Apoptosis Induction in HepG2 and HCT116 Cells by a Novel Quercetin-Zinc (II) Complex: Enhanced Absorption of Quercetin and Zinc (II)
Abstract
:1. Introduction
2. Results and Discussion
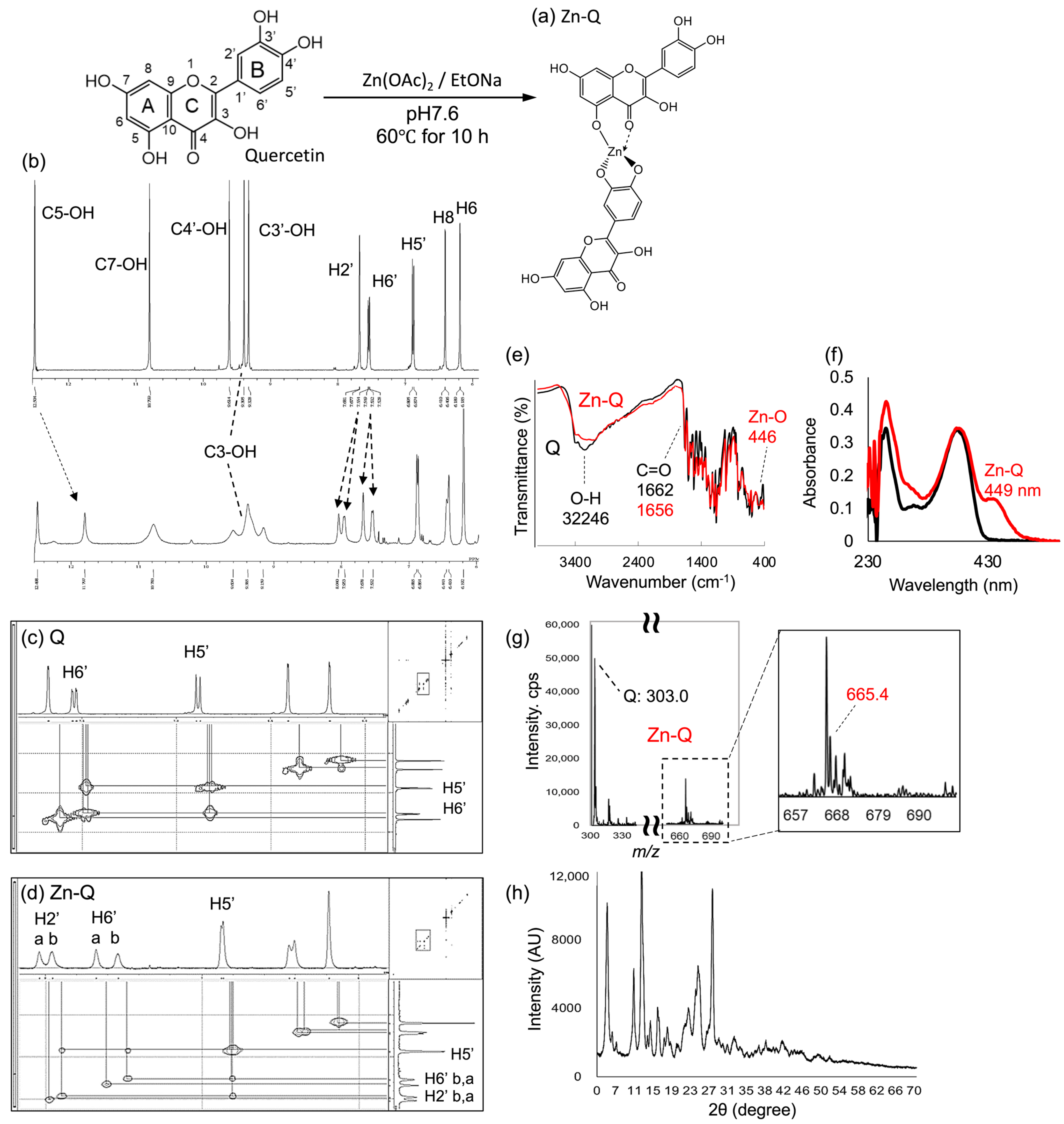
2.1. Spectroscopic Characterization of Zn-Q and Q+Zn
2.1.1. Analysis of Synthetic Zn-Q
2.1.2. Analysis of Predicted Conformational Changes of Quercetin and Zinc Sulfate in Cultured Cell Media
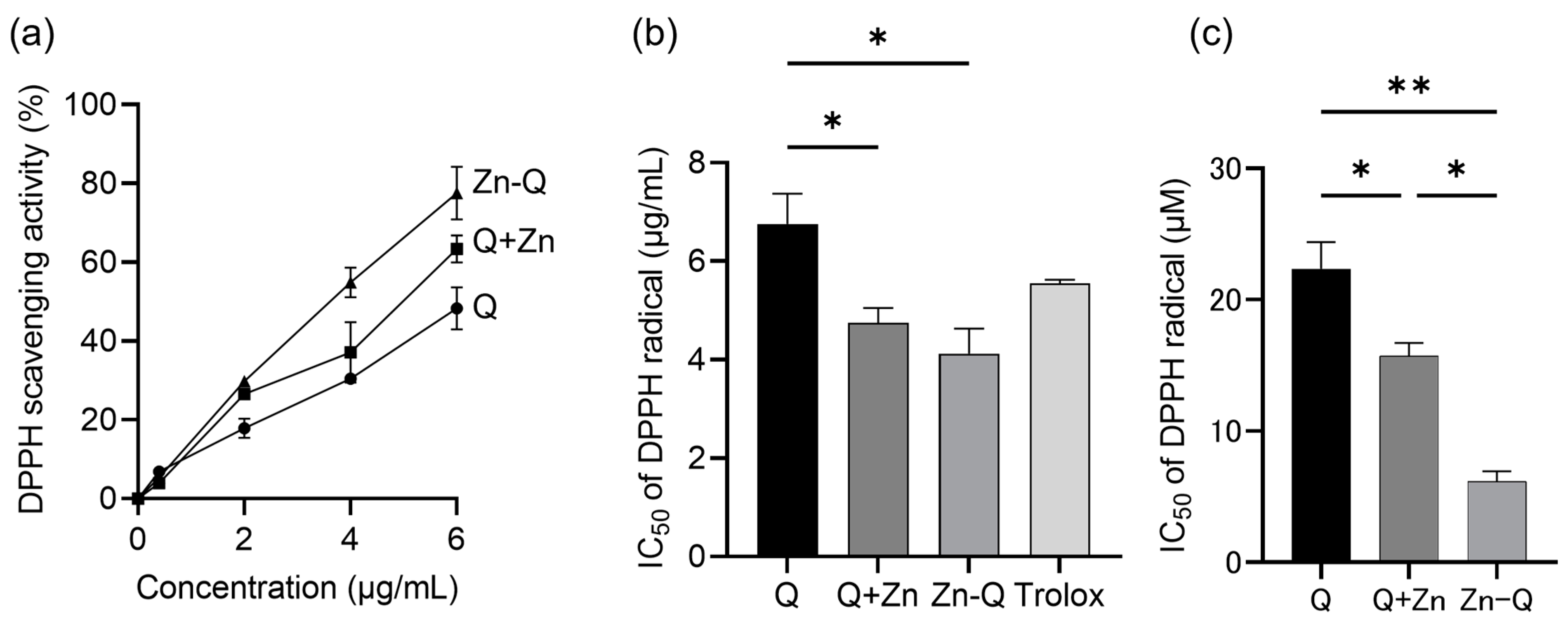
2.2. Antioxidant Activity of Quercetin, Q+Zn, and Zn-Q
2.3. The Antitumor Activity of Quercetin, Q+Zn, and Zn-Q
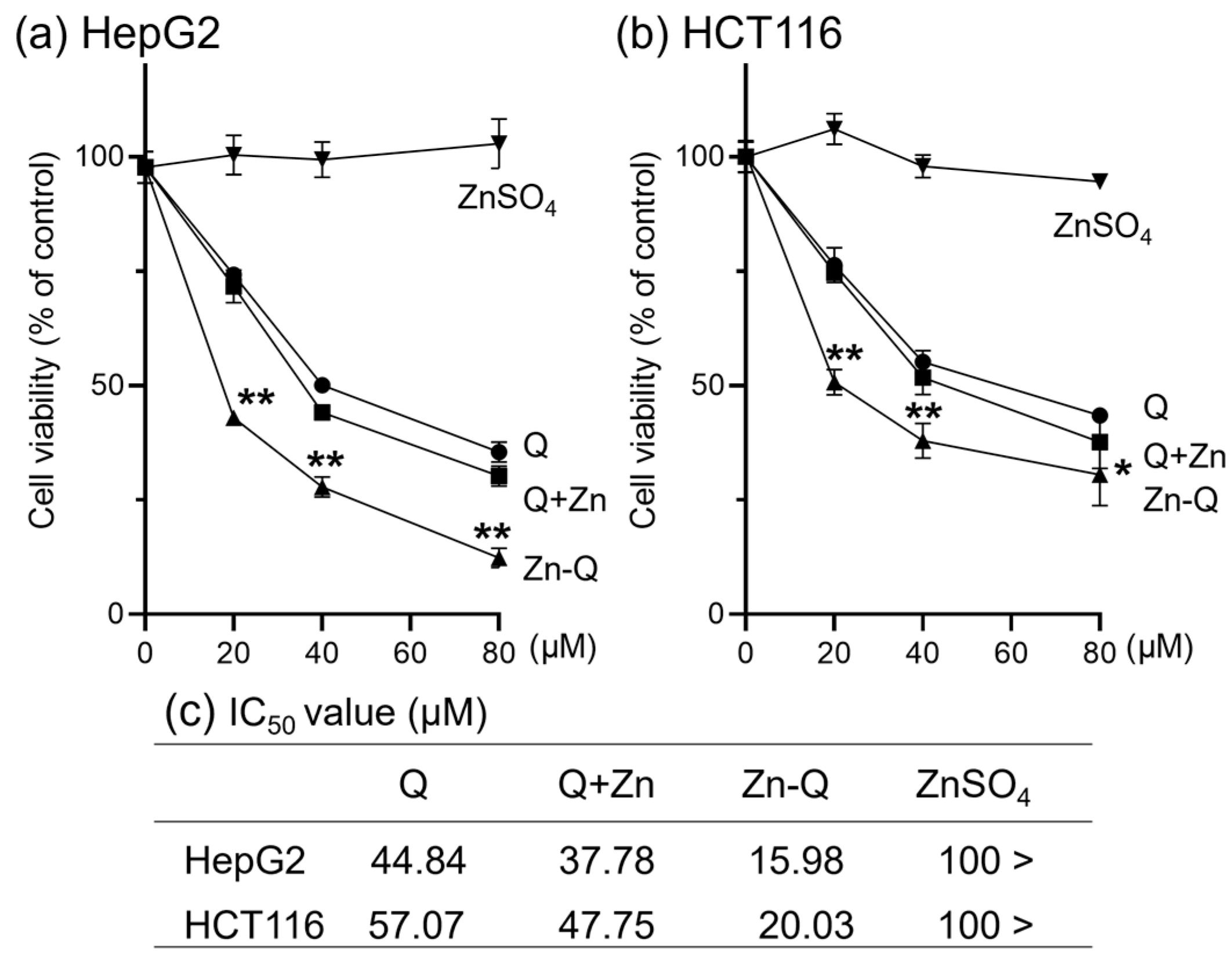
2.3.1. Effect of Quercetin–Zinc (II) Complexes on the Inhibition of HepG2 and HCT116 Cells Proliferation
2.3.2. Apoptosis Induction by Quercetin–Zinc (II) Complexes
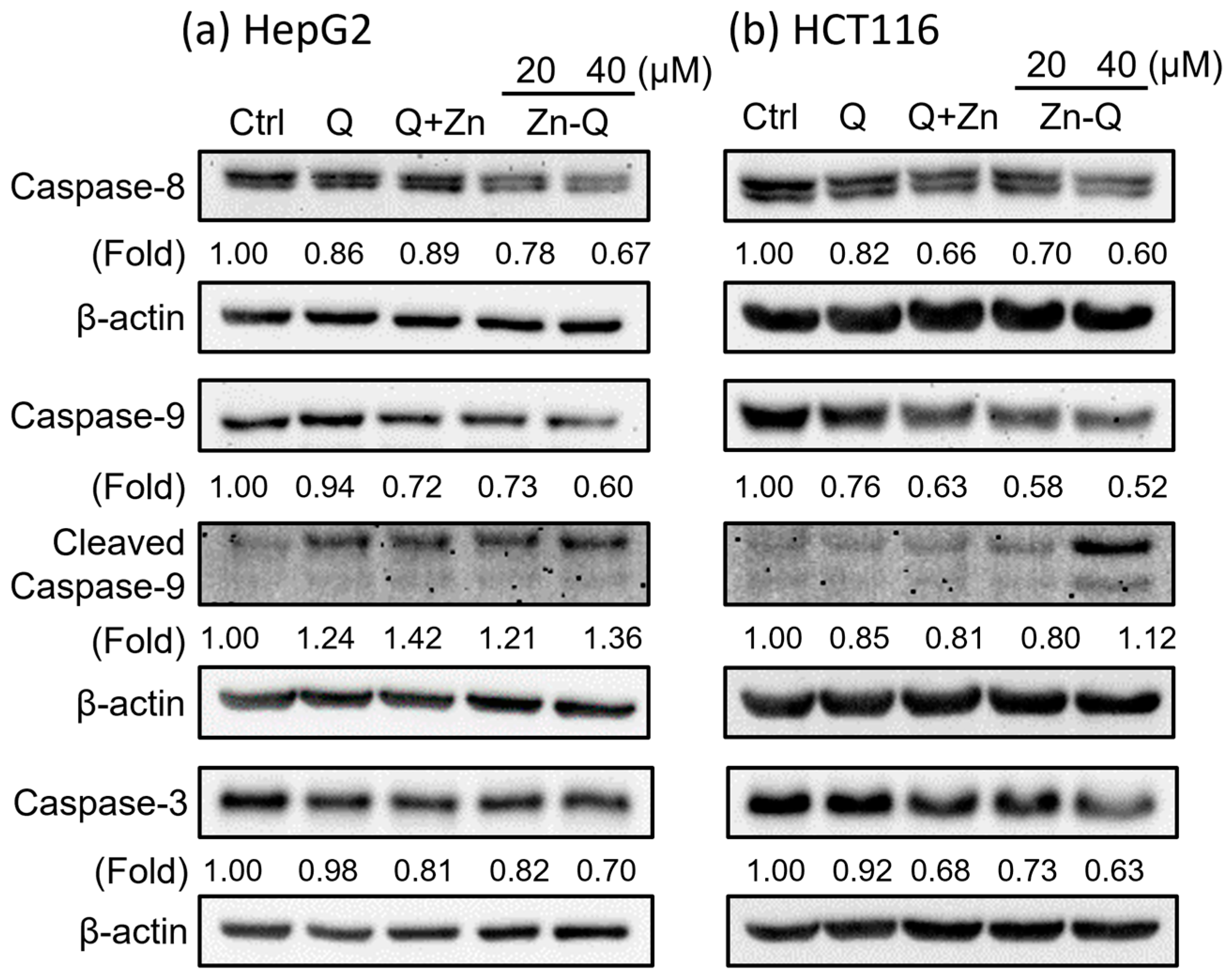
2.3.3. Zn-Q Significantly Induced Activation and Upregulation of Caspase-8, -9, and -3 Proteins
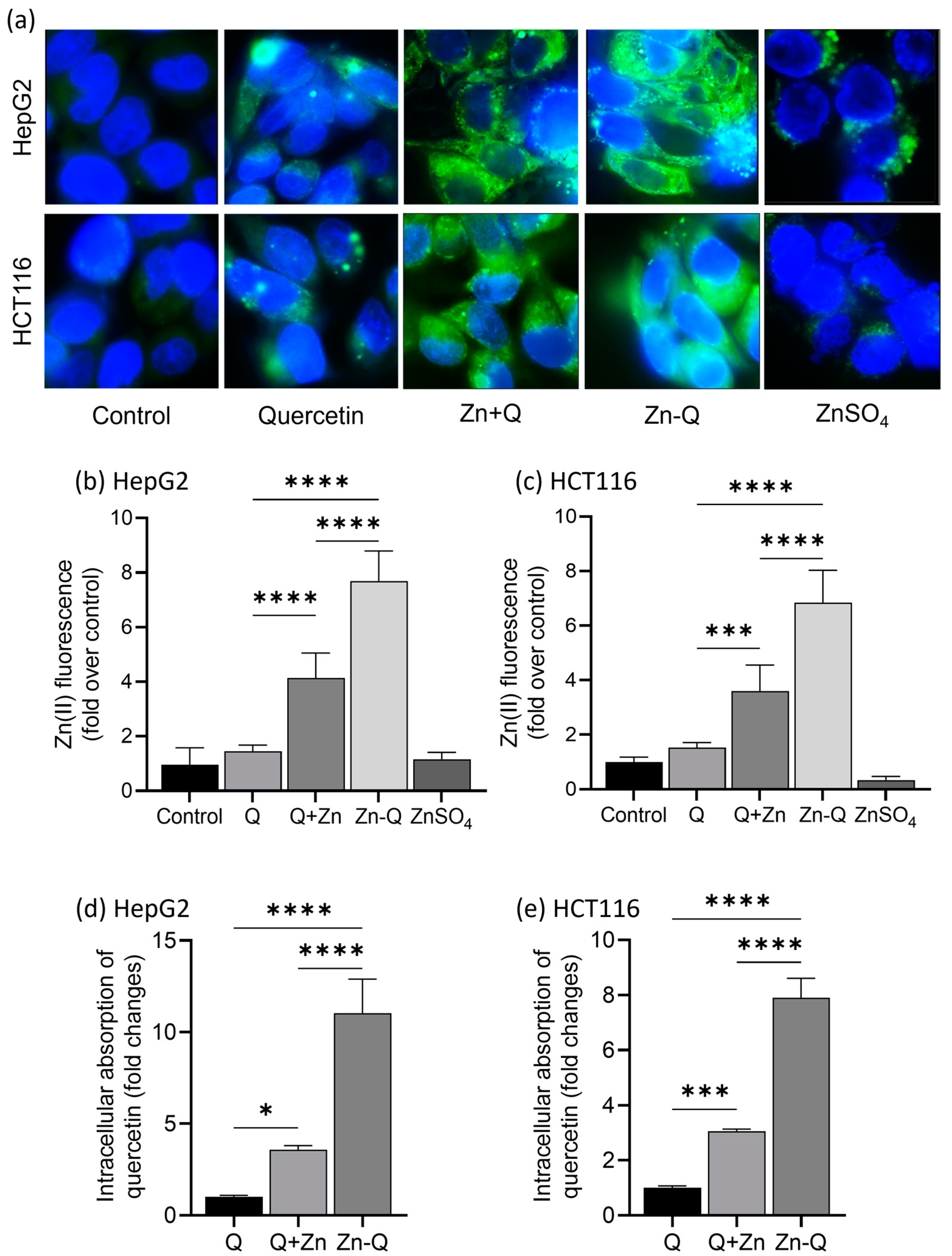
2.4. Intracellular Localization and Absorption of Q+Zn and Zn-Q
3. Materials and Methods
3.1. Reagents
3.2. Synthesis of the Zinc (II)–Quercetin Complex (Zn-Q)
3.3. Structural Characterization of Zn-Q
3.4. Structural Characterization of Q+Zn
3.5. Cell Culture and Sample Treatment
3.6. 2,2-diphenyl-1-picrylhydrazy (DPPH) Free Radical Scavenging Activity
3.7. Cell Viability Assay
3.8. ELISA Detection of DNA Fragmentation
3.9. Apoptosis Detection by Annexin V-FITC/Propidium Iodide Flow Cytometry
3.10. Western Blot Analysis
3.11. Flow Cytometric Measurement of Caspase-3 Activity
3.12. Measurement of Intracellular Zinc Localization and Content by Fluorescent Staining
3.13. Uptake of Quercetin into HepG2 and HCT116 Cells
3.14. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| 1H | Quercetin | Zn-Q |
|---|---|---|
| C5-OH | 12.50 (1H, s) | 11.79 (1H, brs) |
| C7-OH | 10.79 (1H, s) | 10.78 (2H, brs) |
| C4′-OH | 9.61 (1H, s) | 9.60 (1H, brs) |
| C3-OH | 9.39 (1H, s) | 9.38 (2H, brs) |
| C3′-OH | 9.32 (1H, s) | 9.15 (1H, brs) |
| H-2′ | 7.68 (1H, d, J = 2.4 Hz) | 8.04 (1H, brs)/7.93 (1H, brs) |
| H-6′ | 7.54 (1H, dd, J = 3.0/ 3.0 Hz) | 7.67 (1H, brs)/7.53 (1H, brs) |
| H-5′ | 6.88 (1H, d, J = 12.6 Hz) | 6.87 (2H, d, J = 12.6 Hz) |
| H-8 | 6.41 (1H, d, J = 2.4 Hz) | 6.42 (2H, d, J = 19.8 Hz) |
| H-6 | 6.18 (1H, d, J = 3.0 Hz) | 6.19 (2H, s) |
| 1H | Quercetin/DMEM | Q+Zn/DMEM |
| C5-OH | 12.49 (2H, s) | - |
| C7-OH | 10.79 (1H, s) | - |
| C4′-OH | 9.38 (2H, s) | - |
| C3-OH | - | 9.29 (2H, S) |
| C3′-OH | 9.38 (2H, s) | - |
| H-2′ | 7.66 (2H, d, J = 2.4 Hz) | 8.02 (1H, brs)/7.90 (1H, brs) |
| H-6′ | 7.51 (2H, dd, J = 3.0/ 3.0 Hz) | 7.66 (1H, brs)/7.52 (1H, brs) |
| H-5′ | 6.87 (2H, d, J = 12.6 Hz) | 6.85 (2H, d, J = 8.4 Hz) |
