Interaction between Long Noncoding RNAs and Syncytin-1/Syncytin-2 Genes and Transcripts: How Noncoding RNAs May Affect Pregnancy in Patients with Systemic Lupus Erythematosus
Abstract
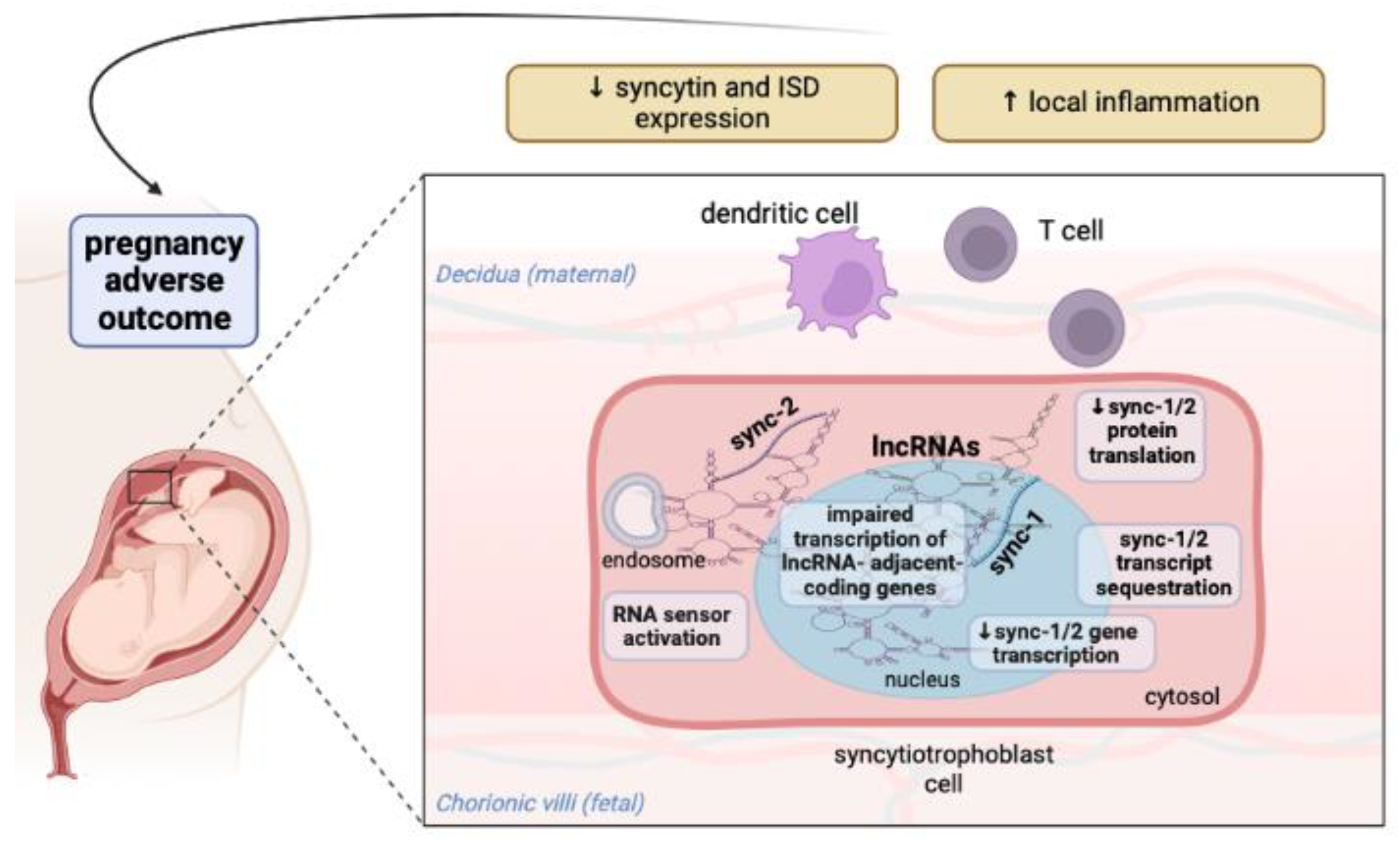
1. Introduction
2. Results
2.1. Syncytin-1 BLASTN Analysis against Human ncRNA Genes
2.2. Syncytin-2 BLASTN Analysis against Human ncRNA Genes
3. Discussion
4. Materials and Methods
4.1. Identification of Human ncRNA Genes with Complementary Sequences to Syncytin-1 and Syncytin-2 Genes
4.2. Analysis of the Molecular Interactions and Biological Function of Human ncRNAs
4.3. Analysis of Polymorphic Variants of Human ncRNAs and Adjacent Coding Genes and Associated Diseases
5. Conclusions
Supplementary Materials
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Barber, M.R.W.; Drenkard, C.; Falasinnu, T.; Hoi, A.; Mak, A.; Kow, N.Y.; Svenungsson, E.; Peterson, J.; Clarke, A.E.; Ramsey-Goldman, R. Global Epidemiology of Systemic Lupus Erythematosus. Nat. Rev. Rheumatol. 2021, 17, 515–532. [Google Scholar] [CrossRef] [PubMed]
- Obrișcă, B.; Sorohan, B.; Tuță, L.; Ismail, G. Advances in Lupus Nephritis Pathogenesis: From Bench to Bedside. Int. J. Mol. Sci. 2021, 22, 3766. [Google Scholar] [CrossRef]
- Aringer, M.; Costenbader, K.; Daikh, D.; Brinks, R.; Mosca, M.; Ramsey-Goldman, R.; Smolen, J.S.; Wofsy, D.; Boumpas, D.T.; Kamen, D.L.; et al. 2019 European League Against Rheumatism/American College of Rheumatology Classification Criteria for Systemic Lupus Erythematosus. Ann. Rheum. Dis. 2019, 78, 1151–1159. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.H.; Choi, S.J.; Ji, J.D.; Song, G.G. Overall and Cause-Specific Mortality in Systemic Lupus Erythematosus: An Updated Meta-Analysis. Lupus 2016, 25, 727–734. [Google Scholar] [CrossRef] [PubMed]
- Castellanos Gutierrez, A.S.; Figueras, F.; Morales-Prieto, D.M.; Schleußner, E.; Espinosa, G.; Baños, N. Placental Damage in Pregnancies with Systemic Lupus Erythematosus: A Narrative Review. Front. Immunol. 2022, 13, 941586. [Google Scholar] [CrossRef] [PubMed]
- Pons-Estel, G.J.; Andreoli, L.; Scanzi, F.; Cervera, R.; Tincani, A. The Antiphospholipid Syndrome in Patients with Systemic Lupus Erythematosus. J. Autoimmun. 2017, 76, 10–20. [Google Scholar] [CrossRef] [PubMed]
- Marx-Deseure, A.; Labreuche, J.; Launay, D.; Depret, S.; Subtil, D. Are Pregnancies with Lupus but without APS of Good Prognosis? Autoimmun. Rev. 2020, 19, 102489. [Google Scholar] [CrossRef]
- Huang, Q.; Chen, H.; Li, J.; Oliver, M.; Ma, X.; Byck, D.; Gao, Y.; Jiang, S.W. Epigenetic and Non-Epigenetic Regulation of Syncytin-1 Expression in Human Placenta and Cancer Tissues. Cell. Signal. 2014, 26, 648–656. [Google Scholar] [CrossRef]
- Lokossou, A.G.; Toudic, C.; Nguyen, P.T.; Elisseeff, X.; Vargas, A.; Rassart, É.; Lafond, J.; Leduc, L.; Bourgault, S.; Gilbert, C.; et al. Endogenous Retrovirus-Encoded Syncytin-2 Contributes to Exosome-Mediated Immunosuppression of T Cells. Biol. Reprod. 2020, 102, 185–198. [Google Scholar] [CrossRef]
- Tolosa, J.M.; Parsons, K.S.; Hansbro, P.M.; Smith, R.; Wark, P.B. The Placental Protein Syncytin-1 Impairs Antiviral Responses and Exaggerates Inflammatory Responses to Influenza. PLoS ONE 2015, 10, e0118629. [Google Scholar] [CrossRef]
- Hummel, J.; Kämmerer, U.; Müller, N.; Avota, E.; Schneider-Schaulies, S. Human Endogenous Retrovirus Envelope Proteins Target Dendritic Cells to Suppress T-Cell Activation. Eur. J. Immunol. 2015, 45, 1748–1759. [Google Scholar] [CrossRef] [PubMed]
- Grandi, N.; Tramontano, E. Human Endogenous Retroviruses Are Ancient Acquired Elements Still Shaping Innate Immune Responses. Front. Immunol. 2018, 9, 2039. [Google Scholar] [CrossRef] [PubMed]
- Greenig, M. HERVs, Immunity, and Autoimmunity: Understanding the Connection. PeerJ 2019, 7, e6711. [Google Scholar] [CrossRef]
- Talotta, R.; Atzeni, F.; Laska, M.J. The Contribution of HERV-E Clone 4-1 and Other HERV-E Members to the Pathogenesis of Rheumatic Autoimmune Diseases. APMIS 2020, 128, 367–377. [Google Scholar] [CrossRef] [PubMed]
- Taheri, M.; Eghtedarian, R.; Dinger, M.E.; Ghafouri-Fard, S. Exploring the Role of Non-Coding Rnas in the Pathophysiology of Systemic Lupus Erythematosus. Biomolecules 2020, 10, 937. [Google Scholar] [CrossRef]
- Statello, L.; Guo, C.J.; Chen, L.L.; Huarte, M. Gene Regulation by Long Non-Coding RNAs and Its Biological Functions. Nat. Rev. Mol. Cell Biol. 2020, 22, 96–118. [Google Scholar] [CrossRef]
- McAninch, D.; Roberts, C.T.; Bianco-Miotto, T. Mechanistic Insight into Long Noncoding RNAs and the Placenta. Int. J. Mol. Sci. 2017, 18, 1371. [Google Scholar] [CrossRef]
- Noh, J.H.; Kim, K.M.; McClusky, W.G.; Abdelmohsen, K.; Gorospe, M. Cytoplasmic Functions of Long Noncoding RNAs. Wiley Interdiscip. Rev. RNA 2018, 9, e1471. [Google Scholar] [CrossRef]
- Santos-Pereira, J.M.; Aguilera, A. R Loops: New Modulators of Genome Dynamics and Function. Nat. Rev. Genet. 2015, 16, 583–597. [Google Scholar] [CrossRef]
