Mmp2 Deficiency Leads to Defective Parturition and High Dystocia Rates in Mice
Abstract
:1. Introduction
2. Results
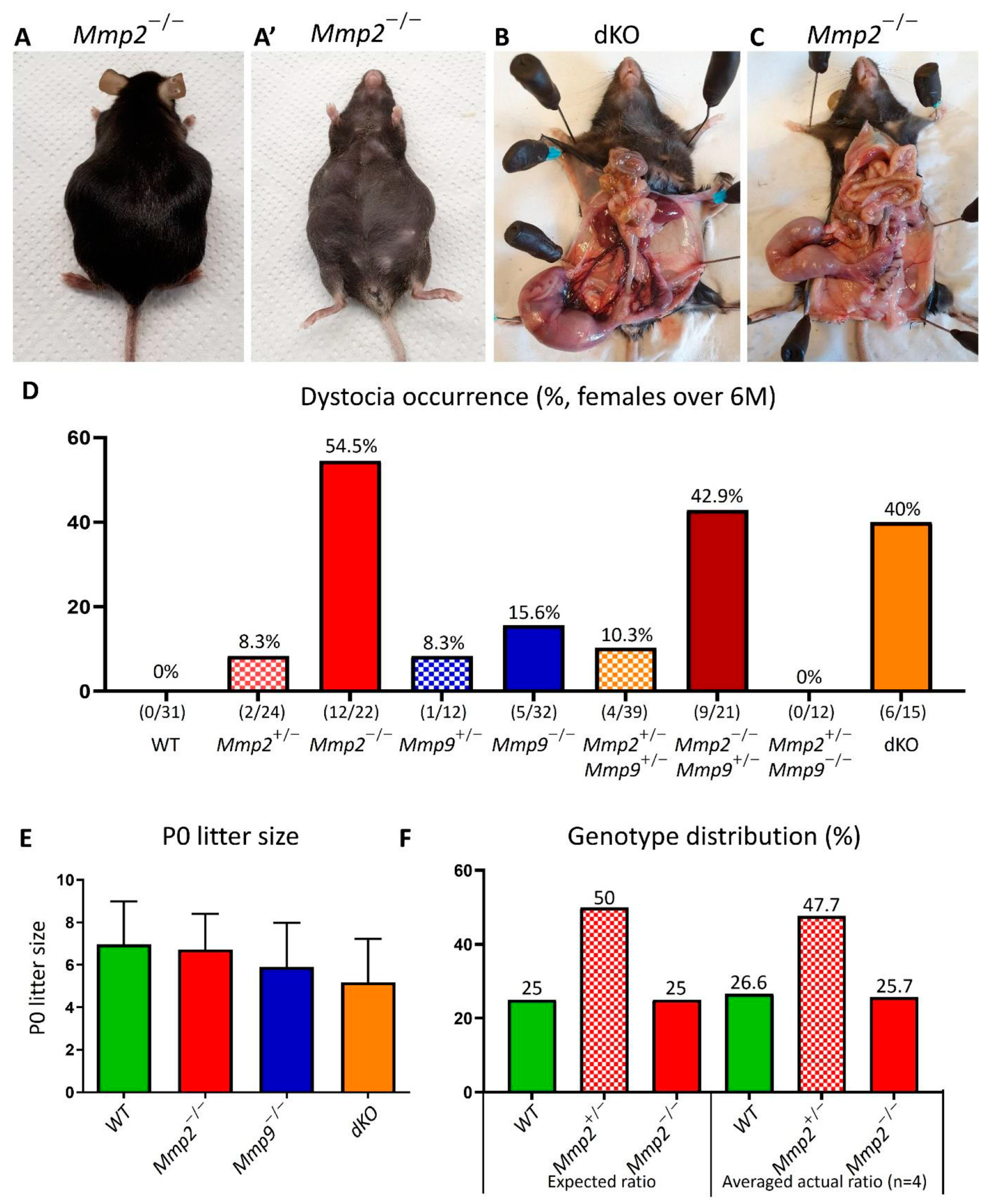
2.1. Mmp2 Loss Results in Defective Parturition Process and Dystocia
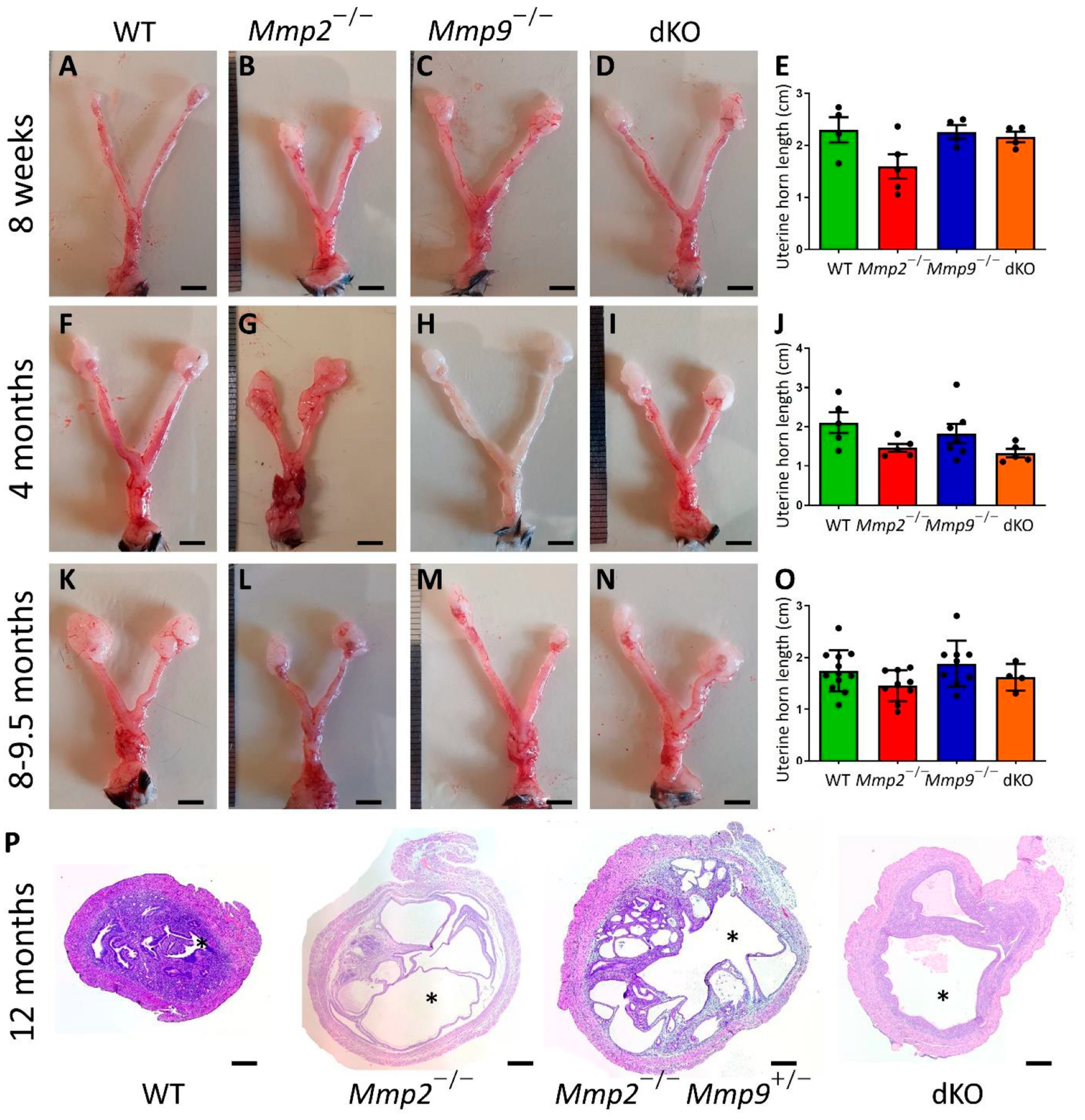
2.2. Mmp2−/− Nulliparous Females Present Relatively Short Uterine Horns
2.3. Mmp2−/− Uterus Demonstrates Enlarged Myometrium, Endometrium and Lumen
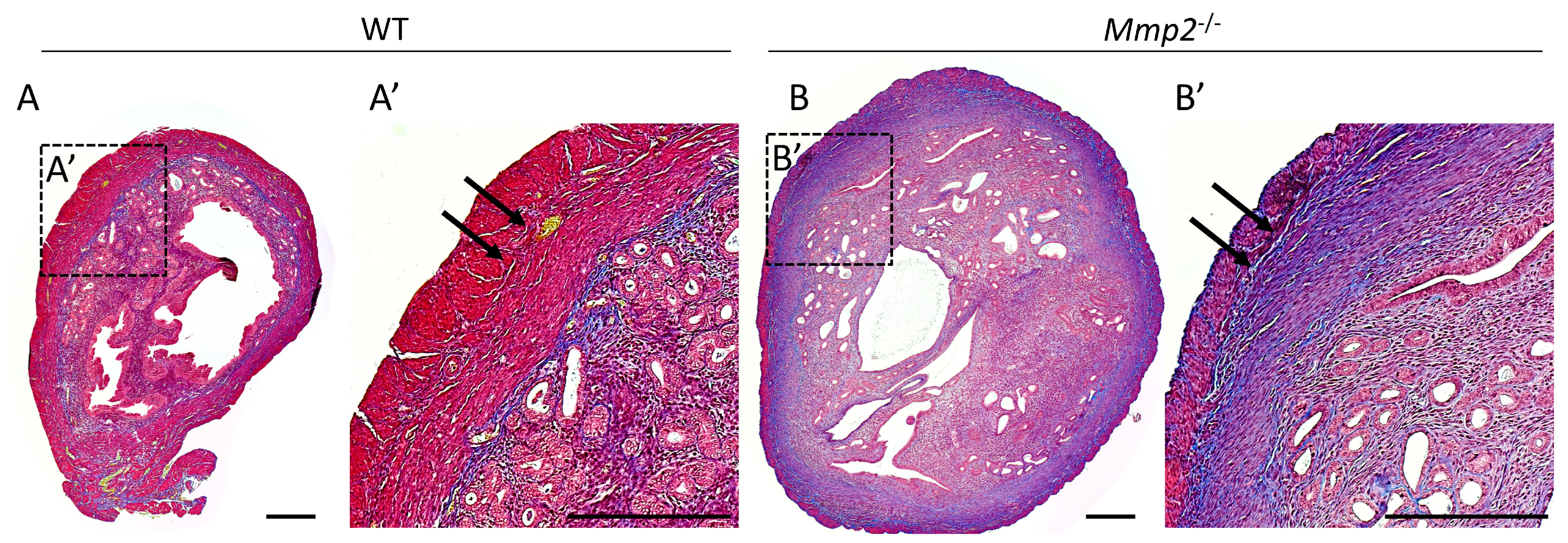
2.4. Mmp2−/− Nulliparous Uterus at 8 M Demonstrates Signs of Myometrial Fibrosis
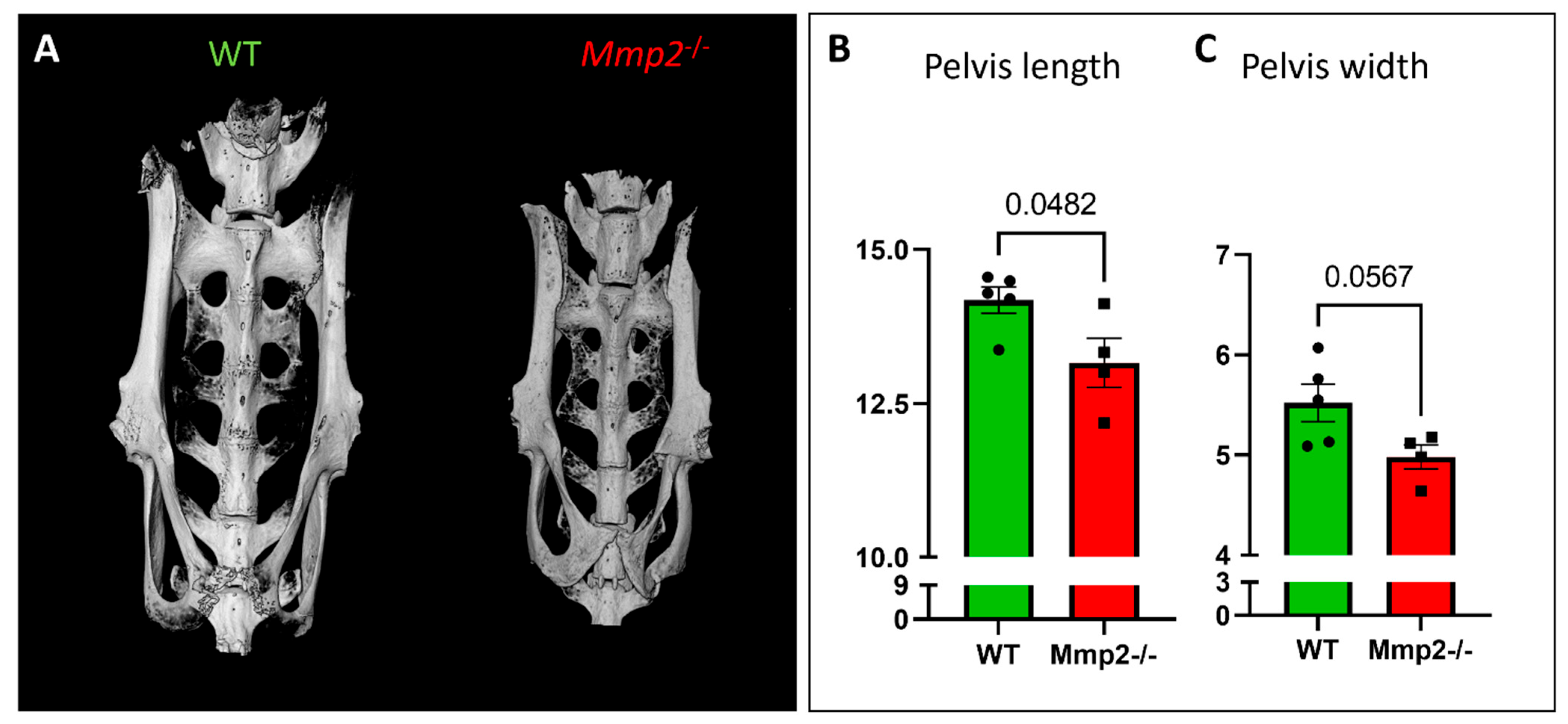
2.5. Morphometric Analysis of the Pelvic Bone in Mmp2−/− Females
3. Discussion
4. Conclusions, Strengths and Limitations
5. Materials and Methods
5.1. Animals
5.2. Dystocia Assessment
5.3. Genotype Distribution and Litter-Size Measurements
5.4. Collection of Uterine Tissues
5.5. Histology
5.6. µCT Scanning and Morphometric Analysis
5.7. Quantification and Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Renthal, N.E.; Williams, K.C.; Montalbano, A.P.; Chen, C.C.; Gao, L.; Mendelson, C.R. Molecular regulation of parturition: A myometrial perspective. Cold Spring Harb. Perspect. Med. 2015, 5, 11. [Google Scholar] [CrossRef]
- Zowalaty, A.E.E.; Ye, X. Seipin deficiency leads to defective parturition in mice. Biol. Reprod. 2017, 97, 378–386. [Google Scholar] [CrossRef]
- Ratajczak, C.K.; Muglia, L.J. Developmental Biology: Model Systems—A Series of Reviews Insights Into Parturition Biology From Genetically Altered Mice. Int. J. Pediatr. Res. 2008, 64, 581–589. [Google Scholar]
- Wood, G.A.; Fata, J.E.; Watson, K.L.M.; Khokha, R. Circulating hormones and estrous stage predict cellular and stromal remodeling in murine uterus. Reproduction 2007, 133, 1035–1044. [Google Scholar] [CrossRef]
