The Orphan GPR50 Receptor Regulates the Aggressiveness of Breast Cancer Stem-like Cells via Targeting the NF-kB Signaling Pathway
Abstract
1. Introduction
2. Results
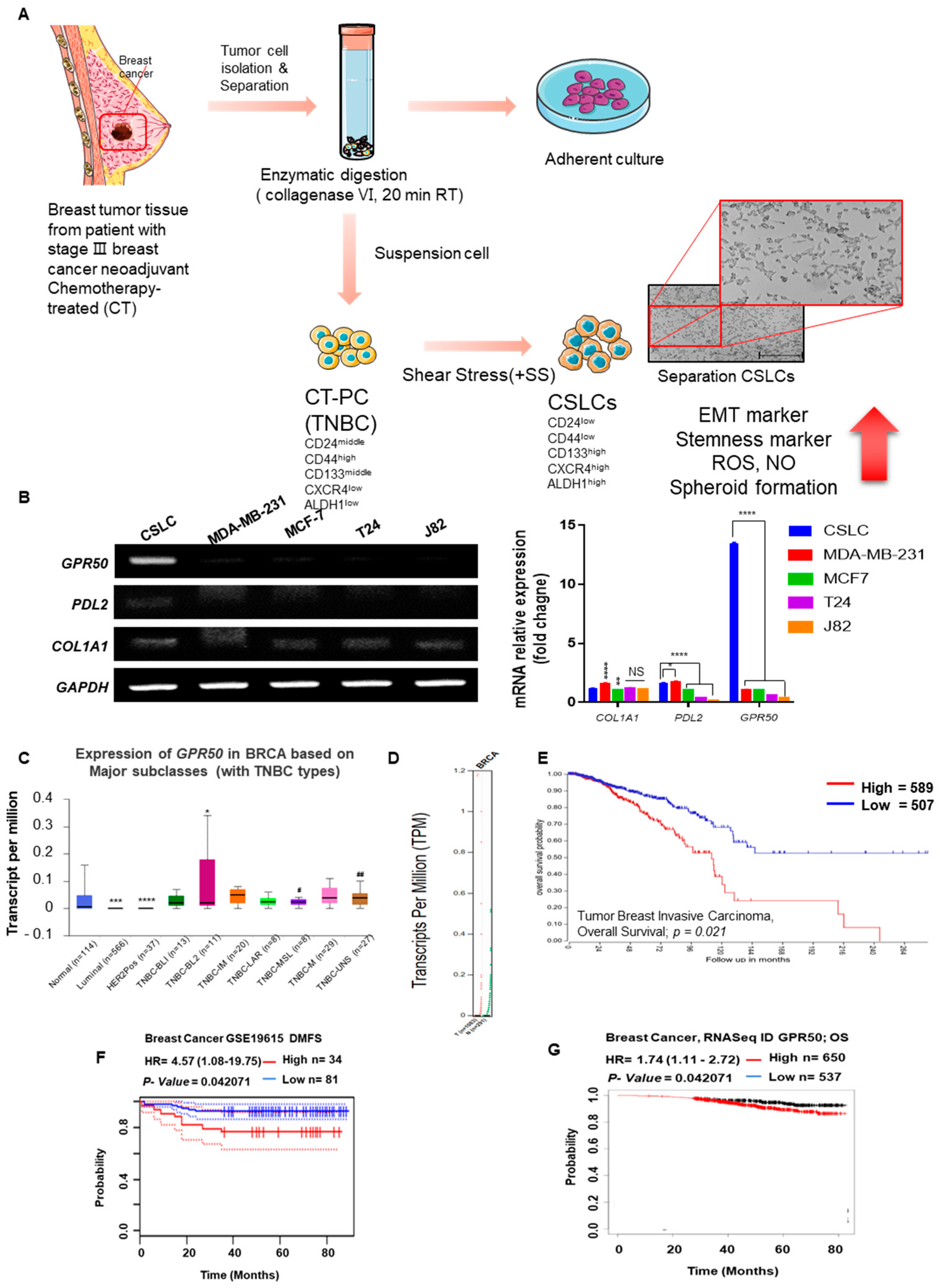
2.1. CSLC Conversion and Gene Expression Changes in Tissues of Breast Cancer Patients Who Received Chemotherapy
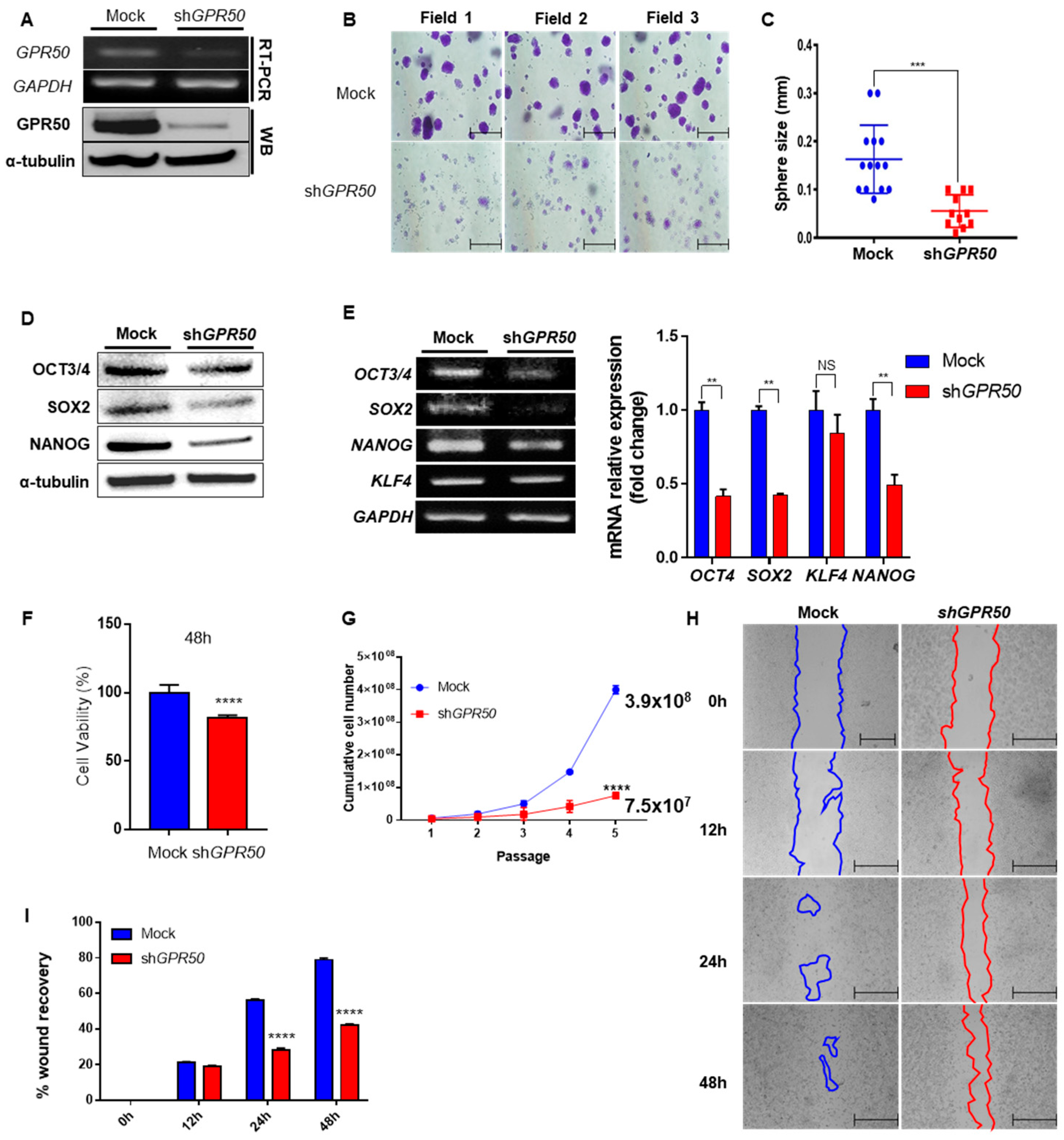
2.2. Change in Cancer Cell Aggressiveness According to the Expression Level of GPR50 in CSLC
2.3. GPR50 Regulates the Activity of NF-kB
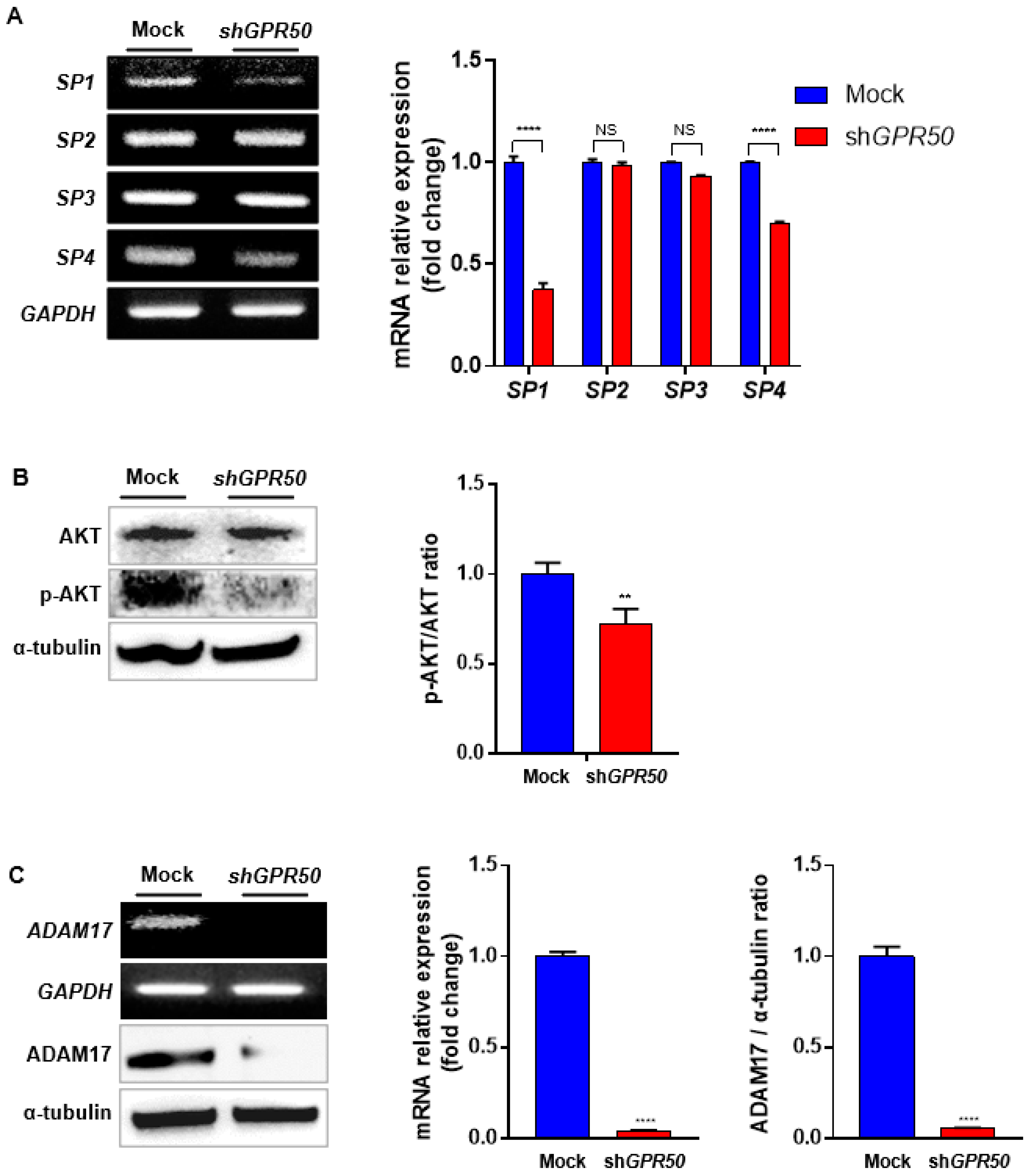
2.4. In CSLC, GPR50 Regulates the Expression of ADAM17 and, Consequently SP1/AKT Signaling
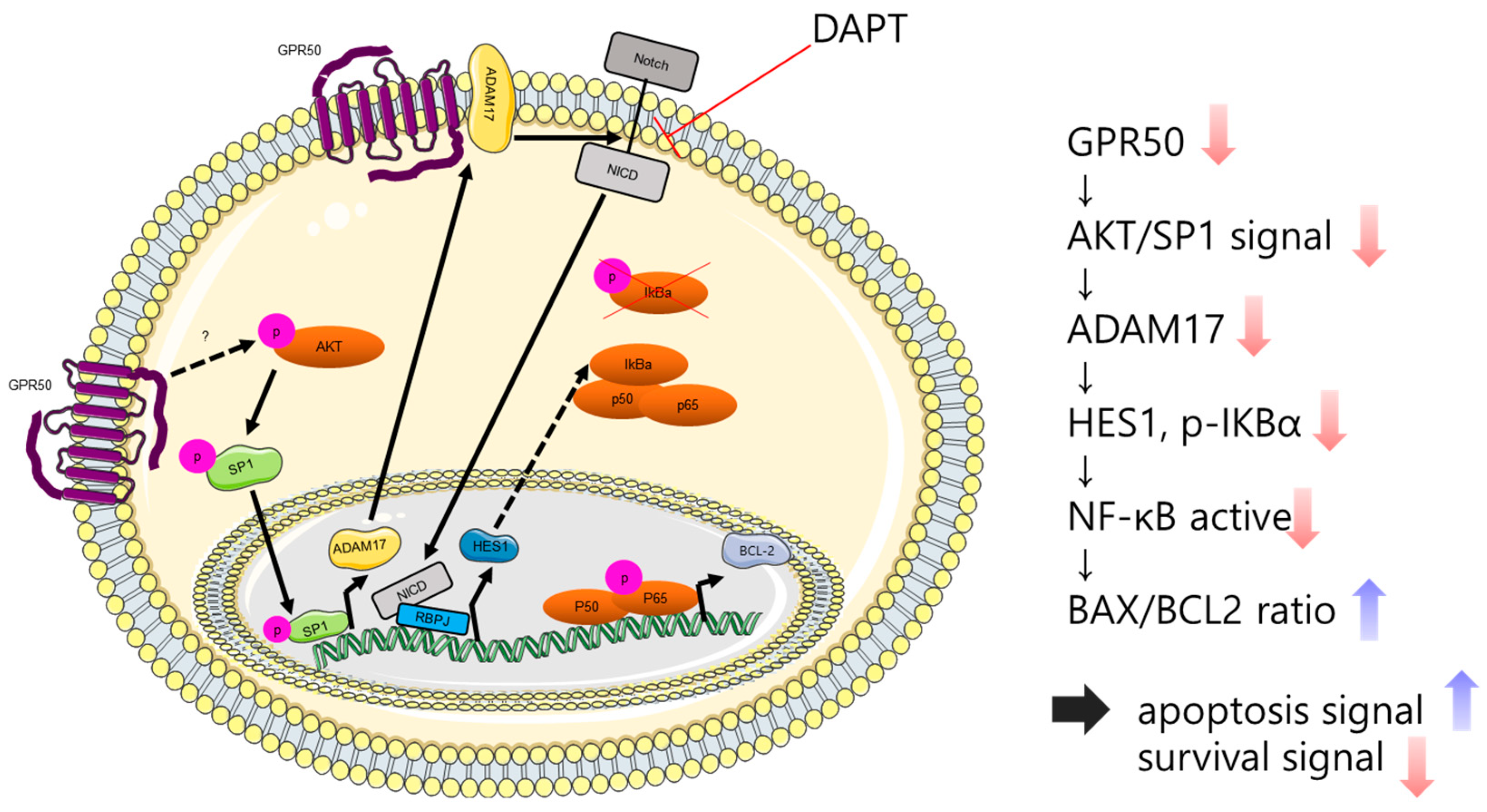
2.5. GPR50 Regulates the Expression of HES1 through the Notch Signaling Pathway in CSLCs
3. Discussion
4. Materials and Methods
4.1. Cell Culture
4.2. Total RNA Extraction and RT-PCR Analysis
4.3. Western Blotting Procedure
4.4. Cell Proliferation, Wound Healing/Migration Assays
4.5. Sphere-Formation Assay
4.6. Luciferase Reporter Assay
4.7. Analysis of GPR50 Protein Expression in Various Types of TNBC
4.8. GEPIA Database Analysis
4.9. Analysis of GPR50 Protein Expression in Various Types of TNBC
4.10. R2: Genomic Analysis and Visualization Platform
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Carol, D.; Rebecca, S.; Priti, B.; Ahmedin, J. Breast cancer statistics, 2013. CA Cancer J. Clin. 2014, 64, 52–62. [Google Scholar]
- Greaney, M.L.; Sprunck-Harrild, K.; Ruddy, K.J.; Ligibel, J.; Barry, W.T.; Baker, E.; Meyer, M.; Emmons, K.M.; Partridge, A.H. Study protocol for Young & Strong: A cluster randomized design to increase attention to unique issues faced by young women with newly diagnosed breast cancer. BMC Public Health 2015, 15, 37. [Google Scholar]
