Donor Heart Preservation: Current Knowledge and the New Era of Machine Perfusion
Abstract
:1. Introduction
2. Cold Cardioplegia and Ischemic Damage
3. Support of the Donor
4. Protection of the Donor Heart
| Extracellular Solutions | Intracellular Solutions |
|---|---|
| Composition proportional to extracellular space | Composition proportional to intracellular space |
| Potassium concentration = 5–30 mmol/L | Potassium concentration = 30–125 mmol/L |
| Sodium concentration ≥ 70 mmol/L | Sodium concentration < 70 mmol/L |
| Asystole due to the increased concentration of potassium in the myocardium | Asystole due to the decrease in the concentration difference of ions on either side of the cell membrane of the myocardial cells |
| Krebs, St Thomas, Celsior | Custodiol, University of Wisconsin, Stanford |
5. Ex Vivo Machine Perfusion as a Novel Strategy to Preserve the Donor Heart
5.1. Normothermic Machine Perfusion: The Organ Care System
5.1.1. Experimental Studies with Organ Care System
5.1.2. Clinical Studies with the Organ Care System
5.2. Hypothermic Machine Perfusion: Ex Vivo Non-Ischemic Heart Preservation (NIHP)
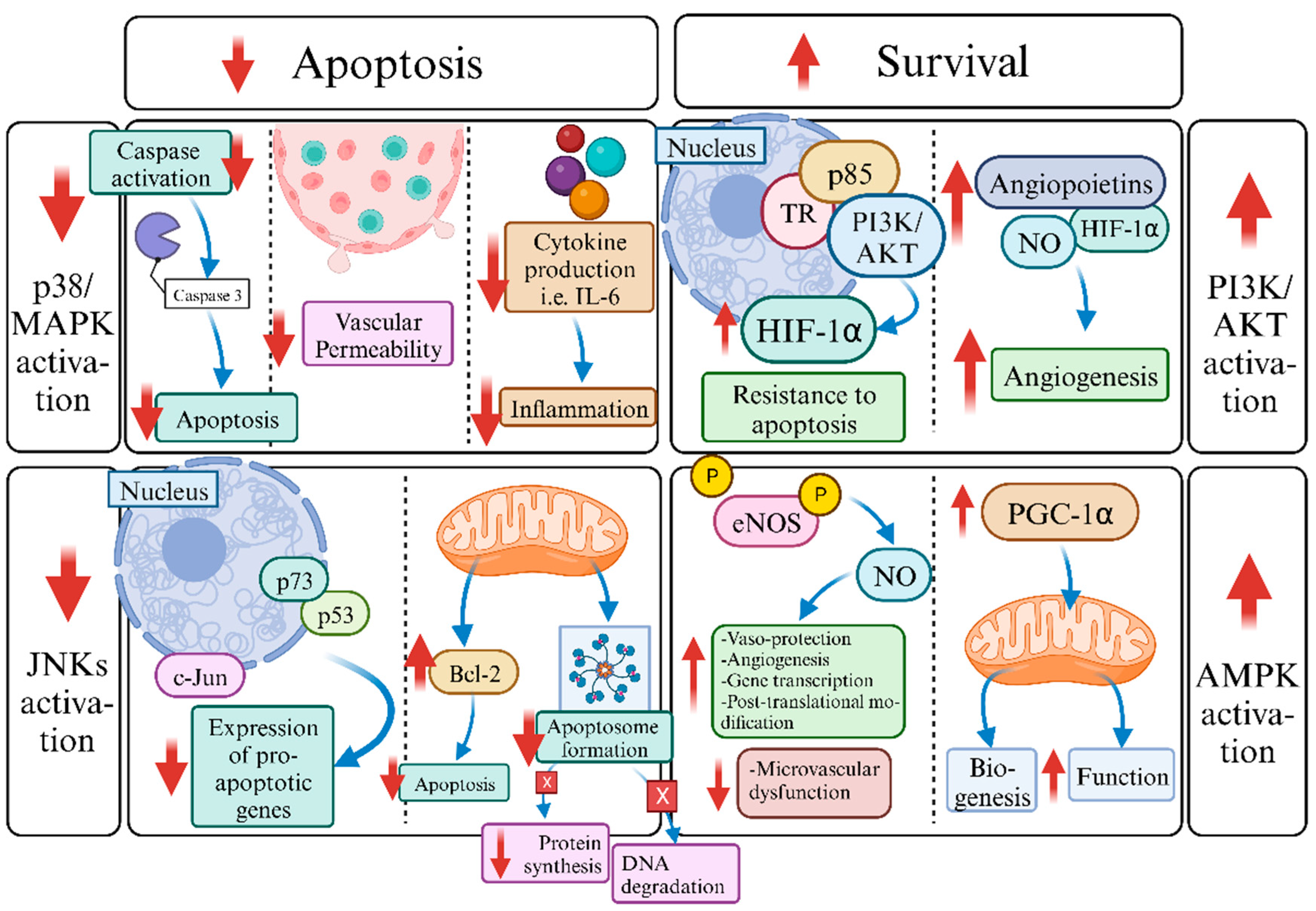
6. The Role of Triiodothyronine (T3) in Machine Normothermic Perfusion: Future Challenges
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hong, C.C.; Macdonald, P.S.; Dhital, K.K. The donor heart and organ perfusion technology. J. Thorac. Dis. 2019, 11 (Suppl. 6), 938–945. [Google Scholar]
- Repse, S.; Pepe, S.; Anderson, J.; McLean, C.; Rosenfeldt, F.L. Cardiac reanimation for donor heart transplantation after circulatory death. J. Heart Lung Transplant. 2010, 7, 747–755. [Google Scholar] [CrossRef] [PubMed]
- Page, A.; Messer, S.; Large, S.R. Heart transplantation after circulatory determined death. Ann. Cardiothorac. Surg. 2018, 1, 75–81. [Google Scholar] [CrossRef] [PubMed]
- Witter, T.; Wittwer, T.; Wahlers, T. Marginal donor grafts in heart transplantation: Lessons learned from 25 years experience. Transpl. Int. 2008, 2, 113–125. [Google Scholar] [CrossRef]
- Jahania, M.S.; Sanchez, J.A.; Narayan, P.; Lasley, R.D.; Mentzer, R.M., Jr. Heart preservation for transplantation: Principles and strategies. Ann. Thorac. Surg. 1999, 5, 1983–1987. [Google Scholar] [CrossRef]
- Rivard, A.L.; Gallegos, R.P.; Bianco, R.W.; Liao, K. The basic science aspect of donor heart preservation: A review. Extra Corpor. Technol. 2004, 3, 269–274. [Google Scholar] [CrossRef]
- Monteagudo Vela, M.; Garcia Saez, D.; Simon, A.R. Current approaches in retrieval and heart preservation. Ann. Cardiothoracic. Surg. 2018, 1, 67–74. [Google Scholar] [CrossRef]
- Taylor, D.O.; Stehlik, J.; Edwards, L.B.; Aurora, P.; Christie, J.D.; Dobbels, F.; Hertz, M. Registry of the International Society for Heart and Lung Transplantation: Twenty-six official adult heart transplant report-2009. J. Heart Lung. Transplant. 2009, 28, 1007–1022. [Google Scholar] [CrossRef]
- Wei, J.; Chen, S.; Xue, S.; Zhu, Q.; Liu, S.; Cui, L.; Hua, X.; Wang, Y. Blockade of inflammation and apoptosis pathways by siRNA prolongs Cold Preservation Time and Protects Donor Hearts in a Porcine Model. Mol. Ther. Nucleic Acids 2017, 9, 428–439. [Google Scholar] [CrossRef]
- Drinkwater, D.C.; Rudis, E.; Laks, H.; Ziv, E.; Marino, J.; Stein, D.; Ardehali, A.; Aharon, A.; Moriguchi, J.; Kobashigawa, J. University of Wisconsin Solution versus Sanford cardioplegic solution and the development of cardiac allograft vasculopathy. J. Heart Lung Transplant. 1995, 5, 891–896. [Google Scholar]
- Goldsmith, K.A.; Demiris, N.; Gooi, J.H.; Sharples, L.D.; Jenkins, D.P.; Dhital, K.K.; Tsui, S.S. Life-years gained by reducing donor heart ischemic times. Transplantation 2009, 87, 243–248. [Google Scholar] [CrossRef] [PubMed]
