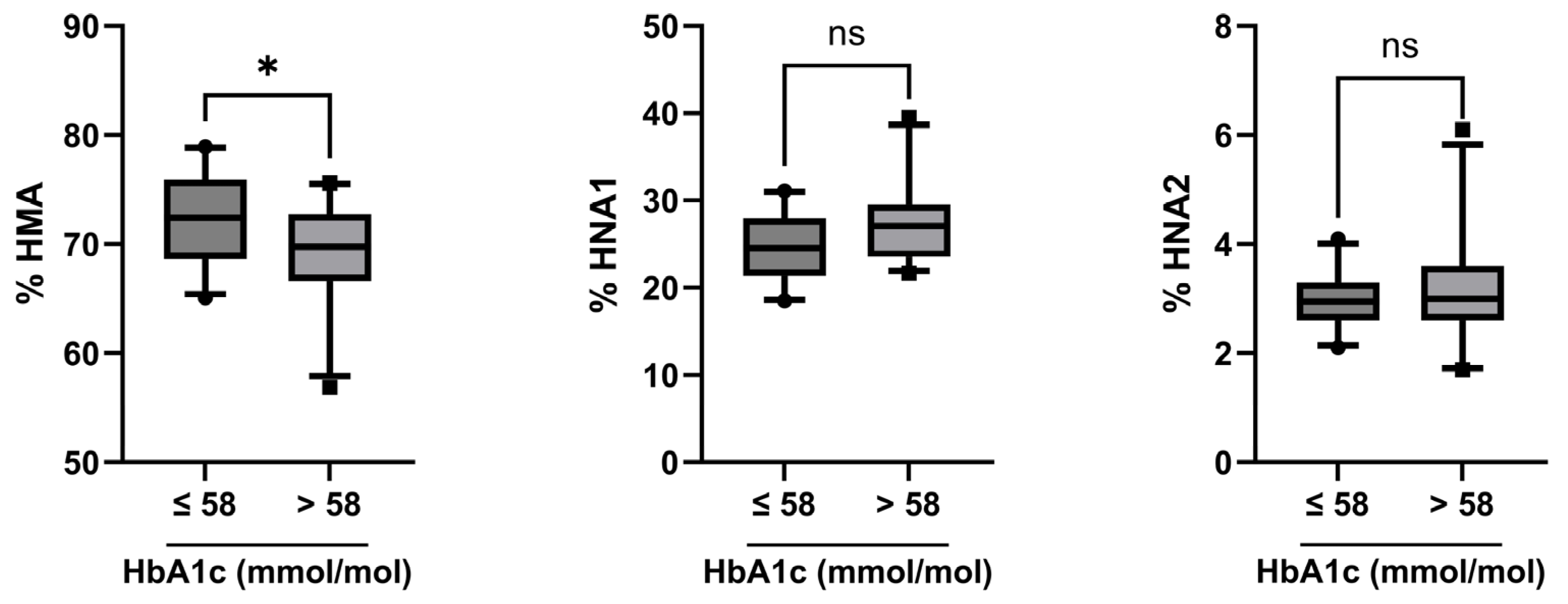
2.3. Albumin Redox State in Relation to Disease Control Status
3. Discussion
4. Materials and Methods
4.1. Study Patients
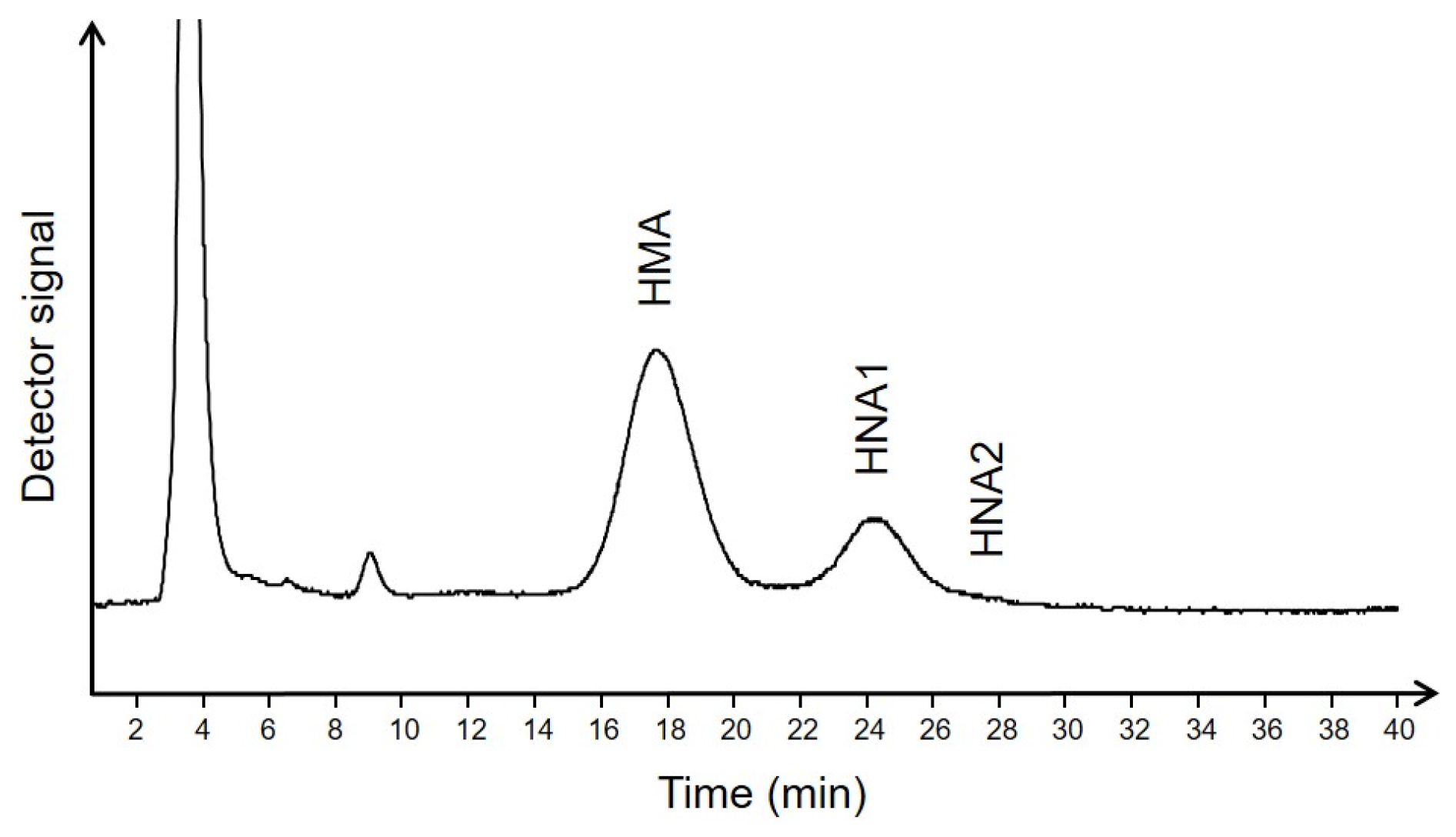
4.2. Determination of Albumin Redox State
4.3. Determination of Albumin Related Diagnostic Laboratory Variables in Plasma
4.4. Statistics
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lin, Y.; Sun, Z. Current views on type 2 diabetes. J. Endocrinol. 2010, 204, 1–11. [Google Scholar] [CrossRef]
- De Ferranti, S.D.; de Boer, I.H.; Fonseca, V.; Fox, C.S.; Golden, S.H.; Lavie, C.J.; Magge, S.N.; Marx, N.; McGuire, D.K.; Orchard, T.J.; et al. Type 1 diabetes mellitus and cardiovascular disease: A scientific statement from the American Heart Association and American Diabetes Association. Diabetes Care 2014, 37, 2843–2863. [Google Scholar] [CrossRef] [PubMed]
- Mazzone, T.; Chait, A.; Plutzky, J. Cardiovascular disease risk in type 2 diabetes mellitus: Insights from mechanistic studies. Lancet 2008, 371, 1800–1809. [Google Scholar] [CrossRef] [PubMed]
- Sies, H.; Berndt, C.; Jones, D.P. Oxidative Stress. Annu. Rev. Biochem. 2017, 86, 715–748. [Google Scholar] [CrossRef]
- International Expert, C. International Expert Committee report on the role of the A1C assay in the diagnosis of diabetes. Diabetes Care 2009, 32, 1327–1334. [Google Scholar] [CrossRef]
- Wu, W.C.; Ma, W.Y.; Wei, J.N.; Yu, T.Y.; Lin, M.S.; Shih, S.R.; Hua, C.H.; Liao, Y.J.; Chuang, L.M.; Li, H.Y. Serum Glycated Albumin to Guide the Diagnosis of Diabetes Mellitus. PLoS ONE 2016, 11, e0146780. [Google Scholar] [CrossRef]
- Nagumo, K.; Tanaka, M.; Chuang, V.T.; Setoyama, H.; Watanabe, H.; Yamada, N.; Kubota, K.; Tanaka, M.; Matsushita, K.; Yoshida, A.; et al. Cys34-cysteinylated human serum albumin is a sensitive plasma marker in oxidative stress-related chronic diseases. PLoS ONE 2014, 9, e85216. [Google Scholar] [CrossRef]
