Comparison of Rheological Properties of Healthy versus Dupuytren Fibroblasts When Treated with a Cell Contraction Inhibitor by Atomic Force Microscope
Abstract
:1. Introduction
2. Results
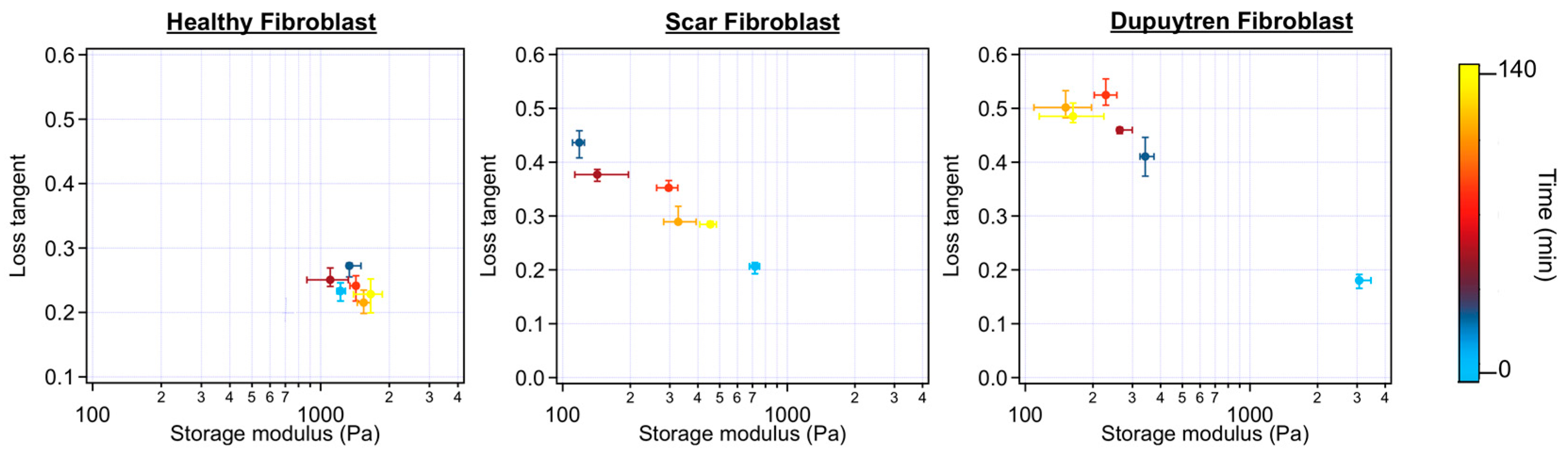
2.1. Rheological Characterization
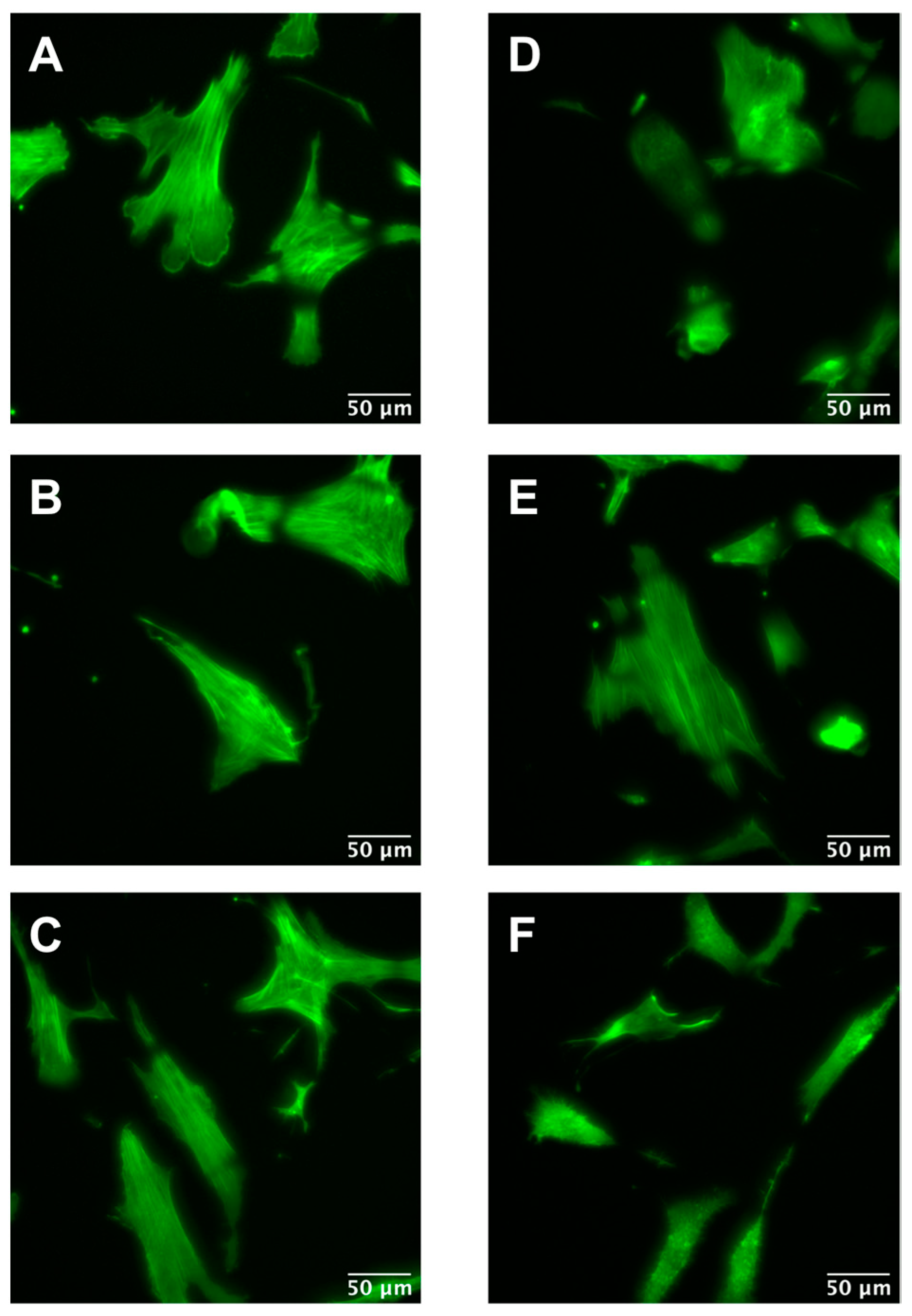
2.2. Cytoskeleton Inhibition
3. Discussion
3.1. Mechanical and Rheological Properties
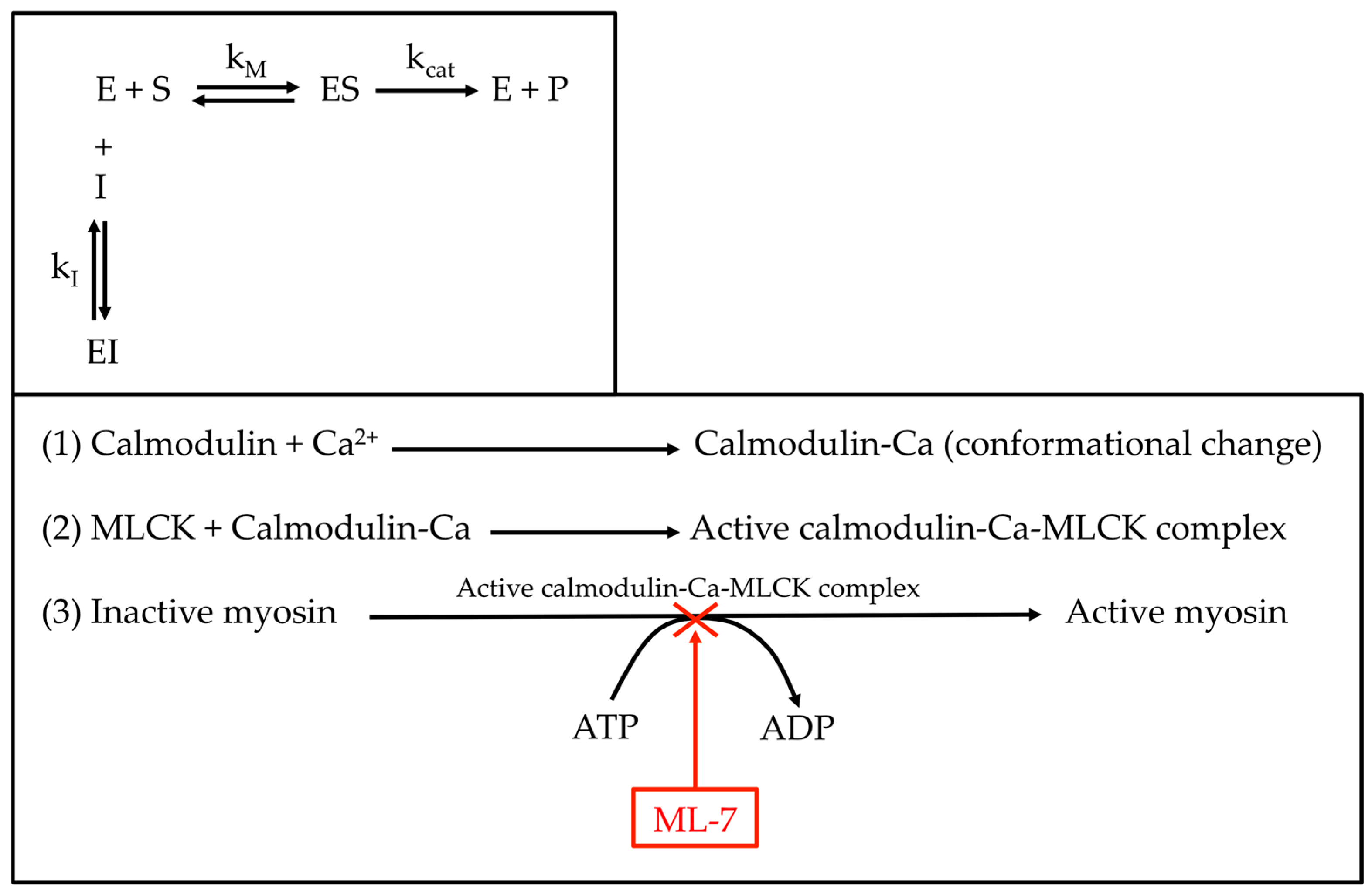
3.2. ML-7 Effect on Cell Mechanics
4. Materials and Methods
4.1. General Materials
4.2. Cell Isolation and Cell Culture
4.3. AFM Experiments
4.4. AFM Data Analysis
4.5. Immunostaining
4.6. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Van Rijssen, A.L.; Gerbrandy, F.S.J.; Linden, H.T.; Klip, H.; Werker, P.M.N. A Comparison of the Direct Outcomes of Percutaneous Needle Fasciotomy and Limited Fasciectomy for Dupuytren’s Disease: A 6-Week Follow-Up Study. J. Hand Surg. Am. 2006, 31, 717–725. [Google Scholar] [CrossRef] [PubMed]
- Abdelrahman, I.; Elmasry, M.; Steinvall, I.; Turesson, C.; Sjöberg, F.; Hansson, T. Needle Fasciotomy or Collagenase Injection in the Treatment of Dupuytren’s Contracture. Plast. Reconstr. Surg.-Glob. Open 2020, 8, e2606. [Google Scholar] [CrossRef] [PubMed]
- Desai, S.S.; Hentz, V.R. The Treatment of Dupuytren Disease. J. Hand Surg. Am. 2011, 36, 936–942. [Google Scholar] [CrossRef] [PubMed]
