Influence of Organ Culture on the Characteristics of the Human Limbal Stem Cell Niche
Abstract
:1. Introduction
2. Results and Discussion
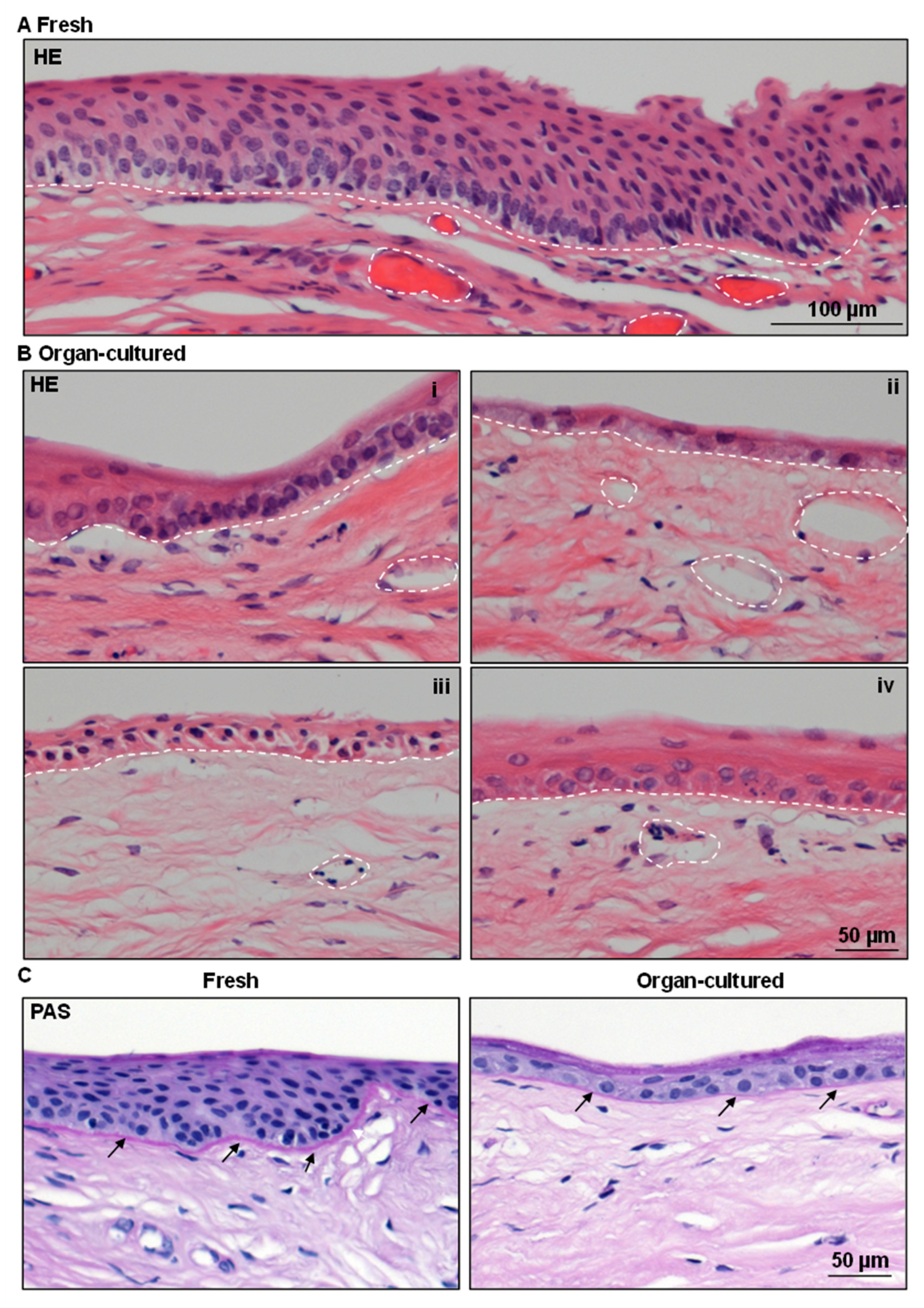
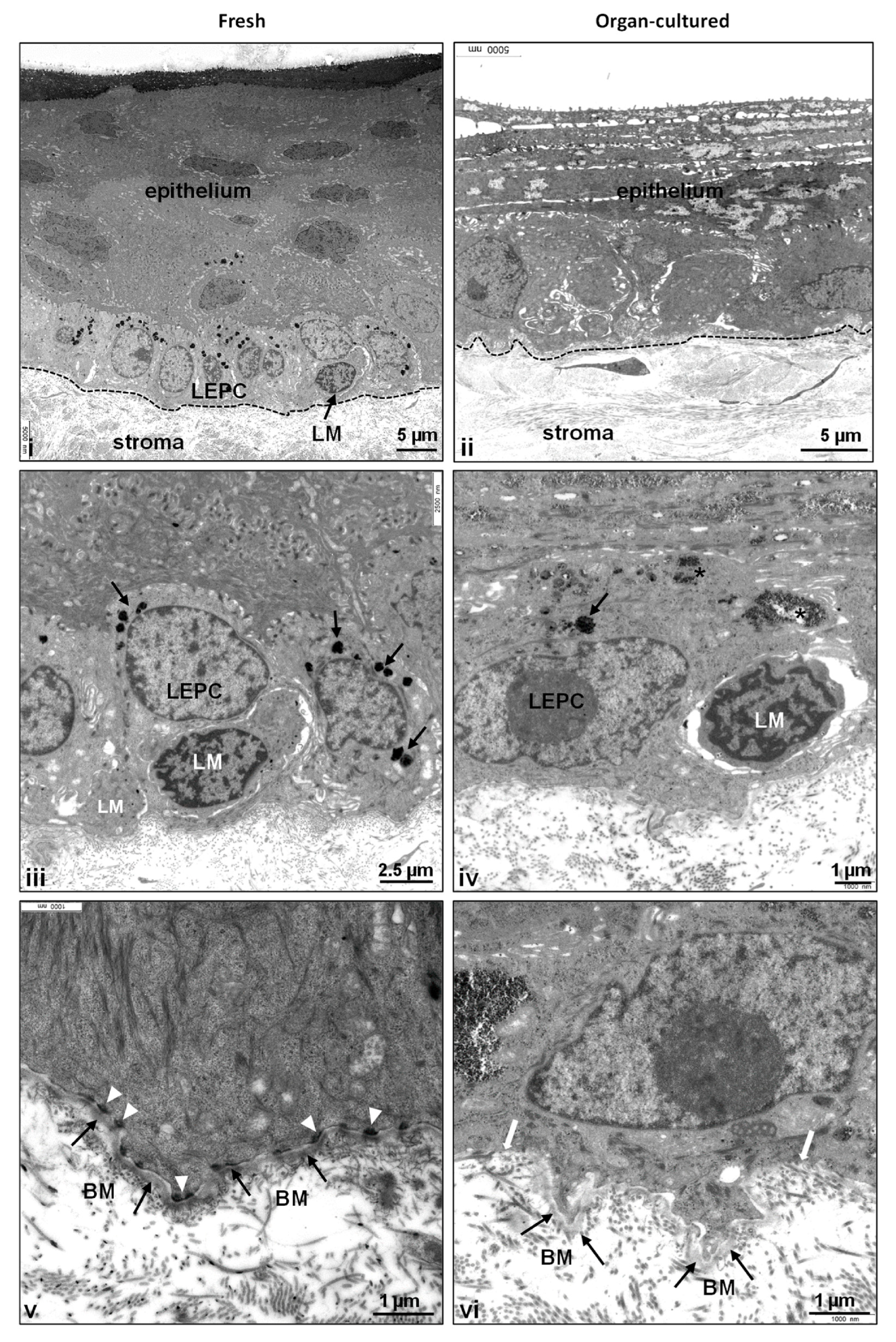
2.1. Architecture of Organ-Cultured Human Corneal Limbus
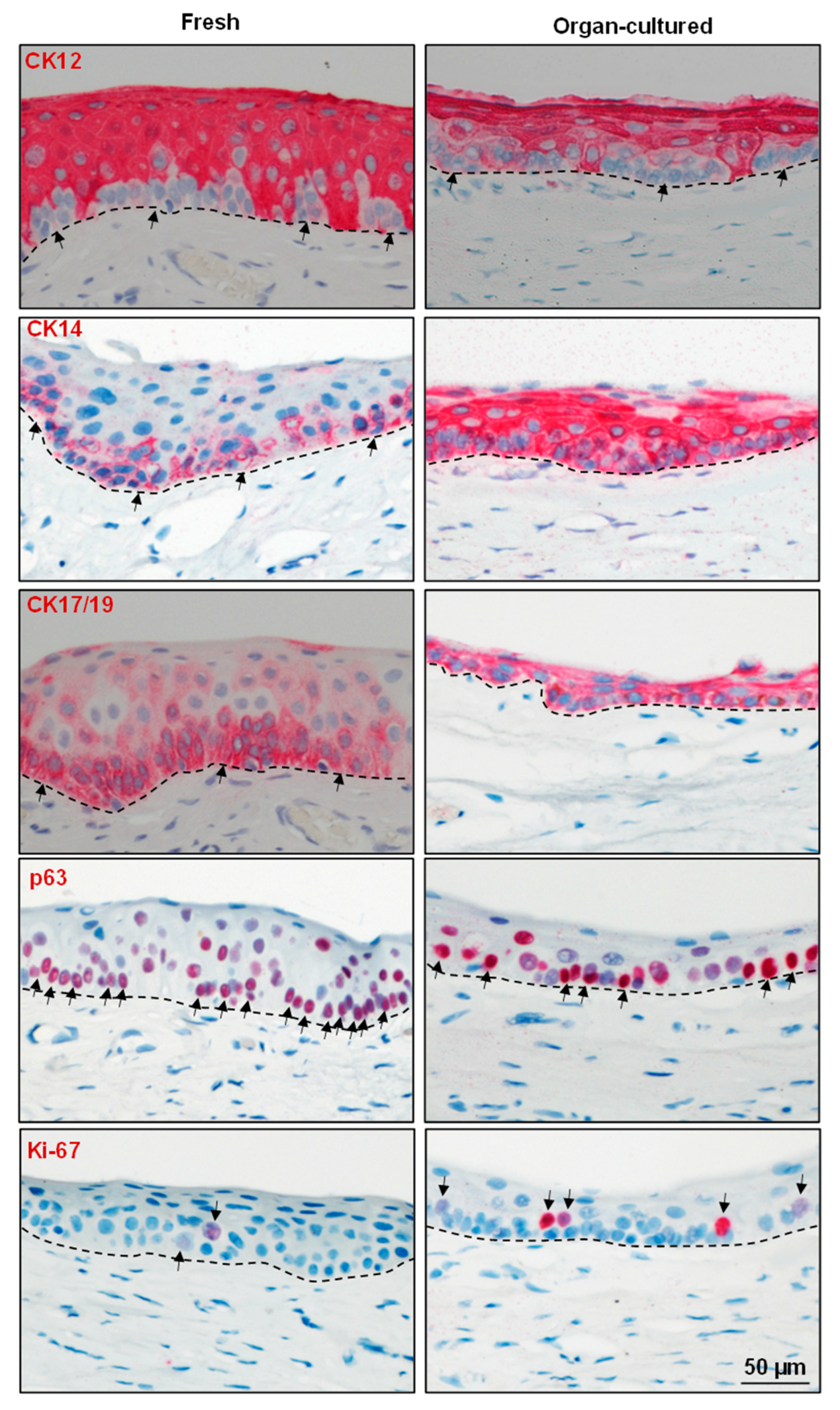
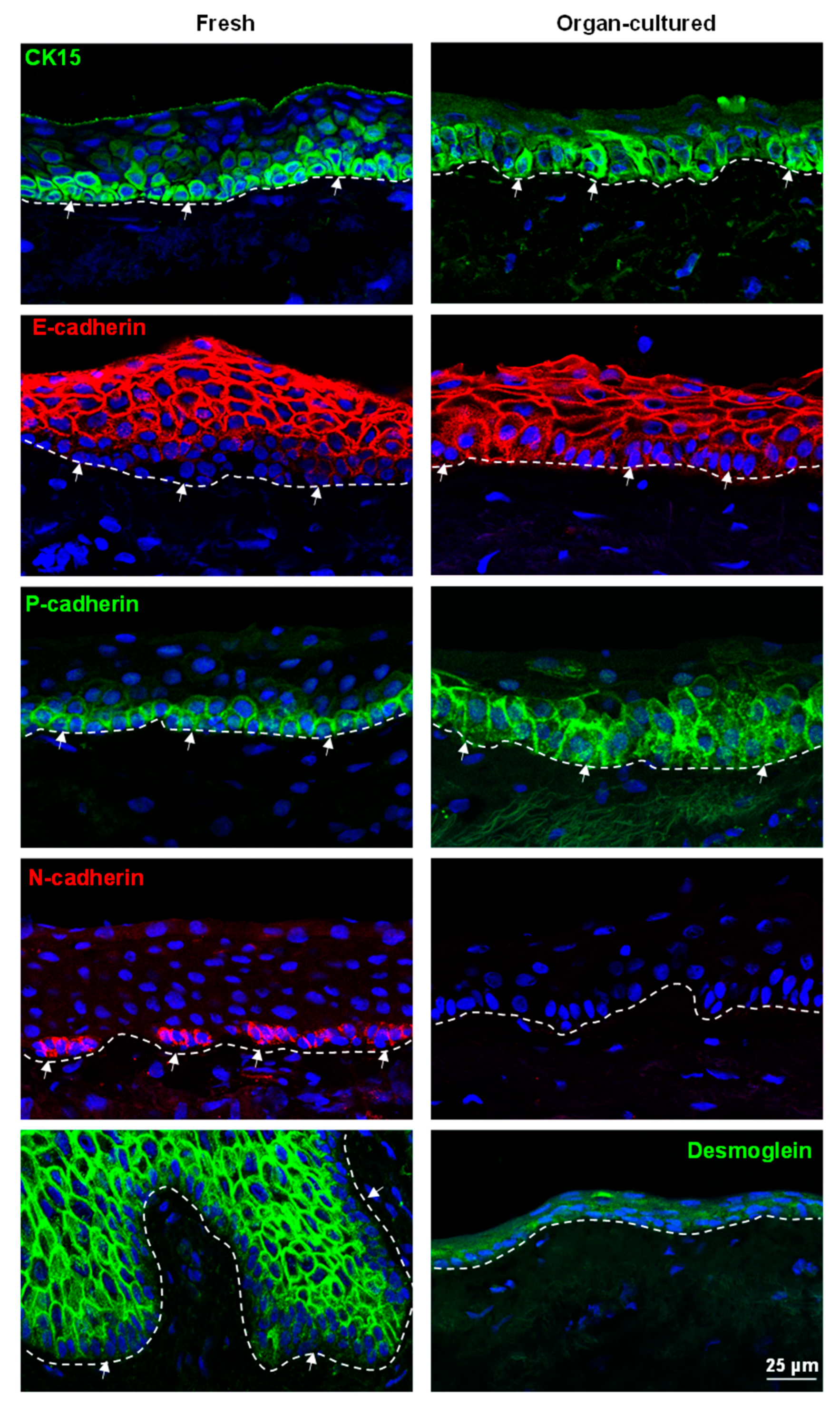
2.2. Immunohistochemical Characterization of the Organ-Cultured Corneal Limbus
2.2.1. Epithelial Progenitor/Differentiation State
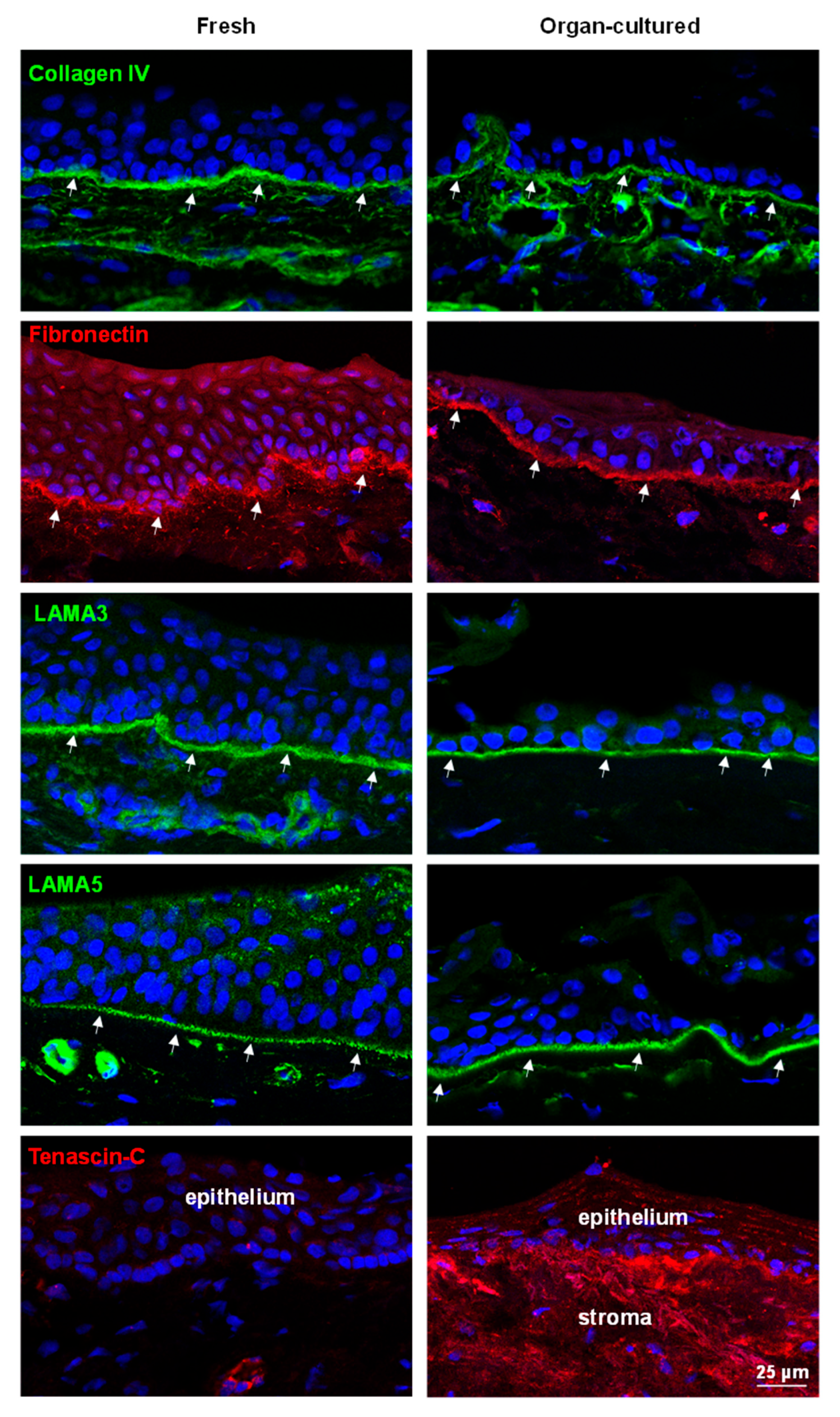
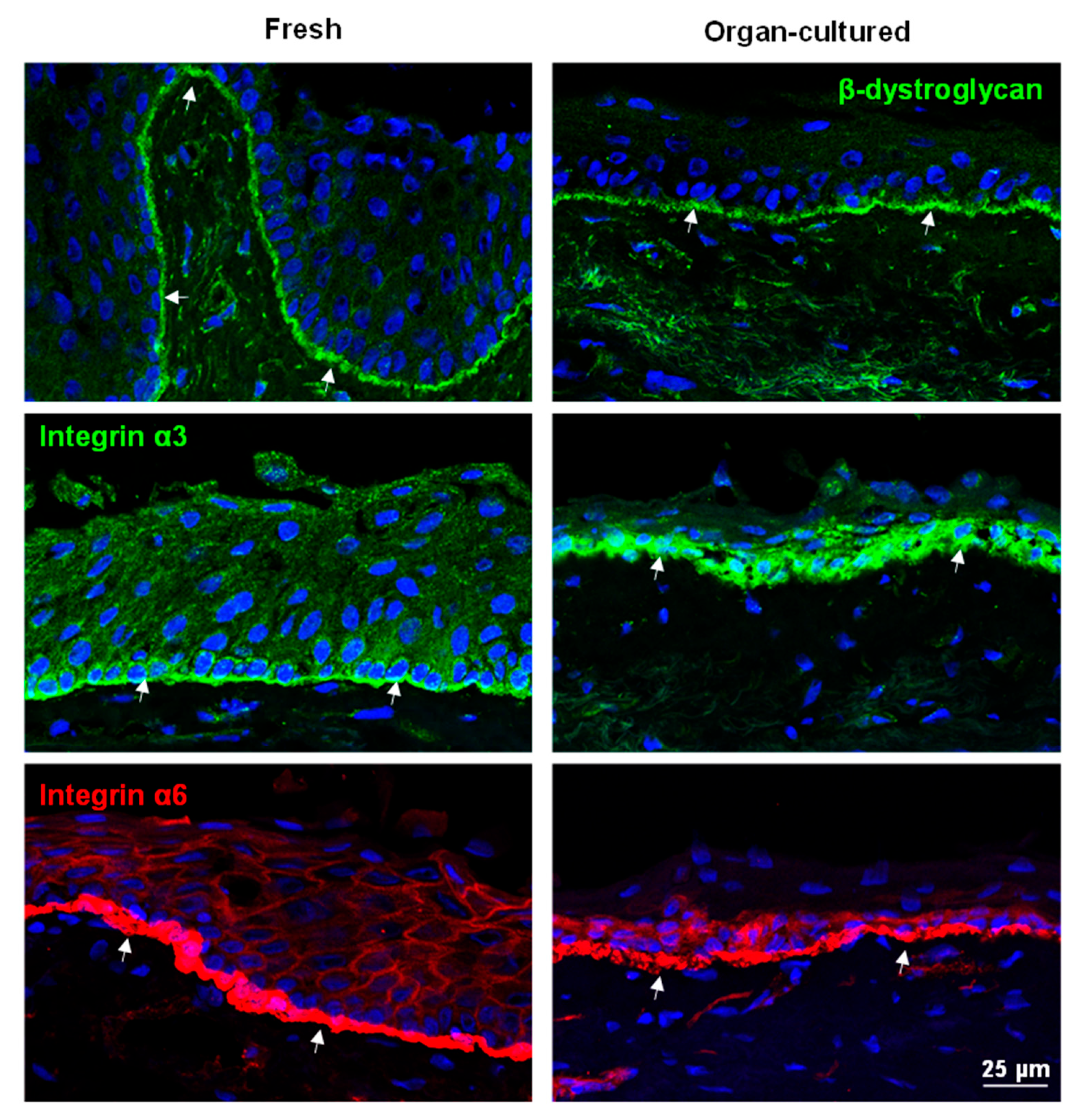
2.2.2. ECM and Associated Molecules
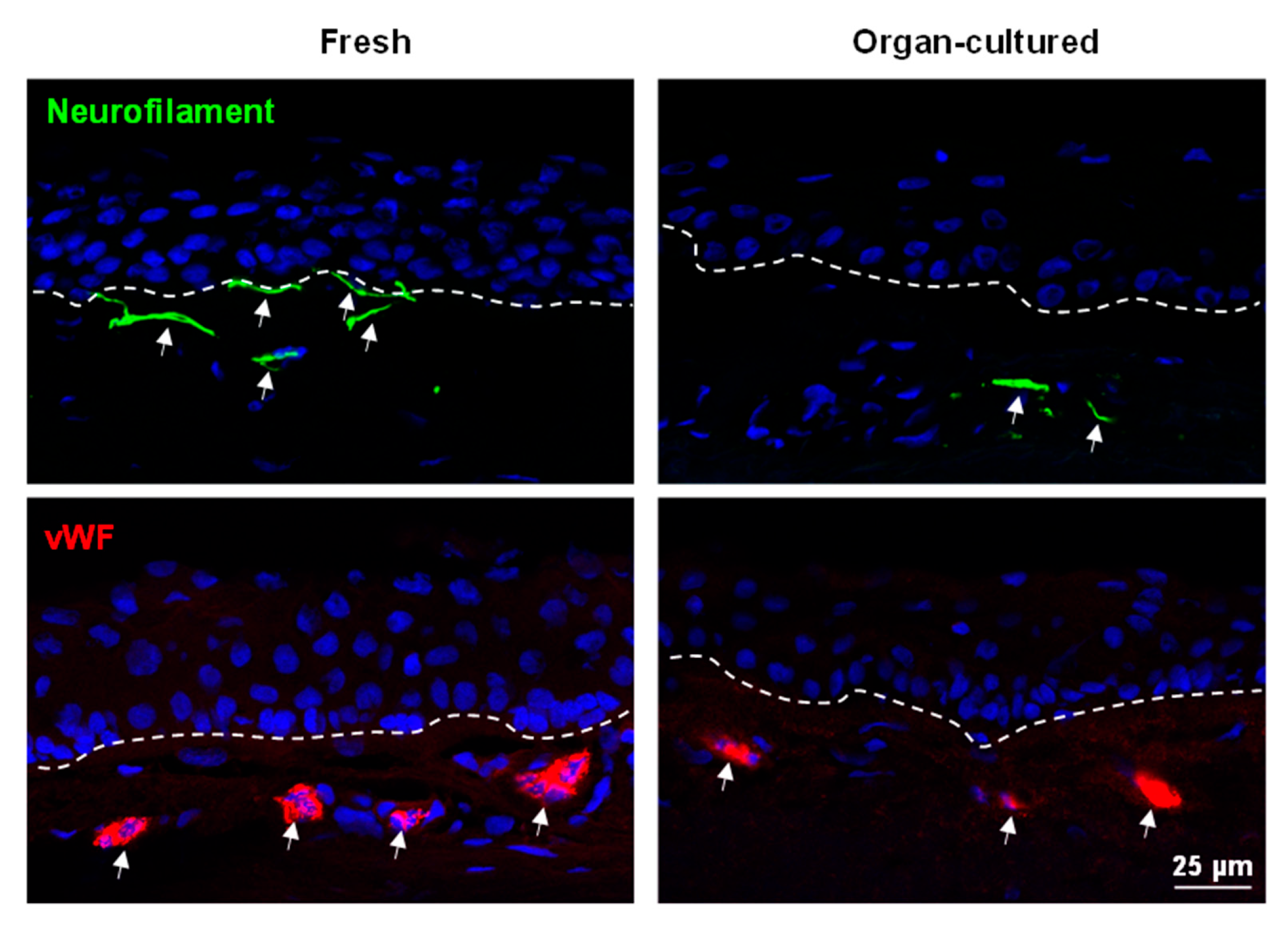
2.2.3. Limbal Niche Cells
3. Materials and Methods
3.1. Human Tissues
3.2. Tissue Preparation and Histology
3.3. Immunostaining of Frozen Sections
3.4. Immunostaining of Paraffin Sections
3.5. Transmission Electron Microscopy
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cotsarelis, G.; Cheng, S.Z.; Dong, G.; Sun, T.T.; Lavker, R.M. Existence of Slow-Cycling Limbal Epithelial Basal Cells That Can Be Preferentially Stimulated to Proliferate: Implications on Epithelial Stem Cells. Cell 1989, 57, 201–209. [Google Scholar] [CrossRef]
- Goldberg, M.F.; Bron, A.J. Limbal Palisades of Vogt. Trans. Am. Ophthalmol. Soc. 1982, 80, 155–171. [Google Scholar] [PubMed]
- Li, Y.; Inoue, T.; Takamatsu, F.; Kobayashi, T.; Shiraishi, A.; Maeda, N.; Ohashi, Y.; Nishida, K. Differences between Niche Cells and Limbal Stromal Cells in Maintenance of Corneal Limbal Stem Cells. Investig. Ophthalmol. Vis. Sci. 2014, 55, 1453–1462. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Zhang, Y.; Cai, S.; Sun, M.; Wang, J.; Li, S.; Li, X.; Tighe, S.; Chen, S.; Xie, H.; et al. Human Limbal Niche Cells Are a Powerful Regenerative Source for the Prevention of Limbal Stem Cell Deficiency in a Rabbit Model. Sci. Rep. 2018, 8, 6566. [Google Scholar] [CrossRef] [PubMed]
