Exploration of the Main Antibiofilm Substance of Lactobacillus plantarum ATCC 14917 and Its Effect against Streptococcus mutans
Abstract
1. Introduction
2. Results
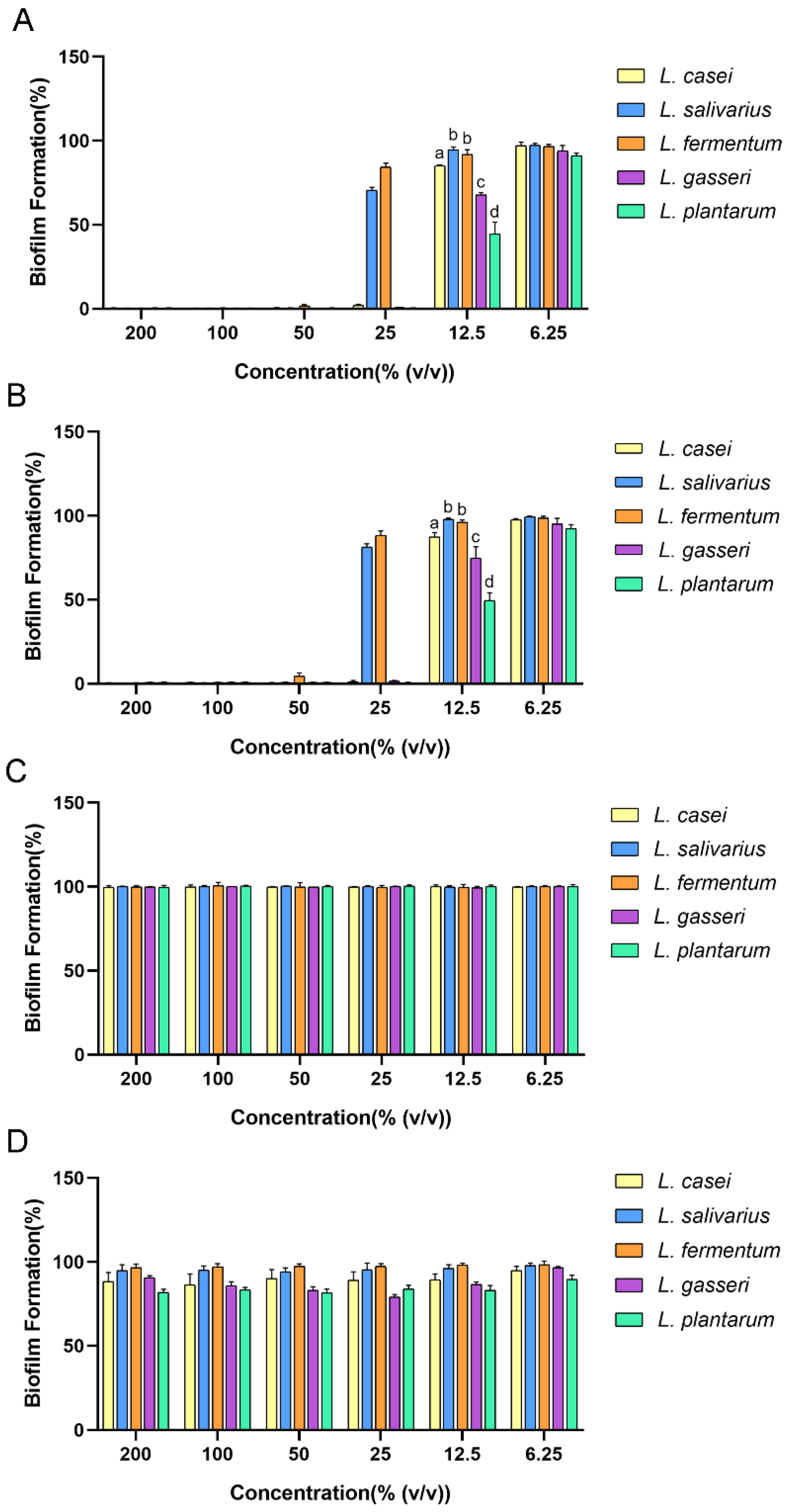
2.1. Antibiofilm Effect of Cell-Free Supernatant (CFS) of Lactobacilli against Streptococcus mutans (S. mutans)
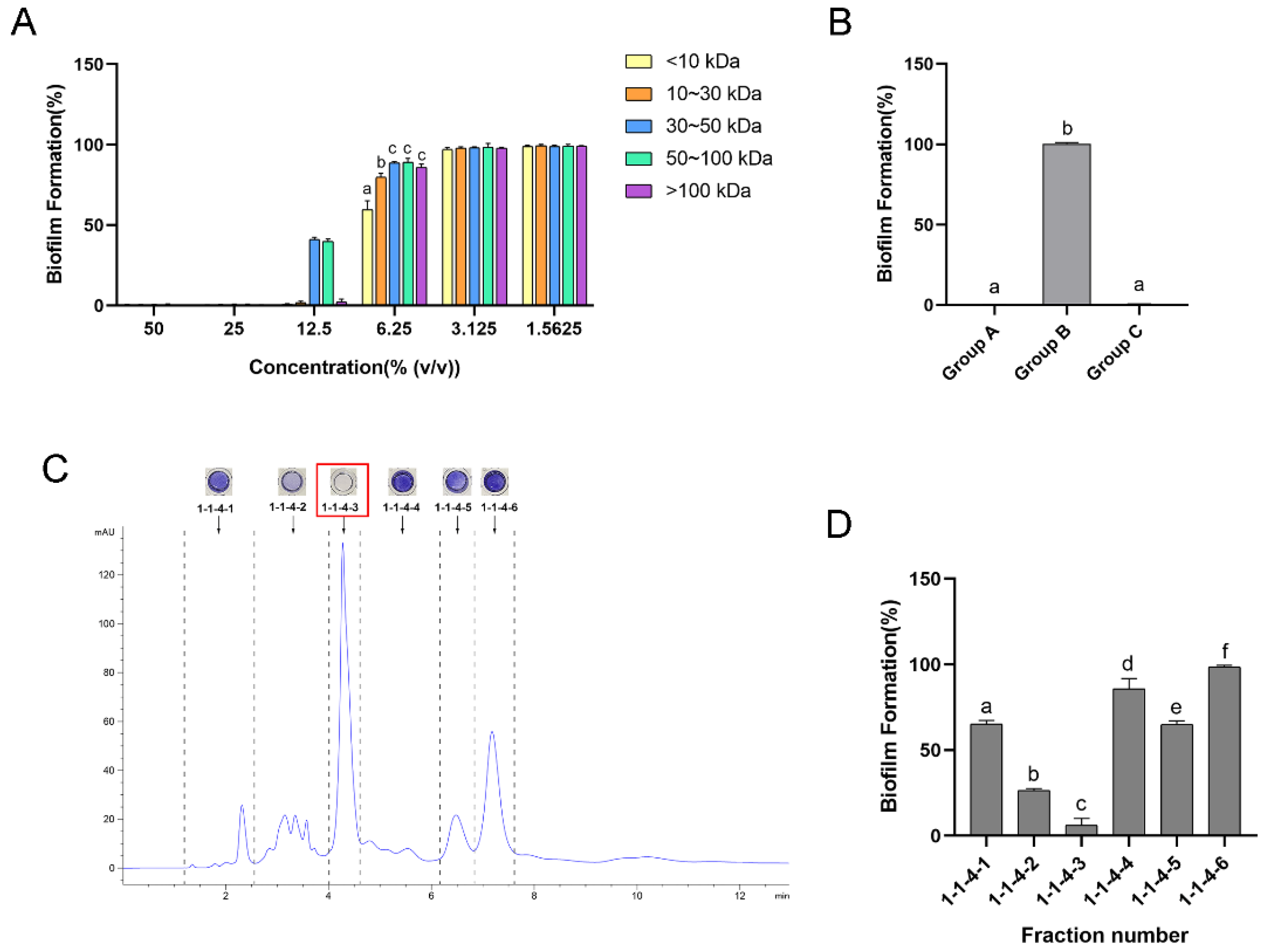
2.2. Isolation of the Main Antibiofilm Component of Lactobacillus plantarum (L. plantarum) CFS
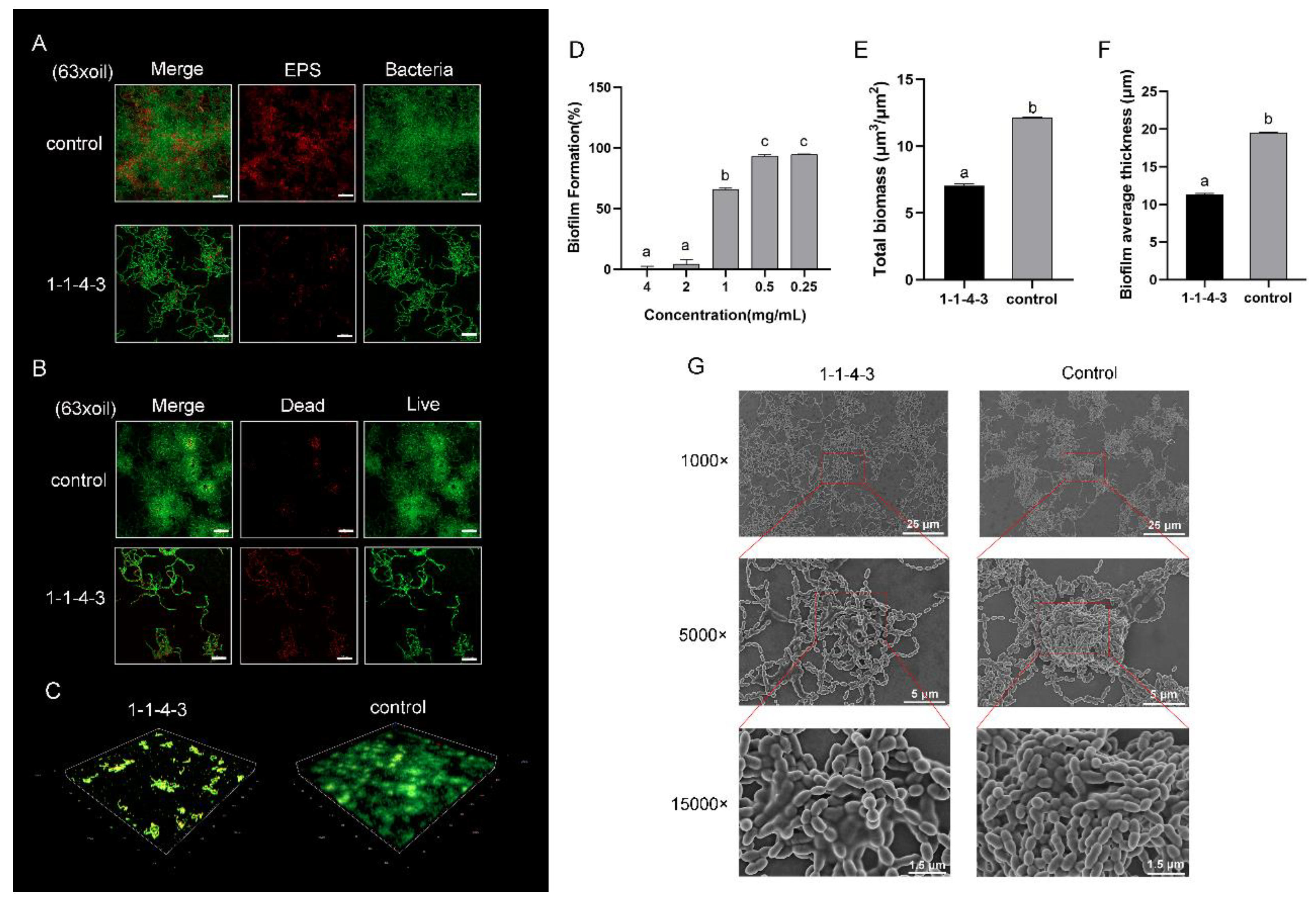
2.3. Antibiofilm Effect of Component 1-1-4-3
2.4. Characterization and Validation of Component 1-1-4-3
2.4.1. 1H and 13C Nuclear Magnetic Resonance (NMR) and Quadrupole Time-of-Flight Mass Spectrometry (Q-TOF-MS) Data for Component 1-1-4-3
2.4.2. Analysis of NMR and Q-TOF-MS Data
2.5. Verification of the Role of Lactic Acid (LA) in the Antibiofilm Activity
2.5.1. Antibiofilm Effect of Component 1-1-4-3, LA (d/l-), Valine (d/l-) and the Mixture of LA and Valine
2.5.2. The Antibiofilm Activity of LA and Low-pH Environment
3. Discussion
4. Materials and Methods
4.1. Bacterial Strains, Culture Conditions and Growth Curves Determination
4.2. Preparation of CFS
4.3. Antibiofilm Assay of CFS on S. mutans
4.4. Ultrafiltration of L. plantarum CFS and Further Investigation of the Influence of pH
4.5. Bioassay-Guided Isolation of the Main Antibiofilm Component
4.6. Examination of Antibiofilm Effect of Component 1-1-4-3
4.7. Structure Elucidation of Component 1-1-4-3
4.7.1. NMR and Q-TOF-MS Analysis of Component 1-1-4-3
4.7.2. NMR Analysis of Valine and LA Standards and HPLC Analysis of Component 1-1-4-3 and Its Standards
4.8. Exploration of the Role of LA in the Antibiofilm Activity
4.8.1. Comparison of the Antibiofilm Effect of Component 1-1-4-3, LA (d/l-), Valine (d/l-) and the Mixture of LA and Valine
4.8.2. Examination of the Antibiofilm Activity of LA and Low pH