- Hadjiargyrou, M.; Delihas, N. The Intertwining of Transposable Elements and Non-Coding RNAs. Int. J. Mol. Sci. 2013, 14, 13307–13328. [Google Scholar] [CrossRef]
- Ogasawara, H.; Okada, M.; Kaneko, H.; Hishikawa, T.; Sekigawa, I.; Hashimoto, H. Possible Role of DNA Hypomethylation in the Induction of SLE: Relationship of the Transcription of Human Endogenous Retroviruses. Clin. Exp. Rheumatol. 2003, 21, 733–738. [Google Scholar] [PubMed]
- Hishikawa, T.; Ogasawara, H.; Kaneko, H.; Shirasawa, T.; Matsuura, Y.; Sekigawa, I.; Takasaki, Y.; Hashimoto, H.; Hirose, S.; Handa, S.; et al. Detection of Antibodies to a Recombinant Gag Protein Derived from Human Endogenous Retrovirus Clone 4-1 in Autoimmune Diseases. Viral Immunol. 1997, 10, 137–147. [Google Scholar] [CrossRef] [PubMed]
- Perl, A.; Colombo, E.; Dai, H.; Agarwal, R.; Mark, K.A.; Banki, K.; Poiesz, B.J.; Phillips, P.E.; Hoch, S.O.; Reveille, J.D.; et al. Antibody Reactivity to the Hres-1 Endogenous Retroviral Element Identifies a Subset of Patients with Systemic Lupus Erythematosus and Overlap Syndromes. Arthritis Rheum. 1995, 38, 1660–1671. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Chen, S.; Li, A.; Shen, K.; Wang, S.; Wang, S.; Wu, P.; Luo, W.; Pan, Q. LncRNA Expression Profiles in Systemic Lupus Erythematosus and Rheumatoid Arthritis: Emerging Biomarkers and Therapeutic Targets. Front. Immunol. 2021, 12, 792884. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, S.; Chen, S.; Du, J.; Lin, J.; Qin, H.; Wang, J.; Liang, J.; Xu, J. Long Noncoding RNA Expression Profile and Association with SLEDAI Score in Monocyte-Derived Dendritic Cells from Patients with Systematic Lupus Erythematosus. Arthritis Res. Ther. 2018, 20, 138. [Google Scholar] [CrossRef]
- Xu, H.; Chen, W.; Zheng, F.; Tang, D.; Liu, D.; Wang, G.; Xu, Y.; Yin, L.; Zhang, X.; Dai, Y. Reconstruction and Analysis of the Aberrant LncRNA–MiRNA–MRNA Network in Systemic Lupus Erythematosus. Lupus 2020, 29, 398–406. [Google Scholar] [CrossRef]
- Narciso, L.; Ietta, F.; Romagnoli, R.; Paulesu, L.; Mantovani, A.; Tait, S. Effects of Bisphenol A on Endogenous Retroviral Envelopes Expression and Trophoblast Fusion in BeWo Cells. Reprod. Toxicol. 2019, 89, 35–44. [Google Scholar] [CrossRef]
- Balada, E.; Ordi-Ros, J.; Vilardell-Tarrés, M. Molecular Mechanisms Mediated by Human Endogenous Retroviruses (HERVs) in Autoimmunity. Rev. Med. Virol. 2009, 19, 273–286. [Google Scholar] [CrossRef]
- Mustelin, T.; Lood, C.; Giltiay, N.V. Sources of Pathogenic Nucleic Acids in Systemic Lupus Erythematosus. Front. Immunol. 2019, 10, 1028. [Google Scholar] [CrossRef]
- Ghafouri-Fard, S.; Poornajaf, Y.; Dashti, F.; Hussen, B.M.; Taheri, M.; Jamali, E. Interaction Between Non-Coding RNAs and Interferons: With an Especial Focus on Type I Interferons. Front. Immunol. 2022, 13, 877243. [Google Scholar] [CrossRef]
- Stearrett, N.; Dawson, T.; Rahnavard, A.; Bachali, P.; Bendall, M.L.; Zeng, C.; Caricchio, R.; Pérez-Losada, M.; Grammer, A.C.; Lipsky, P.E.; et al. Expression of Human Endogenous Retroviruses in Systemic Lupus Erythematosus: Multiomic Integration With Gene Expression. Front. Immunol. 2021, 12, 661437. [Google Scholar] [CrossRef]
- Guzmán-Martín, C.A.; Juárez-Vicuña, Y.; Domínguez-López, A.; González-Ramírez, J.; Amezcua-Guerra, L.M.; Martínez-Martínez, L.A.; Sánchez-Muñoz, F. LncRNAs Dysregulation in Monocytes from Primary Antiphospholipid Syndrome Patients: A Bioinformatic and an Experimental Proof-of-Concept Approach. Mol. Biol. Rep. 2022. [Google Scholar] [CrossRef]
- Sun, Z.; Zhou, Q.; Yang, Y.; Li, L.; Yu, M.; Li, H.; Li, A.; Wang, X.; Jiang, Y. Identification and Ultrasensitive Photoelectrochemical Detection of LncNR_040117: A Biomarker of Recurrent Miscarriage and Antiphospholipid Antibody Syndrome in Platelet-Derived Microparticles. J. Nanobiotechnol. 2022, 20, 396. [Google Scholar] [CrossRef] [PubMed]
- Tan, Y.; Liu, Q.; Li, Z.; Yang, S.; Cui, L. Epigenetics-Mediated Pathological Alternations and Their Potential in Antiphospholipid Syndrome Diagnosis and Therapy. Autoimmun. Rev. 2022, 21, 103130. [Google Scholar] [CrossRef] [PubMed]
- Grandi, N.; Tramontano, E. HERV Envelope Proteins: Physiological Role and Pathogenic Potential in Cancer and Autoimmunity. Front. Microbiol. 2018, 9, 462. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Peng, B.; Klausen, C.; Yi, Y.; Li, Y.; Xiong, S.; von Dadelszen, P.; Leung, P.C.K. NPFF Increases Fusogenic Proteins Syncytin 1 and Syncytin 2 via GCM1 in First Trimester Primary Human Cytotrophoblast Cells. FASEB J. 2020, 34, 9419–9432. [Google Scholar] [CrossRef]
- Knerr, I.; Huppertz, B.; Weigel, C.; Dötsch, J.; Wich, C.; Schild, R.L.; Beckmann, M.W.; Rascher, W. Endogenous Retroviral Syncytin: Compilation of Experimental Research on Syncytin and Its Possible Role in Normal and Disturbed Human Placentogenesis. Mol. Hum. Reprod. 2004, 10, 581–588. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, X.-W.; Li, J.; Brost, B.; Xia, X.-Y.; Chen, H.; Wang, C.-X.; Jiang, S.-W. Decreased Expression and Altered Methylation of Syncytin-1 Gene in Human Placentas Associated with Preeclampsia. Curr. Pharm. Des. 2014, 20, 1796–1802. [Google Scholar] [CrossRef]