- Ordonez, P.; Di Girolamo, N. Limbal Epithelial Stem Cells: Role of the Niche Microenvironment. Stem Cells 2012, 30, 100–107. [Google Scholar] [CrossRef] [PubMed]
- Amin, S.; Jalilian, E.; Katz, E.; Frank, C.; Yazdanpanah, G.; Guaiquil, V.H.; Rosenblatt, M.I.; Djalilian, A.R. The Limbal Niche and Regenerative Strategies. Vision 2021, 5, 43. [Google Scholar] [CrossRef] [PubMed]
- Dziasko, M.A.; Daniels, J.T. Anatomical Features and Cell-Cell Interactions in the Human Limbal Epithelial Stem Cell Niche. Ocul. Surf. 2016, 14, 322–330. [Google Scholar] [CrossRef]
- Klenkler, B.; Sheardown, H. Growth Factors in the Anterior Segment: Role in Tissue Maintenance, Wound Healing and Ocular Pathology. Exp. Eye Res. 2004, 79, 677–688. [Google Scholar] [CrossRef]
- Schlötzer-Schrehardt, U.; Dietrich, T.; Saito, K.; Sorokin, L.; Sasaki, T.; Paulsson, M.; Kruse, F.E. Characterization of Extracellular Matrix Components in the Limbal Epithelial Stem Cell Compartment. Exp. Eye Res. 2007, 85, 845–860. [Google Scholar] [CrossRef]
- Polisetti, N.; Zenkel, M.; Menzel-Severing, J.; Kruse, F.E.; Schlötzer-Schrehardt, U. Cell Adhesion Molecules and Stem Cell-Niche-Interactions in the Limbal Stem Cell Niche. Stem Cells 2016, 34, 203–219. [Google Scholar] [CrossRef]
- Polisetti, N.; Sorokin, L.; Okumura, N.; Koizumi, N.; Kinoshita, S.; Kruse, F.E.; Schlötzer-Schrehardt, U. Laminin-511 and -521-Based Matrices for Efficient Ex Vivo-Expansion of Human Limbal Epithelial Progenitor Cells. Sci. Rep. 2017, 7, 5152. [Google Scholar] [CrossRef] [PubMed]
- Tseng, S.C.G.; He, H.; Zhang, S.; Chen, S.-Y. Niche Regulation of Limbal Epithelial Stem Cells: Relationship between Inflammation and Regeneration. Ocul. Surf. 2016, 14, 100–112. [Google Scholar] [CrossRef] [PubMed]