4.8.3. Examination of the MIC of LA
4.9. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kassebaum, N.J.; Smith, A.; Bernabe, E.; Fleming, T.D.; Reynolds, A.E.; Vos, T.; Murray, C.; Marcenes, W. Global, Regional, and National Prevalence, Incidence, and Disability-Adjusted Life Years for Oral Conditions for 195 Countries, 1990–2015: A Systematic Analysis for the Global Burden of Diseases, Injuries, and Risk Factors. J. Dent. Res. 2017, 96, 380–387. [Google Scholar] [CrossRef] [PubMed]
- Folliero, V.; Dell’Annunziata, F.; Roscetto, E.; Amato, A.; Gasparro, R.; Zannella, C.; Casolaro, V.; De Filippis, A.; Catania, M.R.; Franci, G.; et al. Rhein: A novel antibacterial compound against Streptococcus mutans infection. Microbiol. Res. 2022, 261, 127062. [Google Scholar] [CrossRef] [PubMed]
- Zijnge, V.; van Leeuwen, M.B.; Degener, J.E.; Abbas, F.; Thurnheer, T.; Gmur, R.; Harmsen, H.J. Oral biofilm architecture on natural teeth. PLoS ONE 2010, 5, e9321. [Google Scholar] [CrossRef] [PubMed]
- Svensäter, G.; Borgström, M.; Bowden, G.H.W.; Edwardsson, S.; Malmö, U.; Faculty, O.O. The Acid-Tolerant Microbiota Associated with Plaque from Initial Caries and Healthy Tooth Surfaces. Caries Res. 2003, 37, 395–403. [Google Scholar] [CrossRef] [PubMed]
- Belli, W.A.; Marquis, R.E. Adaptation of Streptococcus mutans and Enterococcus hirae to acid stress in continuous culture. Appl. Environ. Microbiol. 1991, 57, 1134–1138. [Google Scholar] [CrossRef] [PubMed]
- Caufield, P.W.; Schon, C.N.; Saraithong, P.; Li, Y.; Argimon, S. Oral Lactobacilli and Dental Caries: A Model for Niche Adaptation in Humans. J. Dent. Res. 2015, 94, 110S–118S. [Google Scholar] [CrossRef]
- Latifi-Xhemajli, B.; Veronneau, J.; Begzati, A.; Bytyci, A.; Kutllovci, T.; Rexhepi, A. Association between salivary level of infection with Streptococcus mutans/Lactobacilli and caries-risk factors in mothers. Eur. J. Paediatr. Dent. 2016, 17, 70–74. [Google Scholar]
