Evidence for Endogenous Opioid Dependence Related to Latent Sensitization in a Rat Model of Chronic Inflammatory Pain
Abstract
:1. Introduction
2. Results
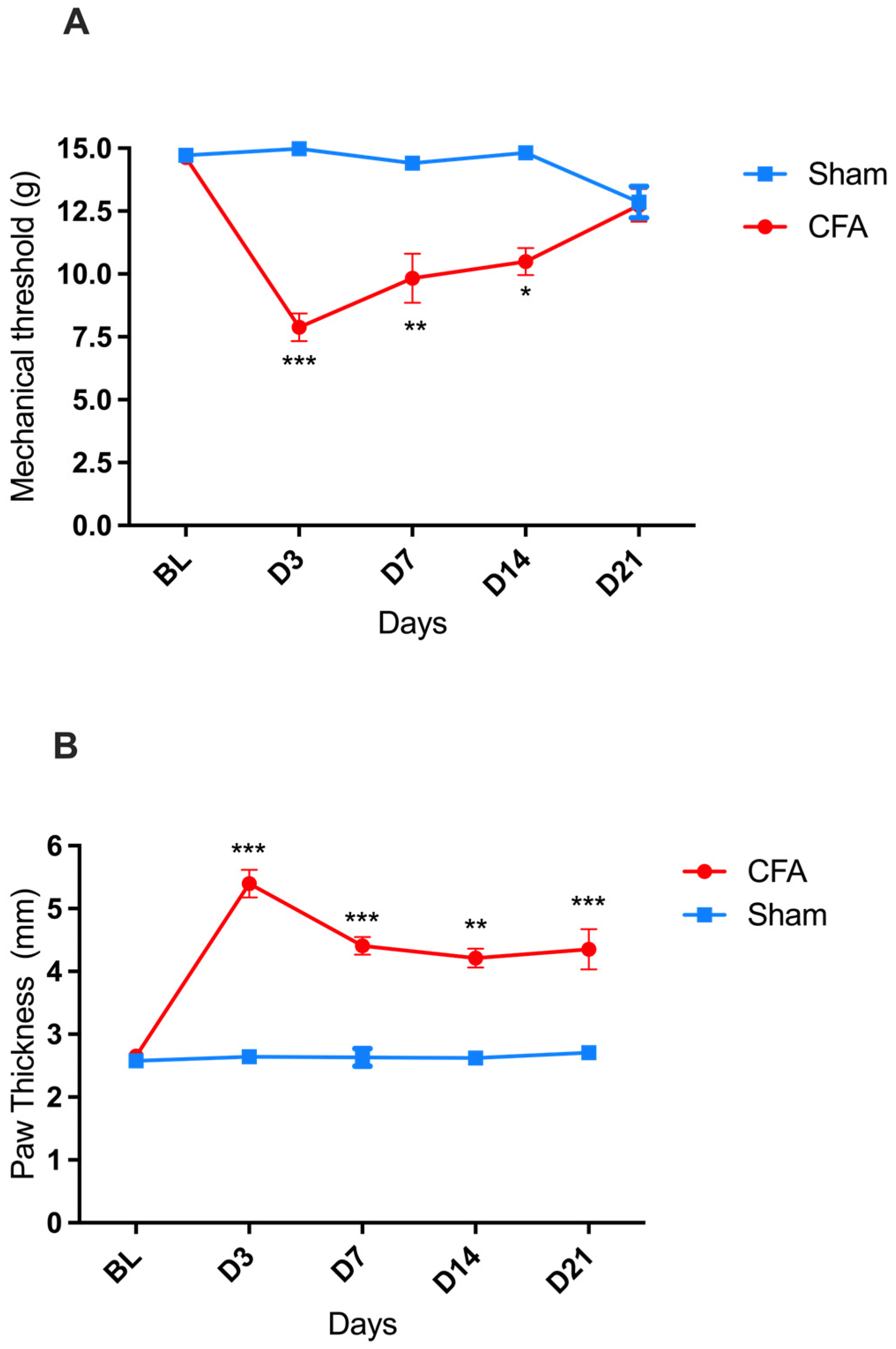
2.1. Time-Course of CFA-Induced Inflammation and Pain in Male Wistar Rats
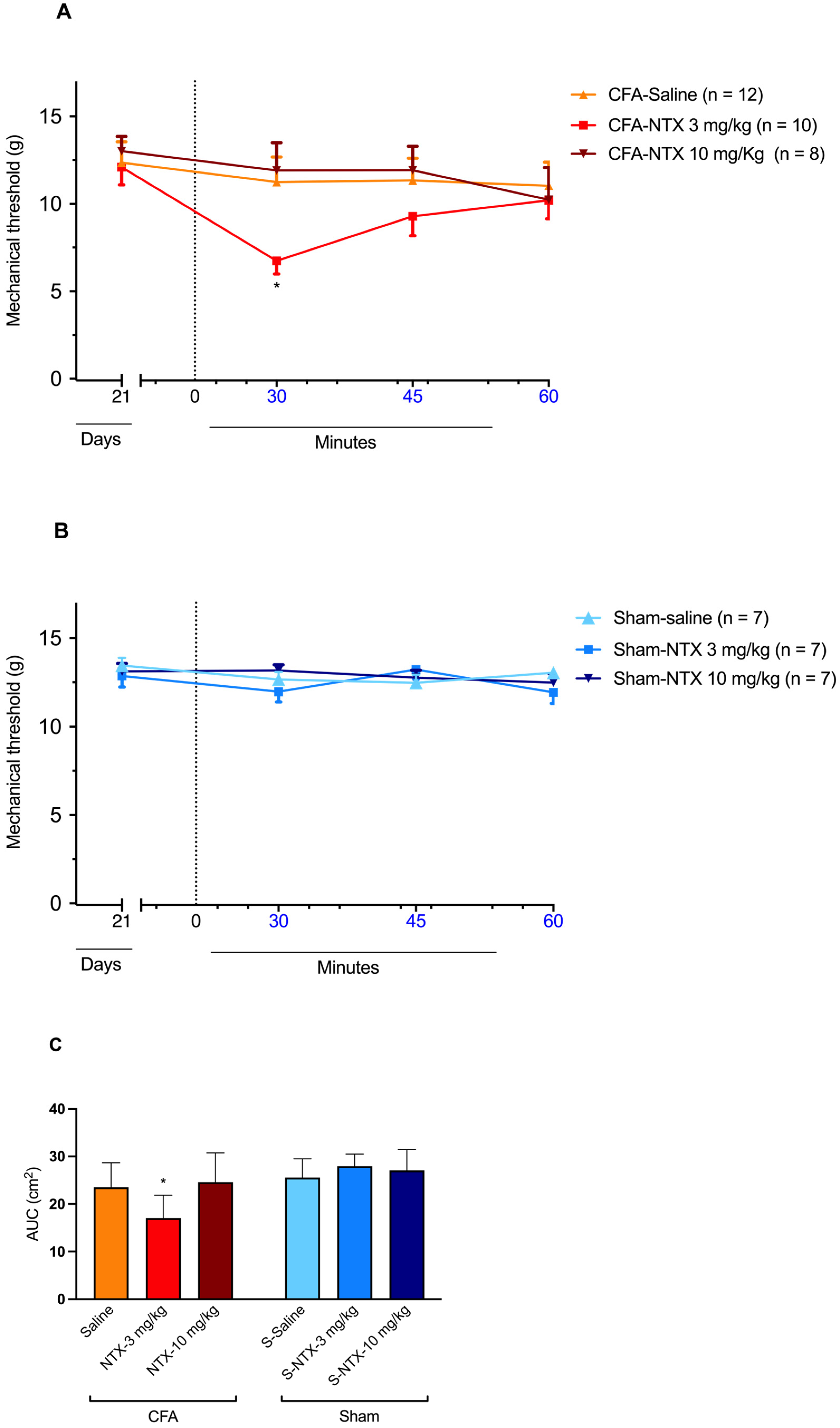
2.2. Effect of NTX Administration on the Paw Mechanical Withdrawal Threshold of Male Wistar Rats at 21 Days Post-CFA Administration (CFA-21d)
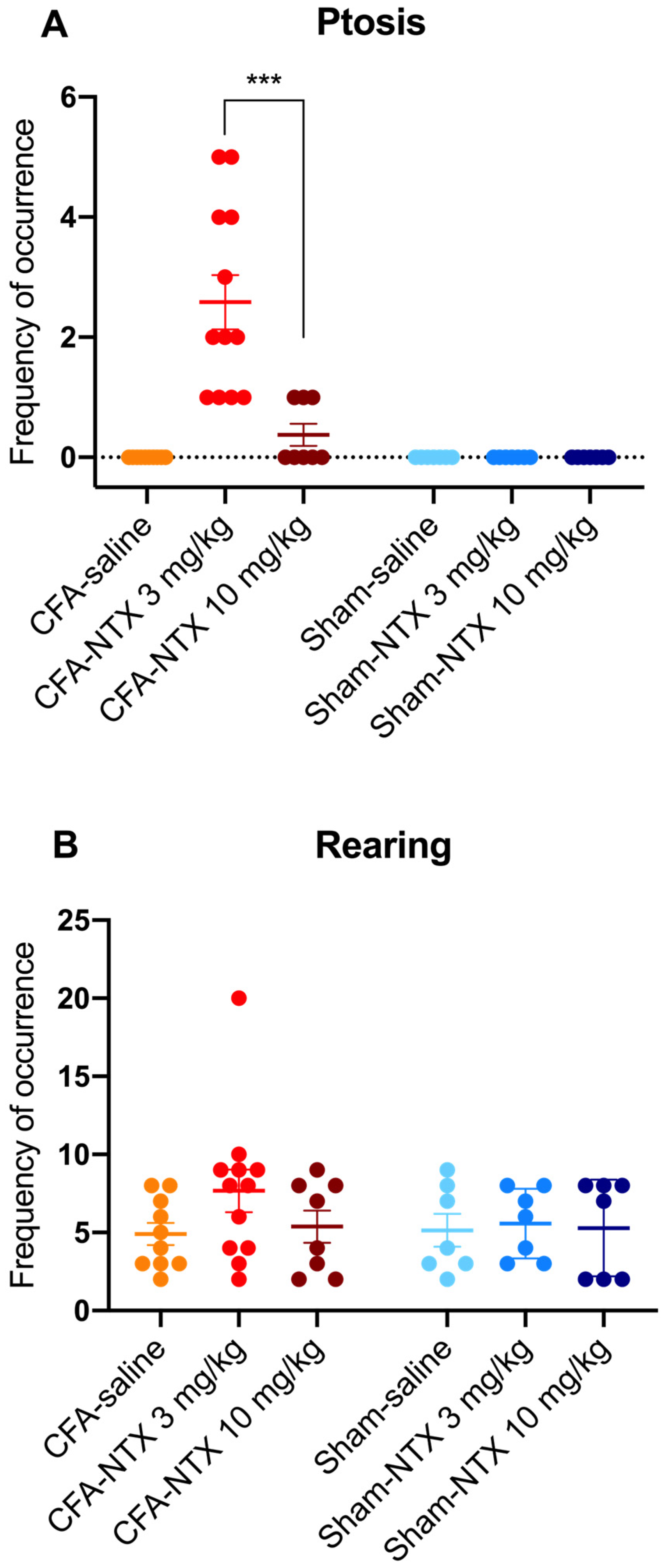
2.3. Effect of NTX Administration on the Behavioral Traits of CFA-21d Male Wistar Rats
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Study Design
4.3. Experimental Procedures
4.3.1. Protocol to Induce the Priming Inflammatory Injury with CFA
4.3.2. Opioid Receptor Inverse Agonist Preparation and Administration
4.3.3. Measurement of the Paw Mechanical Withdrawal Threshold
4.3.4. Measurement of the Paw Thickness
4.3.5. Behavioral Assessment of Endogenous Opioid Withdrawal
4.4. Data Analysis and Statistics
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tsang, A.; Von Korff, M.; Lee, S.; Alonso, J.; Karam, E.; Angermeyer, M.C.; Borges, G.L.; Bromet, E.J.; Demytteneare, K.; de Girolamo, G.; et al. Common chronic pain conditions in developed and developing countries: Gender and age differences and comorbidity with depression-anxiety disorders. J. Pain 2008, 9, 883–891. [Google Scholar] [CrossRef] [PubMed]
- Feizerfan, A.; Sheh, G. Transition from acute to chronic pain. Contin. Educ. Anaesth. Crit. Care Pain 2015, 15, 98–102. [Google Scholar] [CrossRef]
- Gangadharan, V.; Kuner, R. Pain hypersensitivity mechanisms at a glance. Dis. Models Mech. 2013, 6, 889–895. [Google Scholar] [CrossRef] [PubMed]
- Larson, C.M.; Wilcox, G.L.; Fairbanks, C.A. The Study of Pain in Rats and Mice. Comp. Med. 2019, 69, 555–570. [Google Scholar] [CrossRef]