| H-8 | 6.39 (2H, s) | 6.41 (2H, d, J = 1.8 Hz) |
| H-6 | 6.17 (2H, s) | 6.18 (2H, d, J = 1.8 Hz) |

References
- Murakami, A.; Ashida, H.; Terao, J. Multitargeted cancer prevention by quercetin. Cancer Lett. 2008, 269, 315–325. [Google Scholar] [CrossRef]
- Jia, Q.; Cao, H.; Shen, D.; Li, S.; Yan, L.; Chen, C.; Xing, S.; Dou, F. Quercetin protects against atherosclerosis by regulating the expression of PCSK9, CD36, PPARγ, LXRα and ABCA1. Int. J. Mol. Med. 2019, 44, 893–902. [Google Scholar] [CrossRef]
- Hooper, L.; Kroon, P.A.; Rimm, E.B.; Cohn, J.S.; Harvey, I.; Le Cornu, K.A.; Ryder, J.J.; Hall, W.L.; Cassidy, A. Flavonoids, flavonoid-rich foods, and cardiovascular risk: A meta-analysis of randomized controlled trials1. Am. J. Clin. Nutr. 2008, 88, 38–50. [Google Scholar] [CrossRef]
- Xie, J.; Song, W.; Liang, X.; Zhang, Q.; Shi, Y.; Liu, W.; Shi, X. Protective effect of quercetin on streptozotocin-induced diabetic peripheral neuropathy rats through modulating gut microbiota and reactive oxygen species level. Biomed. Pharmacother. 2020, 127, 110147. [Google Scholar] [CrossRef] [PubMed]
- Boots, A.W.; Haenen, G.R.; Bast, A. Health effects of quercetin: From antioxidant to nutraceutical. Eur. J. Pharmacol. 2008, 585, 325–337. [Google Scholar] [CrossRef] [PubMed]
- Maugeri, A.; Calderaro, A.; Patanè, G.T.; Navarra, M.; Barreca, D.; Cirmi, S.; Felice, M.R. Targets Involved in the Anti-Cancer Activity of Quercetin in Breast, Colorectal and Liver Neoplasms. Int. J. Mol. Sci. 2023, 24, 2952. [Google Scholar] [CrossRef]
- Ravichandran, R.; Rajendran, M.; Devapiriam, D. Antioxidant study of quercetin and their metal complex and determination of stability constant by spectrophotometry method. Food Chem. 2014, 146, 472–478. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Arce, E.; Saldias, M. Antioxidant properties of flavonoid metal complexes and their potential inclusion in the development of novel strategies for the treatment against neurodegenerative diseases. Biomed. Pharmacother. 2021, 143, 112236. [Google Scholar] [CrossRef]
- Tan, J.; Wang, B.; Zhu, L. DNA binding, cytotoxicity, apoptotic inducing activity, and molecular modeling study of quercetin zinc(II) complex. Bioorg Med. Chem. 2009, 17, 614–620. [Google Scholar] [CrossRef]
- Tan, J.; Zhu, L.; Wang, B. GC (Guanine-Cytosine)-Selective DNA-Binding and Antitumor Activity of a Quercetin Manganese(II) Complex. Chem. Biodivers. 2011, 8, 1550–1559. [Google Scholar] [CrossRef]
- Sadhukhan, P.; Kundu, M.; Chatterjee, S.; Ghosh, N.; Manna, P.; Das, J.; Sil, P.C. Targeted delivery of quercetin via pH-responsive zinc oxide nanoparticles for breast cancer therapy. Mater. Sci. Eng. C 2019, 100, 129–140. [Google Scholar] [CrossRef]