- Lapirattanakul, J.; Nomura, R.; Okawa, R.; Morimoto, S.; Tantivitayakul, P.; Maudcheingka, T.; Nakano, K.; Matsumoto-Nakano, M. Oral Lactobacilli Related to Caries Status of Children with Primary Dentition. Caries Res. 2020, 54, 194–204. [Google Scholar] [CrossRef]
- Ledder, R.G.; Kampoo, K.; Teanpaisan, R.; McBain, A.J. Oral Microbiota in Severe Early Childhood Caries in Thai Children and Their Families: A Pilot Study. Front. Microbiol. 2018, 9, 2420. [Google Scholar] [CrossRef]
- Wasfi, R.; Abd El-Rahman, O.A.; Zafer, M.M.; Ashour, H.M. ProbioticLactobacillus sp. inhibit growth, biofilm formation and gene expression of caries-inducingStreptococcus mutans. J. Cell. Mol. Med. 2018, 22, 1972–1983. [Google Scholar] [CrossRef]
- Samot, J.; Badet, C. Antibacterial activity of probiotic candidates for oral health. Anaerobe 2013, 19, 34–38. [Google Scholar] [CrossRef] [PubMed]
- Teanpaisan, R.; Piwat, S.; Dahlén, G.; Institutionen, F.O.; Institute, O.O.; Sahlgrenska, A.; Göteborgs, U.; Gothenburg, U.; Sahlgrenska, A. Inhibitory effect of oral Lactobacillus against oral pathogens. Lett. Appl. Microbiol. 2011, 53, 452–459. [Google Scholar] [CrossRef]
- Iwami, Y.; Hata, S.; Takahashi, N.; Yamada, T. Difference in amounts between titratable acid and total carboxylic acids produced by oral streptococci during sugar metabolism. J. Dent. Res. 1989, 68, 16–19. [Google Scholar] [CrossRef] [PubMed]
- Mani-Lopez, E.; Arrioja-Breton, D.; Lopez-Malo, A. The impacts of antimicrobial and antifungal activity of cell-free supernatants from lactic acid bacteria in vitro and foods. Compr. Rev. Food. Sci. Food Saf. 2022, 21, 604–641. [Google Scholar] [CrossRef]