- Morales-Medina, J.C.; Flores, G.; Vallelunga, A.; Griffiths, N.H.; Iannitti, T. Cerebrolysin improves peripheral inflammatory pain: Sex differences in two models of acute and chronic mechanical hypersensitivity. Drug Dev. Res. 2019, 80, 513–518. [Google Scholar] [CrossRef]
- Eckert, W.A., 3rd. Rodent models of persistent pain in drug discovery and development. Curr. Pharm. Biotechnol. 2011, 12, 1590–1595. [Google Scholar] [CrossRef]
- Stein, C.; Millan, M.J.; Herz, A. Unilateral inflammation of the hindpaw in rats as a model of prolonged noxious stimulation: Alterations in behavior and nociceptive thresholds. Pharmacol. Biochem. Behav. 1988, 31, 445–451. [Google Scholar] [CrossRef]
- McCarson, K.E. Models of Inflammation: Carrageenan- or Complete Freund’s Adjuvant (CFA)-Induced Edema and Hypersensitivity in the Rat. Curr. Protoc. Pharmacol. 2015, 70, 4–5. [Google Scholar] [CrossRef]
- Marvizon, J.C.; Walwyn, W.; Minasyan, A.; Chen, W.; Taylor, B.K. Latent sensitization: A model for stress-sensitive chronic pain. Curr. Protoc. Neurosci. 2015, 71, 9–50. [Google Scholar] [CrossRef]
- Morales-Medina, J.C.; Rastogi, A.; Mintz, E.; Caldwell, H.K. Increased immediate early gene activation in the basolateral amygdala following persistent peripheral inflammation. Neuroreport 2020, 31, 724–729. [Google Scholar] [CrossRef]
- Latremoliere, A.; Woolf, C.J. Central sensitization: A generator of pain hypersensitivity by central neural plasticity. J. Pain 2009, 10, 895–926. [Google Scholar] [CrossRef]
- Corder, G.; Doolen, S.; Donahue, R.R.; Winter, M.K.; Jutras, B.L.; He, Y.; Hu, X.; Wieskopf, J.S.; Mogil, J.S.; Storm, D.R.; et al. Constitutive mu-opioid receptor activity leads to long-term endogenous analgesia and dependence. Science 2013, 341, 1394–1399. [Google Scholar] [CrossRef]
- Taylor, B.K.; Corder, G. Endogenous analgesia, dependence, and latent pain sensitization. Curr. Top. Behav. Neurosci. 2014, 20, 283–325. [Google Scholar] [CrossRef]