- Anraku, M.; Chuang, V.T.; Maruyama, T.; Otagiri, M. Redox properties of serum albumin. Biochim. Biophys. Acta 2013, 1830, 5465–5472. [Google Scholar] [CrossRef]
- Peters, T., Jr. All About Albumin: Biochemistry, Genetics, and Medical Applications; Academic Press: Cambridge, MA, USA, 1996. [Google Scholar]
- Hughes, W.L.; Dintzis, H.M. Crystallization of the Mercury Dimers of Human and Bovine Mercaptalbumin. J. Biol. Chem. 1964, 239, 845–849. [Google Scholar] [CrossRef]
- Carballal, S.; Alvarez, B.; Turell, L.; Botti, H.; Freeman, B.A.; Radi, R. Sulfenic acid in human serum albumin. Amino Acids 2007, 32, 543–551. [Google Scholar] [CrossRef]
- Lamprecht, M.; Greilberger, J.F.; Schwaberger, G.; Hofmann, P.; Oettl, K. Single bouts of exercise affect albumin redox state and carbonyl groups on plasma protein of trained men in a workload-dependent manner. J. Appl. Physiol. 2008, 104, 1611–1617. [Google Scholar] [CrossRef] [PubMed]
- Oettl, K.; Stadlbauer, V.; Petter, F.; Greilberger, J.; Putz-Bankuti, C.; Hallstrom, S.; Lackner, C.; Stauber, R.E. Oxidative damage of albumin in advanced liver disease. Biochim. Biophys. Acta 2008, 1782, 469–473. [Google Scholar] [CrossRef] [PubMed]
- Oettl, K.; Birner-Gruenberger, R.; Spindelboeck, W.; Stueger, H.P.; Dorn, L.; Stadlbauer, V.; Putz-Bankuti, C.; Krisper, P.; Graziadei, I.; Vogel, W.; et al. Oxidative albumin damage in chronic liver failure: Relation to albumin binding capacity, liver dysfunction and survival. J. Hepatol. 2013, 59, 978–983. [Google Scholar] [CrossRef]
- Domenicali, M.; Baldassarre, M.; Giannone, F.A.; Naldi, M.; Mastroroberto, M.; Biselli, M.; Laggetta, M.; Patrono, D.; Bertucci, C.; Bernardi, M.; et al. Posttranscriptional changes of serum albumin: Clinical and prognostic significance in hospitalized patients with cirrhosis. Hepatology 2014, 60, 1851–1860. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, Y.; Suzuki, R.; Yasukawa, K.; Oba, K.; Yamauchi, T.; Yatomi, Y.; Kadowaki, T. Oxidized albumin in blood reflects the severity of multiple vascular complications in diabetes mellitus. Metab. Open 2020, 6, 100032. [Google Scholar] [CrossRef] [PubMed]