- Wattanarat, O.; Nirunsittirat, A.; Piwat, S.; Manmontri, C.; Teanpaisan, R.; Pahumunto, N.; Makeudom, A.; Sastraruji, T.; Krisanaprakornkit, S. Significant elevation of salivary human neutrophil peptides 1-3 levels by probiotic milk in preschool children with severe early childhood caries: A randomized controlled trial. Clin. Oral Investig. 2021, 25, 2891–2903. [Google Scholar] [CrossRef] [PubMed]
- Wannun, P.; Piwat, S.; Teanpaisan, R. Purification and characterization of bacteriocin produced by oral Lactobacillus paracasei SD1. Anaerobe 2014, 27, 17–21. [Google Scholar] [CrossRef]
- Yang, K.M.; Kim, J.S.; Kim, H.S.; Kim, Y.Y.; Oh, J.K.; Jung, H.W.; Park, D.S.; Bae, K.H. Lactobacillus reuteri AN417 cell-free culture supernatant as a novel antibacterial agent targeting oral pathogenic bacteria. Sci. Rep. 2021, 11, 1631. [Google Scholar] [CrossRef] [PubMed]
- Rossoni, R.D.; Velloso, M.D.S.; de Barros, P.P.; de Alvarenga, J.A.; Santos, J.D.D.; Santos Prado, A.C.C.D.; Ribeiro, F.D.C.; Anbinder, A.L.; Junqueira, J.C. Inhibitory effect of probiotic Lactobacillus supernatants from the oral cavity on Streptococcus mutans biofilms. Microb. Pathog. 2018, 123, 361–367. [Google Scholar] [CrossRef]
- Fang, F.; Xu, J.; Li, Q.; Xia, X.; Du, G. Characterization of a Lactobacillus brevis strain with potential oral probiotic properties. BMC Microbiol. 2018, 18, 221. [Google Scholar] [CrossRef]
- Jeong, D.; Kim, D.H.; Song, K.Y.; Seo, K.H. Antimicrobial and anti-biofilm activities of Lactobacillus kefiranofaciens DD2 against oral pathogens. J. Oral Microbiol. 2018, 10, 1472985. [Google Scholar] [CrossRef]
- van Zyl, W.F.; Deane, S.M.; Dicks, L.M.T. Molecular insights into probiotic mechanisms of action employed against intestinal pathogenic bacteria. Gut Microbes 2020, 12, 1831339. [Google Scholar] [CrossRef] [PubMed]