- Cooper, A.H.; Hedden, N.S.; Corder, G.; Lamerand, S.R.; Donahue, R.R.; Morales-Medina, J.C.; Selan, L.; Prasoon, P.; Taylor, B.K. Endogenous micro-opioid receptor activity in the lateral and capsular subdivisions of the right central nucleus of the amygdala prevents chronic postoperative pain. J. Neurosci. Res. 2022, 100, 48–65. [Google Scholar] [CrossRef]
- Ellenbroek, B.; Youn, J. Rodent models in neuroscience research: Is it a rat race? Dis. Models Mech. 2016, 9, 1079–1087. [Google Scholar] [CrossRef]
- Mogil, J.S. The translatability of pain across species. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2019, 374, 20190286. [Google Scholar] [CrossRef]
- Morales-Medina, J.C.; Griffiths, N.H.; Flores, G.; Mastranzo, V.M.; Iannitti, T. Cerebrolysin reduces mechanical allodynia in a rodent model of peripheral inflammation. Neurosci. Lett. 2017, 642, 27–30. [Google Scholar] [CrossRef]
- Chen, W.; Marvizon, J.C. Neurokinin 1 receptor activation in the rat spinal cord maintains latent sensitization, a model of inflammatory and neuropathic chronic pain. Neuropharmacology 2020, 177, 108253. [Google Scholar] [CrossRef]
- Grasing, K.; Wang, A.; Schlussman, S. Behavioral measures of anxiety during opiate withdrawal. Behav. Brain Res. 1996, 80, 195–201. [Google Scholar] [CrossRef]
- Iannitti, T.; Di Cerbo, A.; Loschi, A.R.; Rea, S.; Suzawa, M.; Morales-Medina, J.C. Repeated administration of a flavonoid-based formulated extract from citrus peels significantly reduces peripheral inflammation-induced pain in the rat. Food Sci. Nutr. 2020, 8, 3173–3180. [Google Scholar] [CrossRef]
- Walwyn, W.M.; Chen, W.; Kim, H.; Minasyan, A.; Ennes, H.S.; McRoberts, J.A.; Marvizon, J.C. Sustained Suppression of Hyperalgesia during Latent Sensitization by mu-, delta-, and kappa-opioid receptors and alpha2A Adrenergic Receptors: Role of Constitutive Activity. J. Neurosci. 2016, 36, 204–221. [Google Scholar] [CrossRef] [PubMed]
- Rigaud, M.; Gemes, G.; Barabas, M.E.; Chernoff, D.I.; Abram, S.E.; Stucky, C.L.; Hogan, Q.H. Species and strain differences in rodent sciatic nerve anatomy: Implications for studies of neuropathic pain. Pain 2008, 136, 188–201. [Google Scholar] [CrossRef] [PubMed]