- Wang, N.; Weng, J.; Xia, J.; Zhu, Y.; Chen, Q.; Hu, D.; Zhang, X.; Sun, R.; Feng, J.; Minato, N. SIPA1 enhances SMAD2/3 expression to maintain stem cell features in breast cancer cells. Stem Cell Res. 2020, 49, 102099. [Google Scholar] [CrossRef]
- Clarke, M.F.; Dick, J.E.; Dirks, P.B.; Eaves, C.J.; Jamieson, C.H.; Jones, D.L.; Visvader, J.; Weissman, I.; Wahl, G. Cancer stem cells—Perspectives on current status and future directions: AACR Workshop on cancer stem cell. Cancer Res. 2006, 66, 2006–2009. [Google Scholar] [CrossRef] [PubMed]
- Grián-Lis6n, C.; Olivares-Urbano, M.; Jiménez, G. miRNAs asradio-response biomarkersfor breast cancerstem cels. Mol. Oncol. 2020, 14, 556–570. [Google Scholar] [CrossRef]
- Olivares-Urbano, M.A.; Griñán-Lisón, C.; Ríos-Arrabal, S.; Artacho-Cordón, F.; Torralbo, A.I.; López-Ruiz, E.; Marchal, J.A.; Núñez, M.I. Radiation and stemness phenotype may influence individual breast cancer outcomes: The crucial role of mmps and microenvironment. Cancers 2019, 11, 1781. [Google Scholar] [CrossRef]
- Lundstrom, K.; Wagner, R.; Reinhart, C.; Desmyter, A.; Cherouati, N.; Magnin, T.; Zeder-Lutz, G.; Courtot, M.; Prual, C.; André, N. Structural genomics on membrane proteins: Comparison of more than 100 GPCRs in 3 expression systems. J. Struct. Funct. Genom. 2006, 7, 77–91. [Google Scholar] [CrossRef]
- Violin, J.D.; Crombie, A.L.; Soergel, D.G.; Lark, M.W. Biased ligands at G-protein-coupled receptors: Promise and progress. Trends Pharmacol. Sci. 2014, 35, 308–316. [Google Scholar] [CrossRef]