- Stephenson, E.R., Jr.; Jayawant, A.M.; Baumgarten, C.M.; Damiano, R.J., Jr. Cardioplegia-induced cell swelling: Prevention by normothermic infusion. Ann. Thorac. Surg. 2000, 69, 1393–1398. [Google Scholar] [CrossRef] [PubMed]
- Chambers, D.J.; Hearse, D.J. Developments in cardioprotection: “polarized” arrest as an alternative to “depolarized” arrest. Ann. Thorac. Surg. 1999, 68, 1960–1966. [Google Scholar] [CrossRef]
- Robson, S.C.; Candinas, D.; Hancock, W.W.; Wrighton, C.; Winkler, H.; Bach, F.H. Role of endothelial cells in transplantation. Int. Arch. Allergy Immunol. 1995, 106, 305–322. [Google Scholar] [CrossRef]
- Parolari, A.; Rubini, P.; Cannata, A.; Bonati, L.; Alamanni, F.; Tremoli, E.; Biglioli, P. Endothelial damage during myocardial preservation and storage. Ann. Thorac. Surg. 2002, 73, 682–690. [Google Scholar] [CrossRef] [PubMed]
- Menasche, P.; Piwnica, A. Free radicals and myocardial protection: A surgical viewpoint. Ann. Thorac. Surg. 1989, 47, 939–945. [Google Scholar] [CrossRef]
- Sellke, F.W.; Shafique, T.; Johnson, R.G.; Dai, H.B.; Banitt, P.F.; Schoen, F.J.; Weintraub, R.M. Blood and albumin cardioplegia preserve endothelium-dependent microvascular responses. Ann. Thorac. Surg. 1993, 55, 977–985. [Google Scholar] [CrossRef]
- Malinoski, D.J.; Patel, M.S.; Ahmed, O.; Daly, M.C.; Mooney, S.; Graybill, C.O.; Foster, C.E.; Salim, A. United Network for Organ Sharing (UNOS) Region 5 Donor Management Goals (DMG) Workgroup. The impact of meeting donor management goals on the development of delayed graft function in kidney transplant recipients. Am. J. Transplant. 2013, 4, 993–1000. [Google Scholar] [CrossRef]
- Tong, C.K.W.; Khush, K.K. New Approaches to Donor Selection and Preparation in Heart Transplantation. Curr. Treat. Options Cardiovasc. Med. 2021, 5, 28. [Google Scholar] [CrossRef]
- Kilic, A.; Emani, S.; Sai-Sudhakar, C.B.; Higgins, R.S.; Whitson, B.A. Donor selection in heart transplantation. J. Thorac. Dis. 2014, 8, 1097–1104. [Google Scholar]
- Shemie, S.D.; Baker, A.J.; Knoll, G.; Wall, W.; Rocker, G.; Howes, D.; Davidson, J.; Pagliarello, J.; Chambers-Evans, J.; Cockfield, S.; et al. National recommendations for donation after cardiocirculatory death in Canada: Donation after cardiocirculatory death in Canada. CMAJ 2006, 175, S1. [Google Scholar] [CrossRef]
- Ball, I.M.; Hornby, L.; Rochwerg, B.; Weiss, M.J.; Gillrie, C.; Chassé, M.; D’Aragon, F.; Meade, M.O.; Soliman, K.; Ali, A.; et al. Management of the neurologically deceased organ donor: A Canadian clinical practice guideline. CMAJ 2020, 192, E361–E369. [Google Scholar] [CrossRef]
- Novitzky, D.; Cooper, D.K.; Reichart, B. Hemodynamic and metabolic responses to hormonal therapy in brain-dead potential organ donors. Transplantation 1987, 43, 852–854. [Google Scholar] [CrossRef] [PubMed]
- Peled, Y.; Lavee, J.; Kassif, Y.; Arad, M.; Kogan, A.; Peled, A.; Tirosh, A.; Sternik, L.; Ram, E. Donor thyroid hormone therapy is associated with an increased risk of graft dysfunction after heart transplantation. Clin. Transplant. 2020, 34, 13887. [Google Scholar] [CrossRef]
- Zaroff, J.G.; Rosengard, B.R.; Armstrong, W.F.; Babcock, W.D.; D’Alessandro, A.; Dec, G.W.; Edwards, N.M.; Higgins, R.S.; Jeevanandum, V.; Kauffman, M.; et al. Consensus conference report: Maximizing use of organs recovered from the cadaver donor: Cardiac recommendations, March 28–29, 2001, Crystal City, Va. Circulation 2002, 106, 836–841. [Google Scholar] [CrossRef] [PubMed]
- Pantos, C.; Mourouzis, I. Translating thyroid hormone effects into clinical practice: The relevance of thyroid hormone receptor α1 in cardiac repair. Heart. Fail. Rev. 2015, 3, 273–282. [Google Scholar] [CrossRef] [PubMed]
