Abstract
Coronavirus disease-19 (COVID-19) emerged in December 2019 and quickly spread, giving rise to a pandemic crisis. Therefore, it triggered tireless efforts to identify the mechanisms of the disease, how to prevent and treat it, and to limit and hamper its global dissemination. Considering the above, the search for prophylactic approaches has led to a revolution in the reglementary pharmaceutical pipeline, with the approval of vaccines against COVID-19 in an unprecedented way. Moreover, a drug repurposing scheme using regulatory-approved antiretroviral agents is also being pursued. However, their physicochemical characteristics or reported adverse events have sometimes limited their use. Hence, nanotechnology has been employed to potentially overcome some of these challenges, particularly cyclodextrins. Cyclodextrins are cyclic oligosaccharides that present hydrophobic cavities suitable for complexing several drugs. This review, besides presenting studies on the inclusion of antiviral drugs in cyclodextrins, aims to summarize some currently available prophylactic and therapeutic schemes against COVID-19, highlighting those that already make use of cyclodextrins for their complexation. In addition, some new therapeutic approaches are underscored, and the potential application of cyclodextrins to increase their promising application against COVID-19 will be addressed. This review describes the instances in which the use of cyclodextrins promotes increased bioavailability, antiviral action, and the solubility of the drugs under analysis. The potential use of cyclodextrins as an active ingredient is also covered. Finally, toxicity and regulatory issues as well as future perspectives regarding the use of cyclodextrins in COVID-19 therapy will be provided.
1. Introduction
Infectious diseases are responsible for millions of deaths yearly, and the associated economic costs for preventing and treating them are huge. For this reason, it is increasingly important to understand how these diseases evolve to reduce their impact on the socioeconomic landscape and health sector [1].
In 2019, a new coronavirus disease (COVID-19) caused by the SARS-CoV-2 virus emerged, quickly becoming a pandemic. This disease is transmitted from person to person by the inhalation of droplets released during coughing or sneezing. Although