- Wojciech, S.; Ahmad, R.; Belaid-Choucair, Z.; Journé, A.-S.; Gallet, S.; Dam, J.; Daulat, A.; Ndiaye-Lobry, D.; Lahuna, O.; Karamitri, A. The orphan GPR50 receptor promotes constitutive TGFβ receptor signaling and protects against cancer development. Nat. Commun. 2018, 9, 1216. [Google Scholar] [CrossRef]
- Huse, M.; Muir, T.W.; Xu, L.; Chen, Y.-G.; Kuriyan, J.; Massagué, J. The TGFβ receptor activation process: An inhibitor-to substrate-binding switch. Mol. Cell 2001, 8, 671–682. [Google Scholar] [CrossRef]
- Wrana, J.L.; Attisano, L.; Wieser, R.; Ventura, F.; Massagué, J. Mechanism of activation of the TGF-β receptor. Nature 1994, 370, 341–347. [Google Scholar] [CrossRef] [PubMed]
- Franzen, P.; ten Dijke, P.; Ichijo, H.; Yamashita, H.; Schulz, P.; Heldin, C.-H.; Miyazono, K. Cloning of a TGFβ type I receptor that forms a heteromeric complex with the TGFβ type II receptor. Cell 1993, 75, 681–692. [Google Scholar] [CrossRef] [PubMed]
- Fraser, C.C. G Protein–Coupled Receptor Connectivity to NF-κB in Inflammation and Cancer. Int. Rev. Immunol. 2008, 27, 320–350. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.Y.; Saha, S.K.; Kim, K.; Kim, S.; Yang, G.-M.; Kim, B.; Kim, J.-h.; Cho, S.-G. G protein-coupled receptors in stem cell maintenance and somatic reprogramming to pluripotent or cancer stem cells. BMB Rep. 2015, 48, 68. [Google Scholar] [CrossRef] [PubMed]
- Saha, S.K.; Choi, H.Y.; Yang, G.-M.; Biswas, P.K.; Kim, K.; Kang, G.-H.; Gil, M.; Cho, S.-G. GPR50 promotes hepatocellular carcinoma progression via the notch signaling pathway through direct interaction with ADAM17. Mol. Ther.Oncolytics 2020, 17, 332–349. [Google Scholar] [CrossRef]
