Osteobiologies for Spinal Fusion from Biological Mechanisms to Clinical Applications: A Narrative Review
Abstract
:1. Introduction
2. The Mechanisms for Bone Regeneration
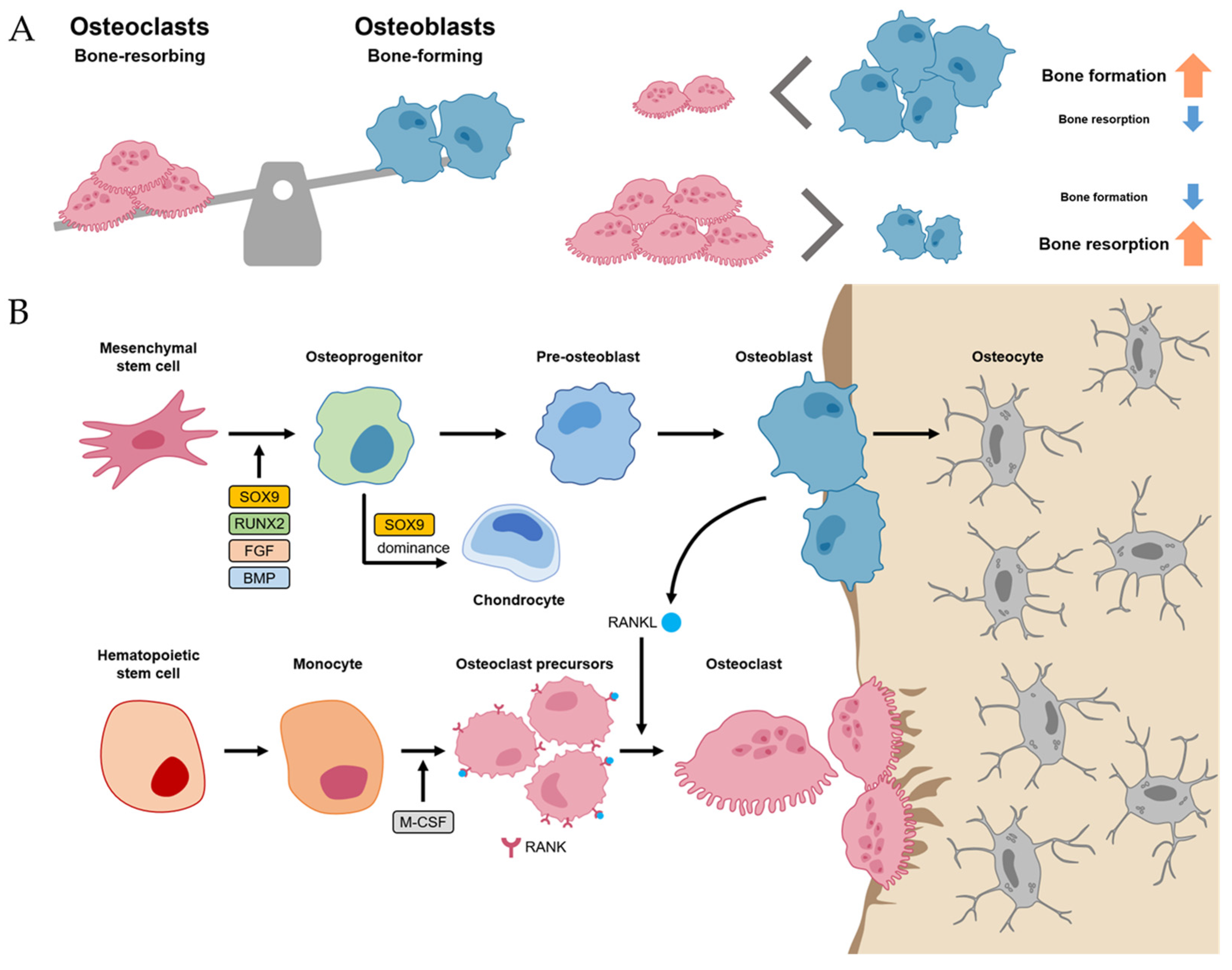
2.1. Bone Homeostasis
2.2. Bone Regeneration Process
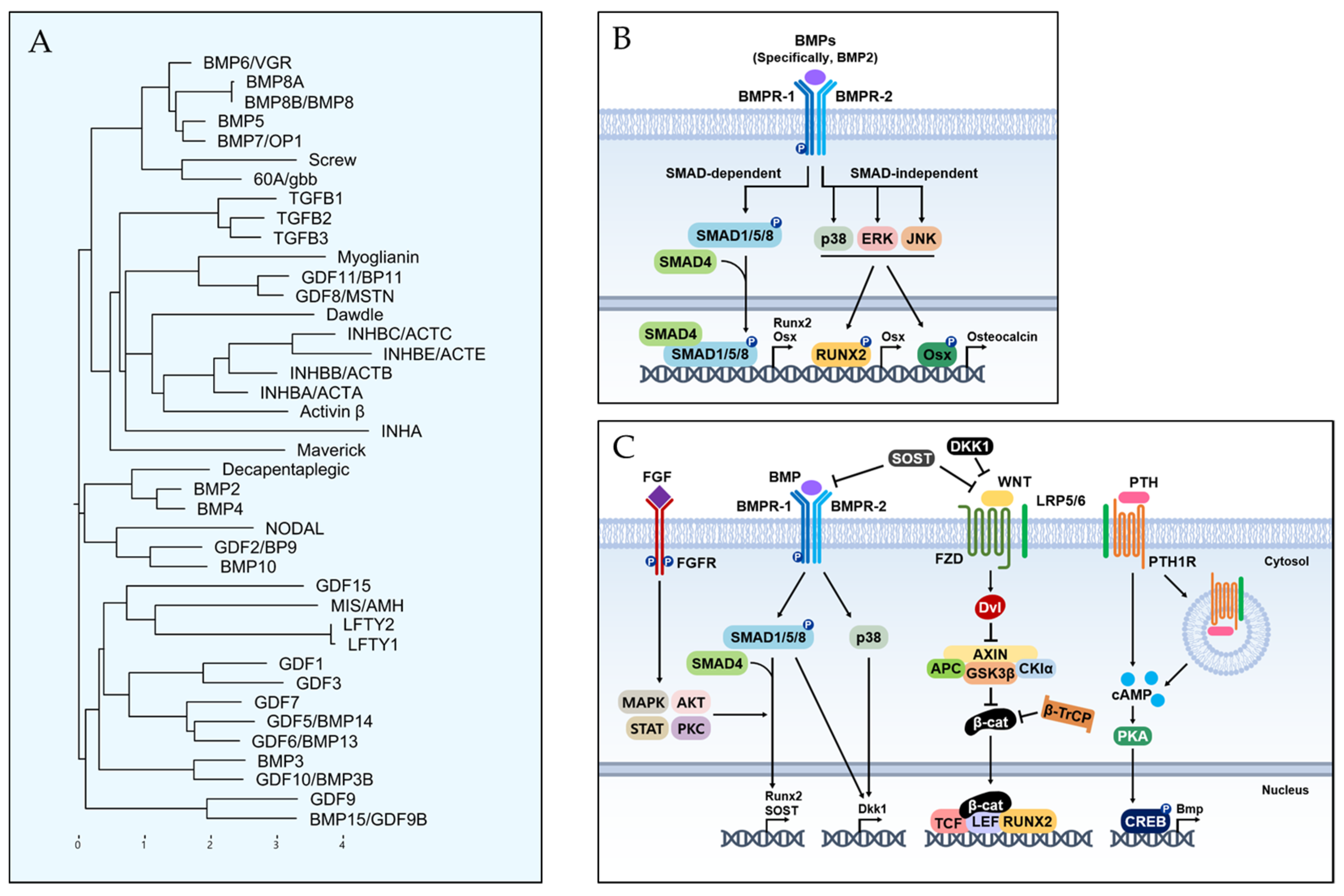
2.3. Signaling Pathways for Bone Formation
3. Bone Morphogenetic Proteins and Their Related Mechanisms
3.1. BMP Lineage
3.2. BMP Signaling Pathway for Osteoblast Differentiation
3.3. Crosstalk between BMP and Other Signaling Pathways
4. Bone Graft Substitutes for Lumbar Interbody Fusions
- Osteoconduction is defined as the physical property of scaffolding, which provides a microstructure to allow for bone ingrowth [39].
- Osteoinduction is defined as the ability to induce the production of osteoblasts, including substances and factors such as BMP [39].