- Parveen, S.; Tabassum, S.; Arjmand, F. Human Topoisomerase I mediated cytotoxicity profile of l-valine-quercetin diorganotin(IV) antitumor drug entities. J. Organomet. Chem. 2016, 823, 23–33. [Google Scholar] [CrossRef]
- Lee, Y.H.; Tuyet, P.T. Synthesis and biological evaluation of quercetin-zinc (II) complex for anti-cancer and anti-metastasis of human bladder cancer cells. Vitr. Cell. Dev. Biol. Anim. 2019, 55, 395–404. [Google Scholar] [CrossRef] [PubMed]
- Zhai, G.; Zhu, W.; Duan, Y.; Qu, W.; Yan, Z. Synthesis, characterization and antitumor activity of the germanium-quercetin complex. Main Group Met. Chem. 2012, 35, 103–109. [Google Scholar] [CrossRef]
- Ren, J.; Meng, S.; Lekka, C.E.; Kaxiras, E. Complexation of Flavonoids with Iron: Structure and Optical Signatures. J. Phys. Chem. B 2008, 112, 1845–1850. [Google Scholar] [CrossRef] [PubMed]
- Uivarosi, V.; Munteanu, A.-C. Flavonoid Complexes as Promising Anticancer Metallodrugs. In Flavonoids-from Biosynthesis to Human Health; Goncalo, C.J., Ed.; IntechOpen: London, UK, 2017. [Google Scholar]
- Tabassum, S.; Zaki, M.; Afzal, M.; Arjmand, F. New modulated design and synthesis of quercetin-Cu(II)/Zn(II)-Sn2(IV) scaffold as anticancer agents: In Vitro DNA binding profile, DNA cleavage pathway and Topo-I activity. Dalton Trans. 2013, 42, 10029–10041. [Google Scholar] [CrossRef]
- Shastrala, K.; Kalam, S.; Damerakonda, K.; Sheshagiri, S.B.B.; Kumar, H.; Guda, R.; Kasula, M.; Bedada, S.K. Synthesis, characterization, and pharmacological evaluation of some metal complexes of quercetin as P-gp inhibitors. Future J. Pharm. Sci. 2021, 7, 1. [Google Scholar] [CrossRef]
- Ojo, O.O.; Fatokun, D.I.; Ejidike, I.P.; Awolope, R.U.; Sanni, S.O. Quercetin Zinc and Iron Metal Complexes Protect against Sodium Arsenite Intoxication in the Hepato-Renal System of Wistar Rats via the Oxidative Stress Pathway. J. Toxicol. 2022, 2022, 6178261. [Google Scholar] [CrossRef]
- Papan, P.; Kantapan, J.; Sangthong, P.; Meepowpan, P.; Dechsupa, N. Iron (III)-Quercetin Complex: Synthesis, Physicochemical Characterization, and MRI Cell Tracking toward Potential Applications in Regenerative Medicine. Contrast Media Mol. Imaging 2020, 2020, 8877862. [Google Scholar] [CrossRef]
- Kantapan, J.; Anukul, N.; Leetrakool, N.; Rolin, G.; Vergote, J.; Dechsupa, N. Iron–Quercetin Complex Preconditioning of Human Peripheral Blood Mononuclear Cells Accelerates Angiogenic and Fibroblast Migration: Implications for Wound Healing. Int. J. Mol. Sci. 2021, 22, 8851. [Google Scholar] [CrossRef]
