Pulsed Electromagnetic Fields Induce Skeletal Muscle Cell Repair by Sustaining the Expression of Proteins Involved in the Response to Cellular Damage and Oxidative Stress
Abstract
:1. Introduction
2. Results
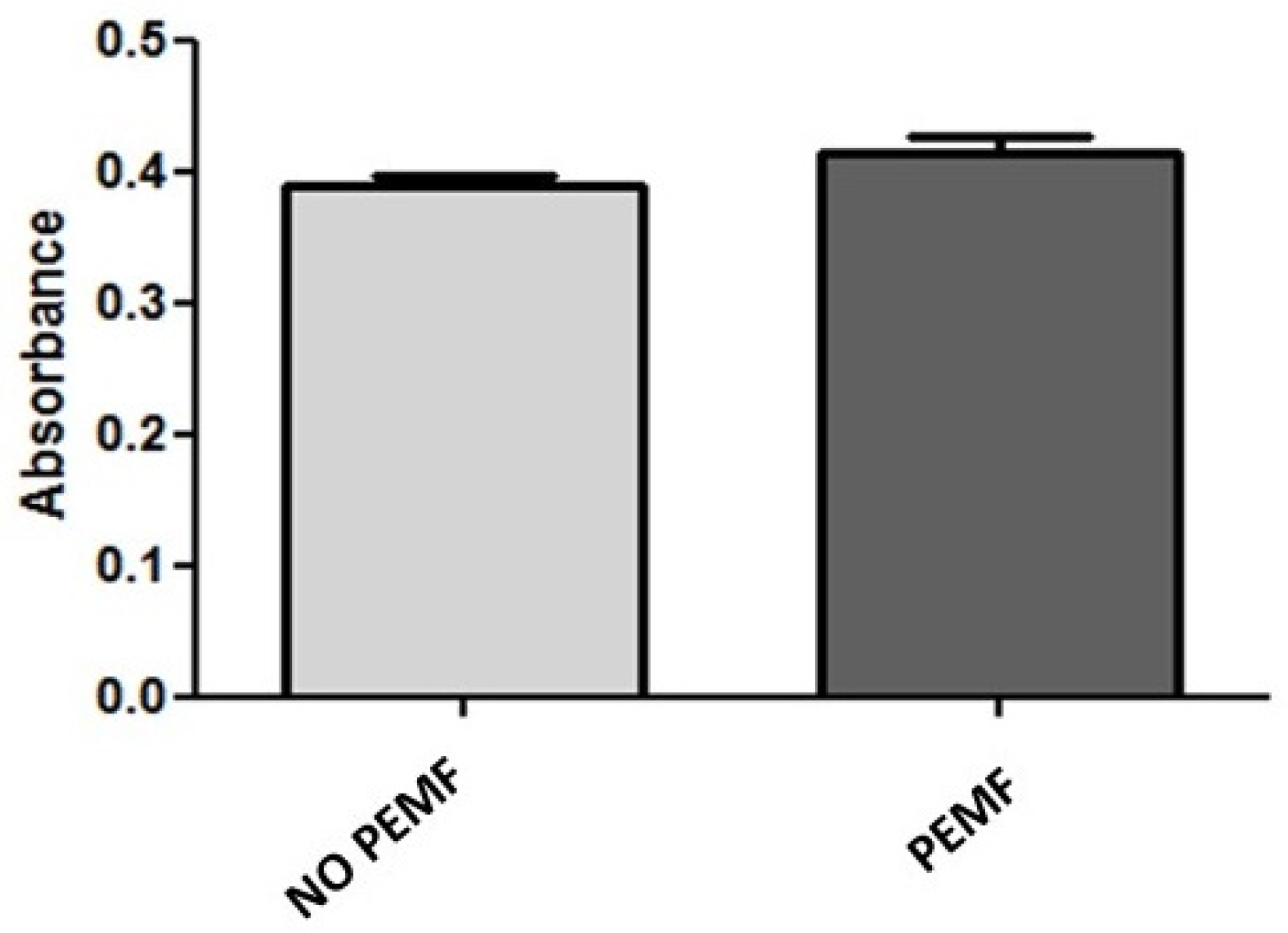
2.1. PEMF Exposure Does Not Alter the Metabolic Activity and Viability of Skeletal Muscle Cells
2.2. PEMF Exposure Stimulates Muscle Cells Proliferation
2.3. Exposure to PEMF Results in Increased MyoD Expression
2.4. Muscle Cells Increase Their Regeneration following Wound Damage When Treated with PEMF
2.5. Exposure to PEMF Correlates with Increased Expression of Proteins Involved in Cellular Stress
3. Discussion
4. Materials and Methods
4.1. Stimulation with Pulsed Electromagnetic Fields (PEMF)
4.2. MTT Assays
4.3. Apoptosis Assays
4.4. Cell Proliferation Assays
4.5. Scratch Assays
4.6. Immunofluorescence Staining
4.7. Proteome Profile
4.8. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Frontera, W.R.; Ochala, J. Skeletal Muscle: A Brief Review of Structure and Function. Calcif. Tissue Int. 2015, 96, 183–195. [Google Scholar] [CrossRef] [PubMed]
- Turner, N.J.; Badylak, S.F. Regeneration of Skeletal Muscle. Cell Tissue Res. 2012, 347, 759–774. [Google Scholar] [CrossRef] [PubMed]
- Le Grand, F.; Rudnicki, M.A. Skeletal Muscle Satellite Cells and Adult Myogenesis. Curr. Opin. Cell Biol. 2007, 19, 628–633. [Google Scholar] [CrossRef] [PubMed]
- Powell, C.A.; Smiley, B.L.; Mills, J.; Vandenburgh, H.H. Mechanical Stimulation Improves Tissue-Engineered Human Skeletal Muscle. Am. J. Physiol. Cell Physiol. 2002, 283, C1557–C1565. [Google Scholar] [CrossRef] [PubMed]
- Aguilar-Agon, K.W.; Capel, A.J.; Martin, N.R.W.; Player, D.J.; Lewis, M.P. Mechanical Loading Stimulates Hypertrophy in Tissue-engineered Skeletal Muscle: Molecular and Phenotypic Responses. J. Cell. Physiol. 2019, 234, 23547. [Google Scholar] [CrossRef] [PubMed]
- Langelaan, M.L.P.; Boonen, K.J.M.; Rosaria-Chak, K.Y.; van der Schaft, D.W.J.; Post, M.J.; Baaijens, F.P.T. Advanced Maturation by Electrical Stimulation: Differences in Response between C2C12 and Primary Muscle Progenitor Cells. J. Tissue Eng. Regen. Med. 2011, 5, 529–539. [Google Scholar] [CrossRef]
- Khodabukus, A.; Madden, L.; Prabhu, N.K.; Koves, T.R.; Jackman, C.P.; Muoio, D.M.; Bursac, N. Electrical Stimulation Increases Hypertrophy and Metabolic Flux in Tissue-Engineered Human Skeletal Muscle. Biomaterials 2019, 198, 259–269. [Google Scholar] [CrossRef]
- Maleiner, B.; Tomasch, J.; Heher, P.; Spadiut, O.; Rünzler, D.; Fuchs, C. The Importance of Biophysical and Biochemical Stimuli in Dynamic Skeletal Muscle Models. Front. Physiol. 2018, 9, 1130. [Google Scholar] [CrossRef]