- Abdul-Al, M.; Kyeremeh, G.K.; Saeinasab, M.; Heidari Keshel, S.; Sefat, F. Stem Cell Niche Microenvironment: Review. Bioengineering 2021, 8, 108. [Google Scholar] [CrossRef] [PubMed]
- Funderburgh, J.L.; Funderburgh, M.L.; Du, Y. Stem Cells in the Limbal Stroma. Ocul. Surf. 2016, 14, 113–120. [Google Scholar] [CrossRef] [PubMed]
- Polisetty, N.; Fatima, A.; Madhira, S.L.; Sangwan, V.S.; Vemuganti, G.K. Mesenchymal Cells from Limbal Stroma of Human Eye. Mol. Vis. 2008, 14, 431–442. [Google Scholar] [PubMed]
- Al-Jaibaji, O.; Swioklo, S.; Connon, C.J. Mesenchymal Stromal Cells for Ocular Surface Repair. Expert. Opin. Biol. Ther. 2019, 19, 643–653. [Google Scholar] [CrossRef] [PubMed]
- Polisetti, N.; Gießl, A.; Zenkel, M.; Heger, L.; Dudziak, D.; Naschberger, E.; Stich, L.; Steinkasserer, A.; Kruse, F.E.; Schlötzer-Schrehardt, U. Melanocytes as Emerging Key Players in Niche Regulation of Limbal Epithelial Stem Cells. Ocul. Surf. 2021, 22, 172–189. [Google Scholar] [CrossRef]
- Polisetti, N.; Sharaf, L.; Schlötzer-Schrehardt, U.; Schlunck, G.; Reinhard, T. Efficient Isolation and Functional Characterization of Niche Cells from Human Corneal Limbus. Int. J. Mol. Sci. 2022, 23, 2750. [Google Scholar] [CrossRef]
- Sejpal, K.; Bakhtiari, P.; Deng, S.X. Presentation, Diagnosis and Management of Limbal Stem Cell Deficiency. Middle East. Afr. J. Ophthalmol. 2013, 20, 5–10. [Google Scholar] [CrossRef]
- Tseng, S.C. Concept and Application of Limbal Stem Cells. Eye 1989, 3 Pt 2, 141–157. [Google Scholar] [CrossRef]
- Calonge, M.; Nieto-Miguel, T.; de la Mata, A.; Galindo, S.; Herreras, J.M.; López-Paniagua, M. Goals and Challenges of Stem Cell-Based Therapy for Corneal Blindness Due to Limbal Deficiency. Pharmaceutics 2021, 13, 1483. [Google Scholar] [CrossRef] [PubMed]