- da Silva, W.M.B.; de Oliveira Pinheiro, S.; Alves, D.R.; de Menezes, J.E.S.A.; Magalhães, F.E.A.; Silva, F.C.O.; Silva, J.; Marinho, E.S.; de Morais, S.M. Synthesis of Quercetin-Metal Complexes, In Vitro and In Silico Anticholinesterase and Antioxidant Evaluation, and In Vivo Toxicological and Anxiolitic Activities. Neurotox. Res. 2020, 37, 893–903. [Google Scholar] [CrossRef]
- Zhou, J.; Wang, L.; Wang, J.; Tang, N. Antioxidative and anti-tumour activities of solid quercetin metal(II) complexes. Transit. Met. Chem. 2001, 26, 57–63. [Google Scholar] [CrossRef]
- Matejczyk, M.; Kalinowska, M.; Swiderski, G.; Lewandowski, W.; Rosochacki, S.J. Cytotoxic And Genotoxic Studies Of Quercetin, Quercetin Sodium Salt and Quercetin Complexes with Nickel (II) And Zinc (II). Acta Pol. Pharm. 2016, 73, 1139–1146. [Google Scholar] [PubMed]
- Bratu, M.M.; Birghila, S.; Miresan, H.; Negreanu-Pirol, T.; Prajitura, C.; Calinescu, M. Biological Activities of Zn(II) and Cu(II) Complexes with Quercetin and Rutin: Antioxidant Properties and UV-Protection Capacity. Rev. Chim. 2014, 65, 544–549. [Google Scholar]
- Chen, X.; Tang, L.J.; Sun, Y.N.; Qiu, P.H.; Liang, G. Syntheses, characterization and antitumor activities of transition metal complexes with isoflavone. J. Inorg. Biochem. 2010, 104, 379–384. [Google Scholar] [CrossRef] [PubMed]
- Kyriakou, E.; Primikyri, A.; Charisiadis, P.; Katsoura, M.; Gerothanassis, I.P.; Stamatis, H.; Tzakos, A.G. Unexpected enzyme-catalyzed regioselective acylation of flavonoid aglycones and rapid product screening. Org. Biomol. Chem. 2012, 10, 1739–1742. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Guo, M. Zinc-Binding Sites on Selected Flavonoids. Biol. Trace Elem. Res. 2014, 161, 223–230. [Google Scholar] [CrossRef]
- Le Nest, G.; Caille, O.; Woudstra, M.; Roche, S.; Guerlesquin, F.; Lexa, D. Zn–polyphenol chelation: Complexes with quercetin, (+)-catechin, and derivatives: I optical and NMR studies. Inorganica Chim. Acta 2004, 357, 775–784. [Google Scholar] [CrossRef]
- Lehr, M.; Paschelke, T.; Trumpf, E.; Vogt, A.M.; Näther, C.; Sönnichsen, F.D.; McConnell, A.J. A Paramagnetic NMR Spectroscopy Toolbox for the Characterisation of Paramagnetic/Spin-Crossover Coordination Complexes and Metal-Organic Cages. Angew. Chem. 2020, 59, 19344–19351. [Google Scholar] [CrossRef]
- Al-Gaashani, R.; Radiman, S.; Tabet, N.; Daud, A.R. Effect of microwave power on the morphology and optical property of zinc oxide nano-structures prepared via a microwave-assisted aqueous solution method. Mater. Chem. Phys. 2011, 125, 846–852. [Google Scholar] [CrossRef]
- Ghosh, N.; Chakraborty, T.; Mallick, S.; Mana, S.; Singha, D.; Ghosh, B.; Roy, S. Synthesis, characterization and study of antioxidant activity of quercetin-magnesium complex. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2015, 151, 807–813. [Google Scholar] [CrossRef]