- Hu, H.; Yang, W.; Zeng, Q.; Chen, W.; Zhu, Y.; Liu, W.; Wang, S.; Wang, B.; Shao, Z.; Zhang, Y. Promising Application of Pulsed Electromagnetic Fields (PEMFs) in Musculoskeletal Disorders. Biomed. Pharmacother. 2020, 131, 110767. [Google Scholar] [CrossRef]
- Waldorff, E.I.; Zhang, N.; Ryaby, J.T. Pulsed Electromagnetic Field Applications: A Corporate Perspective. J. Orthop. Transl. 2017, 9, 60–68. [Google Scholar] [CrossRef]
- Zhang, T.; Zhao, Z.; Wang, T. Pulsed Electromagnetic Fields as a Promising Therapy for Glucocorticoid-Induced Osteoporosis. Front. Bioeng. Biotechnol. 2023, 11, 1103515. [Google Scholar] [CrossRef]
- Muehsam, D.; Lalezari, P.; Lekhraj, R.; Abruzzo, P.; Bolotta, A.; Marini, M.; Bersani, F.; Aicardi, G.; Pilla, A.; Casper, D. Non-Thermal Radio Frequency and Static Magnetic Fields Increase Rate of Hemoglobin Deoxygenation in a Cell-Free Preparation. PLoS ONE 2013, 8, e61752. [Google Scholar] [CrossRef]
- Bragin, D.E.; Statom, G.L.; Hagberg, S.; Nemoto, E.M. Increases in Microvascular Perfusion and Tissue Oxygenation via Pulsed Electromagnetic Fields in the Healthy Rat Brain. J. Neurosurg. 2015, 122, 1239–1247. [Google Scholar] [CrossRef]
- Trofè, A.; Piras, A.; Muehsam, D.; Meoni, A.; Campa, F.; Toselli, S.; Raffi, M. Effect of Pulsed Electromagnetic Fields (PEMFs) on Muscular Activation during Cycling: A Single-Blind Controlled Pilot Study. Healthcare 2023, 11, 922. [Google Scholar] [CrossRef] [PubMed]
- He, W.; Qin, R.; Gao, Y.; Zhou, J.; Wei, J.; Liu, J.; Hou, X.; Ma, H.; Xian, C.J.; Li, X.; et al. The Interdependent Relationship between the Nitric Oxide Signaling Pathway and Primary Cilia in Pulse Electromagnetic Field-stimulated Osteoblastic Differentiation. FASEB J. 2022, 36, e22376. [Google Scholar] [CrossRef] [PubMed]
- Varani, K.; Vincenzi, F.; Pasquini, S.; Blo, I.; Salati, S.; Cadossi, M.; De Mattei, M. Pulsed Electromagnetic Field Stimulation in Osteogenesis and Chondrogenesis: Signaling Pathways and Therapeutic Implications. Int. J. Mol. Sci. 2021, 22, 809. [Google Scholar] [CrossRef]
- Daish, C.; Blanchard, R.; Fox, K.; Pivonka, P.; Pirogova, E. The Application of Pulsed Electromagnetic Fields (PEMFs) for Bone Fracture Repair: Past and Perspective Findings. Ann. Biomed. Eng. 2018, 46, 525–542. [Google Scholar] [CrossRef]
- Parate, D.; Kadir, N.D.; Celik, C.; Lee, E.H.; Hui, J.H.P.; Franco-Obregón, A.; Yang, Z. Pulsed Electromagnetic Fields Potentiate the Paracrine Function of Mesenchymal Stem Cells for Cartilage Regeneration. Stem Cell Res. Ther. 2020, 11, 46. [Google Scholar] [CrossRef]