- Crain, D.A.; Janssen, S.J.; Edwards, T.M.; Heindel, J.; Ho S mei Hunt, P.; Iguchi, T.; Juul, A.; McLachlan, J.A.; Schwartz, J. Female reproductive disorders: The roles of endocrine-disrupting compounds and developmental timing. Fertil Steril. 2008, 90, 911–940. [Google Scholar] [CrossRef]
- Ajayi, A.F.; Akhigbe, R.E. Staging of the estrous cycle and induction of estrus in experimental rodents: An update. Fertil Res. Pract. 2020, 6, 5. [Google Scholar] [CrossRef]
- Novaro, V.; Pustovrh, C.; Colman-Lerner, A.; Radisky, D.; Lo Nostro, F.; Paz, D.; Jawerbaum, A.; González, E. Nitric oxide induces gelatinase A (matrix metalloproteinase 2) during rat embryo implantation. Fertil Steril. 2002, 78, 1278–1287. [Google Scholar] [CrossRef]
- Hrabia, A.; Wolak, D.; Kwaśniewska, M.; Kieronska, A.; Socha, J.K.; Sechman, A. Expression of gelatinases (MMP-2 and MMP-9) and tissue inhibitors of metalloproteinases (TIMP-2 and TIMP-3) in the chicken ovary in relation to follicle development and atresia. Theriogenology 2019, 125, 268–276. [Google Scholar] [CrossRef]
- Zhu, Y. Metalloproteases in gonad formation and ovulation. Gen. Comp. Endocrinol. 2021, 314, 113924. [Google Scholar] [CrossRef]
- Barraza, D.E.; Zampini, R.; Apichela, S.A.; Pacheco, J.I.; Argañaraz, M.E. Modifications of extracellular matrix features in the left and right uterine horns during the embryo pre-implantation period in Vicugna pacos. Theriogenology 2020, 157, 440–448. [Google Scholar] [CrossRef]
- Malemud, C.J. Matrix metalloproteinases (MMPs) in health and disease: An overview. Front Biosci. 2006, 11, 1696. [Google Scholar] [CrossRef]