- Baum, J.; Duffy, H.S. Fibroblasts and Myofibroblasts: What Are We Talking About? J. Cardiovasc. Pharmacol. 2011, 57, 376–379. [Google Scholar] [CrossRef]
- Hinz, B. Myofibroblasts. Exp. Eye Res. 2015, 142, 56–70. [Google Scholar] [CrossRef]
- Bochaton-Piallat, M.L.; Gabbiani, G.; Hinz, B. The Myofibroblast in Wound Healing and Fibrosis: Answered and Unanswered Questions. F1000Research 2016, 5, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Viji Babu, P.K.; Rianna, C.; Belge, G.; Mirastschijski, U.; Radmacher, M. Mechanical and Migratory Properties of Normal, Scar, and Dupuytren’s Fibroblasts. J. Mol. Recognit. 2018, 31, e2719. [Google Scholar] [CrossRef]
- Bisson, M.A.; Beckett, K.S.; McGrouther, D.A.; Grobbelaar, A.O.; Mudera, V. Transforming Growth Factor-β1 Stimulation Enhances Dupuytren’s Fibroblast Contraction in Response to Uniaxial Mechanical Load Within a 3-Dimensional Collagen Gel. J. Hand Surg. Am. 2009, 34, 1102–1110. [Google Scholar] [CrossRef]
- Kloen, P.; Jennings, C.L.; Gebhardt, M.C.; Springfield, D.S.; Mankin, H.J. Transforming Growth Factor-β: Possible Roles in Dupuytren’s Contracture. J. Hand Surg. Am. 1995, 20, 101–108. [Google Scholar] [CrossRef]
- Tanaka, K.; Sano, K.; Tanaka, K.; Kobayashi, M.; Katsumura, K.; Ikeda, T.; Abe, M. Demonstration of Downregulation of α-Smooth Muscle Actin in Interferon-γ-Treated Myofibroblast by a Novel Cell-Capture Enzyme Immunoassay. Int. Immunopharmacol. 2001, 1, 769–775. [Google Scholar] [CrossRef]