- Choi, H.Y.; Yang, G.-M.; Dayem, A.A.; Saha, S.K.; Kim, K.; Yoo, Y.; Hong, K.; Kim, J.-H.; Yee, C.; Lee, K.-M. Hydrodynamic shear stress promotes epithelial-mesenchymal transition by downregulating ERK and GSK3β activities. Breast Cancer Res. 2019, 21, 6. [Google Scholar] [CrossRef]
- Ma, J.; Cao, T.; Cui, Y.; Zhang, F.; Shi, Y.; Xia, J.; Wang, Z.P. miR-223 regulates cell proliferation and invasion via targeting PDS5B in pancreatic cancer cells. Mol. Ther. Nucleic Acids 2019, 14, 583–592. [Google Scholar] [CrossRef]
- Chiba, T.; Suzuki, E.; Yuki, K.; Zen, Y.; Oshima, M.; Miyagi, S.; Saraya, A.; Koide, S.; Motoyama, T.; Ogasawara, S.; et al. Disulfiram eradicates tumor-initiating hepatocellular carcinoma cells in ROS-p38 MAPK pathway-dependent and -independent manners. PLoS ONE 2014, 9, e84807. [Google Scholar] [CrossRef]
- Jeong, M.H.; Kim, H.R.; Park, Y.J.; Chung, K.H. Akt and Notch pathways mediate polyhexamethylene guanidine phosphate-induced epithelial-mesenchymal transition via ZEB2. Toxicol. Appl. Pharmacol. 2019, 380, 114691. [Google Scholar] [CrossRef]
- Verzella, D.; Pescatore, A.; Capece, D.; Vecchiotti, D.; Ursini, M.V.; Franzoso, G.; Alesse, E.; Zazzeroni, F. Life, death, and autophagy in cancer: NF-κB turns up everywhere. Cell Death Dis. 2020, 11, 210. [Google Scholar] [CrossRef]
- Xu, J.; Wu, F.; Tian, D.; Wang, J.; Zheng, Z.; Xia, N. Open reading frame 3 of genotype 1 hepatitis E virus inhibits nuclear factor-κappa B signaling induced by tumor necrosis factor-α in human A549 lung epithelial cells. PLoS ONE 2014, 9, e100787. [Google Scholar] [CrossRef] [PubMed]