- Ruebner, M.; Strissel, P.L.; Ekici, A.B.; Stiegler, E.; Dammer, U.; Goecke, T.W.; Faschingbauer, F.; Fahlbusch, F.B.; Beckmann, M.W.; Strick, R. Reduced Syncytin-1 Expression Levels in Placental Syndromes Correlates with Epigenetic Hypermethylation of the ERVW-1 Promoter Region. PLoS ONE 2013, 8, e56145. [Google Scholar] [CrossRef]
- Mangeney, M.; Renard, M.; Schlecht-Louf, G.; Bouallaga, I.; Heidmann, O.; Letzelter, C.; Richaud, A.; Ducos, B.; Heidmann, T. Placental Syncytins: Genetic Disjunction between the Fusogenic and Immunosuppressive Activity of Retroviral Envelope Proteins. Proc. Natl. Acad. Sci. USA 2007, 104, 20534–20539. [Google Scholar] [CrossRef]
- Perron, H.; Germi, R.; Bernard, C.; Garcia-Montojo, M.; Deluen, C.; Farinelli, L.; Faucard, R.; Veas, F.; Stefas, I.; Fabriek, B.O.; et al. Human Endogenous Retrovirus Type W Envelope Expression in Blood and Brain Cells Provides New Insights into Multiple Sclerosis Disease. Mult. Scler. J. 2012, 18, 1721–1736. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Zhang, P.; Chen, H.; Wang, X.; He, X.; Zhong, J.; Zheng, H.P.; Li, X.; Jakovlić, I.; Zhang, Y.; et al. Whole-Genome Sequencing Identifies Rare Missense Variants of WNT16 and ERVW-1 Causing the Systemic Lupus Erythematosus. Genomics 2022, 114, 110332. [Google Scholar] [CrossRef] [PubMed]
- Munjas, J.; Sopić, M.; Stefanović, A.; Košir, R.; Ninić, A.; Joksić, I.; Antonić, T.; Spasojević-Kalimanovska, V.; Zmrzljak, U.P. Non-Coding RNAs in Preeclampsia—Molecular Mechanisms and Diagnostic Potential. Int. J. Mol. Sci. 2021, 22, 10652. [Google Scholar] [CrossRef] [PubMed]
- Song, X.; Luo, X.; Gao, Q.; Wang, Y.; Gao, Q.; Long, W. Dysregulation of LncRNAs in Placenta and Pathogenesis of Preeclampsia. Curr. Drug Targets 2017, 18, 1165–1170. [Google Scholar] [CrossRef]
- Mohanty, A.F.; Farin, F.M.; Bammler, T.K.; MacDonald, J.W.; Afsharinejad, Z.; Burbacher, T.M.; Siscovick, D.S.; Williams, M.A.; Enquobahrie, D.A. Infant Sex-Specific Placental Cadmium and DNA Methylation Associations. Environ. Res. 2015, 138, 74–81. [Google Scholar] [CrossRef]
- Gormley, M.; Ona, K.; Kapidzic, M.; Garrido-Gomez, T.; Zdravkovic, T.; Fisher, S.J. Preeclampsia: Novel Insights from Global RNA Profiling of Trophoblast Subpopulations. Am. J. Obstet. Gynecol. 2017, 217, e1–e200. [Google Scholar] [CrossRef]
- Akram, K.M.; Kulkarni, N.S.; Brook, A.; Wyles, M.D.; Anumba, D.O.C. Transcriptomic Analysis of the Human Placenta Reveals Trophoblast Dysfunction and Augmented Wnt Signalling Associated with Spontaneous Preterm Birth. SSRN Electron. J. 2022. [Google Scholar] [CrossRef]
- Yu, S.-L.; Kim, T.-H.; Han, Y.-H.; Kang, Y.; Jeong, D.-U.; Lee, D.; Kang, J.; Park, S.-R. Transcriptomic Analysis and Competing Endogenous RNA Network in the Human Endometrium between Proliferative and Mid-secretory Phases. Exp. Ther. Med. 2021, 21, 660. [Google Scholar] [CrossRef]
- Vallot, C.; Patrat, C.; Collier, A.J.; Huret, C.; Casanova, M.; Liyakat Ali, T.M.; Tosolini, M.; Frydman, N.; Heard, E.; Rugg-Gunn, P.J.; et al. XACT Noncoding RNA Competes with XIST in the Control of X Chromosome Activity during Human Early Development. Cell Stem Cell 2017, 20, 102–111. [Google Scholar] [CrossRef]
- Luo, Y.; Li, J.; Zhu, D.; Fan, Y.; Li, S.; Sun, X. High-Resolution Chromosomal Microarray Analysis of Early-Stage Human Embryonic Stem Cells Reveals an Association between X Chromosome Instability and Skewed X Inactivation. Cell Biosci. 2014, 4, 74. [Google Scholar] [CrossRef]
- Shan, S.; Yang, Y.; Jiang, J.; Yang, B.; Yang, Y.; Sun, F.; Zhang, J.; Lin, Y.; Xu, H. Extracellular Vesicle-Derived Long Non-Coding RNA as Circulating Biomarkers for Endometriosis. Reprod. Biomed. Online 2022, 44, 923–933. [Google Scholar] [CrossRef]
- Mortlock, S.; Corona, R.I.; Kho, P.F.; Pharoah, P.; Seo, J.H.; Freedman, M.L.; Gayther, S.A.; Siedhoff, M.T.; Rogers, P.A.W.; Leuchter, R.; et al. A Multi-Level Investigation of the Genetic Relationship between Endometriosis and Ovarian Cancer Histotypes. Cell Rep. Med. 2022, 3, 100542. [Google Scholar] [CrossRef]
- Bhat, M.A.; Sharma, J.B.; Roy, K.K.; Sengupta, J.; Ghosh, D. Genomic Evidence of y Chromosome Microchimerism in the Endometrium during Endometriosis and in Cases of Infertility. Reprod. Biol. Endocrinol. 2019, 17, 22. [Google Scholar] [CrossRef]
- Takei, Y.; Ishikawa, S.; Tokino, T.; Muto, T.; Nakamura, Y. Isolation of a Novel TP53 Target Gene from a Colon Cancer Cell Line Carrying a Highly Regulated Wild-Type TP53 Expression System. Genes Chromosom. Cancer 1998, 23, 1–9. [Google Scholar] [CrossRef]
- Almaani, S.; Prokopec, S.D.; Zhang, J.; Yu, L.; Avila-Casado, C.; Wither, J.; Scholey, J.W.; Alberton, V.; Malvar, A.; Parikh, S.V.; et al. Rethinking Lupus Nephritis Classification on a Molecular Level. J. Clin. Med. 2019, 8, 1524. [Google Scholar] [CrossRef]