- Gu, L.; Zhu, Y.J.; Guo, Z.J.; Xu, X.X.; Xu, W.B. Effect of IFN-γ and Dexamethasone on TGF-β1-Induced Human Fetal Lung Fibroblast-Myofibroblast Differentiation. Acta Pharmacol. Sin. 2004, 25, 1479–1488. [Google Scholar] [PubMed]
- Lowin, T.; Anssar, T.M.; Bäuml, M.; Classen, T.; Schneider, M.; Pongratz, G. Positive and Negative Cooperativity of TNF and Interferon-γ in Regulating Synovial Fibroblast Function and B Cell Survival in Fibroblast/B Cell Co-Cultures. Sci. Rep. 2020, 10, 1–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tanaka, K.; Sano, K.; Yuba, K.; Katsumura, K.; Nakano, T.; Tanaka, K.; Kobayashi, M.; Ikeda, T.; Abe, M. Inhibition of Induction of Myofibroblasts by Interferon γ in a Human Fibroblast Cell Line. Int. Immunopharmacol. 2003, 3, 1273–1280. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, K.; Sano, K.; Nakano, T.; Yuba, K.; Kinoshita, M. Suppression of α Smooth Muscle Actin Expression by IFN-γ in Established Myofibroblast Cell Lines. J. Interf. Cytokine Res. 2007, 27, 835–839. [Google Scholar] [CrossRef] [PubMed]
- Cooper, G. The Cell: A Molecular Approach, 2nd ed.; Sinauer Associates: Sunderland, MA, USA, 2000. Available online: https://www.ncbi.nlm.nih.gov/books/NBK9961 (accessed on 28 November 2022).