- Bukhari, S.B.; Memon, S.; Mahroof-Tahir, M.; Bhanger, M.I. Synthesis, characterization and antioxidant activity copper-quercetin complex. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2009, 71, 1901–1906. [Google Scholar] [CrossRef]
- Hazra, M.; Dasgupta Mandal, D.; Mandal, T.; Bhuniya, S.; Ghosh, M. Designing polymeric microparticulate drug delivery system for hydrophobic drug quercetin. Saudi Pharm. J. 2015, 23, 429–436. [Google Scholar] [CrossRef] [PubMed]
- Trendafilova, I.; Szegedi, A.; Mihály, J.; Momekov, G.; Lihareva, N.; Popova, M. Preparation of efficient quercetin delivery system on Zn-modified mesoporous SBA-15 silica carrier. Mater. Sci. Eng. C 2017, 73, 285–292. [Google Scholar] [CrossRef] [PubMed]
- Cornard, J.P.; Merlin, J.C. Comparison of the chelating power of hydroxyflavones. J. Mol. Struct. 2003, 651–653, 381–387. [Google Scholar] [CrossRef]
- De Souza, L.A.; Tavares, W.M.G.; Lopes, A.P.M.; Soeiro, M.M.; De Almeida, W.B. Structural analysis of flavonoids in solution through DFT 1H NMR chemical shift calculations: Epigallocatechin, Kaempferol and Quercetin. Chem. Phys. Lett. 2017, 676, 46–52. [Google Scholar] [CrossRef]
- De Souza, L.A.; Soeiro, M.M.; De Almeida, W.B. A DFT study of molecular structure and 1H NMR, IR, and UV-Vis spectrum of Zn(II)-kaempferol complexes: A metal-flavonoid complex showing enhanced anticancer activity. Int. J. Quantum Chem. 2018, 118, e25773. [Google Scholar] [CrossRef]
- Catauro, M.; Papale, F.; Bollino, F.; Piccolella, S.; Marciano, S.; Nocera, P.; Pacifico, S. Silica/quercetin sol-gel hybrids as antioxidant dental implant materials. Sci. Technol. Adv. Mater. 2015, 16, 035001. [Google Scholar] [CrossRef]
- De Souza, R.F.; De Giovani, W.F. Synthesis, spectral and electrochemical properties of Al(III) and Zn(II) complexes with flavonoids. Spectrochim. Acta Part. A Mol. Biomol. Spectrosc. 2005, 61, 1985–1990. [Google Scholar] [CrossRef]
- Primikyri, A.; Mazzone, G.; Lekka, C.; Tzakos, A.G.; Russo, N.; Gerothanassis, I.P. Understanding zinc(II) chelation with quercetin and luteolin: A combined NMR and theoretical study. J. Phys. Chem. B 2015, 119, 83–95. [Google Scholar] [CrossRef]
- Raza, A.; Xu, X.; Xia, L.; Xia, C.; Tang, J.; Ouyang, Z. Quercetin-Iron Complex: Synthesis, Characterization, Antioxidant, DNA Binding, DNA Cleavage, and Antibacterial Activity Studies. J. Fluoresc. 2016, 26, 2023–2031. [Google Scholar] [CrossRef]
- de Souza, R.F.V.; De Giovani, W.F. Antioxidant properties of complexes of flavonoids with metal ions. Redox Rep. 2004, 9, 97–104. [Google Scholar] [CrossRef]