- Yap, J.L.Y.; Tai, Y.K.; Fröhlich, J.; Fong, C.H.H.; Yin, J.N.; Foo, Z.L.; Ramanan, S.; Beyer, C.; Toh, S.J.; Casarosa, M.; et al. Ambient and Supplemental Magnetic Fields Promote Myogenesis via a TRPC1-Mitochondrial Axis: Evidence of a Magnetic Mitohormetic Mechanism. FASEB J. 2019, 33, 12853–12872. [Google Scholar] [CrossRef]
- Terrie, L.; Burattini, M.; Van Vlierberghe, S.; Fassina, L.; Thorrez, L. Enhancing Myoblast Fusion and Myotube Diameter in Human 3D Skeletal Muscle Constructs by Electromagnetic Stimulation. Front. Bioeng. Biotechnol. 2022, 10, 892287. [Google Scholar] [CrossRef]
- Bi, J.; Jing, H.; Zhou, C.L.; Gao, P.; Han, F.; Li, G.; Zhang, S. Regulation of Skeletal Myogenesis in C2C12 Cells through Modulation of Pax7, MyoD, and Myogenin via Different Low-Frequency Electromagnetic Field Energies. Technol. Health Care 2022, 30, S371–S382. [Google Scholar] [CrossRef] [PubMed]
- Ong, M.T.Y.; Man, G.C.W.; Lau, L.C.M.; He, X.; Qiu, J.; Wang, Q.; Chow, M.C.S.; Choi, B.C.Y.; Yu, M.; Yung, P.S.H. Effect of Pulsed Electromagnetic Field as an Intervention for Patients with Quadriceps Weakness after Anterior Cruciate Ligament Reconstruction: A Double-Blinded, Randomized-Controlled Trial. Trials 2022, 23, 771. [Google Scholar] [CrossRef] [PubMed]
- Ongaro, A.; Varani, K.; Masieri, F.F.; Pellati, A.; Massari, L.; Cadossi, R.; Vincenzi, F.; Borea, P.A.; Fini, M.; Caruso, A.; et al. Electromagnetic Fields (EMFs) and Adenosine Receptors Modulate Prostaglandin E2 and Cytokine Release in Human Osteoarthritic Synovial Fibroblasts. J. Cell. Physiol. 2012, 227, 2461–2469. [Google Scholar] [CrossRef] [PubMed]
- Ongaro, A.; Pellati, A.; Bagheri, L.; Fortini, C.; Setti, S.; De Mattei, M. Pulsed Electromagnetic Fields Stimulate Osteogenic Differentiation in Human Bone Marrow and Adipose Tissue Derived Mesenchymal Stem Cells. Bioelectromagnetics 2014, 35, 426–436. [Google Scholar] [CrossRef]
- Perucca Orfei, C.; Lovati, A.B.; Lugano, G.; Viganò, M.; Bottagisio, M.; D’Arrigo, D.; Sansone, V.; Setti, S.; de Girolamo, L. Pulsed Electromagnetic Fields Improve the Healing Process of Achilles Tendinopathy: A Pilot Study in a Rat Model. Bone Jt. Res. 2020, 9, 613–622. [Google Scholar] [CrossRef]
- De Mattei, M.; Caruso, A.; Pezzetti, F.; Pellati, A.; Stabellini, G.; Sollazzo, V.; Traina, G.C. Effects of Pulsed Electromagnetic Fields on Human Articular Chondrocyte Proliferation. Connect. Tissue Res. 2001, 42, 269–279. [Google Scholar] [CrossRef] [PubMed]