- Imafuku, T.; Watanabe, H.; Oniki, K.; Yoshida, A.; Kato, H.; Nakano, T.; Tokumaru, K.; Fujita, I.; Arimura, N.; Maeda, H.; et al. Cysteinylated Albumin as a Potential Biomarker for the Progression of Kidney Disease in Patients With Type 2 Diabetes. Diabetes Care 2021, 44, e115–e117. [Google Scholar] [CrossRef]
- Oettl, K.; Reibnegger, G.; Schmut, O. The redox state of human serum albumin in eye diseases with and without complications. Acta Ophthalmol. 2011, 89, e174–e179. [Google Scholar] [CrossRef]
- Fukuhara, S.; Yasukawa, K.; Sato, M.; Ikeda, H.; Inoguchi, Y.; Etoh, T.; Masakado, M.; Umeda, F.; Yatomi, Y.; Yamauchi, T.; et al. Clinical usefulness of human serum nonmercaptalbumin to mercaptalbumin ratio as a biomarker for diabetic complications and disability in activities of daily living in elderly patients with diabetes. Metabolism 2020, 103, 153995. [Google Scholar] [CrossRef]
- Suzuki, E.; Yasuda, K.; Takeda, N.; Sakata, S.; Era, S.; Kuwata, K.; Sogami, M.; Miura, K. Increased oxidized form of human serum albumin in patients with diabetes mellitus. Diabetes Res. Clin. Pract. 1992, 18, 153–158. [Google Scholar] [CrossRef]
- Medina-Navarro, R.; Corona-Candelas, I.; Barajas-Gonzalez, S.; Diaz-Flores, M.; Duran-Reyes, G. Albumin antioxidant response to stress in diabetic nephropathy progression. PLoS ONE 2014, 9, e106490. [Google Scholar] [CrossRef]
- Van Dijk, P.R.; Pasch, A.; van Ockenburg-Brunet, S.L.; Waanders, F.; Eman Abdulle, A.; Muis, M.J.; Hillebrands, J.L.; Bilo, H.J.G.; van Goor, H. Thiols as markers of redox status in type 1 diabetes mellitus. Ther. Adv. Endocrinol. Metab. 2020, 11, 2042018820903641. [Google Scholar] [CrossRef] [PubMed]