- Slief, M.; Levin, J.; Macwana, S.; DeJager, W.; Bourn, R.; Nath, S.; Munroe, M.; Aberle, T.; Gaffney, P.; Merrill, J.; et al. Genetic Associations and Polygenic Risk Assessment in Incomplete Lupus Erythematosus [Abstract]. Arthritis Rheumatol. 2020, 72 (Suppl. 10). [Google Scholar]
- Zeng, H.; Xie, Z.; Liao, C.; Hu, H.; Wang, Z.; Ye, Z. Comprehensive Analysis of Urinary lncRNA and mRNA Expression Profiles In Patients with lupus Nephritis. Res. Sq. 2021, 1–23. [Google Scholar]
- Chen, Z.; Zhang, T.; Mao, K.; Shao, X.; Xu, Y.; Zhu, M.; Zhou, H.; Wang, Q.; Li, Z.; Xie, Y.Y.; et al. A Single-Cell Survey of the Human Glomerulonephritis. J. Cell. Mol. Med. 2021, 25, 4684–4695. [Google Scholar] [CrossRef]
- Li, H.; Sai, L.; Liu, Y.; Freel, C.I.; Wang, K.; Zhou, C.; Zheng, J.; Shu, Q.; Zhao, Y. Systemic Lupus Erythematosus Dysregulates the Expression of Long Noncoding RNAs in Placentas. Arthritis Res. Ther. 2022, 24, 142. [Google Scholar] [CrossRef]
- Kumagai, A.; Itakura, A.; Koya, D.; Kanasaki, K. AMP-Activated Protein (AMPK) in Pathophysiology of Pregnancy Complications. Int. J. Mol. Sci. 2018, 19, 3076. [Google Scholar] [CrossRef]
- Sarkar, M.; Grab, J.; Dodge, J.L.; Gunderson, E.P.; Rubin, J.; Irani, R.A.; Cedars, M.; Terrault, N. Non-Alcoholic Fatty Liver Disease in Pregnancy Is Associated with Adverse Maternal and Perinatal Outcomes. J. Hepatol. 2020, 73, 516–522. [Google Scholar] [CrossRef] [PubMed]
- Herath, R.P.; Siriwardana, S.R.; Ekanayake, C.D.; Abeysekara, V.; Kodithuwakku, S.U.A.; Herath, H.P. Non-Alcoholic Fatty Liver Disease and Pregnancy Complications among Sri Lankan Women: A Cross Sectional Analytical Study. PLoS ONE 2019, 14, e0215326. [Google Scholar] [CrossRef] [PubMed]
- Song, W.; Qiu, J.; Yin, L.; Hong, X.; Dai, W.; Tang, D.; Liu, D.; Dai, Y. Integrated Analysis of Competing Endogenous RNA Networks in Peripheral Blood Mononuclear Cells of Systemic Lupus Erythematosus. J. Transl. Med. 2021, 19, 362. [Google Scholar] [CrossRef]
- Gao, Z.Y.; Su, L.C.; Wu, Q.C.; Sheng, J.E.; Wang, Y.L.; Dai, Y.F.; Chen, A.P.; He, S.S.; Huang, X.; Yan, G.Q. Bioinformatics Analyses of Gene Expression Profile Identify Key Genes and Functional Pathways Involved in Cutaneous Lupus Erythematosus. Clin. Rheumatol. 2022, 41, 437–452. [Google Scholar] [CrossRef]
- Bienertova-Vasku, J.; Bienert, P.; Dostalova, Z.; Chovanec, J.; Vasku, A.; Vasku, V. A Common Variation in the Cannabinoid 1 Receptor (CNR1) Gene Is Associated with Pre-Eclampsia in the Central European Population. Eur. J. Obstet. Gynecol. Reprod. Biol. 2011, 155, 19–22. [Google Scholar] [CrossRef] [PubMed]
- White, W.M.; Brost, B.; Sun, Z.; Rose, C.; Craici, I.; Wagner, S.J.; Turner, S.T.; Garovic, V.D. Genome-Wide Methylation Profiling Demonstrates Hypermethylation in Maternal Leukocyte DNA in Preeclamptic Compared to Normotensive Pregnancies. Hypertens. Pregnancy 2013, 32, 257–269. [Google Scholar] [CrossRef]
- Saeed, M. Novel Linkage Disequilibrium Clustering Algorithm Identifies New Lupus Genes on Meta-Analysis of GWAS Datasets. Immunogenetics 2017, 69, 295–302. [Google Scholar] [CrossRef]
- Yoo, J.Y.; Hyeon, D.Y.; Shin, Y.; Kim, S.M.; You, Y.A.; Kim, D.; Hwang, D.; Kim, Y.J. Integrative Analysis of Transcriptomic Data for Identification of T-Cell Activation-Related MRNA Signatures Indicative of Preterm Birth. Sci. Rep. 2021, 11, 2392. [Google Scholar] [CrossRef]
- Wang, S.; Xin, X.; Luo, W.; Mo, M.; Si, S.; Shao, B.; Shen, Y.; Cheng, H.; Yu, Y. Association of Vitamin D and Gene Variants in the Vitamin D Metabolic Pathway with Preterm Birth. Nutrition 2021, 89, 111349. [Google Scholar] [CrossRef]
- Bodamer, O.A.; Mitterer, G.; Maurer, W.; Pollak, A.; Mueller, M.W.; Schmidt, W.M. Evidence for an Association between Mannose-Binding Lectin 2 (MBL2) Gene Polymorphisms and Pre-Term Birth. Genet. Med. 2006, 8, 518–524. [Google Scholar] [CrossRef]
- Kim, M.-A.; Lee, E.-J.; Yang, W.; Shin, H.-Y.; Kim, Y.-H.; Kim, J.-H. Identification of a Novel Gene Signature in Second-Trimester Amniotic Fluid for the Prediction of Preterm Birth. Sci. Rep. 2022, 12, 3085. [Google Scholar] [CrossRef]
- Christiansen, O.B.; Nielsen, H.S.; Lund, M.; Steffensen, R.; Varming, K. Mannose-Binding Lectin-2 Genotypes and Recurrent Late Pregnancy Losses. Hum. Reprod. 2009, 24, 291–299. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, Y.; Moore, R.; Hess, H.A.; Guo, G.L.; Gonzalez, F.J.; Korach, K.S.; Maronpot, R.R.; Negishi, M. Estrogen Receptor α Mediates 17α-Ethynylestradiol Causing Hepatotoxicity. J. Biol. Chem. 2006, 281, 16625–16631. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.L.; Wang, Y.G.; Gao, G.M.; Feng, L.; Guo, N.; Zhang, C.X. Overexpression of LncRNA TCL6 Promotes Preeclampsia Progression by Regulating PTEN. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 4066–4072. [Google Scholar] [PubMed]