- Tan, I.; Leung, T. Myosin Light Chain Kinases: Division of Work in Cell Migration. Cell Adhes. Migr. 2009, 3, 256–258. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gu, L.Z.; Hu, W.Y.; Antic, N.; Mehta, R.; Turner, J.R.; de Lanerolle, P. Inhibiting Myosin Light Chain Kinase Retards the 600 Growth of Mammary and Prostate Cancer Cells. Eur. J. Cancer 2006, 42, 948–957. [Google Scholar] [CrossRef] [PubMed]
- Cheng, X.; Wang, X.; Wan, Y.; Zhou, Q.; Zhu, H.; Wang, Y. Myosin Light Chain Kinase Inhibitor ML7 Improves Vascular Endothelial Dysfunction via Tight Junction Regulation in a Rabbit Model of Atherosclerosis. Mol. Med. Rep. 2015, 12, 4109–4116. [Google Scholar] [CrossRef] [Green Version]
- Ding, J.; Li, Z.; Li, L.; Ding, Y.; Wang, D.; Meng, S.; Zhou, Q.; Gui, S.; Wei, W.; Zhu, H.; et al. Myosin Light Chain Kinase Inhibitor ML7 Improves Vascular Endothelial Dysfunction and Permeability via the Mitogen-Activated Protein Kinase Pathway in a Rabbit Model of Atherosclerosis. Biomed. Pharmacother. 2020, 128, 110258. [Google Scholar] [CrossRef]
- Xiong, Y.; Wang, C.; Shi, L.; Wang, L.; Zhou, Z.; Chen, D.; Wang, J.; Guo, H. Myosin Light Chain Kinase: A Potential Target for Treatment of Inflammatory Diseases. Front. Pharmacol. 2017, 8, 292. [Google Scholar] [CrossRef] [Green Version]
- Kaneko, K.; Satoh, K.; Masamune, A.; Satoh, A.; Shimosegawa, T. Myosin Light Chain Kinase Inhibitors Can Block Invasion and Adhesion of Human Pancreatic Cancer Cell Lines. Pancreas 2002, 24, 34–41. [Google Scholar] [CrossRef]