- Pantos, C.; Mourouzis, I.; Saranteas, T.; Brozou, V.; Galanopoulos, G.; Kostopanagiotou, G.; Cokkinos, D.V. Acute T3 treatment protects the heart against ischemia-reperfusion injury via TRα1 receptor. Mol. Cell. Biochem. 2011, 353, 235–241. [Google Scholar] [CrossRef]
- Naqvi, N.; Li, M.; Calvert, J.W.; Tejada, T.; Lambert, J.P.; Wu, J.; Kesteven, S.H.; Holman, S.R.; Matsuda, T.; Lovelock, J.D.; et al. A proliferative burst during preadolescence establishes the final cardiomyocyte number. Cell 2014, 157, 795–807. [Google Scholar] [CrossRef]
- Singh, S.S.A.; Das De, S.; Spadaccio, C.; Berry, C.; Al-Attar, N. An overview of different methods of myocardial protection currently employed peri-transplantation. Vessel. Plus 2017, 1, 213–219. [Google Scholar] [CrossRef]
- Minasian, S.M.; Galagudza, M.M.; Dmitriev, Y.V.; Karpov, A.A.; Vlasov, T.D. Preservation of the donor heart: From basic science to clinical studies. Interact Cardiovasc. Thorac. Surg. 2015, 4, 510–519. [Google Scholar] [CrossRef]
- Michel, S.G.; La Muraglia, G.M., 2nd; Madariaga, M.L.; Titus, J.S.; Selig, M.K.; Farkash, E.A.; Allan, J.S.; Anderson, L.M.; Madsen, J.C. Preservation of donor hearts using hypothermic oxygenated perfusion. Ann. Transplant. 2014, 19, 409–416. [Google Scholar] [PubMed]
- Minasian, S.M.; Galagudza, M.M.; Dmitriev, Y.V.; Kurapeev, D.I.; Vlasov, T.D. Myocardial protection against global ischemia with Krebs-Henseleit buffer-based cardioplegic solution. J. Cardiothorac. Surg. 2013, 8, 60. [Google Scholar] [CrossRef] [PubMed]
- Comentale, G.; Giordano, R.; Palma, G. Comparison of the different cardioplegic strategies in cardiac valves surgery: Who wins the “arm-wrestling”? J. Thorac. Dis. 2018, 2, 714–717. [Google Scholar] [CrossRef]
- Bryner, B.S.; Schroder, J.N.; Milano, C.A. Heart transplant advances: Ex vivo organ-preservation systems. JTCVS Open 2021, 8, 123–127. [Google Scholar] [CrossRef]
- Kobashigawa, J.; Zuckermann, A.; Macdonald, P.; Leprince, P.; Esmailian, F.; Luu, M.; Mancini, D.; Patel, J.; Razi, R.; Reichenspurner, H.; et al. Consensus Conference participants. Report from a consensus conference on primary graft dysfunction after cardiac transplantation. J. Heart Lung Transplant. 2014, 33, 327–340. [Google Scholar] [CrossRef]
- Detry, O.; Le Dinh, H.; Noterdaeme, T.; De Roover, A.; Honoré, P.; Squifflet, J.P.; Meurisse, M. Categories of donation after cardiocirculatory death. Transplant. Proc. 2012, 44, 1189–1195. [Google Scholar] [CrossRef]
- Stehlik, J.; Edwards, L.B.; Kucheryavaya, A.Y.; Benden, C.; Christie, J.D.; Dipchand, A.I.; Dobbels, F.; Kirk, R.; Rahmel, A.O.; Hertz, M.I. International Society of Heart and Lung Transplantation. The Registry of the International Society for Heart and Lung Transplantation: 29th official adult heart transplant report-2012. J. Heart Lung Transplant. 2012, 10, 1052–1064. [Google Scholar] [CrossRef] [PubMed]
- Russo, M.J.; Iribarne, A.; Hong, K.N.; Ramlawi, B.; Chen, J.M.; Takayama, H.; Mancini, D.M.; Naka, Y. Factors associated with primary graft failure after heart transplantation. Transplantation 2010, 90, 444–450. [Google Scholar] [CrossRef]
- Stamp, N.L.; Shah, A.; Vincent, V.; Wright, B.; Wood, C.; Pavey, W.; Cokis, C.; Chih, S.; Dembo, L.; Larbalestier, R. Successful Heart Transplant after Ten Hours Out-of-body Time using the TransMedics Organ Care System. Heart. Lung Circ. 2015, 6, 611–613. [Google Scholar] [CrossRef]
- Leprince, P.; Popov, A.F.; Simon, A.R.; Benk, C.; Siepe, M. Ex vivo perfusion of the heart with the use of the Organ Care System. Eur. J. Cardiothorac. Surg. 2016, 49, 1318–1320. [Google Scholar] [CrossRef]