- Osteogenesis is defined as a new bone formation cellular process from the differentiation of the osteoprogenitor cells [39].
4.1. Autogenous Bone Graft Materials (Autografts)
4.2. Allogenous Bone Graft Substitutes (Allografts)
4.3. Demineralized Bone Matrix (DBM)
4.4. Recombinant Human BMP (rhBMP)
5. Current State and Future Aspects of Spinal Fusion
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ferreira, M.L.; de Luca, K.; Haile, L.M.; Steinmetz, J.D.; Culbreth, G.T.; Cross, M.; Kopec, J.A.; Ferreira, P.H.; Blyth, F.M.; Buchbinder, R.; et al. Global, regional, and national burden of low back pain, 1990–2020, its attributable risk factors, and projections to 2050: A systematic analysis of the Global Burden of Disease Study 2021. Lancet Rheumatol. 2023, 5, e316–e329. [Google Scholar] [CrossRef] [PubMed]
- Ravindra, V.M.; Senglaub, S.S.; Rattani, A.; Dewan, M.C.; Hartl, R.; Bisson, E.; Park, K.B.; Shrime, M.G. Degenerative Lumbar Spine Disease: Estimating Global Incidence and Worldwide Volume. Glob. Spine J. 2018, 8, 784–794. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.J.; Yang, J.H.; Chang, D.G.; Lenke, L.G.; Suh, S.W.; Nam, Y.; Park, S.C.; Suk, S.I. Adult Spinal Deformity: A Comprehensive Review of Current Advances and Future Directions. Asian Spine J. 2022, 16, 776–788. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.H.; Ha, K.Y.; Kim, Y.S.; Kim, K.W.; Rhyu, K.W.; Park, J.B.; Shin, J.H.; Kim, Y.Y.; Lee, J.S.; Park, H.Y.; et al. Lumbar Interbody Fusion and Osteobiologics for Lumbar Fusion. Asian Spine J. 2022, 16, 1022–1033. [Google Scholar] [CrossRef]
- Jain, D.; Ray, W.Z.; Vaccaro, A.R. Advances in Techniques and Technology in Minimally Invasive Lumbar Interbody Spinal Fusion. JBJS Rev. 2020, 8, e0171. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.J.; Yang, J.H.; Chang, D.G.; Suk, S.I.; Suh, S.W.; Kim, S.I.; Song, K.S.; Park, J.B.; Cho, W. Proximal Junctional Kyphosis in Adult Spinal Deformity: Definition, Classification, Risk Factors, and Prevention Strategies. Asian Spine J. 2022, 16, 440–450. [Google Scholar] [CrossRef]
- Sherif, S.; Arlet, V. Revision surgery for non-union in adult spinal deformity. Eur. Spine J. 2020, 29 (Suppl. S1), 103–115. [Google Scholar] [CrossRef]
- Tateiwa, D.; Kaito, T. Advances in bone regeneration with growth factors for spinal fusion: A literature review. N. Am. Spine Soc. J. 2023, 13, 100193. [Google Scholar] [CrossRef]
- D’Souza, M.; Macdonald, N.A.; Gendreau, J.L.; Duddleston, P.J.; Feng, A.Y.; Ho, A.L. Graft Materials and Biologics for Spinal Interbody Fusion. Biomedicines 2019, 7, 75. [Google Scholar] [CrossRef]
- Salhotra, A.; Shah, H.N.; Levi, B.; Longaker, M.T. Mechanisms of bone development and repair. Nat. Rev. Mol. Cell Biol. 2020, 21, 696–711. [Google Scholar] [CrossRef]
- Eastell, R.; O’Neill, T.W.; Hofbauer, L.C.; Langdahl, B.; Reid, I.R.; Gold, D.T.; Cummings, S.R. Postmenopausal osteoporosis. Nat. Rev. Dis. Primers 2016, 2, 16069. [Google Scholar] [CrossRef] [PubMed]
- Salazar, V.S.; Gamer, L.W.; Rosen, V. BMP signalling in skeletal development, disease and repair. Nat. Rev. Endocrinol. 2016, 12, 203–221. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Chen, G.; Li, Y.P. TGF-beta and BMP signaling in osteoblast, skeletal development, and bone formation, homeostasis and disease. Bone Res. 2016, 4, 16009. [Google Scholar] [CrossRef]