- Elshikh, M.; Marchant, R.; Banat, I.M. Biosurfactants: Promising bioactive molecules for oral-related health applications. FEMS Microbiol. Lett. 2016, 363, w213. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Chen, X.; Chen, Y.; Jiang, W.; Chen, H. The effect of five probiotic lactobacilli strains on the growth and biofilm formation of Streptococcus mutans. Oral Dis. 2015, 21, e128–e134. [Google Scholar] [CrossRef]
- Busarcevic, M.; Kojic, M.; Dalgalarrondo, M.; Chobert, J.M.; Haertle, T.; Topisirovic, L. Purification of bacteriocin LS1 produced by human oral isolate Lactobacillus salivarius BGHO1. Oral Microbiol. Immunol. 2008, 23, 254–258. [Google Scholar] [CrossRef] [PubMed]
- Munson, M.A.; Banerjee, A.; Watson, T.F.; Wade, W.G. Molecular analysis of the microflora associated with dental caries. J. Clin. Microbiol. 2004, 42, 3023–3029. [Google Scholar] [CrossRef]
- Story, D.A. Bench-to-bedside review: A brief history of clinical acid-base. Crit. Care 2004, 8, 253–258. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.F.; Ding, C.F.; Zhang, L.C.; Wei, X.; Cheng, G.G.; Liu, Y.P.; Zhang, R.P.; Luo, X.D. Alstoscholarisine K, an Antimicrobial Indole from Gall-Induced Leaves of Alstonia scholaris. Org. Lett. 2021, 23, 5782–5786. [Google Scholar] [CrossRef]
- Annang, F.; Perez-Moreno, G.; Gonzalez-Menendez, V.; Lacret, R.; Perez-Victoria, I.; Martin, J.; Cantizani, J.; de Pedro, N.; Choquesillo-Lazarte, D.; Ruiz-Perez, L.M.; et al. Strasseriolides A-D, A Family of Antiplasmodial Macrolides Isolated from the Fungus Strasseria geniculata CF-247251. Org. Lett. 2020, 22, 6709–6713. [Google Scholar] [CrossRef]
- Chen, M.; Wang, R.; Zhao, W.; Yu, L.; Zhang, C.; Chang, S.; Li, Y.; Zhang, T.; Xing, J.; Gan, M.; et al. Isocoumarindole A, a Chlorinated Isocoumarin and Indole Alkaloid Hybrid Metabolite from an Endolichenic Fungus Aspergillus sp. Org. Lett. 2019, 21, 1530–1533. [Google Scholar] [CrossRef]