- Liu, L.P.; Gong, Y.B. LncRNA-TCL6 Promotes Early Abortion and Inhibits Placenta Implantation via the EGFR Pathway. Eur. Rev. Med. Pharmacol. Sci. 2018, 22, 7105–7112. [Google Scholar] [CrossRef] [PubMed]
- Imaizumi, T.; Hayakari, R.; Watanabe, S.; Aizawa, T.; Matsumiya, T.; Yoshida, H.; Tsuruga, K.; Kawaguchi, S.; Tanaka, H. Cylindromatosis (CYLD), a Deubiquitinase, Attenuates Inflammatory Signaling Pathways by Activating Toll-like Receptor 3 in Human Mesangial Cells. Kidney Blood Press. Res. 2018, 42, 942–950. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Z.C.; Xu, W.D.; Lan, Y.Y.; Wang, J.M.; Wu, Q.; Zhou, J.; Huang, A.F. Association of MBL2 Gene Polymorphisms and Systemic Lupus Erythematosus Susceptibility: A Meta-Analysis. Int. J. Rheum. Dis. 2021, 24, 147–158. [Google Scholar] [CrossRef]
- Sandrin-Garcia, P.; Brandão, L.A.C.; Coelho, A.V.C.; Guimarães, R.L.; Pancoto, J.A.T.; Segat, L.; Donadi, E.A.; de Lima-Filho, J.L.; Crovella, S. Mannose Binding Lectin Gene (MBL2) Functional Polymorphisms Are Associated with Systemic Lupus Erythematosus in Southern Brazilians. Hum. Immunol. 2011, 72, 516–521. [Google Scholar] [CrossRef] [PubMed]
- Monticielo, O.A.; Mucenic, T.; Xavier, R.M.; Brenol, J.C.T.; Chies, J.A.B. The Role of Mannose-Binding Lectin in Systemic Lupus Erythematosus. Clin. Rheumatol. 2008, 27, 413–419. [Google Scholar] [CrossRef] [PubMed]
- Baranwal, S.; Wang, Y.; Rathinam, R.; Lee, J.; Jin, L.; McGoey, R.; Pylayeva, Y.; Giancotti, F.; Blobe, G.C.; Alahari, S.K. Molecular Characterization of the Tumor-Suppressive Function of Nischarin in Breast Cancer. J. Natl. Cancer Inst. 2011, 103, 1513–1528. [Google Scholar] [CrossRef]
- Zhang, Q.; Wang, W.; Gao, Q. β-TRCP-Mediated AEBP2 Ubiquitination and Destruction Controls Cisplatin Resistance in Ovarian Cancer. Biochem. Biophys. Res. Commun. 2020, 523, 274–279. [Google Scholar] [CrossRef] [PubMed]
- Mougeot, J.-L.; Noll, B.; Bahrani Mougeot, F. Sjögren’s Syndrome X-Chromosome Dose Effect: An Epigenetic Perspective. Oral Dis. 2019, 25, 372–384. [Google Scholar] [CrossRef] [PubMed]
- Lone, B.A.; Ahmad, F.; Karna, S.K.L.; Pokharel, Y.R. SUPT5H Post-Transcriptional Silencing Modulates PIN1 Expression, Inhibits Tumorigenicity, and Induces Apoptosis of Human Breast Cancer Cells. Cell. Physiol. Biochem. 2020, 54, 928–946. [Google Scholar] [CrossRef] [PubMed]
- Braga, A.C.S.; Carneiro, B.M.; Batista, M.N.; Akinaga, M.M.; Bittar, C.; Rahal, P. Heat Shock Proteins HSPB8 and DNAJC5B Have HCV Antiviral Activity. PLoS ONE 2017, 12, e0188467. [Google Scholar] [CrossRef]
- Hwang, J.Y.; Oh, J.N.; Park, C.H.; Lee, D.K.; Lee, C.K. Dosage Compensation of X-Chromosome Inactivation Center-Linked Genes in Porcine Preimplantation Embryos: Non-Chromosome-Wide Initiation of X-Chromosome Inactivation in Blastocysts. Mech. Dev. 2015, 138, 246–255. [Google Scholar] [CrossRef]
- Hua, Y.; Wang, J.; Yuan, D.L.; Qi, Y.; Tang, Z.; Zhu, X.; Jiang, S.W. A Tag SNP in Syncytin-2 3-UTR Significantly Correlates with the Risk of Severe Preeclampsia. Clin. Chim. Acta 2018, 483, 265–270. [Google Scholar] [CrossRef]
- Yates, A.D.; Achuthan, P.; Akanni, W.; Allen, J.; Allen, J.; Alvarez-Jarreta, J.; Amode, M.R.; Armean, I.M.; Azov, A.G.; Bennett, R.; et al. Ensembl 2020. Nucleic Acids Res. 2019, 48, D682–D688. [Google Scholar] [CrossRef]
- Stelzer, G.; Rosen, N.; Plaschkes, I.; Zimmerman, S.; Twik, M.; Fishilevich, S.; Iny Stein, T.; Nudel, R.; Lieder, I.; Mazor, Y.; et al. The GeneCards Suite: From Gene Data Mining to Disease Genome Sequence Analyses. Curr. Protoc. Bioinforma. 2016, 2016, 1.30.1–1.30.33. [Google Scholar] [CrossRef]
- Jenjaroenpun, P.; Wongsurawat, T.; Yenamandra, S.P.; Kuznetsov, V.A. QmRLFS-Finder: A Model, Web Server and Stand-Alone Tool for Prediction and Analysis of R-Loop Forming Sequences: Table 1. Nucleic Acids Res. 2015, 43, W527–W534. [Google Scholar] [CrossRef]
- Lang, B.; Armaos, A.; Tartaglia, G.G. RNAct: Protein–RNA Interaction Predictions for Model Organisms with Supporting Experimental Data. Nucleic Acids Res. 2019, 47, D601–D606. [Google Scholar] [CrossRef]
- Bateman, A.; Martin, M.J.; Orchard, S.; Magrane, M.; Agivetova, R.; Ahmad, S.; Alpi, E.; Bowler-Barnett, E.H.; Britto, R.; Bursteinas, B.; et al. UniProt: The Universal Protein Knowledgebase in 2021. Nucleic Acids Res. 2021, 49, D480–D489. [Google Scholar] [CrossRef]

| Transcript | LncRNA Gene Hit | Type of Regulatory Region | Adjacent Coding Genes | Coded Protein Function | Associated Disease |
|---|---|---|---|---|---|
| ENST00000653218.1 ENST00000671206.1 ENST00000355189.7 ENST00000656413.1 ENST00000671206.1 | MIR548XHG | promoter | TMPRSS15 | conversion of the pancreatic proenzyme trypsinogen to trypsin | enteropeptidase deficiency; diarrhea; acute pancreatitis; Noonan syndrome 8 |
| ENST00000669402.1 | ENSG00000234426 | CTCF | CNR1 | cannabinoid receptor 1 | cannabis abuse; anxiety; chronic pain |
| RNGTT | bifunctional mRNA-capping | cecum cancer; photokeratitis | |||
| ENST00000605778.1 | HERPUD2-AS1 | promoter | HERPUD2 | endoplasmic reticulum unfolded protein response and spermatogenesis | / |