- Paramasivan, S.; Adav, S.S.; Ngan, S.C.; Dalan, R.; Leow, M.K.; Ho, H.H.; Sze, S.K. Serum albumin cysteine trioxidation is a potential oxidative stress biomarker of type 2 diabetes mellitus. Sci. Rep. 2020, 10, 6475. [Google Scholar] [CrossRef] [PubMed]
- Imai, H.; Hayashi, T.; Negawa, T.; Nakamura, K.; Tomida, M.; Koda, K.; Tajima, T.; Koda, Y.; Suda, K.; Era, S. Strenuous exercise-induced change in redox state of human serum albumin during intensive kendo training. Jpn. J. Physiol. 2002, 52, 135–140. [Google Scholar] [CrossRef] [PubMed]
- Era, S.; Kuwata, K.; Imai, H.; Nakamura, K.; Hayashi, T.; Sogami, M. Age-related change in redox state of human serum albumin. Biochim. Biophys. Acta 1995, 1247, 12–16. [Google Scholar] [CrossRef]
- Costa, M.; Horrillo, R.; Ortiz, A.M.; Perez, A.; Mestre, A.; Ruiz, A.; Boada, M.; Grancha, S. Increased Albumin Oxidation in Cerebrospinal Fluid and Plasma from Alzheimer’s Disease Patients. J. Alzheimers Dis. 2018, 63, 1395–1404. [Google Scholar] [CrossRef]
- Matsuyama, Y.; Hayashi, T.; Terawaki, H.; Negawa, T.; Terada, T.; Okano, Y.; Era, S. Human astrocytes and aortic endothelial cells actively convert the oxidized form of albumin to the reduced form: Reduced albumin might participate in redox regulation of nerve and blood vessel systems. J. Physiol. Sci. 2009, 59, 207–215. [Google Scholar] [CrossRef]
- Harada, D.; Anraku, M.; Fukuda, H.; Naito, S.; Harada, K.; Suenaga, A.; Otagiri, M. Kinetic studies of covalent binding between N-acetyl-L-cysteine and human serum albumin through a mixed-disulfide using an N-methylpyridinium polymer-based column. Drug Metab. Pharmacokinet. 2004, 19, 297–302. [Google Scholar] [CrossRef]
- Hayashi, T.; Suda, K.; Imai, H.; Era, S. Simple and sensitive high-performance liquid chromatographic method for the investigation of dynamic changes in the redox state of rat serum albumin. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2002, 772, 139–146. [Google Scholar] [CrossRef]