- Alba, J.; Barcia, R.; Gutiérrez-Berzal, J.; Ramos-Martínez, J.I. Could inhibition of metalloproteinases be used to block the process of metastasis? Cell Biochem. Funct. 2022, 40, 600–607. [Google Scholar] [CrossRef]
- Scheau, C.; Badarau, I.A.; Costache, R.; Caruntu, C.; Mihai, G.L.; Didilescu, A.C.; Constantin, C.; Neagu, M. The Role of Matrix Metalloproteinases in the Epithelial-Mesenchymal Transition of Hepatocellular Carcinoma. Anal. Cell Pathol. 2019, 2019, 9423907. [Google Scholar] [CrossRef]
- Köhrmann, A.; Kammerer, U.; Kapp, M.; Dietl, J.; Anacker, J. Expression of matrix metalloproteinases (MMPs) in primary human breast cancer and breast cancer cell lines: New findings and review of the literature. BMC Cancer 2009, 9, 1355–1366. [Google Scholar] [CrossRef]
- Hasky-Negev, M.; Simsa, S.; Tong, A.; Genina, O.; Monsonego Ornan, E. Expression of matrix metalloproteinases during vascularization and ossification of normal and impaired avian growth plate. J. Anim. Sci. 2008, 86, 1306–1315. [Google Scholar] [CrossRef]
- Dan, H.; Simsa-Maziel, S.; Hisdai, A.; Sela-Donenfeld, D.; Monsonego Ornan, E. Expression of matrix metalloproteinases during impairment and recovery of the avian growth plate. J. Anim. Sci. 2009, 87, 3544–3555. [Google Scholar] [CrossRef]
- Paiva, K.B.S.; Granjeiro, J.M. Matrix Metalloproteinases in Bone Resorption, Remodeling, and Repair. Prog. Mol. Biol. Transl. Sci. 2017, 148, 203–303. [Google Scholar]
- Aimes, R.T.; Quigley, J.P. Matrix metalloproteinase-2 is an interstitial collagenase. J. Biol. Chem. 1995, 270, 5872–5876. [Google Scholar] [CrossRef]