- De Girolamo, L.; Stanco, D.; Galliera, E.; Viganò, M.; Colombini, A.; Setti, S.; Vianello, E.; Corsi Romanelli, M.M.; Sansone, V. Low Frequency Pulsed Electromagnetic Field Affects Proliferation, Tissue-Specific Gene Expression, and Cytokines Release of Human Tendon Cells. Cell Biochem. Biophys. 2013, 66, 697–708. [Google Scholar] [CrossRef]
- Brighton, C.T.; Wang, W.; Seldes, R.; Zhang, G.; Pollack, S.R. Signal Transduction in Electrically Stimulated Bone Cells. J. Bone Jt. Surg. Am. 2001, 83, 1514–1523. [Google Scholar] [CrossRef]
- Pilla, A.; Fitzsimmons, R.; Muehsam, D.; Wu, J.; Rohde, C.; Casper, D. Electromagnetic Fields as First Messenger in Biological Signaling: Application to Calmodulin-Dependent Signaling in Tissue Repair. Biochim. Biophys. Acta (BBA)—Gen. Subj. 2011, 1810, 1236–1245. [Google Scholar] [CrossRef]
- Diniz, P.; Soejima, K.; Ito, G. Nitric Oxide Mediates the Effects of Pulsed Electromagnetic Field Stimulation on the Osteoblast Proliferation and Differentiation. Nitric Oxide 2002, 7, 18–23. [Google Scholar] [CrossRef]
- Jasti, A.C.; Wetzel, B.J.; Aviles, H.; Vesper, D.N.; Nindl, G.; Johnson, M.T. Effect of a Wound Healing Electromagnetic Field on Inflammatory Cytokine Gene Expression in Rats. Biomed. Sci. Instrum. 2001, 37, 209–214. [Google Scholar] [PubMed]
- Nie, K.; Henderson, A. MAP Kinase Activation in Cells Exposed to a 60 Hz Electromagnetic Field. J. Cell. Biochem. 2003, 90, 1197–1206. [Google Scholar] [CrossRef] [PubMed]
- Goodman, R.; Lin-Ye, A.; Geddis, M.S.; Wickramaratne, P.J.; Hodge, S.E.; Pantazatos, S.P.; Blank, M.; Ambron, R.T. Extremely Low Frequency Electromagnetic Fields Activate the ERK Cascade, Increase Hsp70 Protein Levels and Promote Regeneration in Planaria. Int. J. Radiat. Biol. 2009, 85, 851–859. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.; Gan, Y.; Fu, Y.; Lu, D.; Chiang, H. An Incoherent Magnetic Field Inhibited EGF Receptor Clustering and Phosphorylation Induced by a 50-Hz Magnetic Field in Cultured FL Cells. Cell. Physiol. Biochem. 2008, 22, 507–514. [Google Scholar] [CrossRef]
- Jeon, H.-S.; Kang, S.-Y.; Park, J.-H.; Lee, H.-S. Effects of Pulsed Electromagnetic Field Therapy on Delayed-Onset Muscle Soreness in Biceps Brachii. Phys. Ther. Sport 2015, 16, 34–39. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.-B.; Kim, J.-Y.; Park, S.-W.; Lee, N.-R.; Kim, Y.-H.; Lee, K.-J.; Lee, Y.-H. Effects of PEMFs (Pulsed Electromagnetic Fields) Stimulation on Acupoint in Quadriceps Fatigue Recovery. Int. J. Precis. Eng. Manuf. 2012, 13, 1697–1703. [Google Scholar] [CrossRef]