- Schäfer, A.; Radmacher, M. Influence of Myosin II Activity on Stiffness of Fibroblast Cells. Acta Biomater. 2005, 1, 273–280. [Google Scholar] [CrossRef]
- Lekka, M.; Laidler, P.; Gil, D.; Lekki, J.; Stachura, Z.; Hrynkiewiczk, A.Z. Elasticity of normal and cancerous human bladder cells studied by scanning force microscopy. Eur. Biophys. J. 1999, 28, 312–316. [Google Scholar] [CrossRef]
- Alcaraz, J.; Buscemi, L.; Puig-De-Morales, M.; Colchero, J.; Baró, A.; Navajas, D. Correction of Microrheological Measurements of Soft Samples with Atomic Force Microscopy for the Hydrodynamic Drag on the Cantilever. Langmuir 2002, 18, 716–721. [Google Scholar] [CrossRef]
- Alcaraz, J.; Buscemi, L.; Grabulosa, M.; Trepat, X.; Fabry, B.; Farré, R.; Navajas, D. Microrheology of Human Lung Epithelial Cells Measured by Atomic Force Microscopy. Biophys. J. 2003, 84, 2071–2079. [Google Scholar] [CrossRef] [Green Version]
- Fabry, B.; Maksym, G.N.; Butler, J.P.; Glogauer, M.; Navajas, D.; Fredberg, J.J. Scaling the Microrheology of Living Cells. Phys. Rev. Lett. 2001, 87, 1–4. [Google Scholar] [CrossRef] [Green Version]
- Rico, F.; Roca-Cusachs, P.; Gavara, N.; Farré, R.; Rotger, M.; Navajas, D. Probing Mechanical Properties of Living Cells by Atomic Force Microscopy with Blunted Pyramidal Cantilever Tips. Phys. Rev. E 2005, 72, 021914. [Google Scholar] [CrossRef] [Green Version]
- Hecht, F.M.; Rheinlaender, J.; Schierbaum, N.; Goldmann, W.H.; Fabry, B.; Schäffer, T.E. Imaging Viscoelastic Properties of Live Cells by AFM: Power-Law Rheology on the Nanoscale. Soft Matter 2015, 11, 4584–4591. [Google Scholar] [CrossRef] [Green Version]