- Bolker, J.A. Selection of Models: Evolution and the Choice of Species for Translational Research. Brain Behav. Evol. 2019, 93, 82–91. [Google Scholar] [CrossRef] [PubMed]
- Nagarkar, B.; Jagtap, S. Effect of new polyherbal formulations DF1911, DF2112 and DF2813 on CFA induced inflammation in rat model. BMC Complement. Altern. Med. 2017, 17, 194. [Google Scholar] [CrossRef] [PubMed]
- Noh, A.S.M.; Chuan, T.D.; Khir, N.A.M.; Zin, A.A.M.; Ghazali, A.K.; Long, I.; Ab Aziz, C.B.; Ismail, C.A.N. Effects of different doses of complete Freund’s adjuvant on nociceptive behaviour and inflammatory parameters in polyarthritic rat model mimicking rheumatoid arthritis. PLoS ONE 2021, 16, e0260423. [Google Scholar] [CrossRef]
- Akala, E.O.; Wang, H.; Adedoyin, A. Disposition of naltrexone after intravenous bolus administration in Wistar rats, low-alcohol-drinking rats and high-alcohol-drinking rats. Neuropsychobiology 2008, 58, 81–90. [Google Scholar] [CrossRef]
- Wall, M.E.; Perez-Reyes, M.; Brine, D.R.; Cook, C.E. Naltrexone disposition in man after subcutaneous administration. Drug Metab. Dispos. 1984, 12, 677–682. [Google Scholar]
- Wang, W.; Teresa, M.; Cai, J.; Zhang, C.; Wong, S.; Yan, Z.; Khojasteh, S.C.; Zhang, D. Comparative assessment for rat strain differences in metabolic profiles of 14 drugs in Wistar Han and Sprague Dawley hepatocytes. Xenobiotica 2021, 51, 15–23. [Google Scholar] [CrossRef]
- Gekker, G.; Lokensgard, J.R.; Peterson, P.K. Naltrexone potentiates anti-HIV-1 activity of antiretroviral drugs in CD4+ lymphocyte cultures. Drug Alcohol Depend. 2001, 64, 257–263. [Google Scholar] [CrossRef]
- Johnson, R.E.; Strain, E.C.; Amass, L. Buprenorphine: How to use it right. Drug Alcohol Depend. 2003, 70, S59–S77. [Google Scholar] [CrossRef]