- Dhital, K.; Connellan, M.; Chew, H.; Iyer, A.; Soto, C.; Dinale, A.; Granger, E.; Jansz, P.; Hayward, C.; Jabbour, A.; et al. Rapid retrieval and ex situ portable machine perfusion allows successful cardiac transplantation with donor hearts from controlled donation after circulatory death. J. Heart Lung Transplant. 2017, 36, S16. [Google Scholar] [CrossRef]
- Beuth, J.; Falter, F.; Pinto Ribeiro, R.V.; Badiwala, M.; Meineri, M. New Strategies to Expand and Optimize Heart Donor Pool: Ex Vivo Heart Perfusion and Donation After Circulatory Death: A Review of Current Research and Future Trends. Anesth. Analg. 2019, 128, 406–413. [Google Scholar] [CrossRef] [PubMed]
- Hamed, A.; Tsui, S.; Huber, J.; Lin, R.; Poggio, E.C.; Ardehali, A. A Serum Lactate is highly specific predictor of post cardiac transplant outcomes using the Organ Care System. J. Heart Lung Transplant. 2009, 28, S71. [Google Scholar] [CrossRef]
- White, C.W.; Ambrose, E.; Müller, A.; Li, Y.; Le, H.; Hiebert, B.; Arora, R.; Lee, T.W.; Dixon, I.; Tian, G.; et al. Assessment of donor heart viability during ex vivo heart perfusion. Can. J. Physiol. Pharmacol. 2015, 93, 893–901. [Google Scholar] [CrossRef] [PubMed]
- Rojas, S.V.; Avsar, M.; Ius, F.; Schibilsky, D.; Kaufeld, T.; Benk, C.; Maeding, I.; Berchtold-Herz, M.; Bara, C.; Beyersdorf, F.; et al. Ex-Vivo Preservation with the Organ Care System in High Risk Heart Transplantation. Life 2022, 12, 247. [Google Scholar] [CrossRef]
- Tsukahita, M.; Naka, Y. Organ Care System for Heart Procurement and Strategies to Reduce Primary Graft Failure After Heart Transplant. Oper. Tech. Thorac. Cardiovasc. Surg. 2015, 20, 322–334. [Google Scholar] [CrossRef]
- Iyer, A.; Gao, L.; Doyle, A.; Rao, P.; Jayewardene, D.; Wan, B.; Kumarasinghe, G.; Jabbour, A.; Hicks, M.; Jansz, P.C.; et al. Increasing the tolerance of DCD hearts to warm ischemia by pharmacological postconditioning. Am. J. Transplant. 2014, 8, 1744–1752. [Google Scholar] [CrossRef]
- Phan, K.; Luc, J.G.Y.; Xu, J.; Maltais, S.; Stulak, J.M.; Yan, T.D.; Tchantchaleishvili, V. Utilization and Outcomes of Temporary Mechanical Circulatory Support for Graft Dysfunction After Heart Transplantation. ASAIO J. 2017, 63, 695–703. [Google Scholar] [CrossRef]
- Reich, H.J.; Kobashigawa, J.A.; Aintablian, T.; Ramzy, D.; Kittleson, M.M.; Esmailian, F. Effects of Older Donor Age and Cold Ischemic Time on Long-Term Outcomes of Heart Transplantation. Tex. Heart Inst. J. 2018, 45, 17–22. [Google Scholar] [CrossRef]
- Freed, D.H.; White, C.W. Donor heart preservation: Straight up, or on the rocks? Lancet 2015, 385, 2552–2554. [Google Scholar] [CrossRef]
- Collins, M.J.; Moainie, S.L.; Griffith, B.P.; Poston, R.S. Preserving and evaluating hearts with ex vivo machine perfusion: An avenue to improve early graft performance and expand the donor pool. Eur. J. Cardiothorac. Surg. 2008, 34, 318–325. [Google Scholar] [CrossRef]
- Cooper, D.K.; Wicomb, W.N.; Novitzky, D. Cardiac transplantation following storage of the donor heart by a portable hypothermic perfusion system: The initial clinical experience. Clin. Transpl. 2006, 65, 552–554. [Google Scholar]
- Hassanein, W.H.; Zellos, L.; Tyrrell, T.A.; Healey, N.A.; Crittenden, M.D.; Birjiniuk, V.; Khuri, S.F. Continuous perfusion of donor hearts in the beating state extends preservation time and improves recovery of function. J. Thorac. Cardiovasc. Surg. 1998, 116, 821–830. [Google Scholar] [CrossRef] [PubMed]
- Messer, S.; Ardehali, A.; Tsui, S. Normothermic donor heart perfusion: Current clinical experience and the future. Transpl. Int. 2015, 28, 634–642. [Google Scholar] [CrossRef] [PubMed]