- Rigato, A.; Miyagi, A.; Scheuring, S.; Rico, F. High-Frequency Microrheology Reveals Cytoskeleton Dynamics in Living Cells. Nat. Phys. 2017, 13, 771–775. [Google Scholar] [CrossRef] [Green Version]
- De Sousa, J.S.; Freire, R.S.; Sousa, F.D.; Radmacher, M.; Silva, A.F.B.; Ramos, M.V.; Monteiro-Moreira, A.C.O.; Mesquita, F.P.; Moraes, M.E.A.; Montenegro, R.C.; et al. Double Power-Law Viscoelastic Relaxation of Living Cells Encodes Motility Trends. Sci. Rep. 2020, 10, 4749. [Google Scholar] [CrossRef] [Green Version]
- Rebêlo, L.M.; de Sousa, J.S.; Filho, J.M.; Schäpe, J.; Doschke, H.; Radmacher, M. Microrheology of Cells with Magnetic Force Modulation Atomic Force Microscopy. Soft Matter 2014, 10, 2141–2149. [Google Scholar] [CrossRef]
- Sollich, P. Rheological Constitutive Equation for a Model of Soft Glassy Materials. Phys. Rev. E 1998, 58, 738–759. [Google Scholar] [CrossRef] [Green Version]
- Isambert, H.; Maggs, A.C. Dynamics and Rheology of Actin Solutions. Macromolecules 1996, 29, 1036–1040. [Google Scholar] [CrossRef] [Green Version]
- Tojkander, S.; Gateva, G.; Lappalainen, P. Actin Stress Fibers—Assembly, Dynamics and Biological Roles. J. Cell Sci. 2012, 125, 1855–1864. [Google Scholar] [CrossRef] [Green Version]
- Fletcher, D.A.; Mullins, R.D. Cell Mechanics and the Cytoskeleton. Nature 2010, 463, 485–492. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tomasek, J.; Rayan, G.M. Correlation of α-smooth muscle actin expression and contraction in Dupuytren’s disease fibroblasts. J. Hand Surg. 1995, 20, 450–455. [Google Scholar] [CrossRef]