- Dehghan, G.; Khoshkam, Z. Tin(II)–quercetin complex: Synthesis, spectral characterisation and antioxidant activity. Food Chem. 2012, 131, 422–426. [Google Scholar] [CrossRef]
- Ulusoy, H.G.; Sanlier, N. A minireview of quercetin: From its metabolism to possible mechanisms of its biological activities. Crit. Rev. Food Sci. Nutr. 2020, 60, 3290–3303. [Google Scholar] [CrossRef] [PubMed]
- Vargas, A.J.; Burd, R. Hormesis and synergy: Pathways and mechanisms of quercetin in cancer prevention and management. Nutr. Rev. 2010, 68, 418–428. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.S.; Yao, C.N.; Liu, H.C.; Yu, F.S.; Lin, J.J.; Lu, K.W.; Liao, C.L.; Chueh, F.S.; Chung, J.G. Quercetin induced apoptosis of human oral cancer SAS cells through mitochondria and endoplasmic reticulum mediated signaling pathways. Oncol. Lett. 2018, 15, 9663–9672. [Google Scholar] [CrossRef]
- Ha, E.J.; Kim, K.Y.; Kim, C.E.; Jun, D.Y.; Kim, Y.H. Enhancement of Quercetin-Induced Apoptosis by Cotreatment with Autophagy Inhibitor Is Associated with Augmentation of BAK-Dependent Mitochondrial Pathway in Jurkat T Cells. Oxidative Med. Cell. Longev. 2019, 2019, 1–16. [Google Scholar] [CrossRef]
- Kim, Y.H.; Lee, D.H.; Jeong, J.H.; Guo, Z.S.; Lee, Y.J. Quercetin augments TRAIL-induced apoptotic death: Involvement of the ERK signal transduction pathway. Biochem. Pharmacol. 2008, 75, 1946–1958. [Google Scholar] [CrossRef] [PubMed]
- Granado-Serrano, A.B.; Martín, M.A.; Bravo, L.; Goya, L.; Ramos, S. Quercetin Induces Apoptosis via Caspase Activation, Regulation of Bcl-2, and Inhibition of PI-3-Kinase/Akt and ERK Pathways in a Human Hepatoma Cell Line (HepG2). J. Nutr. 2006, 136, 2715–2721. [Google Scholar] [CrossRef]
- Tanigawa, S.; Fujii, M.; Hou, D.X. Stabilization of p53 is involved in quercetin-induced cell cycle arrest and apoptosis in HepG2 cells. Biosci. Biotechnol. Biochem. 2008, 72, 797–804. [Google Scholar] [CrossRef]
- Khan, I.; Paul, S.; Jakhar, R.; Bhardwaj, M.; Kang, S.C. Novel quercetin derivative TEF induces ER stress and mitochondria-mediated apoptosis in human colon cancer HCT-116 cells. Biomed. Pharmacother. 2016, 84, 789–799. [Google Scholar] [CrossRef] [PubMed]
- Kim, G.T.; Lee, S.H.; Kim, J.I.; Kim, Y.M. Quercetin regulates the sestrin 2-AMPK-p38 MAPK signaling pathway and induces apoptosis by increasing the generation of intracellular ROS in a p53-independent manner. Int. J. Mol. Med. 2014, 33, 863–869. [Google Scholar] [CrossRef] [PubMed]
- Tomas-Hernández, S.; Blanco, J.; Rojas, C.; Roca-Martínez, J.; Ojeda-Montes, M.J.; Beltrán-Debón, R.; Garcia-Vallvé, S.; Pujadas, G.; Arola, L.; Mulero, M. Resveratrol potently counteracts quercetin starvation-induced autophagy and sensitizes HepG2 cancer cells to apoptosis. Mol. Nutr. Food Res. 2018, 62, 1700610. [Google Scholar] [CrossRef]