| SEPTIN7 | organization of the actin cytoskeleton, mitosis, cytokinesis and ciliogenesis | amyotrophic neuralgia; brachial plexopathy; extradural neoplasms; MARCH | |||
| ENST00000685812.2 | ENSG00000289643 | enhancer | BEND4 | DNA-binding activity | retinitis pigmentosa; colon lymphoma |
| SHISA3 | modulation of both Wnt and FGF signaling pathways | / | |||
| ENST00000605778.1 | HERPUD2-AS1 | promoter + promoter flank | HERPUD2 SEPTIN7 | as above | as above |
| ENST00000453774.2 ENST00000671356.1 ENST00000665109.1 ENST00000664286.1 ENST00000621006.1 ENST00000658168.1 ENST00000603129.6 ENST00000671252.1 ENST00000627270.2 ENST00000626601.2 ENST00000616475.4 ENST00000626008.2 | LINC01320 | enhancer | / | / | |
| ENST00000664067.1 | ENSG00000286619 | promoter + promoter flank + CTCF + enhancer | PM20D1 | hydrolase and peptidase activity | transient arthritis; Norwegian scabies |
| ENST00000538329.1 | ENSG00000256084 | enhancer | GRIN2B | subunit of the NMDA receptor ion channel having agonist binding site for glutamate; | epileptic encephalopathy; intellectual developmental disorder; astigmatism |
| ATF7IP | regulation of transcription and chromatin formation | liver, testis, and uterus cancer; optic atrophy; alpha thalassemia-intellectual disability syndrome type 1 |
| Transcript | Gene | Regulatory Sequence | RNA-Binding Protein | Prediction Score |
|---|---|---|---|---|
| ENST00000413763.1 | ENSG00000226854 | no | NISCH | 33.39 |
| ENST00000436786.1 | LINC01239 | no | AEBP2 | 15.07 |
| ENST00000605778.1 | HERPUD2-AS1 | promoter | SUPT5H | 17.65 |
| ENST00000355189.7 | MIR548XHG | promoter | NISCH | 20.06 |
| ENST00000440150.5 | WARS2-AS1 | no | NISCH | 30.96 |
| ENST00000444536.1 | LINC00395 | no | NISCH | 26.5 |
| ENST00000653218.1 | MIR548XHG | promoter | NISCH | 20.06 |
| ENST00000566193.1 | ENSG00000260197 | no | AEBP2 | 11.71 |
| ENST00000621006.1 | LINC01320 | enhancer | AEBP2 | 27.43 |
| ENST00000627270.2 | LINC01320 | enhancer | AEBP2 | 23.97 |
| ENST00000626601.2 | LINC01320 | enhancer | AEBP2 | 22.97 |
| ENST00000616475.4 | LINC01320 | enhancer | AEBP2 | 25.47 |
| ENST00000626008.2 | LINC01320 | enhancer | AEBP2 | 21.37 |
| ENST00000429137.1 | ENSG00000234426 | no | NISCH | 16.79 |
| ENST00000628407.2 | LINC01320 | no | AEBP2 | 19.44 |
| ENST00000625995.2 | LINC01320 | no | NISCH | 23.28 |
| ENST00000433664.1 | LINC00383 | no | AEBP2 | 30.41 |
| ENST00000413763.1 | ENSG00000226854 | no | NISCH | 33.39 |
| ENST00000444770.1 | ENSG00000228566 | no | CHIC1 | 18.28 |
| ENST00000605778.1 | HERPUD2-AS1 | promoter + promoter flank | SUPT5H | 17.65 |
| ENST00000444536.1 | LINC00395 | no | NISCH | 26.5 |
| ENST00000623391.1 | ENSG00000280341 | no | NISCH | 15.93 |
| ENST00000623391.1 | ENSG00000280341 | no | NISCH | 15.93 |
| ENST00000538329.1 | ENSG00000256084 | enhancer | AEBP2 | 22.08 |
| ENST00000440150.5 | WARS2-AS1 | no | NISCH | 30.96 |
| ENST00000621006.1 | LINC01320 | no | AEBP2 | 27.43 |
| ENST00000436786.1 | LINC01239 | no | AEBP2 | 15.07 |
| Transcript | LncRNA Gene Hit | Type of Regulatory Region | Adjacent Coding Genes | Coded Protein Function | Associated Disease |
|---|---|---|---|---|---|
| ENST00000471537.3 | ENSG00000273328 | enhancer | CYP8B1 | cytochrome P450 monooxygenase involved in lipid metabolism and bile acid biosynthesis; | intrahepatic cholestasis of pregnancy; extrahepatic cholestasis; cerebrotindinous xanthomatosis |
| ZNF662 | transcription regulatory activity | / | |||
| ENST00000586185.2 | SCAT1 | enhancer | DNAH17 | sperm motility | spermatogenic failure; infertility; pontocerebellar hypoplasia |
| CYTH1 | membrane trafficking, junctional remodeling, and epithelial polarization through regulation of ARF6 activity | IDDSSBA; ankylosing spondylitis; psoriasis | |||
| ENST00000629723.2 | EIF1B-AS1 | enhancer | EIF1B | translation and protein biosynthesis | uveal melanoma; choroid cancer; transposition of the great arteries |
| ENST00000538640.2 | ENSG00000256504 | enhancer | REP15 | regulation of transferrin receptor recycling from the endocytic recycling compartment | Eiken syndrome; pancreatic adenocarcinoma |
| MRPS35 | mitochondrial ribosomal protein involved in mitochondrial translation and metabolism of proteins | / | |||
| ENST00000657102.1 | LINC02128 | enhancer | CYLD | regulation of inflammation and innate immune response through the control of NF-kB activation | Brooke–Spiegler syndrome; frontotemporal dementia; amyotrophic lateral sclerosis; trichoepithelioma; cylindromatosis; spiradenoma |
| ENST00000507156.1 | ENSG00000248567 | enhancer | GC | vitamin-D-binding protein | rickets; osteomalacia; hepatic encephalopathy; blastomycosis; osteoporosis |
| NPFFR2 | neuropeptide receptor interacting with morphine-modulating peptides | postsurgical hypothyroidism; nutmeg liver | |||
| ENST00000654808.1 ENST00000668157.1 | LINC02672 | enhancer | MBL2 | mannose-binding protein C involved in innate immune defense | MBL and complement deficiency; vulvovaginal candidiasis; rheumatic fever; cystic fibrosis |