- Newman-Tancredi, A.; Cussac, D.; Ormiere, A.M.; Lestienne, F.; Varney, M.A.; Martel, J.C. Bell-shaped agonist activation of 5-HT1A receptor-coupled Galphai3 G-proteins: Receptor density-dependent switch in receptor signaling. Cell Signal. 2019, 63, 109383. [Google Scholar] [CrossRef]
- Gopal, S. Pharmacokinetic/Pharmacodynamic Investigation of the Mechanism of the Bell-Shaped Dose Analgesic Effect Curve of Buprenorphine in Rats: Antagonism by the Metabolite; Temple University: Philadelphia, PA, USA, 2000. [Google Scholar]
- Kaufman, D.L.; Keith, D.E., Jr.; Anton, B.; Tian, J.; Magendzo, K.; Newman, D.; Tran, T.H.; Lee, D.S.; Wen, C.; Xia, Y.R.; et al. Characterization of the murine mu opioid receptor gene. J. Biol. Chem. 1995, 270, 15877–15883. [Google Scholar] [CrossRef]
- Wendel, B.; Hoehe, M.R. The human mu opioid receptor gene: 5′ regulatory and intronic sequences. J. Mol. Med. 1998, 76, 525–532. [Google Scholar] [CrossRef]
- Lee, D.S.; Law, P.Y.; Ln, W.; Loh, H.H.; Song, K.Y.; Choi, H.S. Differential regulation of mouse and human Mu opioid receptor gene depends on the single stranded DNA structure of its promoter and alpha-complex protein 1. Biomed. Rep. 2017, 6, 532–538. [Google Scholar] [CrossRef]
- Pol, O.; Puig, M.M. Expression of opioid receptors during peripheral inflammation. Curr. Top. Med. Chem. 2004, 4, 51–61. [Google Scholar] [CrossRef]
- Nunez, S.; Lee, J.S.; Zhang, Y.; Bai, G.; Ro, J.Y. Role of peripheral mu-opioid receptors in inflammatory orofacial muscle pain. Neuroscience 2007, 146, 1346–1354. [Google Scholar] [CrossRef]
- Aoki, Y.; Mizoguchi, H.; Watanabe, C.; Takeda, K.; Sakurada, T.; Sakurada, S. Potential involvement of mu-opioid receptor dysregulation on the reduced antinociception of morphine in the inflammatory pain state in mice. J. Pharmacol. Sci. 2014, 124, 258–266. [Google Scholar] [CrossRef]
- LaCroix-Fralish, M.L.; Austin, J.S.; Zheng, F.Y.; Levitin, D.J.; Mogil, J.S. Patterns of pain: Meta-analysis of microarray studies of pain. Pain 2011, 152, 1888–1898. [Google Scholar] [CrossRef]