- Ardehali, A.; Esmailian, F.; Deng, M.; Soltesz, E.; Hsich, E.; Naka, Y.; Mancini, D.; Camacho, M.; Zucker, M.; Leprince, P.; et al. Ex-vivo perfusion of donor hearts for human heart transplantation (PROCEED II): A prospective, open-label, multicentre, randomised non-inferiority trial. Lancet 2015, 385, 2577–2584. [Google Scholar] [CrossRef] [PubMed]
- Koerner, M.M.; Ghodsizad, A.; Schulz, U.; El Banayosy, A.; Koerfer, R.; Tenderich, G. Normothermic ex vivo allograft blood perfusion in clinical heart transplantation. Heart. Surg. Forum 2014, 3, 141–145. [Google Scholar] [CrossRef] [PubMed]
- Sponga, S.; Bonetti, A.; Ferrara, V.; Beltrami, A.P.; Isola, M.; Vendramin, I.; Finato, N.; Ortolani, F.; Livi, U. Preservation by cold storage vs ex vivo normothermic perfusion of marginal donor hearts: Clinical, histopathologic, and ultrastructural features. J. Heart Lung Transplant. 2020, 39, 1408–1416. [Google Scholar] [CrossRef]
- Langmuur, S.J.J.; Amesz, J.H.; Veen, K.M.; Bogers, A.J.J.C.; Manintveld, O.C.; Taverne, Y.J.H.J. Normothermic Ex Situ Heart Perfusion With the Organ Care System for Cardiac Transplantation: A Meta-analysis. Transplantation 2022, 106, 1745–1753. [Google Scholar] [CrossRef]
- Sato, T.; Azarbal, B.; Cheng, R.; Esmailian, F.; Patel, J.; Kittleson, M.; Czer, L.; Thottam, M.; Levine, R.; Dimbil, S.; et al. Does ex vivo perfusion lead to more or less intimal thickening in the first-year post-heart transplantation? Clin. Transplant. 2019, 33, e13648. [Google Scholar] [CrossRef]
- Chan, J.L.; Kobashigawa, J.A.; Reich, H.J.; Ramzy, D.; Thottam, M.M.; Yu, Z.; Aintablian, T.L.; Liou, F.; Patel, J.K.; Kittleson, M.M.; et al. Intermediate outcomes with ex-vivo allograft perfusion for heart transplantation. J. Heart Lung Transplant. 2017, 36, 258–263. [Google Scholar] [CrossRef]
- Nilsson, J.; Jernryd, V.; Qin, G.; Paskevicius, A.; Metzsch, C.; Sjöberg, T.; Steen, S. A nonrandomized open-label phase 2 trial of nonischemic heart preservation for human heart transplantation. Nat. Commun. 2020, 11, 2976. [Google Scholar] [CrossRef]
- Kothari, P. Ex-Vivo Preservation of Heart Allografts-An Overview of the Current State. J. Cardiovasc. Dev. Dis. 2023, 10, 105. [Google Scholar] [CrossRef]
- Critchley, W.R.; Stone, J.P.; Liao, Q.; Qin, G.; Risnes, I.; Trafford, A.; Scott, H.; Sjöberg, T.; Steen, S.; Fildes, J.E. Non-ischemic Heart Preservation via Hypothermic Cardioplegic Perfusion Induces Immunodepletion of Donor Hearts Resulting in Diminished Graft Infiltration Following Transplantation. Front. Immunol. 2020, 11, 1621. [Google Scholar] [CrossRef] [PubMed]
- Chew, H.C.; Iyer, A.; Connellan, M.; Scheuer, S.; Villanueva, J.; Gao, L.; Hicks, M.; Harkness, M.; Soto, C.; Dinale, A.; et al. Outcomes of Donation After Circulatory Death Heart Transplantation in Australia. J. Am. Coll. Cardiol. 2019, 73, 1447–1459. [Google Scholar] [CrossRef]
- Pantos, C.I.; Trikas, A.G.; Pissimisis, E.G.; Grigoriou, K.P.; Stougiannos, P.N.; Dimopoulos, A.K.; Linardakis, S.I.; Alexopoulos, N.A.; Evdoridis, C.G.; Gavrielatos, G.D.; et al. Effects of Acute Triiodothyronine Treatment in Patients with Anterior Myocardial Infarction Undergoing Primary Angioplasty: Evidence from a Pilot Randomized Clinical Trial (ThyRepair Study). Thyroid 2022, 32, 714–724. [Google Scholar] [CrossRef] [PubMed]
- Von Hafe, M.; Neves, J.S.; Vale, C.; Borges-Canha, M.; Leite-Moreira, A. The impact of thyroid hormone dysfunction on ischemic heart disease. Endocr. Connect. 2019, 8, 76–90. [Google Scholar] [CrossRef] [PubMed]
- Novitzky, D.; Mi, Z.; Sun, Q.; Collins, J.F.; Cooper, D.K. Thyroid hormone therapy in the management of 63,593 brain-dead organ donors: A retrospective analysis. Transplantation 2014, 98, 1119–1127. [Google Scholar] [CrossRef]