several body systems can be affected, the most impacted is the respiratory system, and symptoms can range from fever to pneumonia. While some patients are asymptomatic or only present mild symptoms, some develop severe symptoms with poor prognosis [2].
Since its beginning, many scientific resources have been devoted to better understanding the virus and finding the best treatment. Several therapies have been developed, including vaccines, antivirals, monoclonal antibodies, and others. Despite vaccines playing a pivotal role in preventing and containing the spread of the virus, antiviral agents have played a key role in treating the disease. However, drawbacks have limited their translation to clinics, namely low bioavailability, and in some cases, adverse events that have been reported.
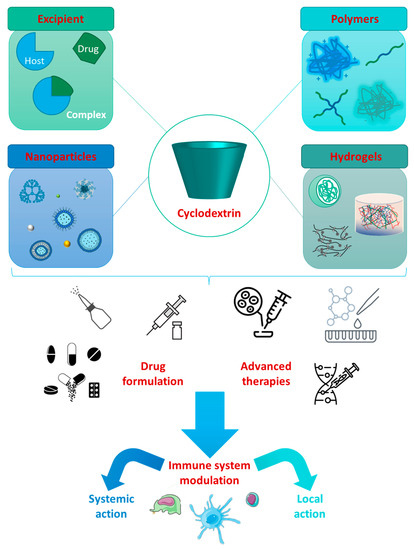
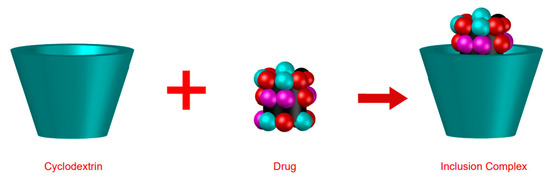
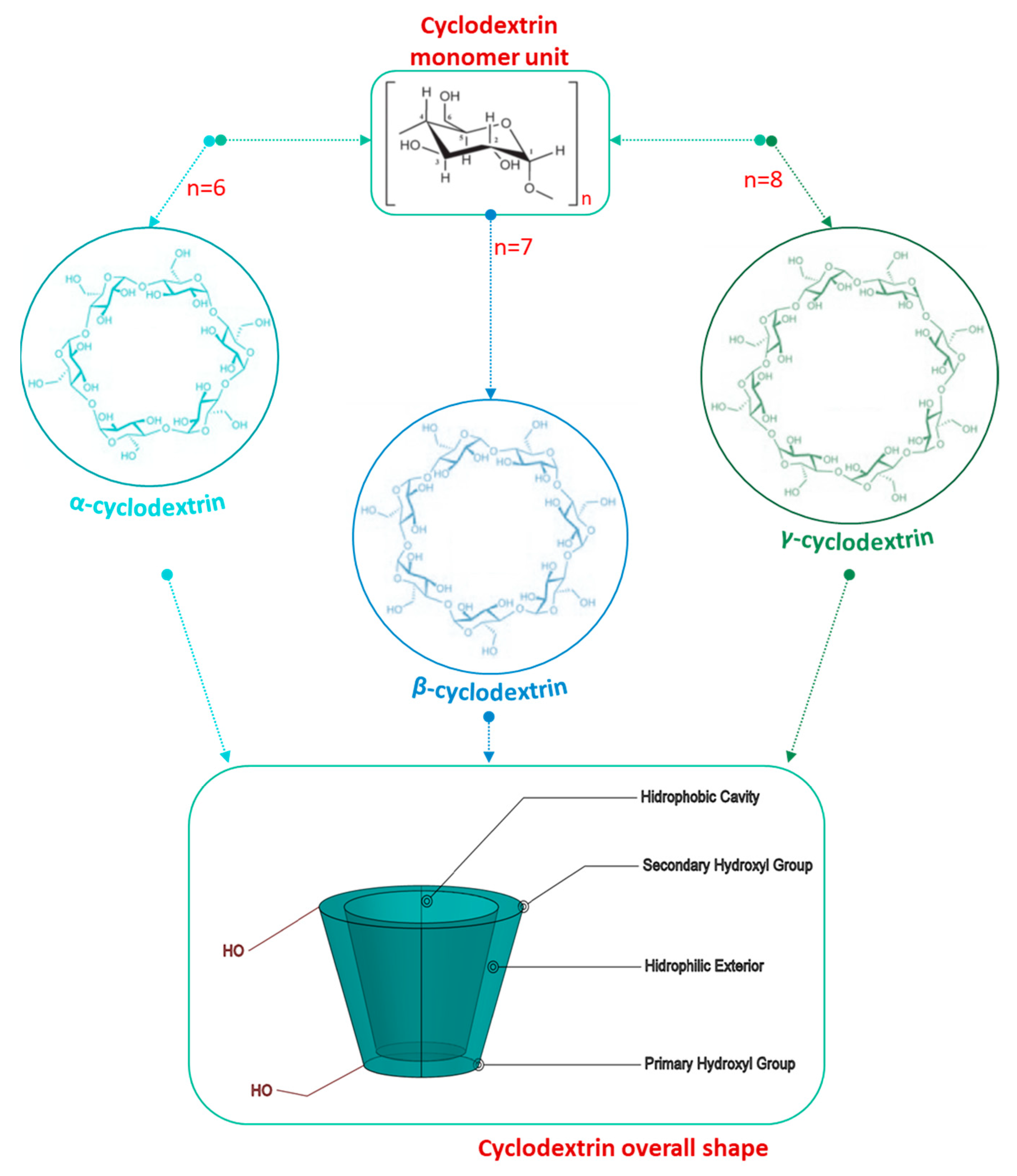
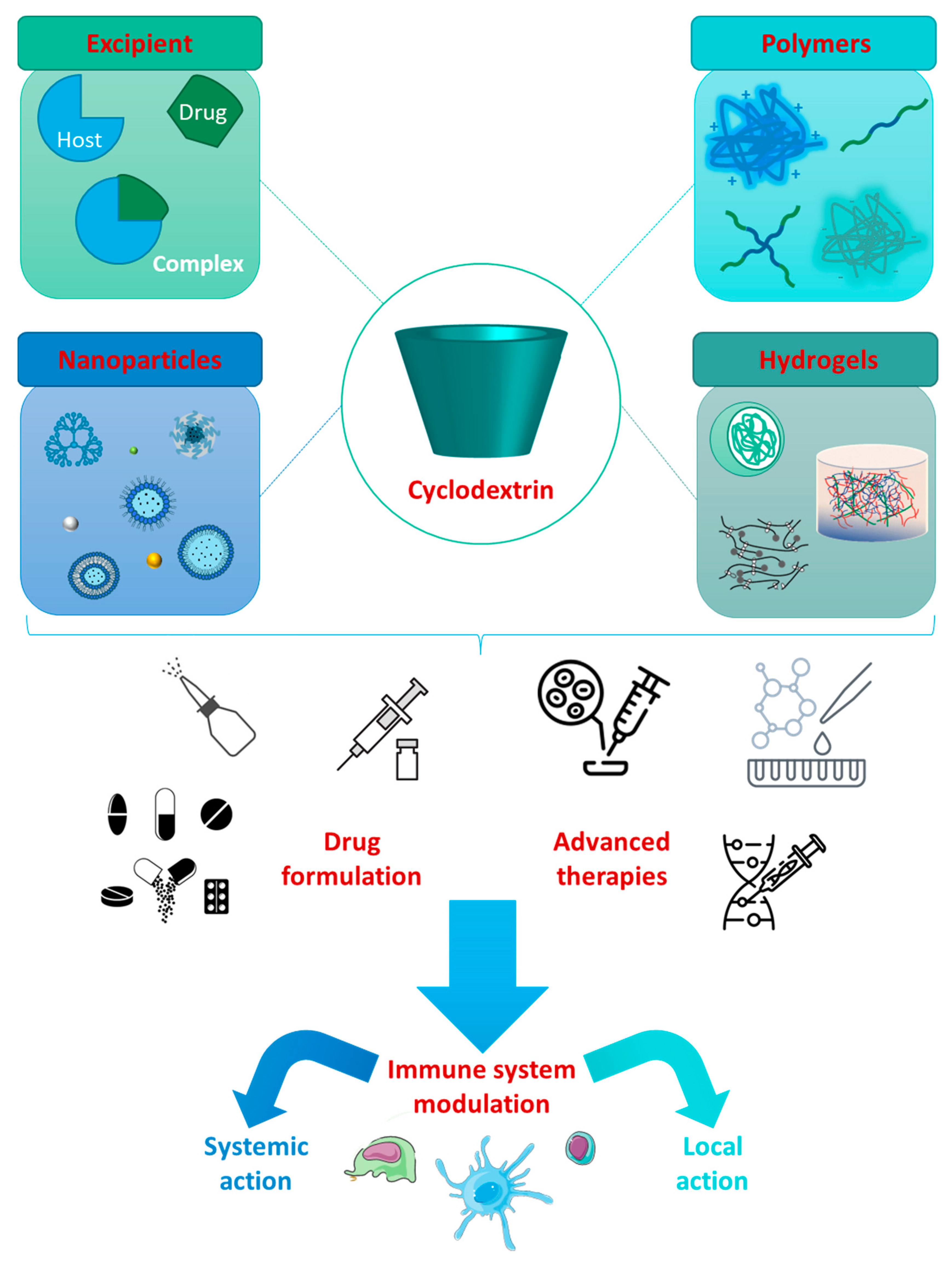
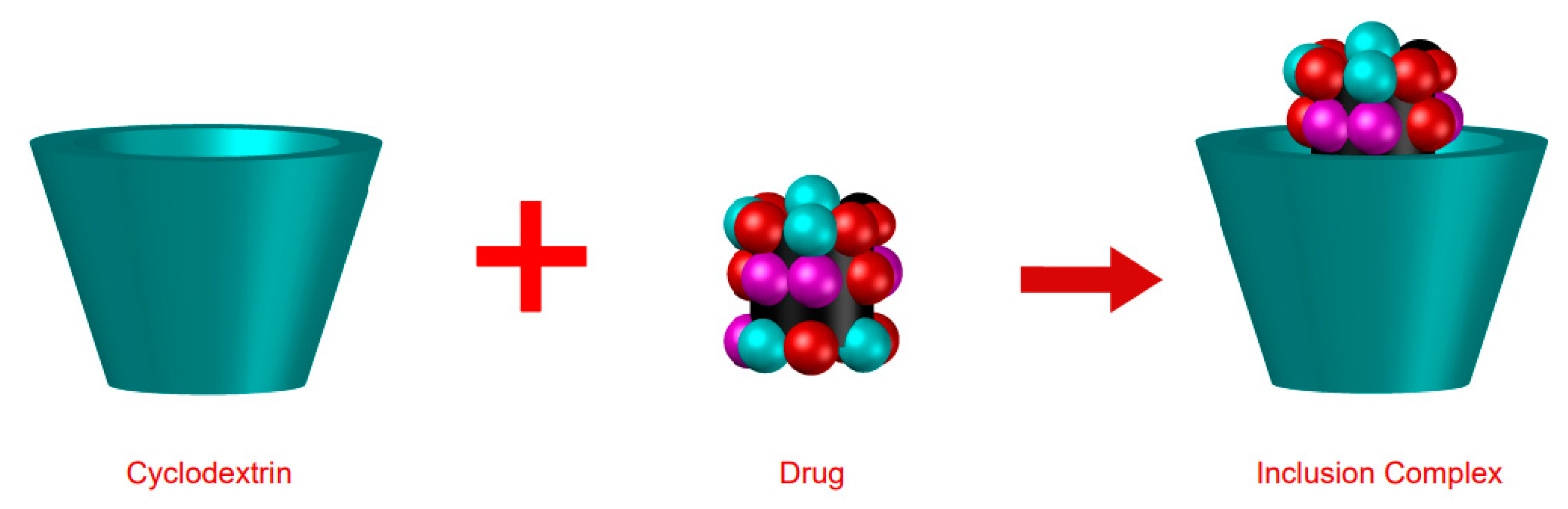
The use of cyclodextrins (CDs) allows the formation of inclusion complexes (ICs) for the benefit of certain drugs, providing more safety and greater efficacy [1,3].