- Ongaro, A.; Pellati, A.; Masieri, F.F.; Caruso, A.; Setti, S.; Cadossi, R.; Biscione, R.; Massari, L.; Fini, M.; De Mattei, M. Chondroprotective Effects of Pulsed Electromagnetic Fields on Human Cartilage Explants. Bioelectromagnetics 2011, 32, 543–551. [Google Scholar] [CrossRef]
- Sollazzo, V.; Massari, L.; Caruso, A.; De Mattei, M.; Pezzetti, F. Effects of Low-Frequency Pulsed Electromagnetic Fields on Human Osteoblast-Like Cells In Wtro. Electro- Magnetobiology 1996, 15, 75–83. [Google Scholar] [CrossRef]
- Sollazzo, V.; Palmieri, A.; Pezzetti, F.; Massari, L.; Carinci, F. Effects of Pulsed Electromagnetic Fields on Human Osteoblastlike Cells (MG-63): A Pilot Study. Clin. Orthop. Relat. Res. 2010, 468, 2260. [Google Scholar] [CrossRef]
- Sakhrani, N.; Stefani, R.M.; Setti, S.; Cadossi, R.; Ateshian, G.A.; Hung, C.T. Pulsed Electromagnetic Field Therapy and Direct Current Electric Field Modulation Promote the Migration of Fibroblast-like Synoviocytes to Accelerate Cartilage Repair In Vitro. Appl. Sci. 2022, 12, 12406. [Google Scholar] [CrossRef]
- Hernández-Hernández, J.M.; García-González, E.G.; Brun, C.E.; Rudnicki, M.A. The Myogenic Regulatory Factors, Determinants of Muscle Development, Cell Identity and Regeneration. Semin. Cell Dev. Biol. 2017, 72, 10–18. [Google Scholar] [CrossRef] [PubMed]
- Kodaka, Y.; Rabu, G.; Asakura, A. Skeletal Muscle Cell Induction from Pluripotent Stem Cells. Stem Cells Int. 2017, 2017, 1376151. [Google Scholar] [CrossRef] [PubMed]
- Zammit, P.S. Function of the Myogenic Regulatory Factors Myf5, MyoD, Myogenin and MRF4 in Skeletal Muscle, Satellite Cells and Regenerative Myogenesis. Semin. Cell Dev. Biol. 2017, 72, 19–32. [Google Scholar] [CrossRef] [PubMed]
- Weidenbusch, M.; Anders, H.-J. Tissue Microenvironments Define and Get Reinforced by Macrophage Phenotypes in Homeostasis or during Inflammation, Repair and Fibrosis. J. Innate Immun. 2012, 4, 463–477. [Google Scholar] [CrossRef] [PubMed]
- Medzhitov, R. Origin and Physiological Roles of Inflammation. Nature 2008, 454, 428–435. [Google Scholar] [CrossRef]
- Zhang, H.; Gong, W.; Wu, S.; Perrett, S. Hsp70 in Redox Homeostasis. Cells 2022, 11, 829. [Google Scholar] [CrossRef]
- Beere, H.M.; Wolf, B.B.; Cain, K.; Mosser, D.D.; Mahboubi, A.; Kuwana, T.; Tailor, P.; Morimoto, R.I.; Cohen, G.M.; Green, D.R. Heat-Shock Protein 70 Inhibits Apoptosis by Preventing Recruitment of Procaspase-9 to the Apaf-1 Apoptosome. Nat. Cell Biol. 2000, 2, 469–475. [Google Scholar] [CrossRef]
- Senf, S.M. Skeletal Muscle Heat Shock Protein 70: Diverse Functions and Therapeutic Potential for Wasting Disorders. Front. Physiol. 2013, 4, 330. [Google Scholar] [CrossRef]