- Wang, X.; Wang, Y.; Gou, W.; Lu, Q.; Peng, J.; Lu, S. Role of mesenchymal stem cells in bone regeneration and fracture repair: A review. Int. Orthop. 2013, 37, 2491–2498. [Google Scholar] [CrossRef] [PubMed]
- Zhou, G.; Zheng, Q.; Engin, F.; Munivez, E.; Chen, Y.; Sebald, E.; Krakow, D.; Lee, B. Dominance of SOX9 function over RUNX2 during skeletogenesis. Proc. Natl. Acad. Sci. USA 2006, 103, 19004–19009. [Google Scholar] [CrossRef]
- Sun, Y.; Li, J.; Xie, X.; Gu, F.; Sui, Z.; Zhang, K.; Yu, T. Macrophage-Osteoclast Associations: Origin, Polarization, and Subgroups. Front. Immunol. 2021, 12, 778078. [Google Scholar] [CrossRef] [PubMed]
- Lademann, F.; Hofbauer, L.C.; Rauner, M. The Bone Morphogenetic Protein Pathway: The Osteoclastic Perspective. Front. Cell Dev. Biol. 2020, 8, 586031. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.C.; Econs, M.J.; DiMeglio, L.A.; Insogna, K.L.; Levine, M.A.; Orchard, P.J.; Miller, W.P.; Petryk, A.; Rush, E.T.; Shoback, D.M.; et al. Diagnosis and Management of Osteopetrosis: Consensus Guidelines From the Osteopetrosis Working Group. J. Clin. Endocrinol. Metab. 2017, 102, 3111–3123. [Google Scholar] [CrossRef]
- Einhorn, T.A.; Gerstenfeld, L.C. Fracture healing: Mechanisms and interventions. Nat. Rev. Rheumatol. 2015, 11, 45–54. [Google Scholar] [CrossRef]
- Ansari, M. Bone tissue regeneration: Biology, strategies and interface studies. Prog. Biomater. 2019, 8, 223–237. [Google Scholar] [CrossRef]
- Perren, S.M.; Rickman, M.; Varghese, V.D.; Koh, A.; Guerado, E.; Giannoudis, P.V.; Rajfer, R.A.; Kilic, A.; Neviaser, A.S.; Schulte, L.M.; et al. Evolution of the internal fixation of long bone fractures. The scientific basis of biological internal fixation: Choosing a new balance between stability and biology. J. Bone Jt. Surg. Br. 2002, 84, 1093–1110. [Google Scholar] [CrossRef]
- Kelly, P.J.; Montgomery, R.J.; Bronk, J.T. Reaction of the circulatory system to injury and regeneration. Clin. Orthop. Relat. Res. 1990, 254, 275–288. [Google Scholar] [CrossRef]
- Tickle, C.; Towers, M. Sonic Hedgehog Signaling in Limb Development. Front. Cell Dev. Biol. 2017, 5, 14. [Google Scholar] [CrossRef] [PubMed]
- Van der Eerden, B.C.; Karperien, M.; Gevers, E.F.; Löwik, C.W.; Wit, J.M. Expression of Indian hedgehog, parathyroid hormone-related protein, and their receptors in the postnatal growth plate of the rat: Evidence for a locally acting growth restraining feedback loop after birth. J. Bone Miner. Res. 2000, 15, 1045–1055. [Google Scholar] [CrossRef]
- Chan, W.C.W.; Tan, Z.; To, M.K.T.; Chan, D. Regulation and Role of Transcription Factors in Osteogenesis. Int. J. Mol. Sci. 2021, 22, 5445. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Xiao, Q.; Xiao, J.; Niu, C.; Li, Y.; Zhang, X.; Zhou, Z.; Shu, G.; Yin, G. Wnt/β-catenin signalling: Function, biological mechanisms, and therapeutic opportunities. Signal Transduct. Target. Ther. 2022, 7, 3. [Google Scholar] [CrossRef]
- Doumpas, N.; Lampart, F.; Robinson, M.D.; Lentini, A.; Nestor, C.E.; Cantù, C.; Basler, K. TCF/LEF dependent and independent transcriptional regulation of Wnt/β-catenin target genes. EMBO J. 2019, 38, e98873. [Google Scholar] [CrossRef]
- Marie, P.J. Fibroblast growth factor signaling controlling osteoblast differentiation. Gene 2003, 316, 23–32. [Google Scholar] [CrossRef]