- Chen, J.; Ortmeier, S.B.; Savinova, O.V.; Nareddy, V.B.; Beyer, A.J.; Wang, D.; Gerdes, A.M. Thyroid hormone induces sprouting angiogenesis in adult heart of hypothyroid mice through the PDGF-Akt pathway. J. Cell. Mol. Med. 2012, 16, 2726–2735. [Google Scholar] [CrossRef] [PubMed]
- Mourouzis, I.; Kounatidis, D.; Brozou, V.; Anagnostopoulos, D.; Katsaouni, A.; Lourbopoulos, A.; Pantos, C. Effects of T3 Administration on Ex Vivo Rat Hearts Subjected to Normothermic Perfusion: Therapeutic Implications in Donor Heart Preservation and Repair. Transpl. Int. 2023, 36, 33–42. [Google Scholar] [CrossRef] [PubMed]
- Cuenda, A.; Rousseau, S. p38 MAP-kinases pathway regulation, function and role in human diseases. Biochim. Biophys. Acta 2007, 1773, 1358–1375. [Google Scholar] [CrossRef]
- Huang, G.; Shi, L.Z.; Chi, H. Regulation of JNK and p38 MAPK in the immune system: Signal integration, propagation and termination. Cytokine 2009, 48, 161–169. [Google Scholar] [CrossRef]
- Oto, T.; Calderone, A.; Li, Z.; Rosenfeldt, F.L.; Pepe, S. p38 Mitogen-activated protein kinase inhibition reduces inflammatory cytokines in a brain-dead transplant donor animal model. Heart. Lung Circ. 2009, 18, 393–400. [Google Scholar] [CrossRef]
- Miller, A.L.; Webb, M.S.; Copik, A.J.; Wang, Y.; Johnson, B.H.; Kumar, R.; Thompson, E.B. p38 Mitogen-activated protein kinase (MAPK) is a key mediator in glucocorticoid-induced apoptosis of lymphoid cells: Correlation between p38 MAPK activation and site-specific phosphorylation of the human glucocorticoid receptor at serine 211. Mol. Endocrinol. 2005, 19, 1569–1583. [Google Scholar] [CrossRef]
- Shvedova, M.; Anfinogenova, Y.; Atochina-Vasserman, E.N.; Schepetkin, I.A.; Atochin, D.N. c-Jun N-Terminal Kinases (JNKs) in Myocardial and Cerebral Ischemia/Reperfusion Injury. Front. Pharmacol. 2018, 9, 715. [Google Scholar] [CrossRef]
- Lin, A. Activation of the JNK signaling pathway: Breaking the brake on apoptosis. Bioessays 2003, 25, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Davis, P.J.; Mousa, S.A.; Lin, H.Y. Nongenomic Actions of Thyroid Hormone: The Integrin Component. Physiol. Rev. 2021, 101, 319–352. [Google Scholar] [CrossRef] [PubMed]
- Chi, H.C.; Tsai, C.Y.; Tsai, M.M.; Yeh, C.T.; Lin, K.H. Molecular functions and clinical impact of thyroid hormone-triggered autophagy in liver-related diseases. J. Biomed. Sci. 2019, 26, 24. [Google Scholar] [CrossRef]
- Greijer, A.E.; Van der Wall, E. The role of hypoxia inducible factor 1 (HIF-1) in hypoxia induced apoptosis. J. Clin. Pathol. 2004, 57, 1009–1014. [Google Scholar] [CrossRef]
- Zou, M.H.; Wu, Y. AMP-activated protein kinase activation as a strategy for protecting vascular endothelial function. Clin. Exp. Pharmacol. Physiol. 2008, 35, 535–545. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Xu, H.; Yan, L.; Ye, L.; Zhang, X.; Tan, B.; Yi, Q.; Tian, J.; Zhu, J. PGC-1α promotes mitochondrial respiration and biogenesis during the differentiation of hiPSCs into cardiomyocytes. Genes Dis. 2020, 8, 891–906. [Google Scholar] [CrossRef]
- Konijnenberg, L.S.F.; Damman, P.; Duncker, D.J.; Kloner, R.A.; Nijveldt, R.; Van Geuns, R.M.; Berry, C.; Riksen, N.P.; Escaned, J.; Van Royen, N. Pathophysiology and diagnosis of coronary microvascular dysfunction in ST-elevation myocardial infarction. Cardiovasc. Res. 2020, 116, 787–805. [Google Scholar] [CrossRef]
- Lee, J.M.; Choi, K.H.; Choi, J.O.; Shin, D.; Park, Y.; Kim, J.; Lee, S.H.; Kim, D.; Yang, J.H.; Cho, Y.H.; et al. Coronary Microcirculatory Dysfunction and Acute Cellular Rejection After Heart Transplantation. Circulation 2021, 144, 1459–1472. [Google Scholar] [CrossRef] [PubMed]