- Aketa, N.; Kasai, M.; Noda, S.; Asano, J.; Kunieda, A.; Kawanishi, S.; Maruyama, Y.; Honda, F. Insights into the Clinical Development of Regenerative Medical Products through a Comparison of Three Cell-Based Products Recently Approved for Limbal Stem Cell Deficiency. Ocul. Surf. 2023, 29, 220–225. [Google Scholar] [CrossRef] [PubMed]
- Reinhard, T.; Spelsberg, H.; Henke, L.; Kontopoulos, T.; Enczmann, J.; Wernet, P.; Berschick, P.; Sundmacher, R.; Böhringer, D. Long-Term Results of Allogeneic Penetrating Limbo-Keratoplasty in Total Limbal Stem Cell Deficiency. Ophthalmology 2004, 111, 775–782. [Google Scholar] [CrossRef] [PubMed]
- Lang, S.J.; Böhringer, D.; Geerling, G.; Reinhard, T. Long-Term Results of Allogenic Penetrating Limbo-Keratoplasty: 20 Years of Experience. Eye 2017, 31, 372–378. [Google Scholar] [CrossRef] [PubMed]
- Shanbhag, S.S.; Saeed, H.N.; Paschalis, E.I.; Chodosh, J. Keratolimbal Allograft for Limbal Stem Cell Deficiency after Severe Corneal Chemical Injury: A Systematic Review. Br. J. Ophthalmol. 2018, 102, 1114–1121. [Google Scholar] [CrossRef] [PubMed]
- Ghareeb, A.E.; Lako, M.; Figueiredo, F.C. Recent Advances in Stem Cell Therapy for Limbal Stem Cell Deficiency: A Narrative Review. Ophthalmol. Ther. 2020, 9, 809–831. [Google Scholar] [CrossRef] [PubMed]
- Robertson, S.Y.T.; Roberts, J.S.; Deng, S.X. Regulation of Limbal Epithelial Stem Cells: Importance of the Niche. Int. J. Mol. Sci. 2021, 22, 11975. [Google Scholar] [CrossRef] [PubMed]
- Romo-Valera, C.; Pérez-Garrastachu, M.; Hernáez-Moya, R.; Rodriguez-Astigarraga, M.; Romano-Ruiz, P.; Etxebarria, J.; Arluzea, J.; Andollo, N. Characterisation of Corneas Following Different Time and Storage Methods for Their Use as a Source of Stem-like Limbal Epithelial Cells. Exp. Eye Res. 2021, 211, 108720. [Google Scholar] [CrossRef]