- Senf, S.M.; Howard, T.M.; Ahn, B.; Ferreira, L.F.; Judge, A.R. Loss of the Inducible Hsp70 Delays the Inflammatory Response to Skeletal Muscle Injury and Severely Impairs Muscle Regeneration. PLoS ONE 2013, 8, 62687. [Google Scholar] [CrossRef]
- Senf, S.M.; Dodd, S.L.; McClung, J.M.; Judge, A.R. Hsp70 Overexpression Inhibits NF-KappaB and Foxo3a Transcriptional Activities and Prevents Skeletal Muscle Atrophy. FASEB J. 2008, 22, 3836–3845. [Google Scholar] [CrossRef]
- Hernando, R.; Manso, R. Muscle Fibre Stress in Response to Exercise: Synthesis, Accumulation and Isoform Transitions of 70-KDa Heat-Shock Proteins. Eur. J. Biochem. 1997, 243, 460–467. [Google Scholar] [CrossRef] [PubMed]
- Broome, C.S.; Kayani, A.C.; Palomero, J.; Dillmann, W.H.; Mestril, R.; Jackson, M.J.; Mcardle, A.; Broome, C.S.; Kayani, A.C.; Palomero, J.; et al. Effect of Lifelong Overexpression of HSP70 in Skeletal Muscle on Age-Related Oxidative Stress and Adaptation after Nondamaging Contractile Activity. FASEB J. 2006, 20, 1549–1551. [Google Scholar] [CrossRef]
- Liskutin, T.; Batey, J.; Li, R.; Schweigert, C.; Mestril, R. Increased Heat Shock Protein Expression Decreases Inflammation in Skeletal Muscle During and after Frostbite Injury. Curr. Mol. Med. 2021, 20, 733–740. [Google Scholar] [CrossRef]
- Fan, W.; Gao, X.K.; Rao, X.S.; Shi, Y.P.; Liu, X.C.; Wang, F.Y.; Liu, Y.F.; Cong, X.X.; He, M.Y.; Xu, S.B.; et al. Hsp70 Interacts with Mitogen-Activated Protein Kinase (MAPK)-Activated Protein Kinase 2 To Regulate P38MAPK Stability and Myoblast Differentiation during Skeletal Muscle Regeneration. Mol. Cell. Biol. 2018, 38, e00211-18. [Google Scholar] [CrossRef] [PubMed]
- Thakur, S.S.; James, J.L.; Cranna, N.J.; Chhen, V.L.; Swiderski, K.; Ryall, J.G.; Lynch, G.S. Expression and Localization of Heat-Shock Proteins during Skeletal Muscle Cell Proliferation and Differentiation and the Impact of Heat Stress. Cell Stress Chaperones 2019, 24, 749. [Google Scholar] [CrossRef] [PubMed]
- Buckingham, M. Myogenic Progenitor Cells and Skeletal Myogenesis in Vertebrates. Curr. Opin. Genet. Dev. 2006, 16, 525–532. [Google Scholar] [CrossRef]
- Park, W. Upregulation of Thioredoxin and Its Reductase Attenuates Arsenic Trioxide-induced Growth Suppression in Human Pulmonary Artery Smooth Muscle Cells by Reducing Oxidative Stress. Oncol. Rep. 2019, 43, 358–367. [Google Scholar] [CrossRef]
- Mitchell, D.A.; Marletta, M.A. Thioredoxin Catalyzes the S-Nitrosation of the Caspase-3 Active Site Cysteine. Nat. Chem. Biol. 2005, 1, 154–158. [Google Scholar] [CrossRef]