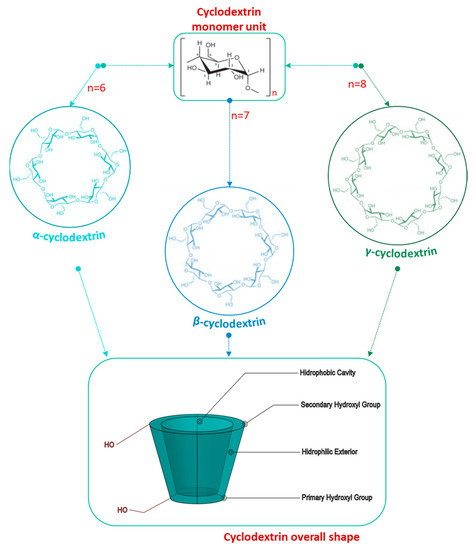
CDs are cyclic oligosaccharides composed of six or more glucose units connected by alpha-1,4 bonds. They have hydrophobic cavities with a hydrophilic exterior which gives them the ability to complex with several drugs, improving their solubility, stability, and bioavailability [3,4]. Due to these versatile properties, the global CDs market size is projected to increase from USD 260 million (2020) to more than USD 390 million (2027), with a compound annual growth rate (CARG) of 5.5 % [5].
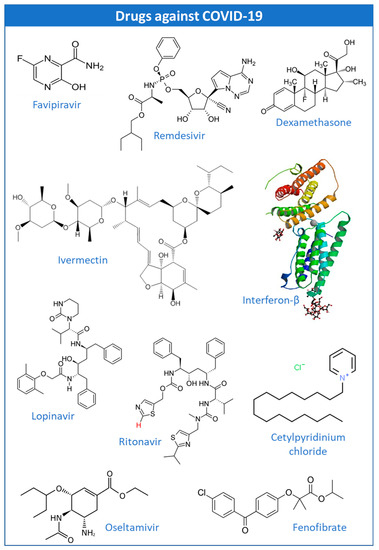
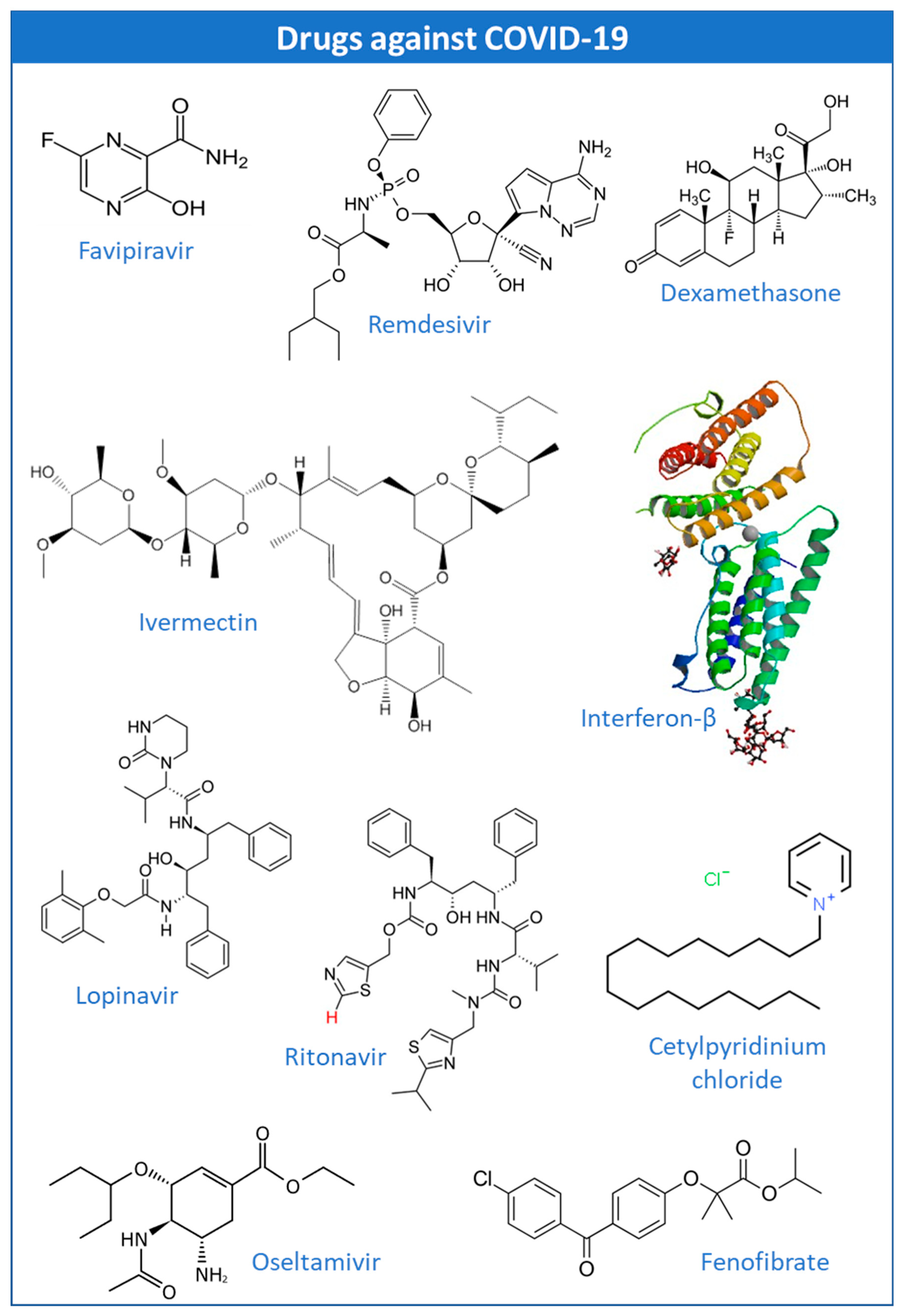
The potential application of CDs in the encapsulation of antiviral drugs such as favipiravir (FPV), remdesivir (REM), dexamethasone (DEX), ivermectin (IVM), hydroxy(chloroquine) (HCQ), interferon-beta (IFN-β), lopinavir/ritonavir (LPV/RTV), oseltamavir (OTV), and fenofibrate have been explored with some promising results. This manuscript brings together the ongoing clinical trials with these drugs, at the time of this review, to provide more detailed information about the main specifications of their use, and to allow a clearer analysis of the feasibility of their incorporation into CDs. In addition, this work provides updated information about the new proposals for therapeutic approaches for the treatment of COVID-19, highlighting new candidate drugs for this purpose: bepridil, glycyrrhizin, plitidepsin, thapsigargin, and polyphenols. Experimental studies of these drugs with CDs are also mentioned as well as the ongoing clinical trials.
This review also aims to raise awareness of the importance of toxicological analysis of CDs, focusing on aspects such as the daily dose administered, the route of administration, and the type of cyclodextrin. These aspects, together with the information collected from clinical and experimental studies, are essential to make available conclusions on the viability of incorporating these drugs into CDs.
Therefore, this review aims to provide a comprehensive overview of the current therapeutic regimen against COVID-19 and the ongoing clinical trials, highlighting the potential use of CDs to overcome some therapeutic failures. Moreover, the potential application of CDs in new therapies was emphasized. Finally, the regulatory landscape of cyclodextrins will be covered.
2. COVID-19 Etiopathology
COVID-19 is an infectious disease declared as a pandemic on 11 March 2020 by the World Health Organization (WHO) [6]. Briefly, COVID-19 is caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), which belongs to a diverse group of coronaviruses characterized by enveloped, single-stranded, positive sense ribonucleic acid (RNA) viruses with a long-range tropism, which gives them the ability to cause overwhelming diseases [7].