- Oettl, K.; Marsche, G. Redox state of human serum albumin in terms of cysteine-34 in health and disease. Methods Enzym. 2010, 474, 181–195. [Google Scholar] [CrossRef]
| Combined Group n = 52 | T1DM n = 26 | T2DM n = 26 | T1DM vs. T2DM p Value | |
|---|---|---|---|---|
| n female (%) | 19 (37) | 10 (38) | 9 (35) | >0.999 |
| age (years) | 56.0 (19–73) | 46.5 (28.5–57.3) | 59.5 (52.5–64.0) | <0.001 |
| diabetes duration (years) | 15.0 (2.0–51.0) | 21.5 (10.5–40.8) | 13.5 (7.8–19.5) | 0.03 |
| HbA1c (mmol/mol) | 58 (50–64) | 56 (45–61) | 60 (56–66) | 0.04 |
| n with HbA1c > 58 mmol/mol (%) | 24 (46%) | 9 (35%) | 15 (58%) | 0.163 |
| Albumin (g/dL) | 3.9 (3.7–4.2) | 3.9 (3.7–4.1) | 3.9 (3.7–4.2) | 0.65 |
| GSP (µmol/L) | 307 (256–398) | 332 (281–464) | 273 (233–377) | 0.12 |
| GSP/Alb (mol/mol) | 0.6 (0.5–0.7) | 0.6 (0.5–0.8) | 0.5 (0.4–0.6) | 0.14 |
| % HMA | 72.0 (68.3–74.8) | 73.2 (70.8–76.2) | 69.1 (66.7–72.4) | <0.001 |
| % HNA1 | 25.6 (23.2–28.3) | 23.4 (21.3–26.1) | 27.3 (25.3–29.6) | <0.001 |
| % HNA2 | 3.0 (2.6–3.4) | 3.0 (2.5–3.5) | 3.0 (2.6–3.4) | 0.64 |
| T1DM n = 17 | T2DM n = 23 | p Value | |
|---|---|---|---|
| % HMA | 72.5 (68.7–75.2) | 69.6 (68.1–72.4) | 0.12 |
| % HNA1 | 23.9 (22.3–27.9) | 27.2 (24.8–29.2) | 0.07 |
| % HNA2 | 3.1 (2.5–3.7) | 2.8 (2.6–3.3) | 0.45 |
| Age | ||||
|---|---|---|---|---|
| Combined Group | T1DM | T2DM | ||
| HMA | r | −0.57 | −0.59 | −0.29 |
| p | <0.0001 | 0.002 | 0.159 | |
| HNA1 | r | 0.53 | 0.55 | 0.17 |
| p | <0.0001 | 0.004 | 0.418 | |
| HNA2 | r | 0.37 | 0.34 | 0.46 |
| p | 0.007 | 0.093 | 0.017 | |
| Disease Duration | ||||
|---|---|---|---|---|
| Combined Group | T1DM | T2DM | ||
| HMA | r | −0.26 | −0.40 | −0.26 |
| p | 0.066 | 0.048 | 0.206 | |
| HNA1 | r | 0.13 | 0.34 | 0.22 |
| p | 0.376 | 0.102 | 0.281 | |
| HNA2 | r | 0.31 | 0.41 | 0.03 |
| p | 0.025 | 0.040 | 0.894 | |
| Combined Group n = 52 | HbA1c ≤ 58 mmol/mol n = 28 | HbA1c > 58 mmol/mol n = 24 | HbA1c ≤ 58 vs. >58 mmol/mol p Value | |
|---|---|---|---|---|