- Grgurevic, L.; Pecina, M.; Vukicevic, S.; Marshall, R. Urist and the discovery of bone morphogenetic proteins. Int. Orthop. 2017, 41, 1065–1069. [Google Scholar] [CrossRef]
- Schmierer, B.; Hill, C.S. TGFbeta-SMAD signal transduction: Molecular specificity and functional flexibility. Nat. Rev. Mol. Cell Biol. 2007, 8, 970–982. [Google Scholar] [CrossRef]
- Dereeper, A.; Guignon, V.; Blanc, G.; Audic, S.; Buffet, S.; Chevenet, F.; Dufayard, J.F.; Guindon, S.; Lefort, V.; Lescot, M.; et al. Phylogeny.fr: Robust phylogenetic analysis for the non-specialist. Nucleic Acids Res. 2008, 36, W465–W469. [Google Scholar] [CrossRef]
- Hinck, A.P. Structural studies of the TGF-βs and their receptors—Insights into evolution of the TGF-β superfamily. FEBS Lett. 2012, 586, 1860–1870. [Google Scholar] [CrossRef] [PubMed]
- Kamiya, N.; Kobayashi, T.; Mochida, Y.; Yu, P.B.; Yamauchi, M.; Kronenberg, H.M.; Mishina, Y. Wnt inhibitors Dkk1 and Sost are downstream targets of BMP signaling through the type IA receptor (BMPRIA) in osteoblasts. J. Bone Miner. Res. 2010, 25, 200–210. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Oyajobi, B.O.; Harris, S.E.; Chen, D.; Tsao, C.; Deng, H.W.; Zhao, M. Wnt/β-catenin signaling activates bone morphogenetic protein 2 expression in osteoblasts. Bone 2013, 52, 145–156. [Google Scholar] [CrossRef]
- Kuhn, L.T.; Ou, G.; Charles, L.; Hurley, M.M.; Rodner, C.M.; Gronowicz, G. Fibroblast growth factor-2 and bone morphogenetic protein-2 have a synergistic stimulatory effect on bone formation in cell cultures from elderly mouse and human bone. J. Gerontol. A Biol. Sci. Med. Sci. 2013, 68, 1170–1180. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Cui, Y.; Luan, J.; Zhou, X.; Liu, Z.; Han, J. Fibroblast growth factor-2 inhibits mineralization of osteoblast-like Saos-2 cells by inhibiting the functioning of matrix vesicles. Drug Discov. Ther. 2014, 8, 42–47. [Google Scholar] [CrossRef] [PubMed]
- De Jong, D.S.; Steegenga, W.T.; Hendriks, J.M.; van Zoelen, E.J.; Olijve, W.; Dechering, K.J. Regulation of Notch signaling genes during BMP2-induced differentiation of osteoblast precursor cells. Biochem. Biophys. Res. Commun. 2004, 320, 100–107. [Google Scholar] [CrossRef]
- Yu, B.; Zhao, X.; Yang, C.; Crane, J.; Xian, L.; Lu, W.; Wan, M.; Cao, X. Parathyroid hormone induces differentiation of mesenchymal stromal/stem cells by enhancing bone morphogenetic protein signaling. J. Bone Miner. Res. 2012, 27, 2001–2014. [Google Scholar] [CrossRef]
- Albrektsson, T.; Johansson, C. Osteoinduction, osteoconduction and osseointegration. Eur. Spine J. 2001, 10 (Suppl. S2), S96–S101. [Google Scholar] [CrossRef]
- Whitehead, J.; Kothambawala, A.; Leach, J.K. Morphogen Delivery by Osteoconductive Nanoparticles Instructs Stromal Cell Spheroid Phenotype. Adv. Biosyst. 2019, 3, 1900141. [Google Scholar] [CrossRef]
- Elkhenany, H.; Bourdo, S.; Hecht, S.; Donnell, R.; Gerard, D.; Abdelwahed, R.; Lafont, A.; Alghazali, K.; Watanabe, F.; Biris, A.S.; et al. Graphene nanoparticles as osteoinductive and osteoconductive platform for stem cell and bone regeneration. Nanomedicine 2017, 13, 2117–2126. [Google Scholar] [CrossRef]
- Zou, F.; Jiang, J.; Lv, F.; Xia, X.; Ma, X. Preparation of antibacterial and osteoconductive 3D-printed PLGA/Cu(I)@ZIF-8 nanocomposite scaffolds for infected bone repair. J. Nanobiotechnol. 2020, 18, 39. [Google Scholar] [CrossRef] [PubMed]