Viruses are known to enter the host cell with the help of receptors that mediate endocytosis. In the case of SARS-CoV-2, it has been reported that the spike protein is responsible for binding to the angiotensin-converting enzyme 2 (ACE-2) receptor on the host cell surface, which is the entry point for the virus. However, SARS-CoV-2 entry is not only dependent on the binding of the spike protein to the ACE-2 receptor, but it also requires the priming of the spike protein by the serine-2 transmembrane protease (TMPRSS2), which is crucial for the fusion of the virus to the host cell membrane. This synergy between the ACE-2 receptor and TMPRSS2 is necessary for the virus to enter into the host. The expression of TMPRSS2 is much higher than the ACE-2 receptor, suggesting that the latter is the limiting factor for SARS-CoV-2 during the early stage of infection [8,9].
Evidence has shown that the first target of the virus is the respiratory system. However, it can cause alterations in different body organs. Although some patients are asymptomatic or have mild to moderate symptoms, a percentage can develop severe illness [10]. The most common complications are acute respiratory failure syndrome (ARDS), septic shock, and sepsis. Risk factors such as age and comorbidities such as chronic diseases are related to severe illness and mortality [2].
Several symptoms have been associated with this disease, such as fever, cough, difficulty in breathing, headache, fatigue, sore throat, rhinorrhea, anorexia, myalgias, diarrhea, and in severe cases, pneumonia. The primary transmission mode is from person to person, through inhalation of the droplets released when coughing or sneezing. In general, symptomatic people are more contagious. However, transmission is also possible through fomites [2].
Moreover, it is valuable to mention that viruses are susceptible to mutations leading to the potential development of new variants. In response to the emergence of new SARS-CoV-2 variants, WHO has classified the variants according to the Greek alphabet (e.g., Alpha, Beta, Gamma, and Delta).
The strains of interest present mutations on the spike protein, which often results in altered virus comportment and may lead to immune escape [2,7,11].
Therefore, comprehensive knowledge of the current prophylactic and treatment schemes for COVID-19 is of the utmost importance to preparing efforts to fight disease dissemination.