| n hypertension (%) | 26 (50) | 13 (46) | 13 (54) | 0.78 |
| n coronary heart disease (%) | 5 (10) | 2 (7) | 3 (13) | 0.65 |
| n myocardial infection (%) | 1 (2) | 1 (4) | 0 (0) | >0.99 |
| n TIA (%) | 2 (4) | 1 (4) | 1 (4) | >0.99 |
| n heart failure (%) | 1 (2) | 1 (4) | 0 (0) | >0.99 |
| n PTCA/CABG (%) | 3 (6) | 2 (7) | 1 (4) | >0.99 |
| n stroke (%) | 2 (4) | 1 (4) | 1 (4) | >0.99 |
| n liver disease (%) | 7 (13) | 3 (11) | 4 (17) | 0.69 |
| n history of cancer (%) | 4 (8) | 2 (7) | 2 (8) | >0.99 |
| n retinopathy (%) | 12 (23) | 5 (18) | 7 (29) | 0.51 |
| n polyneuropathy (%) | 16 (31) | 7 (25) | 9 (38) | 0.38 |
| eGFR, mL/min/1.73m2 | 90.7 (80.2–102.0) | 99.3 (84.6–111.3) | 86.1 (68.3–94.1) | 0.02 |
| hemoglobin, g/dL | 14.8 (14.0–16.0) | 14.5 (13.6–15.6) | 14.5 (13.7–15.9) | >0.99 |
| Liver Disease n = 7 | No Liver Disease n = 19 | p Value | |
|---|---|---|---|
| ALBUMIN (mg/dL) | 4.0 (3.6–4.3) | 3.9 (3.8–4.2) | 0.92 |
| % HMA | 69.9 (68.1–72.6) | 68.6 (66.6–72.4) | 0.68 |
| % HNA1 | 27.3 (24.8–29.2) | 27.3 (25.5–29.7) | 0.92 |
| % HNA2 | 2.8 (2.6–3.3) | 3.2 (2.6–3.6) | 0.54 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Paar, M.; Cvirn, G.; Hoerl, G.; Reibnegger, G.; Sourij, H.; Sourij, C.; Kojzar, H.; Oettl, K. Albumin of People with Diabetes Mellitus Is More Reduced at Low HbA1c. Int. J. Mol. Sci. 2023, 24, 16256. https://doi.org/10.3390/ijms242216256
Paar M, Cvirn G, Hoerl G, Reibnegger G, Sourij H, Sourij C, Kojzar H, Oettl K. Albumin of People with Diabetes Mellitus Is More Reduced at Low HbA1c. International Journal of Molecular Sciences. 2023; 24(22):16256. https://doi.org/10.3390/ijms242216256
Chicago/Turabian StylePaar, Margret, Gerhard Cvirn, Gerd Hoerl, Gilbert Reibnegger, Harald Sourij, Caren Sourij, Harald Kojzar, and Karl Oettl. 2023. "Albumin of People with Diabetes Mellitus Is More Reduced at Low HbA1c" International Journal of Molecular Sciences 24, no. 22: 16256. https://doi.org/10.3390/ijms242216256