- Le-Bel, G.; Desjardins, P.; Gross, C.; Cortez Ghio, S.; Couture, C.; Germain, L.; Guérin, S.L. Influence of the Postmortem/Storage Time of Human Corneas on the Properties of Cultured Limbal Epithelial Cells. Cells 2022, 11, 2716. [Google Scholar] [CrossRef]
- Shanmuganathan, V.A.; Rotchford, A.P.; Tullo, A.B.; Joseph, A.; Zambrano, I.; Dua, H.S. Epithelial Proliferative Potential of Organ Cultured Corneoscleral Rims; Implications for Allo-Limbal Transplantation and Eye Banking. Br. J. Ophthalmol. 2006, 90, 55–58. [Google Scholar] [CrossRef]
- Pels, E.; Schuchard, Y. Organ-Culture Preservation of Human Corneas. Doc. Ophthalmol. 1983, 56, 147–153. [Google Scholar] [CrossRef] [PubMed]
- Andersen, J.; Ehlers, N. The Influence of Donor Age and Post Mortem Time on Corneal Graft Survival and Thickness When Employing Banked Donor Material (A Five-Year Follow-Up). Acta Ophthalmol. 1988, 66, 313–317. [Google Scholar] [CrossRef]
- Polisetti, N.; Sharaf, L.; Martin, G.; Schlunck, G.; Reinhard, T. P-Cadherin Is Expressed by Epithelial Progenitor Cells and Melanocytes in the Human Corneal Limbus. Cells 2022, 11, 1975. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, X.; Schlunck, G.; Wolf, J.; Rosmus, D.-D.; Bleul, T.; Luo, R.; Böhringer, D.; Wieghofer, P.; Lange, C.; Reinhard, T.; et al. Time- and Stimulus-Dependent Characteristics of Innate Immune Cells in Organ-Cultured Human Corneal Tissue. J. Innate Immun. 2021, 14, 98–111. [Google Scholar] [CrossRef] [PubMed]
- Ardjomand, N.; Berghold, A.; Reich, M.E. Loss of Corneal Langerhans Cells during Storage in Organ Culture Medium, Optisol and McCarey-Kaufman Medium. Eye 1998, 12, 134–138. [Google Scholar] [CrossRef] [PubMed]