- Sohur, U.S.; Dixit, M.N.; Chen, C.-L.; Byrom, M.W.; Kerr, L.D. Rel/NF-κΒ Represses bcl-2 Transcription in pro-B Lymphocytes. Gene Expr. J. Liver Res. 1999, 8, 219–229. [Google Scholar]
- Baek, N.; Seo, O.W.; Kim, M.; Hulme, J.; An, S.S.A. Monitoring the effects of doxorubicin on 3D-spheroid tumor cells in real-time. OncoTargets Ther. 2016, 9, 7207. [Google Scholar] [CrossRef] [PubMed]
- Szalad, A.; Katakowski, M.; Zheng, X.; Jiang, F.; Chopp, M. Transcription factor Sp1 induces ADAM17 and contributes to tumor cell invasiveness under hypoxia. J. Exp. Clin. Cancer Res. 2009, 28, 129. [Google Scholar] [CrossRef]
- Murthy, A.; Shao, Y.W.; Narala, S.R.; Molyneux, S.D.; Zúñiga-Pflücker, J.C.; Khokha, R. Notch activation by the metalloproteinase ADAM17 regulates myeloproliferation and atopic barrier immunity by suppressing epithelial cytokine synthesis. Immunity 2012, 36, 105–119. [Google Scholar] [CrossRef]
- Lehal, R.; Zaric, J.; Vigolo, M.; Urech, C.; Frismantas, V.; Zangger, N.; Cao, L.; Berger, A.; Chicote, I.; Loubéry, S. Pharmacological disruption of the Notch transcription factor complex. Proc. Natl. Acad. Sci. USA 2020, 117, 16292–16301. [Google Scholar] [CrossRef]
- Espinosa, L.; Cathelin, S.; D’Altri, T.; Trimarchi, T.; Statnikov, A.; Guiu, J.; Rodilla, V.; Inglés-Esteve, J.; Nomdedeu, J.; Bellosillo, B. The Notch/Hes1 pathway sustains NF-κB activation through CYLD repression in T cell leukemia. Cancer cell 2010, 18, 268–281. [Google Scholar] [CrossRef]