- Tan, J.; Zhu, L.; Wang, B. From GC-rich DNA binding to the repression of survivin gene for quercetin nickel (II) complex: Implications for cancer therapy. Biometals 2010, 23, 1075–1084. [Google Scholar] [CrossRef] [PubMed]
- Tan, J.; Wang, B.; Zhu, L. DNA binding and oxidative DNA damage induced by a quercetin copper (II) complex: Potential mechanism of its antitumor properties. JBIC J. Biol. Inorg. Chem. 2009, 14, 727–739. [Google Scholar] [CrossRef]
- Naso, L.; Valcarcel, M.; Villacé, P.; Roura-Ferrer, M.; Salado, C.; Ferrer, E.G.; Williams, P.A. Specific antitumor activities of natural and oxovanadium (IV) complexed flavonoids in human breast cancer cells. New J. Chem. 2014, 38, 2414–2421. [Google Scholar] [CrossRef]
- Jun, T.; Bochu, W.; Liancai, Z. Hydrolytic cleavage of DNA by quercetin manganese(II) complexes. Colloids Surf. B: Biointerfaces 2007, 55, 149–152. [Google Scholar] [CrossRef]
- Arif, H.; Rehmani, N.; Farhan, M.; Ahmad, A.; Hadi, S.M. Mobilization of Copper ions by Flavonoids in Human Peripheral Lymphocytes Leads to Oxidative DNA Breakage: A Structure Activity Study. Int. J. Mol. Sci. 2015, 16, 26754–26769. [Google Scholar] [CrossRef]
- Kang, J.; Zhuo, L.; Lu, X.; Liu, H.; Zhang, M.; Wu, H. Electrochemical investigation on interaction between DNA with quercetin and Eu–Qu3 complex. J. Inorg. Biochem. 2004, 98, 79–86. [Google Scholar] [CrossRef]
- Sreenivasulu, K.; Raghu, P.; Nair, K.M. Polyphenol-rich beverages enhance zinc uptake and metallothionein expression in Caco-2 cells. J. Food Sci. 2010, 75, H123–H128. [Google Scholar] [CrossRef]
- Kim, E.Y.; Pai, T.K.; Han, O. Effect of bioactive dietary polyphenols on zinc transport across the intestinal Caco-2 cell monolayers. J. Agric. Food Chem. 2011, 59, 3606–3612. [Google Scholar] [CrossRef] [PubMed]
- Bebe, F.N.; Panemangalore, M. Biosafety of flavonoids in rats: Effects on copper and zinc homeostasis and interaction with low-level pesticide exposure. Biol. Trace Elem. Res. 2009, 129, 200–212. [Google Scholar] [CrossRef] [PubMed]
- Clergeaud, G.; Dabbagh-Bazarbachi, H.; Ortiz, M.; Fernández-Larrea, J.B.; O’Sullivan, C.K. A simple liposome assay for the screening of zinc ionophore activity of polyphenols. Food Chem. 2016, 197, 916–923. [Google Scholar] [CrossRef] [PubMed]
- Dabbagh-Bazarbachi, H.; Clergeaud, G.; Quesada, I.M.; Ortiz, M.; O’Sullivan, C.K.; Fernandez-Larrea, J.B. Zinc ionophore activity of quercetin and epigallocatechin-gallate: From Hepa 1–6 cells to a liposome model. J. Agric. Food Chem. 2014, 62, 8085–8093. [Google Scholar] [CrossRef] [PubMed]