- Haug, K.; Azqueta, A.; Johnsen-Soriano, S.; Shahdadfar, A.; Drolsum, L.K.; Moe, M.C.; Røger, M.T.; Romero, F.J.; Collins, A.R.; Nicolaissen, B. Donor Cornea Transfer from Optisol GS to Organ Culture Storage: A Two-Step Procedure to Increase Donor Tissue Lifespan. Acta Ophthalmol. 2013, 91, 219–225. [Google Scholar] [CrossRef] [PubMed]
- Wolf, J.; Kammrath Betancor, P.; Maier, P.; Heinzelmann, S.U.; Jiang, J.; Lange, C.; Reinhard, T.; Schlunck, G.; Lapp, T. Transcriptional Profiling Provides New Insights into Organ Culture-Induced Changes in Human Donor Corneas. Int. J. Mol. Sci. 2022, 23, 14507. [Google Scholar] [CrossRef]
- Armitage, W.J.; Jones, M.N.A.; Zambrano, I.; Carley, F.; Tole, D.M. The Suitability of Corneas Stored by Organ Culture for Penetrating Keratoplasty and Influence of Donor and Recipient Factors on 5-Year Graft Survival. Investig. Ophthalmol. Vis. Sci. 2014, 55, 784–791. [Google Scholar] [CrossRef]
- Mason, S.L.; Stewart, R.M.K.; Sheridan, C.M.; Keshtkar, F.; Rooney, P.; Austin, E.; Schlötzer-Schrehardt, U.; Kruse, F.E.; Kaye, S.B. Yield and Viability of Human Limbal Stem Cells from Fresh and Stored Tissue. Investig. Ophthalmol. Vis. Sci. 2016, 57, 3708–3713. [Google Scholar] [CrossRef]
- Figueira, E.; Di Girolamo, N.; Coroneo, M.; Wakefield, D. The Phenotype of Limbal Epithelial Stem Cells. Investig. Ophthalmol. Vis. Sci. 2007, 48, 144–156. [Google Scholar] [CrossRef]
- Schlötzer-Schrehardt, U.; Kruse, F.E. Identification and Characterization of Limbal Stem Cells. Exp. Eye Res. 2005, 81, 247–264. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, R.; Yamato, M.; Sugiyama, H.; Sumide, T.; Yang, J.; Okano, T.; Tano, Y.; Nishida, K. N-Cadherin Is Expressed by Putative Stem/Progenitor Cells and Melanocytes in the Human Limbal Epithelial Stem Cell Niche. Stem Cells 2007, 25, 289–296. [Google Scholar] [CrossRef] [PubMed]