- Avci, N.G.; Ebrahimzadeh-Pustchi, S.; Akay, Y.M.; Esquenazi, Y.; Tandon, N.; Zhu, J.-J.; Akay, M. NF-κB inhibitor with Temozolomide results in significant apoptosis in glioblastoma via the NF-κB (p65) and actin cytoskeleton regulatory pathways. Sci. Rep. 2020, 10, 13352. [Google Scholar] [CrossRef]
- Levoye, A.; Dam, J.; Ayoub, M.A.; Guillaume, J.L.; Couturier, C.; Delagrange, P. The orphan GPR50 receptor specifically inhibits MT1 melatonin receptor function through heterodimerization. EMBO J. 2006, 25, 3012–3023. [Google Scholar] [CrossRef]
- Ahmad, R.; Lahuna, O.; Sidibe, A.; Daulat, A.; Zhang, Q.; Luka, M.; Guillaume, J.-L.; Gallet, S.; Guillonneau, F.; Hamroune, J. GPR50-Ctail cleavage and nuclear translocation: A new signal transduction mode for G protein-coupled receptors. Cell. Mol. Life Sci. 2020, 77, 5189–5205. [Google Scholar] [CrossRef]
- Kim, W.; Khan, S.K.; Gvozdenovic-Jeremic, J.; Kim, Y.; Dahlman, J.; Kim, H.; Park, O.; Ishitani, T.; Jho, E.-h.; Gao, B. Hippo signaling interactions with Wnt/β-catenin and Notch signaling repress liver tumorigenesis. J. Clin. Investig. 2017, 127, 137–152. [Google Scholar] [CrossRef] [PubMed]
- Dieter, S.M.; Ball, C.R.; Hoffmann, C.M.; Nowrouzi, A.; Herbst, F.; Zavidij, O.; Abel, U.; Arens, A.; Weichert, W.; Brand, K. Distinct types of tumor-initiating cells form human colon cancer tumors and metastases. Cell Stem Cell 2011, 9, 357–365. [Google Scholar] [CrossRef] [PubMed]
- Zhou, B.-B.S.; Zhang, H.; Damelin, M.; Geles, K.G.; Grindley, J.C.; Dirks, P.B. Tumour-initiating cells: Challenges and opportunities for anticancer drug discovery. Nat. Rev. Drug Discov. 2009, 8, 806–823. [Google Scholar] [CrossRef] [PubMed]