- Patterson, M.L.; Atkinson, S.J.; Knäuper, V.; Murphy, G. Specific collagenolysis by gelatinase A, MMP-2, is determined by the hemopexin domain and not the fibronectin-like domain. FEBS Lett. 2001, 503, 158–162. [Google Scholar] [CrossRef]
- Monsonego-Ornan, E.; Kosonovsky, J.; Bar, A.; Roth, L.; Fraggi-Rankis, V.; Simsa, S.; Kohl, A.; Sela-Donenfeld, D. Matrix metalloproteinase 9/gelatinase B is required for neural crest cell migration. Dev. Biol. 2012, 364, 162–177. [Google Scholar] [CrossRef]
- Kalev-Altman, R.; Hanael, E.; Zelinger, E.; Blum, M.; Monsonego-Ornan, E.; Sela-Donenfeld, D. Conserved role of matrix metalloproteases 2 and 9 in promoting the migration of neural crest cells in avian and mammalian embryos. FASEB J. 2020, 34, 5240–5261. [Google Scholar] [CrossRef]
- Duong, T.D.; Erickson, C.A. MMP-2 Plays an Essential Role in Producing Epithelial-Mesenchymal Transformations in the Avian Embryo. Dev. Dyn. 2004, 229, 42–53. [Google Scholar] [CrossRef]
- Kalev-Altman, R.; Janssen, J.N.; Ben-Haim, N.; Levy, T.; Shitrit-Tovli, A.; Milgram, J.; Shahar, R.; Sela-Donenfeld, D.; Monsonego-Ornan, E. The gelatinases, matrix metalloproteinases 2 and 9, play individual roles in skeleton development. Matrix Biol. 2022, 113, 100–121. [Google Scholar] [CrossRef]
- Curry, T.E., Jr.; Osteen, K.G. The Matrix Metalloproteinase System: Changes, Regulation, and Impact throughout the Ovarian and Uterine Reproductive Cycle. Endocr. Rev. 2003, 24, 428–465. [Google Scholar] [CrossRef]
- Nothnick, W.B. Disruption of the Tissue Inhibitor of Metalloproteinase-1 Gene in Reproductive-Age Female Mice Is Associated with Estrous Cycle Stage-Specific Increases in Stromelysin Messenger RNA Expression and Activity. Biol. Reprod. 2001, 65, 1780–1788. [Google Scholar] [CrossRef]