- Pellegrini, G.; Dellambra, E.; Golisano, O.; Martinelli, E.; Fantozzi, I.; Bondanza, S.; Ponzin, D.; McKeon, F.; De Luca, M. P63 Identifies Keratinocyte Stem Cells. Proc. Natl. Acad. Sci. USA 2001, 98, 3156–3161. [Google Scholar] [CrossRef] [PubMed]
- Senoo, M.; Pinto, F.; Crum, C.P.; McKeon, F. P63 Is Essential for the Proliferative Potential of Stem Cells in Stratified Epithelia. Cell 2007, 129, 523–536. [Google Scholar] [CrossRef] [PubMed]
- Takács, L.; Tóth, E.; Berta, A.; Vereb, G. Stem Cells of the Adult Cornea: From Cytometric Markers to Therapeutic Applications. Cytom. A 2009, 75, 54–66. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; de Paiva, C.S.; Luo, L.; Kretzer, F.L.; Pflugfelder, S.C.; Li, D. Characterization of Putative Stem Cell Phenotype in Human Limbal Epithelia. Stem Cells 2004, 22, 355–366. [Google Scholar] [CrossRef] [PubMed]
- Higa, K.; Shimmura, S.; Miyashita, H.; Kato, N.; Ogawa, Y.; Kawakita, T.; Shimazaki, J.; Tsubota, K. N-Cadherin in the Maintenance of Human Corneal Limbal Epithelial Progenitor Cells In Vitro. Investig. Ophthalmol. Vis. Sci. 2009, 50, 4640–4645. [Google Scholar] [CrossRef]
- Arai, F.; Hirao, A.; Hosokawa, K.; Suda, T. Role of Cell Adhesion in the Maintenance of Hematopoietic Stem Cells in the Bone Marrow Niche. Blood 2004, 104, 669. [Google Scholar] [CrossRef]
- Preservation of Human Limbal Epithelial Progenitor Cells on Carbodiimide Cross-Linked Amniotic Membrane via Integrin-Linked Kinase-Mediated Wnt Activation—ScienceDirect. Available online: https://www.sciencedirect.com/science/article/pii/S174270611530218X?via%3Dihub (accessed on 22 November 2023).