- Kaltschmidt, C.; Banz-Jansen, C.; Benhidjeb, T.; Beshay, M.; Förster, C.; Greiner, J.; Hamelmann, E.; Jorch, N.; Mertzlufft, F.; Pfitzenmaier, J. A role for NF-κB in organ specific cancer and cancer stem cells. Cancers 2019, 11, 655. [Google Scholar] [CrossRef] [PubMed]
- Urban, M.; Baeuerle, P. The role of the p50 and p65 subunits of NF-kappa B in the recognition of cognate sequences. New Biol. 1991, 3, 279–288. [Google Scholar]
- Wang, W.; Nag, S.A.; Zhang, R. Targeting the NFκB signaling pathways for breast cancer prevention and therapy. Curr. Med. Chem. 2015, 22, 264–289. [Google Scholar] [CrossRef]
- Saha, S.; Choi, H.; Kim, B.; Dayem, A.; Yang, G.; Kim, K.; Yin, Y.; Cho, S. KRT19 directly interacts with β-catenin/RAC1 complex to regulate NUMB-dependent NOTCH signaling pathway and breast cancer properties. Oncogene 2017, 36, 332–349. [Google Scholar] [CrossRef]
- Chen, S.; Yang, L.; Pan, A.; Duan, S.; Li, M.; Li, P.; Huang, J.; Gao, X.; Huang, X.; Lin, Y. Inhibitory Effect on the Hepatitis B Cells through the Regulation of miR-122-MAP3K2 signal pathway. An. Acad. Bras. Ciências 2019, 91, e20180941. [Google Scholar] [CrossRef]
- Shaw, F.L.; Harrison, H.; Spence, K.; Ablett, M.P.; Simões, B.M.; Farnie, G.; Clarke, R.B. A detailed mammosphere assay protocol for the quantification of breast stem cell activity. J. Mammary Gland. Biol. Neoplasia 2012, 17, 111–117. [Google Scholar] [CrossRef]