- Lo, W.C.; Tsai, L.W.; Yang, Y.S.; Chan, R.W.Y. Understanding the Future Prospects of Synergizing Minimally Invasive Transforaminal Lumbar Interbody Fusion Surgery with Ceramics and Regenerative Cellular Therapies. Int. J. Mol. Sci. 2021, 22, 3638. [Google Scholar] [CrossRef] [PubMed]
- Park, J.B.; Yang, J.H.; Chang, D.G.; Suk, S.I.; Suh, S.W.; Kim, G.U.; Choi, J.Y.; Seo, J.Y.; Park, H.Y.; Kim, S.I.; et al. Comparison of Union Rates Between Autogenous Iliac Crest Bone Graft and Local Bone Graft as Fusion Materials in Lumbar Fusion Surgery: An Evaluation of Up to 3-Level Fusion. World Neurosurg. 2020, 139, e286–e292. [Google Scholar] [CrossRef]
- Sengupta, D.K.; Truumees, E.; Patel, C.K.; Kazmierczak, C.; Hughes, B.; Elders, G.; Herkowitz, H.N. Outcome of local bone versus autogenous iliac crest bone graft in the instrumented posterolateral fusion of the lumbar spine. Spine 2006, 31, 985–991. [Google Scholar] [CrossRef] [PubMed]
- Putzier, M.; Strube, P.; Funk, J.F.; Gross, C.; Mönig, H.J.; Perka, C.; Pruss, A. Allogenic versus autologous cancellous bone in lumbar segmental spondylodesis: A randomized prospective study. Eur. Spine J. 2009, 18, 687–695. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Yang, L.; Yang, X.G.; Wang, F.; Feng, J.T.; Hua, K.C.; Li, Q.; Hu, Y.C. Demineralized Bone Matrix Carriers and their Clinical Applications: An Overview. Orthop. Surg. 2019, 11, 725–737. [Google Scholar] [CrossRef] [PubMed]
- Schizas, C.; Triantafyllopoulos, D.; Kosmopoulos, V.; Tzinieris, N.; Stafylas, K. Posterolateral lumbar spine fusion using a novel demineralized bone matrix: A controlled case pilot study. Arch. Orthop. Trauma. Surg. 2008, 128, 621–625. [Google Scholar] [CrossRef]
- Epstein, N.E.; Epstein, J.A. SF-36 outcomes and fusion rates after multilevel laminectomies and 1 and 2-level instrumented posterolateral fusions using lamina autograft and demineralized bone matrix. J. Spinal Disord. Tech. 2007, 20, 139–145. [Google Scholar] [CrossRef]
- Faundez, A.; Tournier, C.; Garcia, M.; Aunoble, S.; Le Huec, J.C. Bone morphogenetic protein use in spine surgery-complications and outcomes: A systematic review. Int. Orthop. 2016, 40, 1309–1319. [Google Scholar] [CrossRef]
- Carreon, L.Y.; Glassman, S.D.; Djurasovic, M.; Campbell, M.J.; Puno, R.M.; Johnson, J.R.; Dimar, J.R., 2nd. RhBMP-2 versus iliac crest bone graft for lumbar spine fusion in patients over 60 years of age: A cost-utility study. Spine 2009, 34, 238–243. [Google Scholar] [CrossRef]
- Shahlaie, K.; Kim, K.D. Occipitocervical fusion using recombinant human bone morphogenetic protein-2: Adverse effects due to tissue swelling and seroma. Spine 2008, 33, 2361–2366. [Google Scholar] [CrossRef]
- Rihn, J.A.; Makda, J.; Hong, J.; Patel, R.; Hilibrand, A.S.; Anderson, D.G.; Vaccaro, A.R.; Albert, T.J. The use of RhBMP-2 in single-level transforaminal lumbar interbody fusion: A clinical and radiographic analysis. Eur. Spine J. 2009, 18, 1629–1636. [Google Scholar] [CrossRef]
- Comer, G.C.; Smith, M.W.; Hurwitz, E.L.; Mitsunaga, K.A.; Kessler, R.; Carragee, E.J. Retrograde ejaculation after anterior lumbar interbody fusion with and without bone morphogenetic protein-2 augmentation: A 10-year cohort controlled study. Spine J. 2012, 12, 881–890. [Google Scholar] [CrossRef]