- Vollet, M.G.; Belleville, M.P.; Lacour, S.; Dupas, H.M. Membrane Fractionation of Protein Hydrolysates from By-Products: Recovery of Valuable Compounds from Spent Yeasts. Membranes 2020, 11, 23. [Google Scholar] [CrossRef]
- Gilar, M.; Olivova, P.; Daly, A.E.; Gebler, J.C. Orthogonality of Separation in Two-Dimensional Liquid Chromatography. Anal. Chem. 2005, 77, 6426–6434. [Google Scholar] [CrossRef] [PubMed]
- Giddings, J.C. Sample dimensionality: A predictor of order-disorder in component peak distribution in multidimensional separation. J. Chromatogr. A 1995, 703, 3–15. [Google Scholar] [CrossRef]
- Boateng, I.D. Evaluating the status quo of deep eutectic solvent in food chemistry. Potentials and limitations. Food Chem. 2023, 406, 135079. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Jiang, X.; Yang, Q.; Zhang, Y.; Wang, C.; Huang, R. Inhibition of Streptococcus mutans Biofilm Formation by the Joint Action of Oxyresveratrol and Lactobacillus casei. Appl. Environ. Microbiol. 2022, 88, e243621. [Google Scholar] [CrossRef]
- Hansen, A.L.; Kupče, E.; Li, D.; Bruschweiler-Li, L.; Wang, C.; Brüschweiler, R. 2D NMR-Based Metabolomics with HSQC/TOCSY NOAH Supersequences. Anal. Chem. 2021, 93, 6112–6119. [Google Scholar] [CrossRef]
- Chen, C.C.; Lai, C.C.; Huang, H.L.; Su, Y.T.; Chiu, Y.H.; Toh, H.S.; Chiang, S.R.; Chuang, Y.C.; Lu, Y.C.; Tang, H.J. Antimicrobial ability and mechanism analysis of Lactobacillus species against carbapenemase-producing Enterobacteriaceae. J. Microbiol. Immunol. Infect. 2021, 54, 447–456. [Google Scholar] [CrossRef]
- Cao, Y.; Zhou, Y.; Chen, D.; Wu, R.; Guo, L.; Lin, H. Proteomic and metabolic characterization of membrane vesicles derived from Streptococcus mutans at different pH values. Appl. Microbiol. Biotechnol. 2020, 104, 9733–9748. [Google Scholar] [CrossRef] [PubMed]