| ENST00000688783.1 | PURPL | promoter | / | / | |
| ENST00000610630.1 | ENSG00000275409 | enhancer | SUDS3 | repression of transcription | / |
| ENST00000656094.1 | LINC00964 | promoter + CTCF | ZNF572 | transcriptional regulation | posterior myocardial infarction |
| ENST00000657673.1 ENST00000659262.1 ENST00000654139.1 ENST00000666470.1 ENST00000660299.1 ENST00000654721.1 ENST00000656453.1 ENST00000667101.1 ENST00000671217.1 ENST00000663502.1 | LINC02318 | enhancer | GLRX5 | mitochondrial iron–sulfur cluster transfer | sideroblastic anemia; childhood-onset spasticity with hyperglycinemia |
| TCL6 | modulation of the EGFR/AKT pathway at least in placental tissue | lymphoma; leukemia; renal cell carcinoma | |||
| ENST00000661539.1 ENST00000654770.1 ENST00000657454.1 ENST00000661856.1 ENST00000519241.6 ENST00000517611.1 ENST00000519160.5 ENST00000521132.1 ENST00000520929.1 ENST00000655105.1 | LINC01606 | CTCF | BPNT2 | Golgi-resident adenosine 3’,5’-bisphosphate 3’-phosphatase with 3’-nucleotidase activity | chondrodysplasia with joint dislocations; ring dermoid of the cornea |
| Transcript | Gene | Regulatory Sequence | RNA-Binding Protein | Prediction Score |
| ENST00000471537.3 | ENSG00000273328 | no | CHIC1 | 11.96 |
| ENST00000471537.3 | ENSG00000273328 | no | CHIC1 | 11.96 |
| ENST00000496604.5 | ENSG00000273328 | no | NISCH | 18.28 |
| ENST00000471537.3 | ENSG00000273328 | no | CHIC1 | 11.96 |
| ENST00000444770.1 | ENSG00000228566 | no | CHIC1 | 18.28 |
| ENST00000471537.3 | ENSG00000273328 | enhancer | CHIC1 | 11.96 |
| ENST00000426240.5 | LINC02263 | no | AEBP2 | 33 |
| ENST00000522213.5 | ENSG00000254367 | no | NISCH | 20.7 |
| ENST00000626008.2 | ENSG00000256504 | enhancer | AEBP2 | 16.19 |
| ENST00000438428.1 | LINC01732 | no | DNAJC5B | 16.73 |
| ENST00000435023.1 | LINC01732 | no | NISCH | 18.9 |
| ENST00000562167.1 | ENSG00000261400 | no | NISCH | 14.36 |
| ENST00000507156.1 | ENSG00000248567 | enhancer | CHIC1 | 24.39 |
| ENST00000456446.1 | ENSG00000226681 | no | AEBP2 | 21.7 |
| ENST00000556346.1 | LINC02318 | no | NISCH | 19.51 |
| ENST00000610630.1 | ENSG00000275409 | enhancer | AEBP2 | 27.62 |
| ENST00000562167.1 | ENSG00000261400 | no | NISCH | 14.36 |
| ENST00000423197.2 | LINC01777 | no | NISCH | 26.26 |
| ENST00000635002.1 | LINC01777 | no | NISCH | 33.73 |
| ENST00000306533.8 | ENSG00000255689 | no | AEBP2 | 9.82 |
| ENST00000517611.1 | LINC01606 | CTCF | NISCH | 15.33 |
| ENST00000519160.5 | LINC01606 | CTCF | AEBP2 | 19.36 |
| ENST00000521132.1 | LINC01606 | CTCF | NISCH | 16.57 |
| ENST00000520929.1 | LINC01606 | CTCF | NISCH | 23.5 |
| Syncytin-1 | Aligned lncRNA Gene | Potential Contribution to SLE Pathogenesis | Potential Contribution to Pregnancy Complications |
| TP53TG1 | Hypo-expressed in glomerulosclerosis kidney samples according to a molecular signature study of 51 patients with lupus nephritis [55] | Demethylated in female cadmium-exposed placenta according to a genome-wide DNA methylation study of placental tissue from 24 women [45] | |
| XACT | Unknown | Hyper-expressed in both human preimplantation embryos and naive human embryonic stem cells; competes with XIST and prevents X chromosome silencing and functional nullisomy during early human development according to a single-cell RNA-sequencing analysis of more than 100 human embryos [49]; XACT loss of heterozygosity potentially affecting the inactivation of the skewed X chromosome and leading to X chromosome instability in human embryonic stem cells revealed by a high-resolution chromosome microarray analysis of 105 human embryos and derived human embryonic stem cells [50] | |
| MIR548XHG | Unknown | Overexpressed in plasma extracellular vesicles from women with endometriosis according to an RNA-sequencing study of 85 patients and 86 controls [51] | |
| LINC01239 | Associated with incomplete lupus erythematosus according to a GWAS of 335 patients and 236 controls [56]; Upregulated in morning urine samples from 3 LN patients compared to 3 healthy controls [57] | Dysregulated in patients with epithelial ovarian cancer and endometriosis according to a ChIP-sequencing and ATAC-sequencing analysis of a large cohort of endometriosis and epithelial ovarian cancer patients [52] | |
| TTTY14 | Unknown | Hyper-expressed in endometrial samples from infertile women as a phenomenon of male microchimerism according to a transcriptomic profiling study of 60 fertile and infertile participants without endometriosis and 60 fertile and infertile participants with endometriosis [53] | |
| LINC01320 | Associated with the inflammatory proximal tubule histologic subtype observed in kidney samples from patients with glomerulonephritis, including one case of LN, according to a single-cell RNA-sequencing study [58] | Upregulated in the endometrium during the implantation window according to an RNA-sequencing analysis of 30 fertile women [48]; Dysregulated in syncytiotrophoblasts, invasive cytotrophoblasts, and endovascular cytotrophoblasts isolated from placental tissue of 4 women with severe preeclampsia and 4 women with uninfected preterm birth according to a global transcriptional profiling study [46] | |