- Tang, H.; Kim, M.; Lee, M.; Baumann, K.; Olguin, F.; He, H.; Wang, Y.; Jiang, B.; Fang, S.; Zhu, J.; et al. Overexpression of Thioredoxin-2 Attenuates Age-related Muscle Loss by Suppressing Mitochondrial Oxidative Stress and Apoptosis. JCSM Rapid Commun. 2022, 5, 130–145. [Google Scholar] [CrossRef]
- Chen, B.; Nelin, V.E.; Locy, M.L.; Jin, Y.; Tipple, T.E. Thioredoxin-1 Mediates Hypoxia-Induced Pulmonary Artery Smooth Muscle Cell Proliferation. Am. J. Physiol.-Lung Cell. Mol. Physiol. 2013, 305, L389–L395. [Google Scholar] [CrossRef]
- Das, K.C.; Lewis-Molock, Y.; White, C.W. Elevation of Manganese Superoxide Dismutase Gene Expression by Thioredoxin. Am. J. Respir. Cell Mol. Biol. 1997, 17, 713–726. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Van Remmen, H.; Csete, M. Sod2 Overexpression Preserves Myoblast Mitochondrial Mass and Function, but Not Muscle Mass with Aging. Aging Cell 2009, 8, 296–310. [Google Scholar] [CrossRef] [PubMed]
- Kozakowska, M.; Pietraszek-Gremplewicz, K.; Jozkowicz, A.; Dulak, J. The Role of Oxidative Stress in Skeletal Muscle Injury and Regeneration: Focus on Antioxidant Enzymes. J. Muscle Res. Cell Motil. 2015, 36, 377–393. [Google Scholar] [CrossRef]
- Togliatto, G.; Trombetta, A.; Dentelli, P.; Cotogni, P.; Rosso, A.; Tschöp, M.H.; Granata, R.; Ghigo, E.; Brizzi, M.F. Unacylated Ghrelin Promotes Skeletal Muscle Regeneration Following Hindlimb Ischemia via SOD-2–Mediated MiR-221/222 Expression. J. Am. Heart Assoc. 2013, 2, e000376. [Google Scholar] [CrossRef]
- Taler-Verčič, A.; Goličnik, M.; Bavec, A. The Structure and Function of Paraoxonase-1 and Its Comparison to Paraoxonase-2 and -3. Molecules 2020, 25, 5980. [Google Scholar] [CrossRef] [PubMed]
- Shi, S.; Buck, T.M.; Kinlough, C.L.; Marciszyn, A.L.; Hughey, R.P.; Chalfie, M.; Brodsky, J.L.; Kleyman, T.R. Regulation of the Epithelial Na+ Channel by Paraoxonase-2. J. Biol. Chem. 2017, 292, 15927–15938. [Google Scholar] [CrossRef] [PubMed]
- Sulaiman, D.; Li, J.; Devarajan, A.; Cunningham, C.M.; Li, M.; Fishbein, G.A.; Fogelman, A.M.; Eghbali, M.; Reddy, S.T. Paraoxonase 2 Protects against Acute Myocardial Ischemia-Reperfusion Injury by Modulating Mitochondrial Function and Oxidative Stress via the PI3K/Akt/GSK-3β RISK Pathway. J. Mol. Cell. Cardiol. 2019, 129, 154–164. [Google Scholar] [CrossRef]
- Nagarajan, A.; Dogra, S.K.; Sun, L.; Gandotra, N.; Ho, T.; Cai, G.; Cline, G.; Kumar, P.; Cowles, R.A.; Wajapeyee, N. Paraoxonase 2 Facilitates Pancreatic Cancer Growth and Metastasis by Stimulating GLUT1-Mediated Glucose Transport. Mol. Cell 2017, 67, 685–701.e6. [Google Scholar] [CrossRef]