- Van Engelen, E.; Breeveld-Dwarkasing, V.N.A.; Taverne, M.A.M.; Everts, M.E.; van der Weijden, G.C.; Rutten, V.P.M.G. MMP-2 expression precedes the final ripening process of the bovine cervix. Mol. Reprod Dev. 2008, 75, 1669–1677. [Google Scholar] [CrossRef]
- Vadillo-Ortega, F.; Gonzalez-Avila, G.; Furth, E.E.; Lei, H.; Muschel, R.J.; Stetler-Stevenson, W.G.; Strauss, J.F. 92-kd Type IV Collagenase (Matrix Metalloproteinase-9) Activity in Human Amniochorion Increases with Labor. Am. J. Pathol. 1995, 146, 148–156. [Google Scholar]
- Ekambaram, G.; Kumar, S.K.S.; Joseph, L.D. Comparative study on the estimation of estrous cycle in mice by visual and vaginal lavage method. J. Clin. Diagn. Res. 2017, 11, AC05–AC07. [Google Scholar] [CrossRef]
- Pang, S.C.; Janzen-Pang, J.; Tse, M.Y.; Croy, B.A.; Lima, P.D.A. The Guide to Investigation of Mouse Pregnancy; Croy, B.A., Yamada, A.T., DeMayo, F.J., Adamson, S.L., Eds.; Academic Press: Boston, MA, USA, 2014; pp. 3–19. [Google Scholar]
- Bertolin, K.; Murphy, B.D. The Guide to Investigation of Mouse Pregnancy; Croy, B.A., Yamada, A.T., DeMayo, F.J., Adamson, S.L., Eds.; Academic Press: Boston, MA, USA, 2014; pp. 85–94. [Google Scholar]
- Bertolin, K.; Murphy, B.D. The Guide to Investigation of Mouse Pregnancy; Croy, B.A., Yamada, A.T., DeMayo, F.J., Adamson, S.L., Eds.; Academic Press: Boston, MA, USA, 2014; pp. 475–477. [Google Scholar]
- Islam, M.A.; Getz, M.; Macklin, P.; Versypt, A.N.F. An agent-based modeling approach for lung fibrosis in response to COVID-19. bioRxiv 2022. Available online: http://www.ncbi.nlm.nih.gov/pubmed/36238719 (accessed on 12 October 2023).