| Syncytin-2 | XACT | Unknown | As above |
| LINC00320 | Unknown | Upregulated in spontaneous preterm placenta from 20 women compared to spontaneous term placenta from 20 control subjects according to a transcriptomic RNA-sequencing analysis [47] | |
| TP53TG1 | As above | As above |
| Syncytin-1 | Aligned lncRNA Gene | Adjacent Protein-coding Gene | Data Supporting an Association with SLE | Data Supporting an Association with Pregnancy Adverse Outcomes |
| ENSG00000234426 | CNR1 | Unknown | Increased risk of preeclampsia according to a case-control study genotyping 115 preeclamptic women and 145 healthy pregnant controls [65] | |
| ENSG00000256084 | GRIN2B | Included among the new SLE genes according to an OASIS analysis of 6077 subjects [67] | Hypermetilated in leukocytes of preeclamptic women compared with normotensive pregnant women according to a genome-wide methylation profiling study of 28 participants [66] | |
| Syncytin-2 | LINC02128 | CYLD | Overexpressed in kidney samples from 4 patients with class II and class IV LN according to ISN/RPS 2003 criteria [76] | Candidate predictor for preterm birth according to an mRNA-sequencing analysis of 88 Korean preterm births and 118 control subjects [68] |
| ENSG00000248567 | GC | Unknown | SNPs associated with risk of preterm birth according to a prospective cohort study genotyping 3465 pregnant women, of whom 202 were preterm [69] | |
| LINC02672 | MBL2 | Association of A/B and A/O polymorphisms with SLE susceptibility, and protective effect of allele H according to a meta-analysis of 7194 SLE patients and 7401 healthy controls [77]; Association between the MBL2 O allele and low MBL producer genotypes and increased SLE risk according to a genotyping study of 34 Brazilian SLE patients and 101 controls [78] | Higher frequency of codon 52 polymorphism in preterm birth cases compared with term controls and association of MBL2 O/O genotype with risk of preterm birth according to a genotyping study of 204 DNA blood samples [70]; Association between MBL2 genotypes leading to MBL deficiency and recurrent late pregnancy loss independent of LAC positivity according to a genotyping study of 75 patients and 104 controls [72] | |
| ENSG00000273328 | ZNF572 | Unknown | Upregulated in amniotic fluid supernatant samples from 21 preterm birth patients compared to term birth controls according to a sequencing and qPCR study [71] | |
| ENSG00000273328 | CYP8B1 | Unknown | Undergoes ERα-induced downregulation in mice, leading to impaired bile acid biosynthesis and potential risk of intrahepatic cholestasis in pregnancy [73] | |
| LINC02318 | TCL6 | Unknown | Overexpressed in 42 placental tissues from women with preeclampsia compared with controls and hypo-expressed in preeclamptic pregnancies with lower urine protein levels, normal blood pressure, and higher newborn weight according to a qRT-PCR study [74];Overexpressed in placental tissue in threatened abortion pregnancy compared with normal pregnancy and in spontaneous abortion pregnancy compared with induced abortion pregnancy according to a qRT-PCR study of 30 women with spontaneous abortion, 30 women with induced abortion, and 30 control subjects with normal pregnancy [75] | |
| ENSG00000248567 | NPFFR2 | Unknown | Hyper-expressed in placental tissue during the first trimester and in placental samples from preeclamptic women and indirectly associated with the expression of syncytin-1 and syncytin-2 in human cytotrophoblast cells [36] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Talotta, R. Interaction between Long Noncoding RNAs and Syncytin-1/Syncytin-2 Genes and Transcripts: How Noncoding RNAs May Affect Pregnancy in Patients with Systemic Lupus Erythematosus. Int. J. Mol. Sci. 2023, 24, 2259. https://doi.org/10.3390/ijms24032259
Talotta R. Interaction between Long Noncoding RNAs and Syncytin-1/Syncytin-2 Genes and Transcripts: How Noncoding RNAs May Affect Pregnancy in Patients with Systemic Lupus Erythematosus. International Journal of Molecular Sciences. 2023; 24(3):2259. https://doi.org/10.3390/ijms24032259
Chicago/Turabian StyleTalotta, Rossella. 2023. "Interaction between Long Noncoding RNAs and Syncytin-1/Syncytin-2 Genes and Transcripts: How Noncoding RNAs May Affect Pregnancy in Patients with Systemic Lupus Erythematosus" International Journal of Molecular Sciences 24, no. 3: 2259. https://doi.org/10.3390/ijms24032259
APA StyleTalotta, R. (2023). Interaction between Long Noncoding RNAs and Syncytin-1/Syncytin-2 Genes and Transcripts: How Noncoding RNAs May Affect Pregnancy in Patients with Systemic Lupus Erythematosus. International Journal of Molecular Sciences, 24(3), 2259. https://doi.org/10.3390/ijms24032259