- Lubelski, D.; Abdullah, K.G.; Nowacki, A.S.; Alvin, M.D.; Steinmetz, M.P.; Chakka, S.; Li, Y.; Gajewski, N.; Benzel, E.C.; Mroz, T.E. Urological complications following use of recombinant human bone morphogenetic protein-2 in anterior lumbar interbody fusion: Presented at the 2012 Joint Spine Section Meeting: Clinical article. J Neurosurg Spine 2013, 18, 126–131. [Google Scholar] [CrossRef] [PubMed]
- Owens, K.; Glassman, S.D.; Howard, J.M.; Djurasovic, M.; Witten, J.L.; Carreon, L.Y. Perioperative complications with rhBMP-2 in transforaminal lumbar interbody fusion. Eur. Spine J. 2011, 20, 612–617. [Google Scholar] [CrossRef] [PubMed]
- Jain, A.; Hassanzadeh, H.; Strike, S.A.; Skolasky, R.L.; Riley, L.H., 3rd. rhBMP use in cervical spine surgery: Associated factors and in-hospital complications. J. Bone Jt. Surg. Am. 2014, 96, 617–623. [Google Scholar] [CrossRef] [PubMed]
- Tannoury, C.A.; An, H.S. Complications with the use of bone morphogenetic protein 2 (BMP-2) in spine surgery. Spine J. 2014, 14, 552–559. [Google Scholar] [CrossRef] [PubMed]
- Dahabreh, Z.; Calori, G.M.; Kanakaris, N.K.; Nikolaou, V.S.; Giannoudis, P.V. A cost analysis of treatment of tibial fracture nonunion by bone grafting or bone morphogenetic protein-7. Int. Orthop. 2009, 33, 1407–1414. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, C.; Ge, X. Clinical and radiographic outcomes of stand-alone oblique lateral interbody fusion in the treatment of adult degenerative scoliosis: A retrospective observational study. BMC Musculoskelet. Disord. 2022, 23, 1133. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.M.; You, K.H.; Kang, M.S.; Kim, J.H.; Park, H.J. Oblique Lumbar Interbody Fusion with Selective Biportal Endoscopic Posterior Decompression for Multilevel Lumbar Degenerative Diseases. Asian Spine J. 2023, 17, 392–400. [Google Scholar] [CrossRef] [PubMed]
- Fujibayashi, S.; Takemoto, M.; Ishii, K.; Funao, H.; Isogai, N.; Otsuki, B.; Shimizu, T.; Nakamura, T.; Matsuda, S. Multicenter Prospective Study of Lateral Lumbar Interbody Fusions Using Bioactive Porous Titanium Spacers without Bone Grafts. Asian Spine J. 2022, 16, 890–897. [Google Scholar] [CrossRef] [PubMed]
- Mittal, S.; Sudhakar, P.V.; Ahuja, K.; Ifthekar, S.; Yadav, G.; Sinha, S.; Goyal, N.; Verma, V.; Sarkar, B.; Kandwal, P. Deformity Correction with Interbody Fusion Using Lateral versus Posterior Approach in Adult Degenerative Scoliosis: A Systematic Review and Observational Meta-analysis. Asian Spine J. 2023, 17, 431–451. [Google Scholar] [CrossRef] [PubMed]
- De Stefano, F.A.; Elarjani, T.; Burks, J.D.; Burks, S.S.; Levi, A.D. Dose Adjustment Associated Complications of Bone Morphogenetic Protein: A Longitudinal Assessment. World Neurosurg. 2021, 156, e64–e71. [Google Scholar] [CrossRef]
- Blanco, J.F.; Villarón, E.M.; Pescador, D.; da Casa, C.; Gómez, V.; Redondo, A.M.; López-Villar, O.; López-Parra, M.; Muntión, S.; Sánchez-Guijo, F. Autologous mesenchymal stromal cells embedded in tricalcium phosphate for posterolateral spinal fusion: Results of a prospective phase I/II clinical trial with long-term follow-up. Stem Cell Res. Ther. 2019, 10, 63. [Google Scholar] [CrossRef]