- Lee, J.M.; Kim, I.S.; Kim, H.; Lee, J.S.; Kim, K.; Yim, H.Y.; Jeong, J.; Kim, J.H.; Kim, J.-Y.; Lee, H. RORα attenuates Wnt/β-catenin signaling by PKCα-dependent phosphorylation in colon cancer. Mol. Cell 2010, 37, 183–195. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Biswas, P.K.; Park, S.R.; An, J.; Lim, K.M.; Dayem, A.A.; Song, K.; Choi, H.Y.; Choi, Y.; Park, K.S.; Shin, H.J.; et al. The Orphan GPR50 Receptor Regulates the Aggressiveness of Breast Cancer Stem-like Cells via Targeting the NF-kB Signaling Pathway. Int. J. Mol. Sci. 2023, 24, 2804. https://doi.org/10.3390/ijms24032804
Biswas PK, Park SR, An J, Lim KM, Dayem AA, Song K, Choi HY, Choi Y, Park KS, Shin HJ, et al. The Orphan GPR50 Receptor Regulates the Aggressiveness of Breast Cancer Stem-like Cells via Targeting the NF-kB Signaling Pathway. International Journal of Molecular Sciences. 2023; 24(3):2804. https://doi.org/10.3390/ijms24032804
Chicago/Turabian StyleBiswas, Polash Kumar, Sang Rok Park, Jongyub An, Kyung Min Lim, Ahmed Abdal Dayem, Kwonwoo Song, Hye Yeon Choi, Yujin Choi, Kyoung Sik Park, Hyun Jin Shin, and et al. 2023. "The Orphan GPR50 Receptor Regulates the Aggressiveness of Breast Cancer Stem-like Cells via Targeting the NF-kB Signaling Pathway" International Journal of Molecular Sciences 24, no. 3: 2804. https://doi.org/10.3390/ijms24032804
APA StyleBiswas, P. K., Park, S. R., An, J., Lim, K. M., Dayem, A. A., Song, K., Choi, H. Y., Choi, Y., Park, K. S., Shin, H. J., Kim, A., Gil, M., Saha, S. K., & Cho, S.-G. (2023). The Orphan GPR50 Receptor Regulates the Aggressiveness of Breast Cancer Stem-like Cells via Targeting the NF-kB Signaling Pathway. International Journal of Molecular Sciences, 24(3), 2804. https://doi.org/10.3390/ijms24032804