3. COVID-19 Prevention and Treatment Approaches
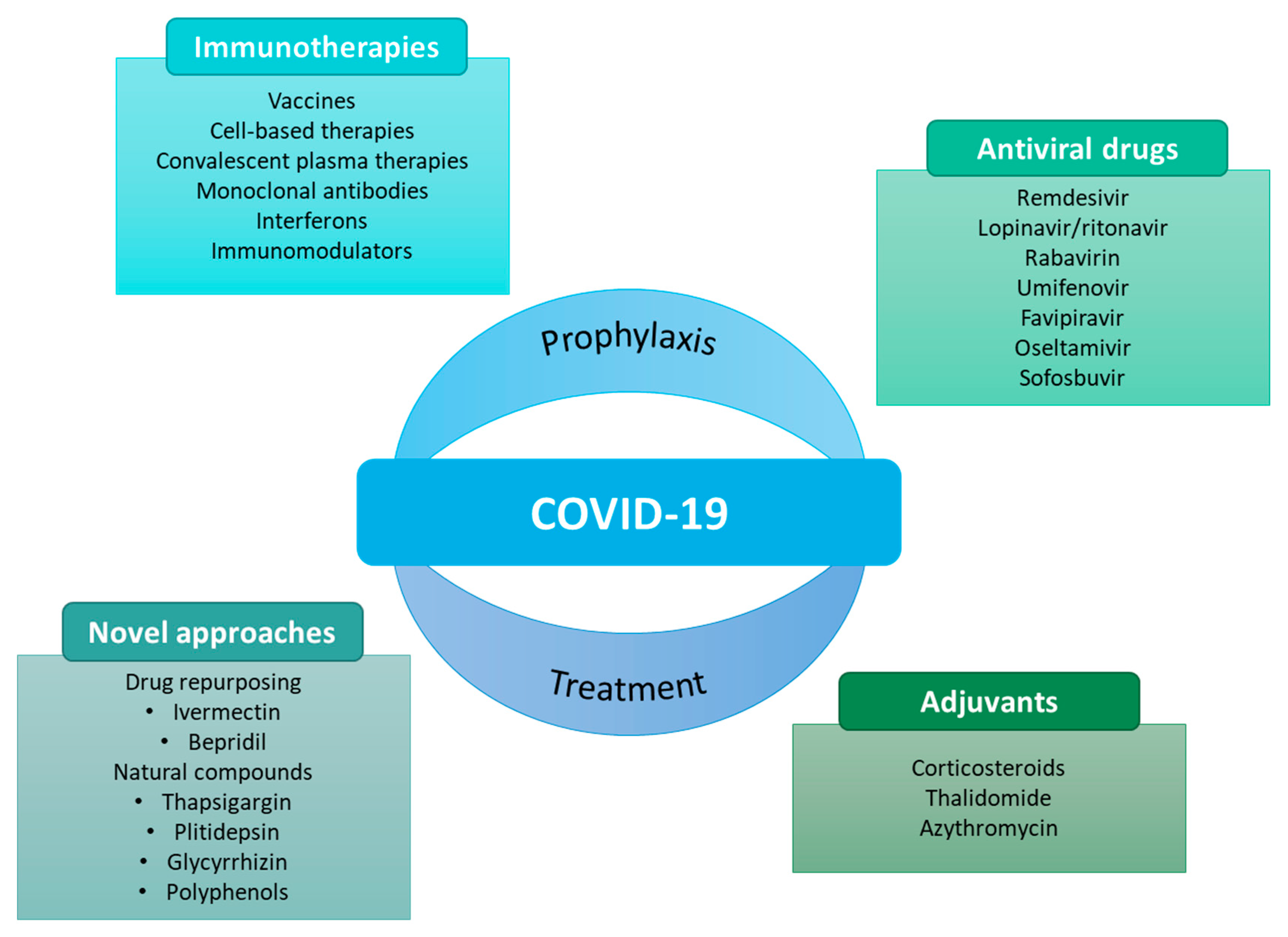
As the pandemic evolved, so did the search for potential prophylactic and therapeutic agents (Figure 1).
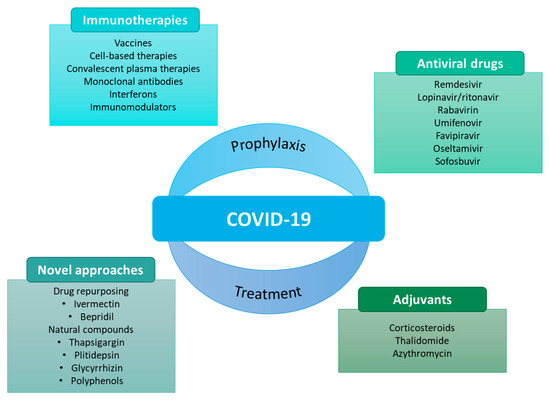
Figure 1.
Some examples of prophylactic and therapeutic approaches against COVID-19 [12].
Vaccines have undoubtedly conquered the research space to prevent SARS-CoV-2 because of their advantages in the prophylaxis of COVID-19. Besides that, some therapies have also been considered against COVID-19. Many of these therapies have emerged from drug repurposing. Repurposing of a drug consists of using an existing medicine with a new therapeutic purpose beyond its primary indications [7,12,13,14,15].