- Donadelli, M.; Dalla Pozza, E.; Scupoli, M.T.; Costanzo, C.; Scarpa, A.; Palmieri, M. Intracellular zinc increase inhibits p53(-/-) pancreatic adenocarcinoma cell growth by ROS/AIF-mediated apoptosis. Biochim. Biophys. Acta 2009, 1793, 273–280. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.A.; Tarahovsky, Y.S.; Yagolnik, E.A.; Kuznetsova, S.M.; Muzafarov, E.N. Lipophilicity of flavonoid complexes with iron(II) and their interaction with liposomes. Biochem. Biophys. Res. Commun. 2013, 431, 680–685. [Google Scholar] [CrossRef] [PubMed]
- Tarahovsky, Y.S.; Kim, Y.A.; Yagolnik, E.A.; Muzafarov, E.N. Flavonoid–membrane interactions: Involvement of flavonoid–metal complexes in raft signaling. Biochim. Biophys. Acta (BBA)—Biomembr. 2014, 1838, 1235–1246. [Google Scholar] [CrossRef]
- Xie, K.; He, X.; Chen, K.; Chen, J.; Sakao, K.; Hou, D.X. Antioxidant Properties of a Traditional Vine Tea, Ampelopsis grossedentata. Antioxidant 2019, 8, 295. [Google Scholar] [CrossRef]
- Yano, S.; Wu, S.; Sakao, K.; Hou, D.X. Wasabi 6-(methylsulfinyl)hexyl isothiocyanate induces apoptosis in human colorectal cancer cells through p53-independent mitochondrial dysfunction pathway. Biofactors 2018, 44, 361–368. [Google Scholar] [CrossRef]
- Wong, C.C.; Williamson, G. Inhibition of hydroxycinnamic acid sulfation by flavonoids and their conjugated metabolites. Biofactors 2013, 39, 644–651. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nakamura, M.; Urakawa, D.; He, Z.; Akagi, I.; Hou, D.-X.; Sakao, K. Apoptosis Induction in HepG2 and HCT116 Cells by a Novel Quercetin-Zinc (II) Complex: Enhanced Absorption of Quercetin and Zinc (II). Int. J. Mol. Sci. 2023, 24, 17457. https://doi.org/10.3390/ijms242417457
Nakamura M, Urakawa D, He Z, Akagi I, Hou D-X, Sakao K. Apoptosis Induction in HepG2 and HCT116 Cells by a Novel Quercetin-Zinc (II) Complex: Enhanced Absorption of Quercetin and Zinc (II). International Journal of Molecular Sciences. 2023; 24(24):17457. https://doi.org/10.3390/ijms242417457
Chicago/Turabian StyleNakamura, Mizuki, Daigo Urakawa, Ziyu He, Isao Akagi, De-Xing Hou, and Kozue Sakao. 2023. "Apoptosis Induction in HepG2 and HCT116 Cells by a Novel Quercetin-Zinc (II) Complex: Enhanced Absorption of Quercetin and Zinc (II)" International Journal of Molecular Sciences 24, no. 24: 17457. https://doi.org/10.3390/ijms242417457
APA StyleNakamura, M., Urakawa, D., He, Z., Akagi, I., Hou, D.-X., & Sakao, K. (2023). Apoptosis Induction in HepG2 and HCT116 Cells by a Novel Quercetin-Zinc (II) Complex: Enhanced Absorption of Quercetin and Zinc (II). International Journal of Molecular Sciences, 24(24), 17457. https://doi.org/10.3390/ijms242417457