- Assinder, S.J.; Eynstone, L.V.J.; Shellis, R.P.; Dibdin, G.H. Inhibition of acid production in Streptococcus mutans R9: Inhibition constants and reversibility. FEMS Microbiol. Lett. 1995, 134, 287–292. [Google Scholar] [CrossRef]
- Ma, Q.; Pan, Y.; Chen, Y.; Yu, S.; Huang, J.; Liu, Y.; Gong, T.; Zhang, Q.; Sun, Q.; Zou, J.; et al. Acetylation of Lactate Dehydrogenase Negatively Regulates the Acidogenicity of Streptococcus mutans. mBio 2022, 13, e201322. [Google Scholar] [CrossRef]
- Zou, H.; Wu, Z.; Xian, M.; Liu, H.; Cheng, T.; Cao, Y. Not only osmoprotectant: Betaine increased lactate dehydrogenase activity and L-lactate production in lactobacilli. Bioresour. Technol. 2013, 148, 591–595. [Google Scholar] [CrossRef]
- Sivamaruthi, B.S.; Kesika, P.; Chaiyasut, C. A Review of the Role of Probiotic Supplementation in Dental Caries. Probiotics Antimicrob. Proteins 2020, 12, 1300–1309. [Google Scholar] [CrossRef] [PubMed]
- O’Toole, G.A.; Kolter, R. Initiation of biofilm formation in Pseudomonas fluorescens WCS365 proceeds via multiple, convergent signalling pathways: A genetic analysis. Mol. Microbiol. 1998, 28, 449–461. [Google Scholar] [CrossRef]
- Wang, Z.; Xue, R.; Cui, J.; Wang, J.; Fan, W.; Zhang, H.; Zhan, X. Antibacterial activity of a polysaccharide produced from Chaetomium globosum CGMCC 6882. Int. J. Biol. Macromol. 2019, 125, 376–382. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Cui, G.; Cui, Y.; Chen, D.; Lin, H. Small molecule targeting amyloid fibrils inhibits Streptococcus mutans biofilm formation. AMB Express 2021, 11, 171. [Google Scholar] [CrossRef] [PubMed]
- CLSI Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically; Approved Standard—Ninth Edition; CLSI Document: Wayne, PA, USA, 2012.
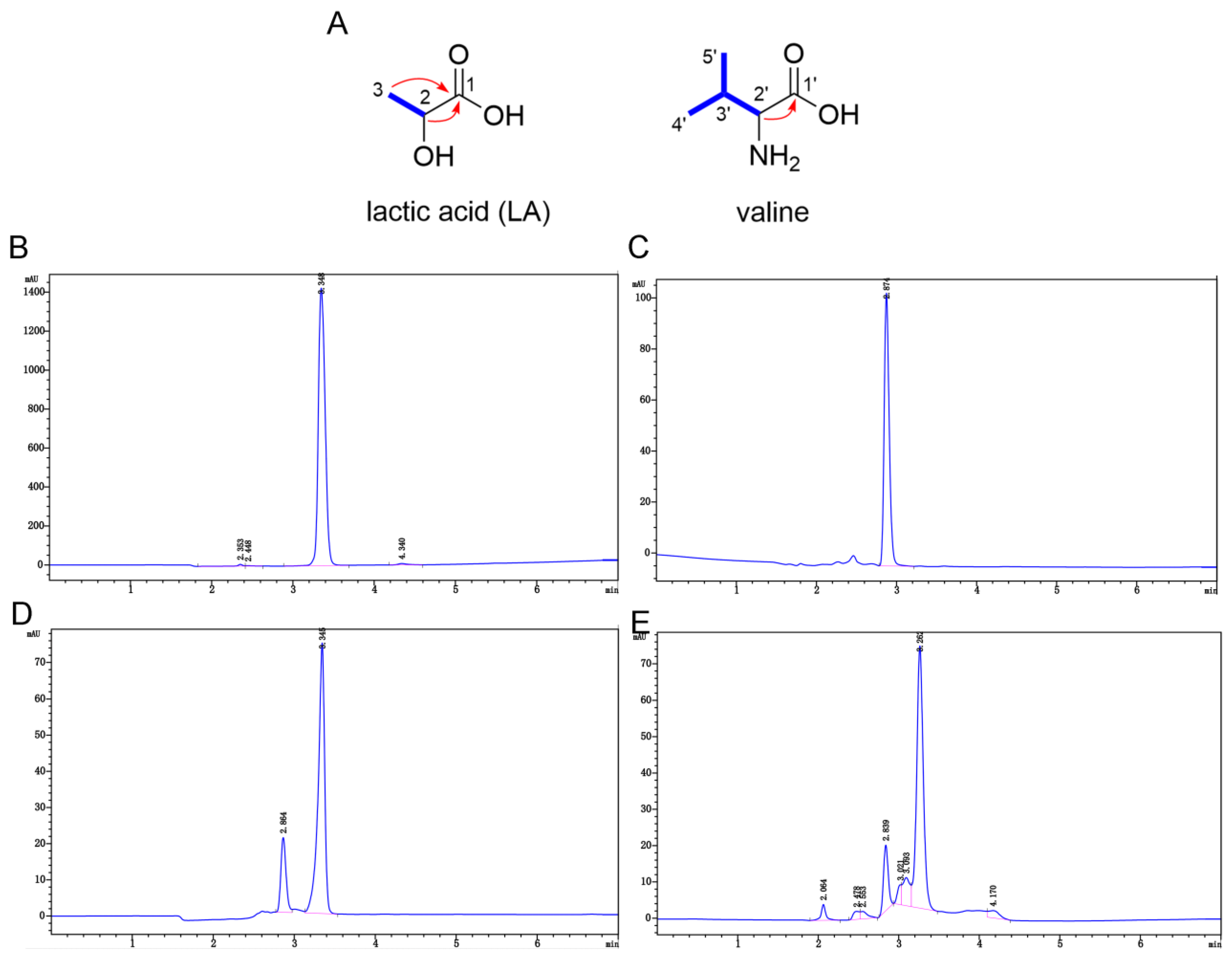

| Position | 1-1-4-3 | LA + Valine | LA | Valine | ||||
|---|---|---|---|---|---|---|---|---|
| δC, Type | δH, Mult. (J in Hz) | δC, Type | δH, Mult. (J in Hz) | δC, Type | δH, Mult. (J in Hz) | δC, Type | δH, Mult. (J in Hz) | |