- Hadeed, J.G.; Bond, J.E.; Selim, M.A.; Bergeron, A.; Levin, S.; Levinson, H. Calcium-dependent signaling in Dupuytren’s disease. Hand 2011, 6, 159–164. [Google Scholar] [CrossRef] [Green Version]
- Sayadi, L.R.; Alhunayan, D.; Sarantopoulos, N.; Kong, C.; Condamoor, S.; Sayadi, J.; Banyard, D.A.; Shaterian, A.; Leis, A.; Evans, G.R.D.; et al. The molecular pathogenesis of Dupuytren disease. Ann. Plast. Surg. 2019, 83, 594–600. [Google Scholar] [CrossRef]
- Lyapunova, E.; Nikituk, A.; Bayandin, Y.; Naimark, O.; Rianna, C.; Radmacher, M. Passive Microrheology of Normal and Cancer Cells after ML7 Treatment by Atomic Force Microscopy. AIP Conf. Proc. 2016, 1760, 020046. [Google Scholar] [CrossRef]
- Shi, J.; Takahashi, S.; Jin, X.H.; Li, Y.Q.; Ito, Y.; Mori, Y.; Inoue, R. Myosin Light Chain Kinase-Independent Inhibition by ML-9 of Murine TRPC6 Channels Expressed in HEK293 Cells. Br. J. Pharmacol. 2007, 152, 122–131. [Google Scholar] [CrossRef] [Green Version]
- Isemura, M.; Mita, T.; Satoh, K.; Narumi, K.; Motomiya, M. Myosin Light Chain Kinase Inhibitors ML-7 and ML-9 Inhibit Mouse Lung Carcinoma Cell Attachment to the Fibronectin Substratum. Cell Biol. Int. Rep. 1991, 15, 965–972. [Google Scholar] [CrossRef]
- Burridge, K.; Chrzanowska-Wodnicka, M. Focal adhesions, contractility, and signaling. Ann. Rev. Cell Dev. Biol. 1996, 12, 463–518. [Google Scholar] [CrossRef] [PubMed]
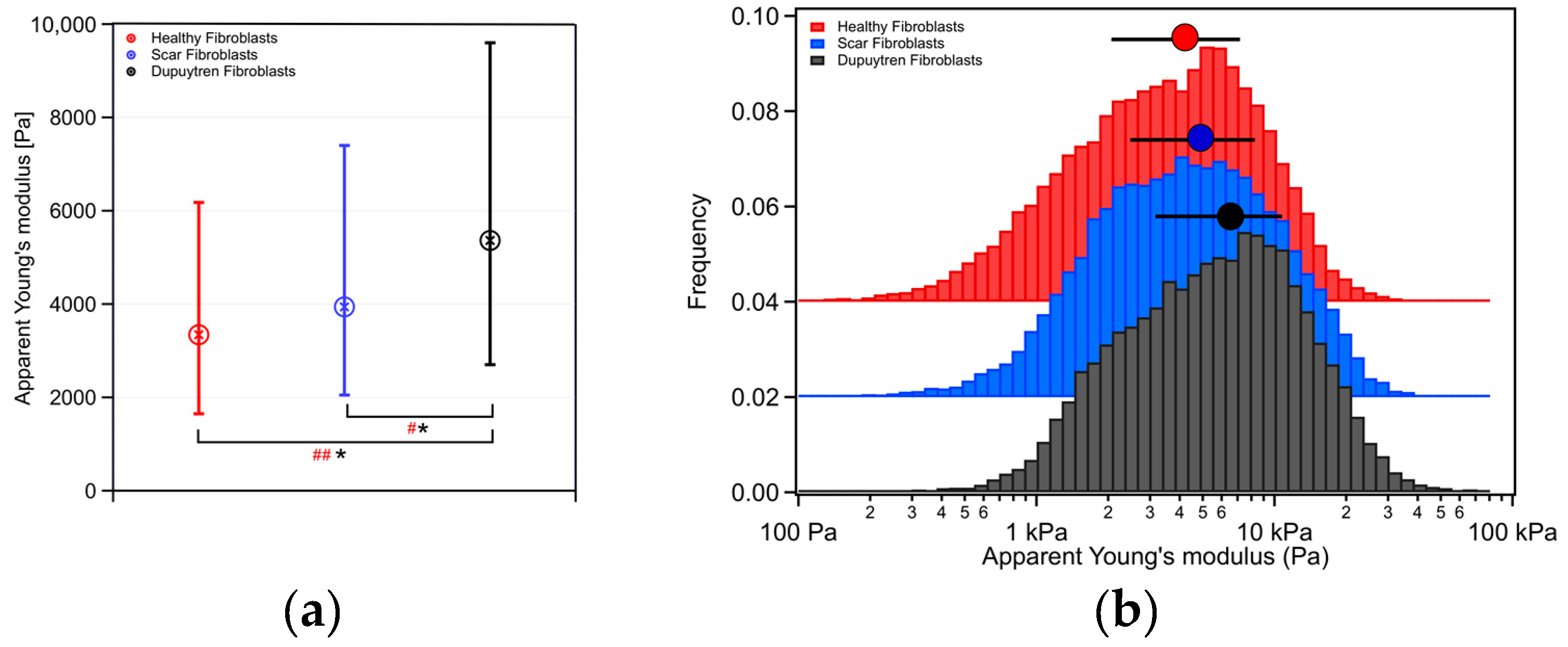
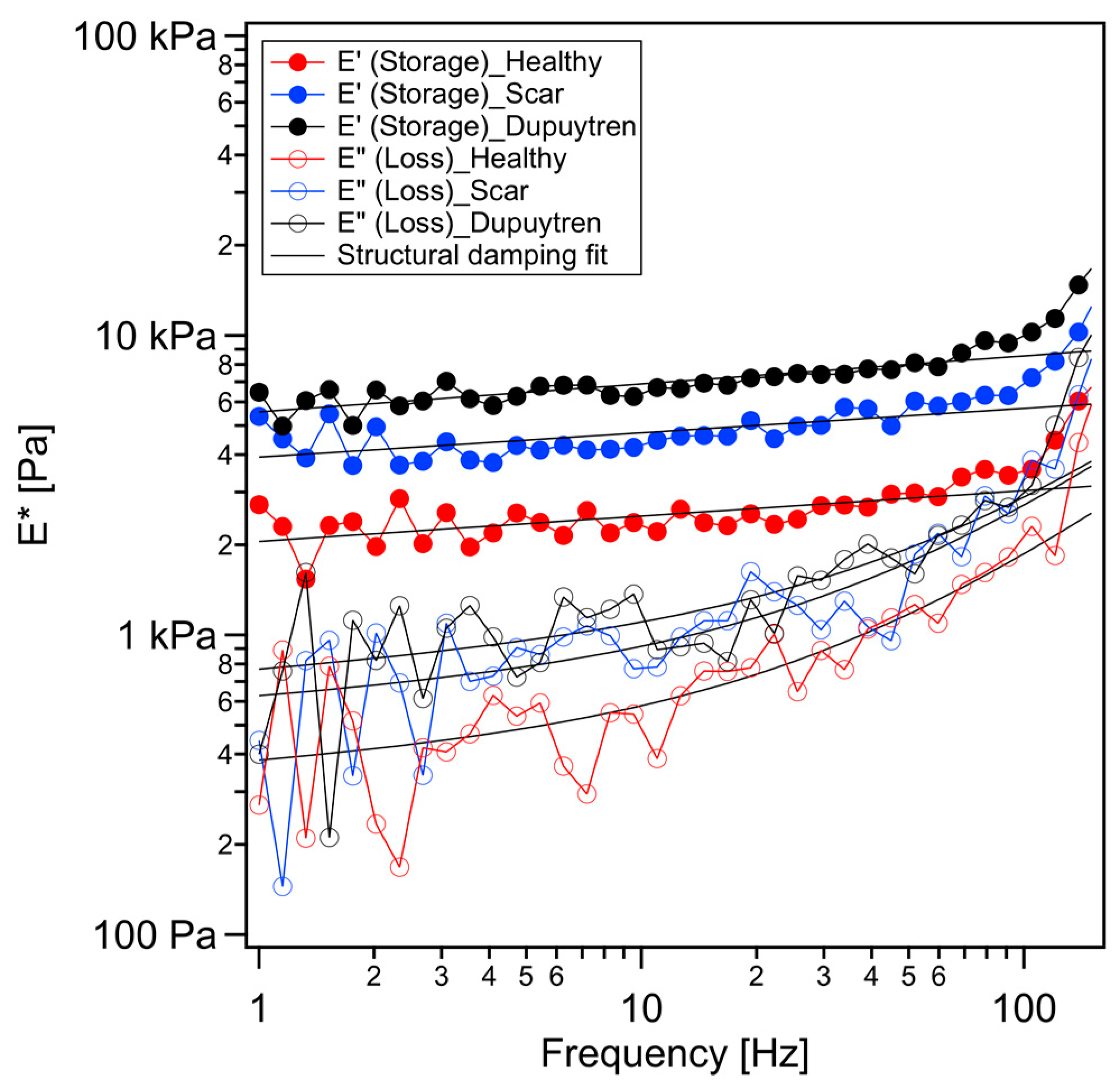
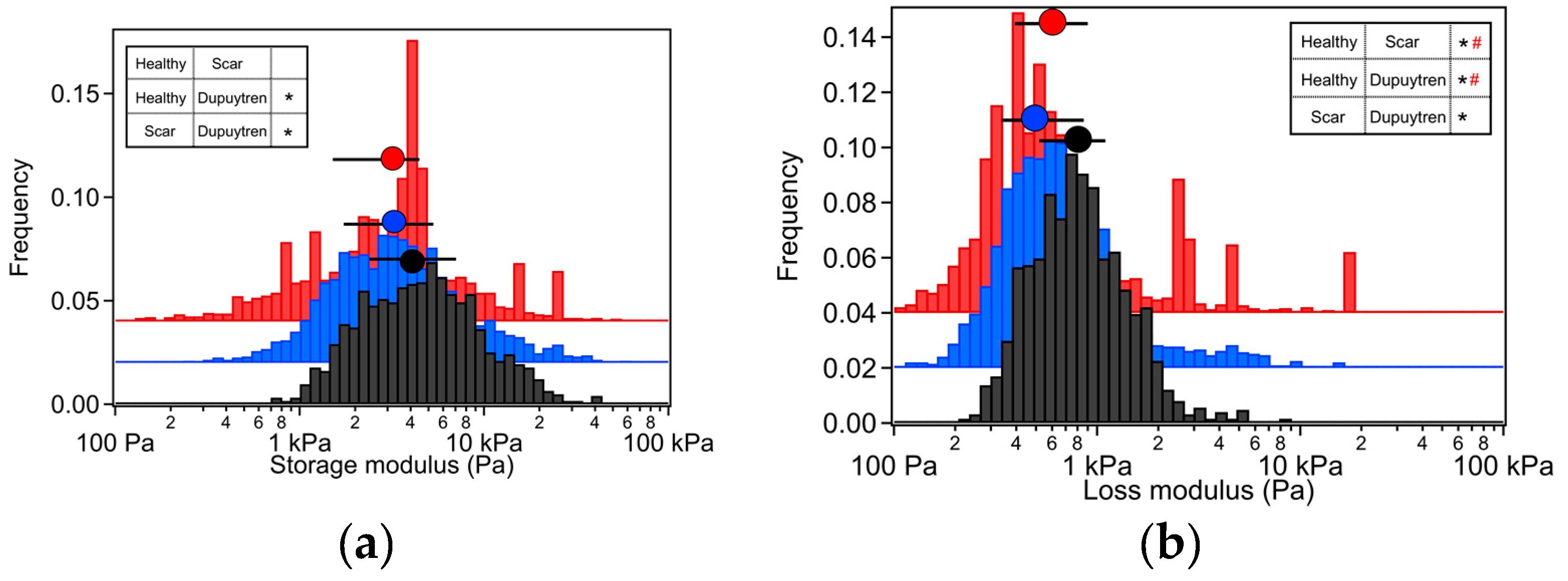
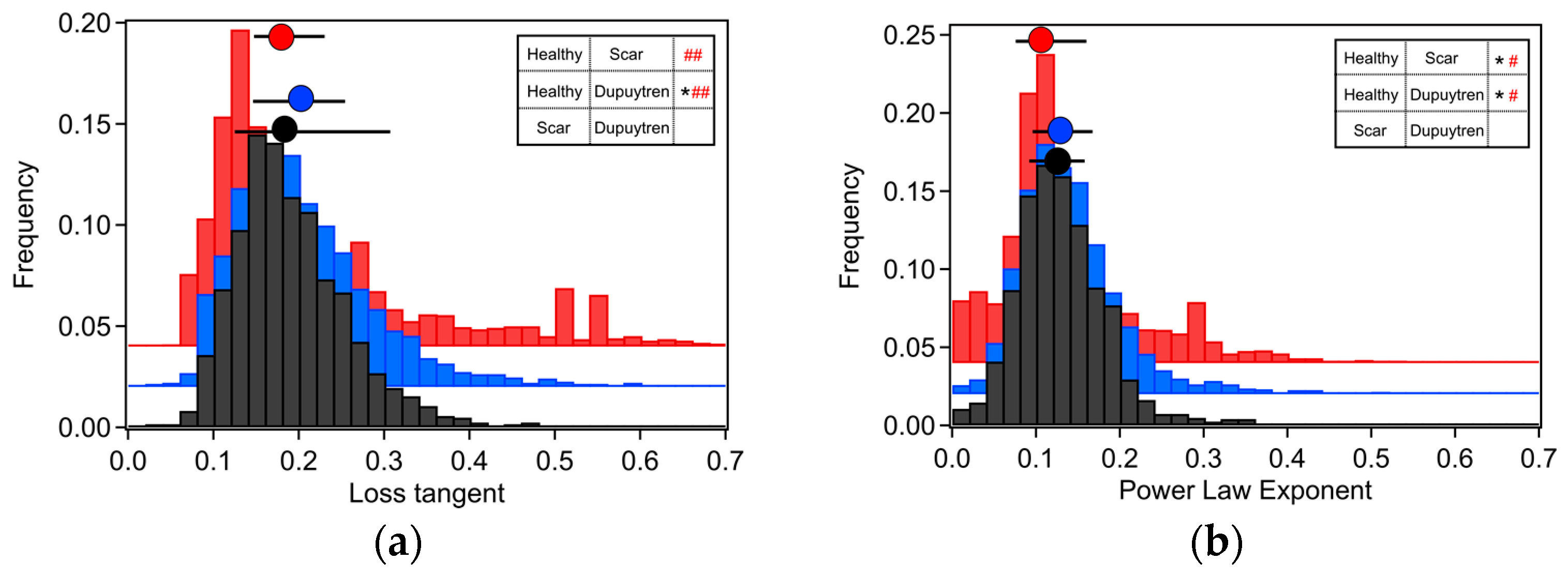


| Apparent Young’s Modulus (Pa) | Storage Modulus (Pa) | Loss Modulus (Pa) | Loss Tangent | Power Law Exponent | |