- Blackwood, C.A.; McCoy, M.T.; Ladenheim, B.; Cadet, J.L. Escalated Oxycodone Self-Administration and Punishment: Differential Expression of Opioid Receptors and Immediate Early Genes in the Rat Dorsal Striatum and Prefrontal Cortex. Front. Neurosci. 2019, 13, 1392. [Google Scholar] [CrossRef]
- Brejchova, J.; Holan, V.; Svoboda, P. Expression of Opioid Receptors in Cells of the Immune System. Int. J. Mol. Sci. 2020, 22, 315. [Google Scholar] [CrossRef]
- Mannelli, P.; Peindl, K.S.; Wu, L.T. Pharmacological enhancement of naltrexone treatment for opioid dependence: A review. Subst. Abuse Rehabil. 2011, 2011, 113–123. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Custodio-Patsey, L.; Donahue, R.R.; Fu, W.; Lambert, J.; Smith, B.N.; Taylor, B.K. Sex differences in kappa opioid receptor inhibition of latent postoperative pain sensitization in dorsal horn. Neuropharmacology 2020, 163, 107726. [Google Scholar] [CrossRef] [PubMed]
- Lutfy, K.; Cowan, A. Buprenorphine: A unique drug with complex pharmacology. Curr. Neuropharmacol. 2004, 2, 395–402. [Google Scholar] [CrossRef] [PubMed]
- Malspeis, L.; Bathala, M.S.; Ludden, T.M.; Bhat, H.B.; Frank, S.G.; Sokoloski, T.D.; Morrison, B.E.; Reuning, R.H. Metabolic reduction of naltrexone. I. Synthesis, separation and characterization of naloxone and naltrexone reduction products and qualitative assay of urine and bile following administration of naltrexone, alpha-naltrexol, or beta-naltrexol. Res. Commun. Chem. Pathol. Pharmacol. 1975, 12, 43–65. [Google Scholar]
- Dayton, H.E.; Inturrisi, C.E. The urinary excretion profiles of naltrexone in man, monkey, rabbit, and rat. Drug Metab. Dispos. 1976, 4, 474–478. [Google Scholar]
- Besson, M.; Suarez, S.; Changeux, J.-P.; Granon, S. A low dose of nicotine is sufficient to produce nicotine withdrawal in mice. Health 2010, 2, 1–7. [Google Scholar]
- Uddin, O.; Jenne, C.; Fox, M.E.; Arakawa, K.; Keller, A.; Cramer, N. Divergent profiles of fentanyl withdrawal and associated pain in mice and rats. Pharmacol. Biochem. Behav. 2021, 200, 173077. [Google Scholar] [CrossRef]