Table 1 summarizes the currently available therapies against COVID-19 and their main underlying action mechanisms.

Table 1.
Potential therapeutic agents used in the treatment of COVID-19.
3.1. Vaccines
The spread of COVID-19 has mobilized research and development (R&D) efforts. Therefore, several approaches for vaccine development against COVID-19 have been tested, such as inactivated virus, live attenuated, recombinant protein, adenovirus vector, influenza virus vector, as well as mRNA and DNA vaccines. As a revolutionary innovation, mRNA vaccine technology has uniquely controlled the COVID-19 pandemic [19].
Briefly, mRNA vaccines are composed of a vehicle, particularly lipid nanoparticles, that enables the delivery of a nucleic acid molecule encoding the antigen of interest. In the case of SARS-CoV-2, the spike protein is delivered into the target cell in the human host, allowing the host cell to produce the target protein and express the antigen to elicit an immune response [19].
Currently, two mRNA-based vaccines are approved against COVID-19: Comirnaty (BNT162b2) and Spikevax (mRNA-1273) [20]. According to the literature, mRNA technology is desirable as it works as a template for protein translation and does not require bioreactors. It reduces the risk of bacterial contamination and makes scaling up less challenging. Moreover, mRNA vaccines reduce the risk of immunogenicity compared to other viral vector-based modalities. However, the dependency on cold-chain storage and transport may hamper their global applications [20].
3.2. Antiviral Drugs
Given the clinical picture presented by patients with SARS-CoV-2, another potential therapy is antiviral drugs such as remdesivir (REM), favipiravir (FPV), and lopinavir/ritonavir, which will be described in more detail later in the manuscript.
In brief, these drugs can inhibit the entry of the virus by targeting the type-II transmembrane serine protease (TMPRSS2) and the ACE-2 receptor. They may also interfere with endocytosis or with the action of RNA-dependent RNA polymerase (RdRp) and the SARS-CoV-2 3-chymotrypsin-like protease (3CLpro) through fusion inhibitors.
Despite the promising prospects for this therapeutic class, some groups of antiviral drugs remain to be explored to treat COVID-19 [21,22].
3.3. Convalescent Plasma
The convalescent plasma of patients who have recovered from COVID-19 presents neutralizing antibodies in its constitution, which can fight infection by minimizing the inflammatory response [17]. The reduction in the inflammatory response may happen due to viremia suppression contributing to prophylaxis and recovery.
The administration of passive antibodies may be an option to achieve rapid immunity [14].. In theory, the administration of convalescent plasma should be completed at an early stage for superior efficacy [23]. However, its application continues to be controversial [24].
3.4. Monoclonal Antibodies
Monoclonal antibodies (mAbs) have effectively prevented and treated various viral infections [25]. Currently, potent neutralizing mAbs have been investigated against COVID-19, by targeting the receptor-binding domain (RBD) of the spike glycoprotein of SARS-CoV-2, blocking the binding between the S protein and the host receptor, ACE2 [26,27]. Moreover, other neutralizing mAbs can mediate viral activity by targeting nonblocking epitopes of the RBD or N-terminal domain (NTD) of the spike protein [28,29]. Some neutralizing antibodies studied against COVID-19 have been reviewed previously [30].
However, the emergence of new virus strains with mutations in the protein epitopes may hinder the application of these selective immunotherapies.
Therefore, to overcome mutational virus escape, cocktail therapies aiming at administering antibodies targeting multiple epitopes on the spike protein have been investigated. Nevertheless, these treatment approaches may be challenging and considerably increase manufacturing costs [31].
Recently, the emergence of bispecific mAbs (bsAbs) has gained interest for the treatment of COVID-19 as one molecule can target two different antigen-binding sites [31]. However, the use of these approaches remains to be fully explored.
Although applying mAb-based interventions against SARS-CoV-2 may require periodic updates due to the shifting antigenic landscape, the potential passive immunization in persons with a high risk of ineffective responses is a significant leap forward in the fight against viral evolution [32].
3.5. Interferons
Interferons (IFNs) induce the encoding of several proteins that can inhibit viral replication by decreasing cellular metabolism, interfering with the membrane formation necessary for virus replication, and inducing the release of cytokines that promote adaptive immunity. There are three families of IFNs, but only type I and type III are produced when the immune system detects the presence of viral nucleic acids. IFN-α belongs to type I, as well as INF-β, and fights coronaviruses by inhibiting virus replication [17,25].
According to Sodeifian et al. [33], it is paramount to establish the best time window to prescribe this type of treatment, as evidence has revealed that the administration of INF before the viral peak and the inflammatory phase of the illness could offer a highly protective effect. On the contrary, the administration of IFN during the inflammatory and severe phase of the disease may cause immunopathology and long-lasting harm for patients [33].