- Vincenzi, F.; Targa, M.; Corciulo, C.; Gessi, S.; Merighi, S.; Setti, S.; Cadossi, R.; Goldring, M.B.; Borea, P.A.; Varani, K. Pulsed Electromagnetic Fields Increased the Anti-Inflammatory Effect of A2A and A3 Adenosine Receptors in Human T/C-28a2 Chondrocytes and HFOB 1.19 Osteoblasts. PLoS ONE 2013, 8, e65561. [Google Scholar] [CrossRef]
- Vincenzi, F.; Ravani, A.; Pasquini, S.; Merighi, S.; Gessi, S.; Setti, S.; Cadossi, R.; Borea, P.A.; Varani, K. Pulsed Electromagnetic Field Exposure Reduces Hypoxia and Inflammation Damage in Neuron-like and Microglial Cells. J. Cell. Physiol. 2017, 232, 1200–1208. [Google Scholar] [CrossRef]
- Suarez-Arnedo, A.; Figueroa, F.T.; Clavijo, C.; Arbeláez, P.; Cruz, J.C.; Muñoz-Camargo, C. An Image J Plugin for the High Throughput Image Analysis of In Vitro Scratch Wound Healing Assays. PLoS ONE 2020, 15, e0232565. [Google Scholar] [CrossRef] [PubMed]






Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maiullari, S.; Cicirelli, A.; Picerno, A.; Giannuzzi, F.; Gesualdo, L.; Notarnicola, A.; Sallustio, F.; Moretti, B. Pulsed Electromagnetic Fields Induce Skeletal Muscle Cell Repair by Sustaining the Expression of Proteins Involved in the Response to Cellular Damage and Oxidative Stress. Int. J. Mol. Sci. 2023, 24, 16631. https://doi.org/10.3390/ijms242316631
Maiullari S, Cicirelli A, Picerno A, Giannuzzi F, Gesualdo L, Notarnicola A, Sallustio F, Moretti B. Pulsed Electromagnetic Fields Induce Skeletal Muscle Cell Repair by Sustaining the Expression of Proteins Involved in the Response to Cellular Damage and Oxidative Stress. International Journal of Molecular Sciences. 2023; 24(23):16631. https://doi.org/10.3390/ijms242316631
Chicago/Turabian StyleMaiullari, Silvia, Antonella Cicirelli, Angela Picerno, Francesca Giannuzzi, Loreto Gesualdo, Angela Notarnicola, Fabio Sallustio, and Biagio Moretti. 2023. "Pulsed Electromagnetic Fields Induce Skeletal Muscle Cell Repair by Sustaining the Expression of Proteins Involved in the Response to Cellular Damage and Oxidative Stress" International Journal of Molecular Sciences 24, no. 23: 16631. https://doi.org/10.3390/ijms242316631
APA StyleMaiullari, S., Cicirelli, A., Picerno, A., Giannuzzi, F., Gesualdo, L., Notarnicola, A., Sallustio, F., & Moretti, B. (2023). Pulsed Electromagnetic Fields Induce Skeletal Muscle Cell Repair by Sustaining the Expression of Proteins Involved in the Response to Cellular Damage and Oxidative Stress. International Journal of Molecular Sciences, 24(23), 16631. https://doi.org/10.3390/ijms242316631