- Wang, G.B.; Wu, L.Z.; Yu, P.; Li, Y.J.; Ping, X.J.; Cui, C.L. Multiple 100 Hz electroacupuncture treatments produced cumulative effect on the suppression of morphine withdrawal syndrome: Central preprodynorphin mRNA and p-CREB implicated. Peptides 2011, 32, 713–721. [Google Scholar] [CrossRef]
- Moradi, S.; Charkhpour, M.; Ghavimi, H.; Motahari, R.; Ghaderi, M.; Hassanzadeh, K. Gap junction blockers: A potential approach to attenuate morphine withdrawal symptoms. J. Biomed. Sci. 2013, 20, 77. [Google Scholar] [CrossRef]
- Moayeri, A.; Azimi, M.; Karimi, E.; Aidy, A.; Abbasi, N. Attenuation of Morphine Withdrawal Syndrome by Prosopis Farcta Extract and Its Bioactive Component Luteolin in Comparison with Clonidine in Rats. Med. Sci. Monit. Basic Res. 2018, 24, 151–158. [Google Scholar] [CrossRef]
- Pinelli, A.; Trivulzio, S. Quantitative evaluation of opioid withdrawal signs in rats repeatedly treated with morphine and injected with naloxone, in the absence or presence of the antiabstinence agent clonidine. J. Pharmacol. Toxicol. Methods 1997, 38, 117–131. [Google Scholar] [CrossRef]
- Mogil, J.S. Sex differences in pain and pain inhibition: Multiple explanations of a controversial phenomenon. Nat. Rev. Neurosci. 2012, 13, 859–866. [Google Scholar] [CrossRef]
- Becker, J.B.; Perry, A.N.; Westenbroek, C. Sex differences in the neural mechanisms mediating addiction: A new synthesis and hypothesis. Biol. Sex Differ. 2012, 3, 14. [Google Scholar] [CrossRef]
- Townsend, E.A.; Kim, R.K.; Robinson, H.L.; Marsh, S.A.; Banks, M.L.; Hamilton, P.J. Opioid withdrawal produces sex-specific effects on fentanyl-vs.-food choice and mesolimbic transcription. Biol. Psychiatry Glob. Open Sci. 2021, 1, 112–122. [Google Scholar] [CrossRef]
- Paton, K.F.; Luo, D.; La Flamme, A.C.; Prisinzano, T.E.; Kivell, B.M. Sex Differences in Kappa Opioid Receptor Agonist Mediated Attenuation of Chemotherapy-Induced Neuropathic Pain in Mice. Front. Pharmacol. 2022, 13, 813562. [Google Scholar] [CrossRef]