- Braicu, C.; Buse, M.; Busuioc, C.; Drula, R.; Gulei, D.; Raduly, L.; Rusu, A.; Irimie, A.; Atanasov, A.G.; Slaby, O.; et al. A Comprehensive Review on MAPK: A Promising Therapeutic Target in Cancer. Cancers 2019, 11, 1618. [Google Scholar] [CrossRef] [PubMed]
- Mourouzis, I.S.; Lourbopoulos, A.I.; Trikas, A.G.; Tseti, I.K.; Pantos, C.I. Triiodothyronine prevents tissue hypoxia in experimental sepsis: Potential therapeutic implications. Intensive Care Med. Exp. 2021, 9, 17. [Google Scholar] [CrossRef] [PubMed]
| Favorable Characteristics | Unfavorable Characteristics |
|---|---|
| 1. Age up to 65 years old 2. Cold ischemia time up to 4–6 h 3. By-passable one- or two-coronary-vessel disease 4. Correctable valvular dysfunction determined by heart ultrasound | 1. Prolonged hospitalization 2. History of chest trauma 3. Undersizing or oversizing by more than 20% body weight 4. Open cardiac massage 5. Elevation of myocardial enzyme levels 6. Cardiopulmonary resuscitation >5 min 7. Transient hypotension >30 min 8. High-dose vasopressor requirement 9. Wall motion abnormalities by heart ultrasound 10. Long distance procurement >1000 miles 11. Persistent conduction disturbances |
| Standard monitoring | 1. Urine catheter to straight drainage, strict intake and output 2. Nasogastric tube to straight drainage 3. Vital signs every hour 4. Pulse oximetry, 3-lead-ECG 5. Central venous pressure 6. Arterial line pressure 7. Optional PACs |
| Laboratory investigations | 1. Arterial blood gases, electrolytes, and glucose every 4 h and as needed 2. Complete blood count every 8 h 3. Blood urea nitrogen and creatinine every 6 h 4. Urine analysis 5. AST, ALT, bilirubin (total and direct), INR (or PT), and PTT every 6 h |
| Hemodynamic monitoring and therapy | General targets: heart rate 60–120 bpm, systolic blood pressure > 100 mm Hg, mean arterial pressure ≥ 70 mm Hg. 1. Fluid resuscitation to maintain normovolemia, central venous pressure 6–10 mm Hg 2. If arterial blood pressure is ≥160/90 mm Hg, then: -Wean inotropes and vasopressors -If necessary, start either nitroprusside 0.5–5.0 μg/kg per minute or esmolol 100–500 μg/kg bolus followed by 100–300 μg/kg per minute 3. Serum lactate every 2–4 h 4. Mixed venous oximetry every 2–4 h; titrate therapy to MVO2 ≥ 60 mm Hg |
| Agents for hemodynamic support | 1. Dopamine: ≤10 μg/kg/min 2. Vassopresin: ≤2.4 U/h (0.04 U/min) 3. Norepinephrine/epinephrine/phenylephrine (caution with doses > 0.2 μg/kg/min) |
| Indications for PACs | 1. 2-dimensional echo ejection fraction ≤ 40% and/or 2. Dopamine > 10 μg/kg/min and/or 3. Vasopressor support (not including vasopressin if part of hormonal therapy) and/or 4. Escalation of supports |
| Glycemia and nutrition | 1. Routine intravenous dextrose infusions 2. Initiate/continue enteral feeding as tolerated 3. Continue parenteral nutrition if already initiated 4. Initiate and titrate insulin infusion to maintain serum glucose level at 4–8 mmol/L |
| Fluid and electrolyte targets | 1. Urine output 0.5–3 mL/kg/h 2. Serum Na 130–150 mM 3. Normal ranges for potassium, calcium, magnesium, and phosphate |
| Hematology | 1. Optimum hemoglobin: 90–100 g/L for unstable donors, lowest acceptable level is 70 g/L 2. For platelets, INR and PTT, there are no predefined targets; transfuse in case of clinically relevant bleeding 3. No other specific transfusion requirements |
| Microbiology | 1. Daily blood cultures 2. Daily urine cultures 3. Daily endotracheal tube cultures 4. Administer antibiotics for presumed or proven infection |