- Gan, Y.; Dai, K.; Zhang, P.; Tang, T.; Zhu, Z.; Lu, J. The clinical use of enriched bone marrow stem cells combined with porous beta-tricalcium phosphate in posterior spinal fusion. Biomaterials 2008, 29, 3973–3982. [Google Scholar] [CrossRef]
- Zhou, T.; Yuan, Z.; Weng, J.; Pei, D.; Du, X.; He, C.; Lai, P. Challenges and advances in clinical applications of mesenchymal stromal cells. J. Hematol. Oncol. 2021, 14, 24. [Google Scholar] [CrossRef]
- Wang, Y.; Yi, H.; Song, Y. The safety of MSC therapy over the past 15 years: A meta-analysis. Stem Cell Res. Ther. 2021, 12, 545. [Google Scholar] [CrossRef]
- Fay, F.S.; Carrington, W.; Fogarty, K.E. Three-dimensional molecular distribution in single cells analysed using the digital imaging microscope. J. Microsc. 1989, 153 Pt 2, 133–149. [Google Scholar] [CrossRef]


| Class | Potential Properties | ||
|---|---|---|---|
| Osteogenic | Osteoconductive | Osteoinductive | |
| Autograft (e.g., Cancellous bone of ICBG) | +++ | +++ | +++ |
| Allograft (e.g., Freeze-dried bone, DBM) | − | + | +/− (allobone) ++ (DBM) |
| Growth-factor-based substitutes (e.g., rhBMP-7) | − | − | ++ |
| Class | Class | Functions |
|---|---|---|
| BMP-1 | Metalloprotease | Regulation of the formation of the extracellular matrix (ECM) via acting on procollagen I, II, and III |
| BMP-2 | TGP-β family | Key role in osteoblast differentiation, cartilage and bone morphogenesis, heart formation |
| BMP-3 | TGP-β family | Ostegenin, inhibition of osteogenesis |
| BMP-4 | TGP-β family | Regulation of the formation of teeth, limbs, and bone from mesoderms. An important role in fracture repair |
| BMP-5 | TGP-β family | Cartilage development |
| BMP-6 | TGP-β family | Osteoblast differentiation, chondrogenesis A role in joint integrity in adults |
| BMP-7 | TGP-β family | Osteogenic protein-1 (OP-1), osteoblast differentiation, development of kidney and eye |
| BMP-8 | TGP-β family | Osteogenic protein-1 (OP-1), bone and cartilage development |
| Class | Mechanisms | Efficacy | Limitations |
|---|---|---|---|
| ICBG | All capabilities | Traditional gold standard | Donor site morbidity |
| Allobone | Osteoconductive capabilities | Nearly not limited to the graft amounts | Infection risk of HBV or HCV |
| DBM | Osteoinductive and osteoconductive capabilities | Non-inferior to autoBG | Higher rates of spinal collapse |
| BMP | Stimulation of osteogenic differentiation of MSCs and new bone formation (i.e., osteoinduction) | Superior fusion rate in BMP/autoBG | Dosage and safety concerns |
| MSCs | Enhancement of spinal fusion by osteogenic effect | Not proven | Not proven |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Keum, B.-R.; Kim, H.J.; Kim, G.-H.; Chang, D.-G. Osteobiologies for Spinal Fusion from Biological Mechanisms to Clinical Applications: A Narrative Review. Int. J. Mol. Sci. 2023, 24, 17365. https://doi.org/10.3390/ijms242417365
Keum B-R, Kim HJ, Kim G-H, Chang D-G. Osteobiologies for Spinal Fusion from Biological Mechanisms to Clinical Applications: A Narrative Review. International Journal of Molecular Sciences. 2023; 24(24):17365. https://doi.org/10.3390/ijms242417365
Chicago/Turabian StyleKeum, Byeong-Rak, Hong Jin Kim, Gun-Hwa Kim, and Dong-Gune Chang. 2023. "Osteobiologies for Spinal Fusion from Biological Mechanisms to Clinical Applications: A Narrative Review" International Journal of Molecular Sciences 24, no. 24: 17365. https://doi.org/10.3390/ijms242417365