|---|---|---|---|---|---|
| Healthy Fib. | 3345 | 3260 | 473 | 0.18 | 0.11 |
| Scar Fib. | 3940 | 3024 | 576 | 0.19 | 0.13 |
| Dupuytren Fib. | 5364 | 4260 | 742 | 0.18 | 0.12 |
| Healthy Fibroblasts | Scar Fibroblasts | Dupuytren Fibroblasts | |
|---|---|---|---|
| 1 µM ML-7 (n = 15) | Dead: 20% | Dead: 0% | Dead: 13% |
| Recovery: 20% | Recovery: 20% | Recovery: 47% | |
| No change: 60% | No change: 80% | No change: 40% | |
| 3 µM ML-7 (n = 20) | Dead: 39% | Dead: 39% | Dead: 60% |
| Recovery: 34% | Recovery: 25% | Recovery: 10% | |
| No change: 27% | No change: 36% | No change: 30% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pérez-Domínguez, S.; López-Alonso, J.; Lafont, F.; Radmacher, M. Comparison of Rheological Properties of Healthy versus Dupuytren Fibroblasts When Treated with a Cell Contraction Inhibitor by Atomic Force Microscope. Int. J. Mol. Sci. 2023, 24, 2043. https://doi.org/10.3390/ijms24032043
Pérez-Domínguez S, López-Alonso J, Lafont F, Radmacher M. Comparison of Rheological Properties of Healthy versus Dupuytren Fibroblasts When Treated with a Cell Contraction Inhibitor by Atomic Force Microscope. International Journal of Molecular Sciences. 2023; 24(3):2043. https://doi.org/10.3390/ijms24032043
Chicago/Turabian StylePérez-Domínguez, Sandra, Javier López-Alonso, Frank Lafont, and Manfred Radmacher. 2023. "Comparison of Rheological Properties of Healthy versus Dupuytren Fibroblasts When Treated with a Cell Contraction Inhibitor by Atomic Force Microscope" International Journal of Molecular Sciences 24, no. 3: 2043. https://doi.org/10.3390/ijms24032043