- LaTowsky, B.; Robertson, D.W.; Capelli, C.C. Long-term improvement in the appearance of hypertrophic scars following a single treatment with acoustic subcision—A single center proof-of-concept study. Lasers Surg Med. 2022, 54, 1251–1260. [Google Scholar] [CrossRef]
- Devocelle, A.; Lecru, L.; Ferlicot, S.; Bessede, T.; Candelier, J.J.; Giron-michel, J.; François, H. Il-15 prevents renal fibrosis by inhibiting collagen synthesis: A new pathway in chronic kidney disease? Int. J. Mol. Sci. 2021, 22, 11698. [Google Scholar] [CrossRef]
- Xu, Q.X.; Zhang, W.Q.; Liu, X.Z.; Yan, W.K.; Lu, L.; Song, S.S.; Wei, S.W.; Liu, Y.N.; Kang, J.W.; Su, R.W. Notch1 signaling enhances collagen expression and fibrosis in mouse uterus. BioFactors 2021, 47, 852–864. [Google Scholar] [CrossRef]
- Bataller, R.; Brenner, D.A. Liver fibrosis. J. Clin. Investig. 2005, 115, 209–218. [Google Scholar] [CrossRef]
- Greenlee, K.J.; Corry, D.B.; Engler, D.A.; Matsunami, R.K.; Tessier, P.; Cook, R.G.; Werb, Z.; Kheradmand, F. Proteomic Identification of In Vivo Substrates for Matrix Metalloproteinases 2 and 9 Reveals a Mechanism for Resolution of Inflammation. J. Immunol. 2006, 177, 7312–7321. [Google Scholar] [CrossRef]
- Rodríguez, D.; Morrison, C.J.; Overall, C.M. Matrix metalloproteinases: What do they not do? New substrates and biological roles identified by murine models and proteomics. Biochim. Et Biophys. Acta (BBA) Mol. Cell Res. 2010, 1803, 39–54. [Google Scholar] [CrossRef]
- Maharaj, D. Assessing Cephalopelvic Disproportion: Back to the Basics. Obstet. Gynecol. Surv. 2010, 65, 387–395. [Google Scholar] [CrossRef]
- Gaudet, L.; Ferraro, Z.M.; Wen, S.W.; Walker, M. Maternal obesity and occurrence of fetal macrosomia: A systematic review and meta-analysis. Biomed. Res. Int. 2014, 2014, 640291. [Google Scholar] [CrossRef]
- Panwar, P.; Butler, G.S.; Jamroz, A.; Azizi, P.; Overall, C.M.; Brömme, D. Aging-associated modifications of collagen affect its degradation by matrix metalloproteinases. Matrix Biol. 2018, 65, 30–44. [Google Scholar] [CrossRef]
- López-Otín, C.; Overall, C.M. Protease degradomics: A new challenge for proteomics. Nat. Rev. Mol. Cell Biol. 2002, 3, 509–519. [Google Scholar] [CrossRef]
- Read, C.P.; Word, R.A.; Ruscheinsky, M.A.; Timmons, B.C.; Mahendroo, M.S. Cervical remodeling during pregnancy and parturition: Molecular characterization of the softening phase in mice. Reproduction 2007, 134, 327–340. [Google Scholar] [CrossRef]
- Mahendroo, M. Cervical remodeling in term and preterm birth: Insights from an animal model. Reproduction 2012, 143, 429–438. [Google Scholar] [CrossRef]
- Reeves, C.V.; Wang, X.; Charles-Horvath, P.C.; Vink, J.Y.; Borisenko, V.Y.; Young, J.A.T.; Kitajewski, J.K. Anthrax toxin receptor 2 functions in ecm homeostasis of the murine reproductive tract and promotes MMP activity. PLoS ONE 2012, 7, e34862. [Google Scholar] [CrossRef]
- Peters, D.E.; Zhang, Y.; Molinolo, A.A.; Miller-Randolph, S.; Szabo, R.; Bugge, T.H.; Leppla, S.H.; Liu, S. Capillary morphogenesis protein-2 is required for mouse parturition by maintaining uterine collagen homeostasis. Biochem. Biophys Res. Commun. 2012, 422, 393–397. [Google Scholar] [CrossRef]
- Sang, Y.J.; Wang, Q.; Zheng, F.; Hua, Y.; Wang, X.Y.; Zhang, J.Z.; Li, K.; Wang, H.Q.; Zhao, Y.; Zhu, M.S.; et al. Ggps1 deficiency in the uterus results in dystocia by disrupting uterine contraction. J. Mol. Cell Biol. 2021, 13, 116–127. [Google Scholar] [CrossRef]