- Zhan, B.; Ma, H.Y.; Wang, J.L.; Liu, C.B. Sex differences in morphine-induced behavioral sensitization and social behaviors in ICR mice. Dongwuxue Yanjiu 2015, 36, 103–108. [Google Scholar] [CrossRef]
- Smith, J.C. A Review of Strain and Sex Differences in Response to Pain and Analgesia in Mice. Comp. Med. 2019, 69, 490–500. [Google Scholar] [CrossRef]
- Hsieh, T.; Vaickus, M.H.; Remick, D.G. Enhancing Scientific Foundations to Ensure Reproducibility: A New Paradigm. Am. J. Pathol. 2018, 188, 6–10. [Google Scholar] [CrossRef]
- Kilkenny, C.; Browne, W.J.; Cuthill, I.C.; Emerson, M.; Altman, D.G. Improving bioscience research reporting: The ARRIVE guidelines for reporting animal research. Osteoarthr. Cartil. 2012, 20, 256–260. [Google Scholar] [CrossRef]
- Chaplan, S.R.; Bach, F.W.; Pogrel, J.W.; Chung, J.M.; Yaksh, T.L. Quantitative assessment of tactile allodynia in the rat paw. J. Neurosci. Methods 1994, 53, 55–63. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Morales-Medina, J.C.; Pugliese, N.; Di Cerbo, A.; Zizzadoro, C.; Iannitti, T. Evidence for Endogenous Opioid Dependence Related to Latent Sensitization in a Rat Model of Chronic Inflammatory Pain. Int. J. Mol. Sci. 2023, 24, 2812. https://doi.org/10.3390/ijms24032812
Morales-Medina JC, Pugliese N, Di Cerbo A, Zizzadoro C, Iannitti T. Evidence for Endogenous Opioid Dependence Related to Latent Sensitization in a Rat Model of Chronic Inflammatory Pain. International Journal of Molecular Sciences. 2023; 24(3):2812. https://doi.org/10.3390/ijms24032812
Chicago/Turabian StyleMorales-Medina, Julio César, Nicola Pugliese, Alessandro Di Cerbo, Claudia Zizzadoro, and Tommaso Iannitti. 2023. "Evidence for Endogenous Opioid Dependence Related to Latent Sensitization in a Rat Model of Chronic Inflammatory Pain" International Journal of Molecular Sciences 24, no. 3: 2812. https://doi.org/10.3390/ijms24032812