| 1 | 21.0, CH3 | 1.22, d (6.9) | 20.9, CH3 | 1.23, d (6.9) | 20.9, CH3 | 1.23, d (6.8) | ||
| 2 | 66.3, CH | 4.02, q (6.8) | 66.2, CH | 4.03, q (6.8) | 66.3, CH | 4.04, q (6.8) | ||
| 3 | 176.9, C | 176.8, C | 176.8, C | |||||
| 1′ | 18.1, CH3 | 0.89, d (7.0) | 18.2, CH3 | 0.91, d (7.0) | 18.0, CH3 | 0.87, d (7.0) | ||
| 2′ | 29.5, CH | 2.16–2.09, m | 29.5, CH | 2.17–2.10, m | 29.5, CH | 2.19–2.06, m | ||
| 3′ | 19.3, CH3 | 0.93, d (7.0) | 19.0, CH3 | 0.94, d (7.0) | 19.5, CH3 | 0.91, d (7.0) | ||
| 4′ | 59.9, CH | 3.12, d (3.6) | 59.4, CH | 3.28, d (3.9) | 60.2, CH | 3.00, d (3.5) | ||
| 5′ | 170.0, C | 170.3, C | 170.4, C | |||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liang, J.; Zhou, Y.; Tang, G.; Wu, R.; Lin, H. Exploration of the Main Antibiofilm Substance of Lactobacillus plantarum ATCC 14917 and Its Effect against Streptococcus mutans. Int. J. Mol. Sci. 2023, 24, 1986. https://doi.org/10.3390/ijms24031986
Liang J, Zhou Y, Tang G, Wu R, Lin H. Exploration of the Main Antibiofilm Substance of Lactobacillus plantarum ATCC 14917 and Its Effect against Streptococcus mutans. International Journal of Molecular Sciences. 2023; 24(3):1986. https://doi.org/10.3390/ijms24031986
Chicago/Turabian StyleLiang, Jingheng, Yan Zhou, Guihua Tang, Ruixue Wu, and Huancai Lin. 2023. "Exploration of the Main Antibiofilm Substance of Lactobacillus plantarum ATCC 14917 and Its Effect against Streptococcus mutans" International Journal of Molecular Sciences 24, no. 3: 1986. https://doi.org/10.3390/ijms24031986
APA StyleLiang, J., Zhou, Y., Tang, G., Wu, R., & Lin, H. (2023). Exploration of the Main Antibiofilm Substance of Lactobacillus plantarum ATCC 14917 and Its Effect against Streptococcus mutans. International Journal of Molecular Sciences, 24(3), 1986. https://doi.org/10.3390/ijms24031986