- Lu, R.J.; Zhao, G.Z.; Jiang, R.; He, S.; Xu, H.; He, J.M.; Sun, Y.; Wu, M.N.; Ran, J.H.; Chen, D.L.; et al. Brusatol Inhibits Proliferation and Metastasis of Colorectal Cancer by Targeting and Reversing the RhoA/ROCK1 Pathway. Biomed Res. Int. 2022, 2022, 7132159. [Google Scholar] [CrossRef]
- Skrzypiec-Spring, M.; Sapa-Wojciechowska, A.; Rak-Pasikowska, A.; Kaczorowski, M.; Bil-Lula, I.; Hałoń, A.; Szeląg, A. The Protective Effect of Simvastatin on the Systolic Function of the Heart in the Model of Acute Ischemia and Reperfusion Is Due to Inhibition of the RhoA Pathway and Independent of Reduction of MMP-2 Activity. Biomolecules 2022, 12, 1291. [Google Scholar] [CrossRef]
- Bonafé, G.A.; Boschiero, M.N.; Sodré, A.R.; Ziegler, J.V.; Rocha, T.; Ortega, M.M. Natural Plant Compounds: Does Caffeine, Dipotassium Glycyrrhizinate, Curcumin, and Euphol Play Roles as Antitumoral Compounds in Glioblastoma Cell Lines? Front. Neurol. 2022, 12, 784330. [Google Scholar] [CrossRef]
- Lee, P.P.H.; Hwang, J.J.; Mead, L.; Ip, M.M. Functional role of matrix metalloproteinases (MMPS) in mammary epithelial cell development. J. Cell Physiol. 2001, 188, 75–88. [Google Scholar] [CrossRef]
- Sorrell, D.A.; Szymanowska, M.; Boutinaud, M.; Robinson, C.; Clarkson, R.W.E.; Stein, T.; Flint, D.J.; Kolb, A.F. Regulation of genes encoding proteolytic enzymes during mammary gland development. J. Dairy Res. 2005, 72, 433–441. [Google Scholar] [CrossRef]
- Wiseman, B.S.; Sternlicht, M.D.; Lund, L.R.; Alexander, C.M.; Mott, J.; Bissell, M.J.; Soloway, P.; Itohara, S.; Werb, Z.J. Site-specific inductive and inhibitory activities of MMP-2 and MMP-3 orchestrate mammary gland branching morphogenesis. Cell Biol. 2003, 162, 1123–1133. [Google Scholar] [CrossRef]
- Karsenty, G. The complexities of skeletal biology. Nature 2003, 423, 316–318. [Google Scholar] [CrossRef]
- Zelzer, E.; Olsen, B.R. The Genetic Basis for Skeletal Diseases. Nature 2003, 423, 343–348. [Google Scholar] [CrossRef]
- Kobayashi, T.; Kronenberg, H.M. Overview of skeletal development. Methods Mol. Biol. 2014, 1130, 3–12. [Google Scholar]
- Vu, T.H.; Shipley, J.M.; Bergers, G.; Berger, L.E.; Helms, J.A.; Hanahan, D.; Shapiro, S.D.; Senior, R.M.; Werb, Z. MMP-9/Gelatinase B Is a Key Regulator of Growth Plate Angiogenesis and Apoptosis of Hypertrophic Chondrocytes. Cell 1998, 93, 411–422. [Google Scholar] [CrossRef]
- Sternlicht, M.D.; Werb, Z. How Matrix Metalloproteinases Regulate Cell Behavior. Annu. Rev. Cell Dev. Biol. 2001, 17, 463–516. [Google Scholar] [CrossRef]
- Soloway, P.; Alexander, C.; Werb, Z.; Jaenisch, R. Targeted mutagenesis of Timp-1 reveals that lung tumor invasion is influenced by Timp-1 genotype of the tumor but not by that of the host. Oncogene 1996, 13, 2307–2314. [Google Scholar]
- Dubois, B.; Arnold, B.; Opdenakker, G.J. Gelatinase B deficiency impairs reproduction. Clin. Investig. 2000, 106, 627–628. [Google Scholar] [CrossRef]
- Rudolph-Owen, L.A.; Hulboy, D.L.; Wilson, C.L.; Mudgett, J.; Matrisian, L.M. Coordinate Expression of Matrix Metalloproteinase Family Members in the Uterus of Normal, Matrilysin-Deficient, and Stromelysin-1-Deficient Mice. Endocrinology 1997, 138, 4902–4911. [Google Scholar] [CrossRef]
- Kleifeld, O.; Doucet, A.; Auf Dem Keller, U.; Prudova, A.; Schilling, O.; Kainthan, R.K.; Starr, A.E.; Foster, L.J.; Kizhakkedathu, J.N.; Overall, C.M. Isotopic labeling of terminal amines in complex samples identifies protein N-termini and protease cleavage products. Nat. Biotechnol. 2010, 28, 281–288. [Google Scholar] [CrossRef]