| Diabetes insipidus | A. Defined as: 1. Urine output > 4 mL/kg/h associated with 2. Rising serum sodium ≥ 145 mmol/L and/or 3. Rising serum osmolarity ≥ 300 mosM and/or 4. Decreasing urine osmolarity ≤ 200 mosM B. Therapy (to be titrated to urine output ≤ 3 mL/kg/h): 1. Intravenous vasopressin infusion at ≤2.4 U/h and/or 2. Intermittent DDAVP 1–4 μg IV, then 1–2 μg IV every 6 h (there is no true upper limit for dose; should be titrated to desired urine output rate) |
| Combined hormonal therapy | A. Defined as: 1. T4: 20 μg IV bolus followed by 10 μg/h IV infusion (or 100 μg IV bolus followed by 50 μg IV every 12 h) or T3: 4 mcg IV bolus followed by 3 mcg/h IV infusion 2. Vasopressin: 1 U IV bolus followed by 2.4 U/h IV infusion 3. Methylprednisolone: 15 mg/kg (≤1 g) IV every 24 h B. Indications: 1. 2-dimensional echo ejection fraction ≤ 40% or 2. Hemodynamic instability (includes shock, unresponsive to restoration of normovolemia and requiring vasoactive support (dopamine > 10 μg/min or any vasopressor agent) 3. Consideration should be given to its use in all donors |
| Heart-specific follow-up | 1. 12-lead ECG 2. Troponin I every 12 h 3. 2-dimensional echocardiography: 1. Should only be performed after fluid and hemodynamic resuscitation 2. If ejection fraction ≤ 40% then, insert PACs and titrate therapy to the following targets: 1. PCWP: 6–10 mm Hg 2. Cardiac index: >2–4 L/minute/m2 3. SVR: 80–1200 dynes/sec/cm−5 4. LVSWI > 15 g/kg/min 3. PAC data are relevant for hemodynamic therapy and evaluation for suitability for heart transplantation independent of echo findings 4. Consider repeat echocardiography at 6–12 h intervals 4. Coronary angiography: A. Indications: 1. History of cocaine use 2. Male > 55 years or female > 60 years 3. Male > 40 years or female > 45 years in the presence of 2 or more risk factors 4. ≥3 risk factors of any age B. Risk factors: 1. Smoking 2. Hypertension 3. Diabetes 4. Hyperlipidemia 5. Body mass index > 32 6. Family history of the disease 7. History of coronary artery disease 8. Ischemia on ECG 9. Anterolateral regional wall motion abnormalities on ECG 10. 2-dimensional echo assessment of ejection fraction ≤ 40% C. Precautions: 1. Ensure normovolemia 2. Administer prophylactic NAC, 600–1000 mg enterally twice daily (first dose as soon as angiography indicated) or IV 150 mg/kg in 500 mL normal saline over 30 min immediately before contrast agent followed by 50 mg/kg in 500 mL normal saline over 4 h 3.Use low-risk radiocontrast agent (non-ionic or iso-osmolar), using minimum radiocontrast volume, no ventriculogram |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kounatidis, D.; Brozou, V.; Anagnostopoulos, D.; Pantos, C.; Lourbopoulos, A.; Mourouzis, I. Donor Heart Preservation: Current Knowledge and the New Era of Machine Perfusion. Int. J. Mol. Sci. 2023, 24, 16693. https://doi.org/10.3390/ijms242316693
Kounatidis D, Brozou V, Anagnostopoulos D, Pantos C, Lourbopoulos A, Mourouzis I. Donor Heart Preservation: Current Knowledge and the New Era of Machine Perfusion. International Journal of Molecular Sciences. 2023; 24(23):16693. https://doi.org/10.3390/ijms242316693
Chicago/Turabian StyleKounatidis, Dimitris, Vassiliki Brozou, Dimitris Anagnostopoulos, Constantinos Pantos, Athanasios Lourbopoulos, and Iordanis Mourouzis. 2023. "Donor Heart Preservation: Current Knowledge and the New Era of Machine Perfusion" International Journal of Molecular Sciences 24, no. 23: 16693. https://doi.org/10.3390/ijms242316693
APA StyleKounatidis, D., Brozou, V., Anagnostopoulos, D., Pantos, C., Lourbopoulos, A., & Mourouzis, I. (2023). Donor Heart Preservation: Current Knowledge and the New Era of Machine Perfusion. International Journal of Molecular Sciences, 24(23), 16693. https://doi.org/10.3390/ijms242316693