- Ljubimov, A.V.; Burgeson, R.E.; Butkowski, R.J.; Michael, A.F.; Sun, T.T.; Kenney, M.C. Human Corneal Basement Membrane Heterogeneity: Topographical Differences in the Expression of Type IV Collagen and Laminin Isoforms. Lab. Investig. 1995, 72, 461–473. [Google Scholar]
- Mei, H.; Gonzalez, S.; Deng, S.X. Extracellular Matrix Is an Important Component of Limbal Stem Cell Niche. J. Funct. Biomater. 2012, 3, 879–894. [Google Scholar] [CrossRef]
- Polisetti, N.; Gießl, A.; Li, S.; Sorokin, L.; Kruse, F.E.; Schlötzer-Schrehardt, U. Laminin-511-E8 Promotes Efficient in Vitro Expansion of Human Limbal Melanocytes. Sci. Rep. 2020, 10, 11074. [Google Scholar] [CrossRef] [PubMed]
- Tenascin-C: A Key Regulator in Angiogenesis during Wound Healing—PMC. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC9687801/ (accessed on 4 November 2023).
- Maseruka, H.; Ridgway, A.; Tullo, A.; Bonshek, R. Developmental Changes in Patterns of Expression of Tenascin-C Variants in the Human Cornea. Investig. Ophthalmol. Vis. Sci. 2000, 41, 4101–4107. [Google Scholar]
- Schlötzer-Schrehardt, U.; Latta, L.; Gießl, A.; Zenkel, M.; Fries, F.N.; Käsmann-Kellner, B.; Kruse, F.E.; Seitz, B. Dysfunction of the Limbal Epithelial Stem Cell Niche in Aniridia-Associated Keratopathy. Ocul. Surf. 2021, 21, 160–173. [Google Scholar] [CrossRef] [PubMed]
- Maseruka, H.; Bonshek, R.E.; Tullo, A.B. Tenascin-C Expression in Normal, Inflamed, and Scarred Human Corneas. Br. J. Ophthalmol. 1997, 81, 677–682. [Google Scholar] [CrossRef]
- Tian, M.; Jacobson, C.; Gee, S.H.; Campbell, K.P.; Carbonetto, S.; Jucker, M. Dystroglycan in the Cerebellum Is a Laminin Alpha 2-Chain Binding Protein at the Glial-Vascular Interface and Is Expressed in Purkinje Cells. Eur. J. Neurosci. 1996, 8, 2739–2747. [Google Scholar] [CrossRef] [PubMed]
- Teniente-De Alba, C.; Martínez-Vieyra, I.; Vivanco-Calixto, R.; Galván, I.J.; Cisneros, B.; Cerecedo, D. Distribution of Dystrophin- and Utrophin-Associated Protein Complexes (DAPC/UAPC) in Human Hematopoietic Stem/Progenitor Cells. Eur. J. Haematol. 2011, 87, 312–322. [Google Scholar] [CrossRef] [PubMed]
- Xi, R. Anchoring Stem Cells in the Niche by Cell Adhesion Molecules. Cell Adhes. Migr. 2009, 3, 396–401. [Google Scholar] [CrossRef]
- Dziasko, M.A.; Tuft, S.J.; Daniels, J.T. Limbal Melanocytes Support Limbal Epithelial Stem Cells in 2D and 3D Microenvironments. Exp. Eye Res. 2015, 138, 70–79. [Google Scholar] [CrossRef]
- Al-Aqaba, M.A.; Dhillon, V.K.; Mohammed, I.; Said, D.G.; Dua, H.S. Corneal Nerves in Health and Disease. Prog. Retin. Eye Res. 2019, 73, 100762. [Google Scholar] [CrossRef]
- Lang, S.J.; Werner, N.; Böhringer, D.; Maier, P.; Reinhard, T. Influence of Graft Vascularization on Graft Survival Following Homologous Limbo-Keratoplasty. Int. Ophthalmol. 2022, 42, 3053–3059. [Google Scholar] [CrossRef]
- Mendez, M.G.; Kojima, S.-I.; Goldman, R.D. Vimentin Induces Changes in Cell Shape, Motility, and Adhesion during the Epithelial to Mesenchymal Transition. FASEB J. 2010, 24, 1838–1851. [Google Scholar] [CrossRef] [PubMed]
- Yu, F.X.; Guo, J.; Zhang, Q. Expression and Distribution of Adhesion Molecule CD44 in Healing Corneal Epithelia. Investig. Ophthalmol. Vis. Sci. 1998, 39, 710–717. [Google Scholar]
- Gillette, T.E.; Chandler, J.W.; Greiner, J.V. Langerhans Cells of the Ocular Surface. Ophthalmology 1982, 89, 700–711. [Google Scholar] [CrossRef] [PubMed]
- Lange, C.; Wolf, J.; Auw-Haedrich, C.; Schlecht, A.; Boneva, S.; Lapp, T.; Horres, R.; Agostini, H.; Martin, G.; Reinhard, T.; et al. Expression of the COVID-19 Receptor ACE2 in the Human Conjunctiva. J. Med. Virol. 2020, 92, 2081–2086. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Polisetti, N.; Martin, G.; Ulrich, E.; Glegola, M.; Schlötzer-Schrehardt, U.; Schlunck, G.; Reinhard, T. Influence of Organ Culture on the Characteristics of the Human Limbal Stem Cell Niche. Int. J. Mol. Sci. 2023, 24, 16856. https://doi.org/10.3390/ijms242316856
Polisetti N, Martin G, Ulrich E, Glegola M, Schlötzer-Schrehardt U, Schlunck G, Reinhard T. Influence of Organ Culture on the Characteristics of the Human Limbal Stem Cell Niche. International Journal of Molecular Sciences. 2023; 24(23):16856. https://doi.org/10.3390/ijms242316856
Chicago/Turabian StylePolisetti, Naresh, Gottfried Martin, Eva Ulrich, Mateusz Glegola, Ursula Schlötzer-Schrehardt, Günther Schlunck, and Thomas Reinhard. 2023. "Influence of Organ Culture on the Characteristics of the Human Limbal Stem Cell Niche" International Journal of Molecular Sciences 24, no. 23: 16856. https://doi.org/10.3390/ijms242316856