- Ghazalpour, A.; Bennett, B.; Petyuk, V.A.; Orozco, L.; Hagopian, R.; Mungrue, I.N.; Farber, C.R.; Sinsheimer, J.; Kang, H.M.; Furlotte, N.; et al. Comparative analysis of proteome and transcriptome variation in mouse. PLoS Genet. 2011, 7, e1001393. [Google Scholar] [CrossRef]
- Itoh, T.; Ikeda, T.; Gomi, H.; Nakao, S.; Suzuki, T.; Itohara, S.J. Unaltered secretion of β-amyloid precursor protein in gelatinase a (matrix metalloproteinase 2)-deficient mice. Biol. Chem. 1997, 272, 22389–22392. [Google Scholar] [CrossRef]
- Mosig, R.A.; Dowling, O.; DiFeo, A.; Ramirez, M.C.M.; Parker, I.C.; Abe, E.; Diouri, J.; Aqeel, A.; Al Wylie, J.D.; Oblander, S.A.; et al. Loss of MMP-2 disrupts skeletal and craniofacial development and results in decreased bone mineralization, joint erosion and defects in osteoblast and osteoclast growth. Hum. Mol. Genet. 2007, 16, 1113–1123. [Google Scholar] [CrossRef]
- Schouten, I.; Bernys-Karolys, A.; Schneider, P.; Dror, T.; Ofer, L.; Shimoni, C.; Nissim-Eliraz, E.; Shpigel, N.Y.; Schlesinger, S. Mesenchymal stromal cells modulate infection and inflammation in the uterus and mammary gland. BMC Vet. Res. 2023, 19, 64. [Google Scholar] [CrossRef]
- Zhou, T.; Hu, M.; Pearlman, A.; Rohan, L.C. Expression, regulation, and function of drug transporters in cervicovaginal tissues of a mouse model used for microbicide testing. Biochem. Pharmacol. 2016, 116, 162–175. [Google Scholar] [CrossRef]
- Travinsky-Shmul, T.; Beresh, O.; Zaretsky, J.; Griess-Fishheimer, S.; Rozner, R.; Kalev-Altman, R.; Penn, S.; Shahar, R.; Monsonego-Ornan, E. Ultra-Processed Food Impairs Bone Quality, Increases Marrow Adiposity and Alters Gut Microbiome in Mice. Foods 2021, 10, 3107. [Google Scholar] [CrossRef]
- Rozner, R.; Vernikov, J.; Griess-Fishheimer, S.; Travinsky, T.; Penn, S.; Schwartz, B.; Mesilati-Stahy, R.; Argov-Argaman, N.; Shahar, R.; Monsonego-Ornan, E. The role of omega-3 polyunsaturated fatty acids from different sources in bone development. Nutrients 2020, 12, 3494. [Google Scholar] [CrossRef]
- Weisinger, K.; Kohl, A.; Kayam, G.; Monsonego-Ornan, E.; Sela-Donenfeld, D. Expression of hindbrain boundary markers is regulated by FGF3. Biol. Open. 2012, 1, 67–74. [Google Scholar] [CrossRef]
- Bouxsein, M.L.; Boyd, S.K.; Christiansen, B.A.; Guldberg, R.E.; Jepsen, K.J.; Müller, R.J. Guidelines for assessment of bone microstructure in rodents using micro–computed tomography. Bone Miner Res. 2010, 25, 1468–1486. [Google Scholar]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kalev-Altman, R.; Becker, G.; Levy, T.; Penn, S.; Shpigel, N.Y.; Monsonego-Ornan, E.; Sela-Donenfeld, D. Mmp2 Deficiency Leads to Defective Parturition and High Dystocia Rates in Mice. Int. J. Mol. Sci. 2023, 24, 16822. https://doi.org/10.3390/ijms242316822
Kalev-Altman R, Becker G, Levy T, Penn S, Shpigel NY, Monsonego-Ornan E, Sela-Donenfeld D. Mmp2 Deficiency Leads to Defective Parturition and High Dystocia Rates in Mice. International Journal of Molecular Sciences. 2023; 24(23):16822. https://doi.org/10.3390/ijms242316822
Chicago/Turabian StyleKalev-Altman, Rotem, Gal Becker, Tamar Levy, Svetlana Penn, Nahum Y. Shpigel, Efrat Monsonego-Ornan, and Dalit Sela-Donenfeld. 2023. "Mmp2 Deficiency Leads to Defective Parturition and High Dystocia Rates in Mice" International Journal of Molecular Sciences 24, no. 23: 16822. https://doi.org/10.3390/ijms242316822