3.6. Corticosteroids
Corticosteroids are readily available agents extensively used as anti-inflammatory agents against respiratory infections. However, evidence has suggested that no clear benefits have been observed regarding their application in SARS and MERS patients. Therefore, their application in the initial phase of the COVID-19 pandemic was not recommended [34].
Later, due to preliminary data demonstrating lower mortality in patients with COVID-19 treated with corticosteroids, the use of corticosteroids for treating patients with severe or critical COVID-19 has been recommended [35].
Corticosteroids may play a pleiotropic role in different pathophysiological components in severe COVID-19 [36]. One of the studied drugs is dexamethasone (DEX), a synthetic glucocorticoid (detailed later) [17].
5. New Candidates for COVID-19 Treatment
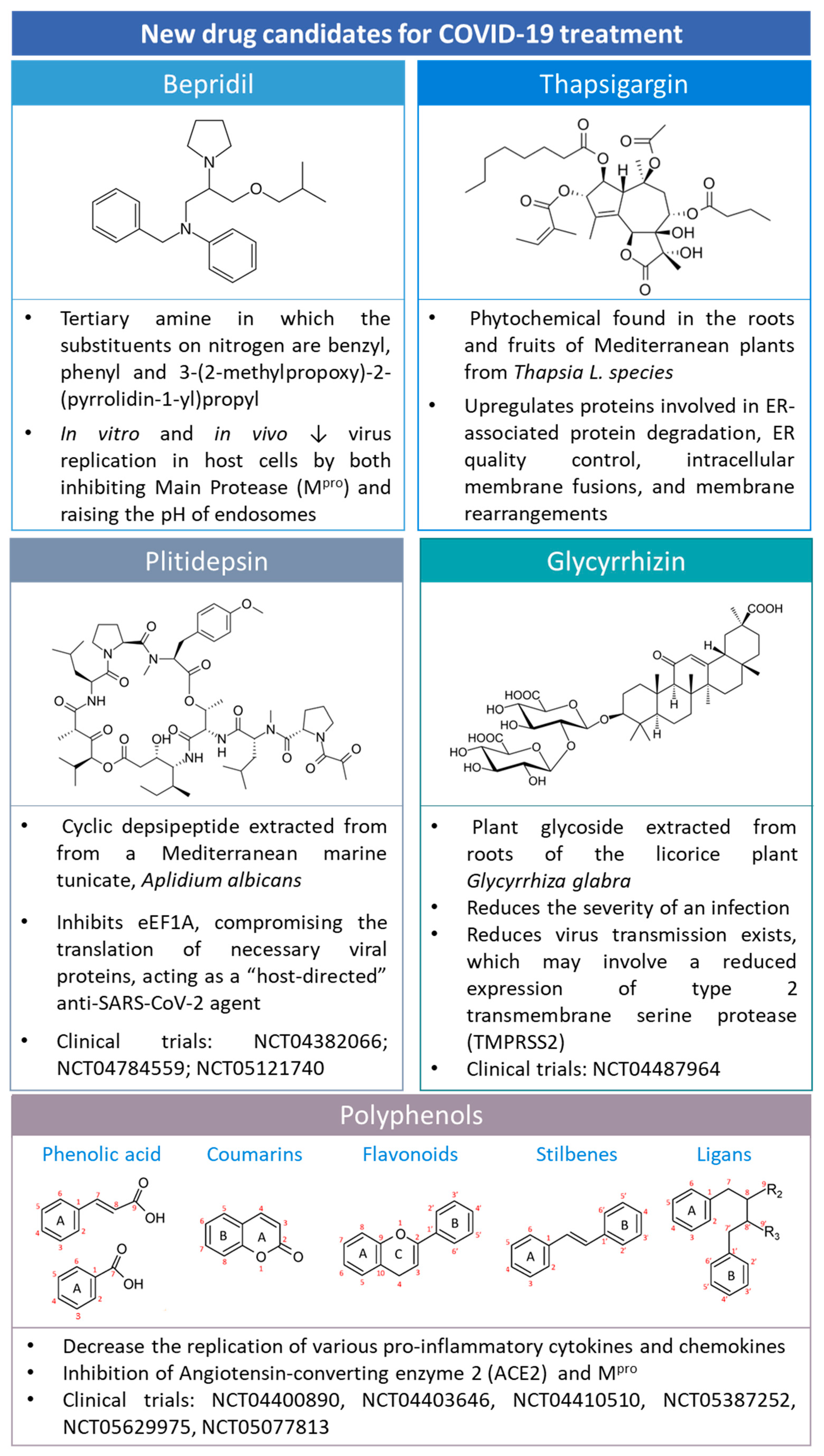
COVID-19 has gained huge attention due to its socioeconomic impact and health repercussions. Therefore, the scientific community has combined its efforts to identify new treatment approaches for COVID-19. Some of them are summarized in Figure 9.

Figure 9.
The chemical structure of drugs that are candidates for COVID-19 treatment, e.g., bepridil, thapsigargin, plitidepsin, glycyrrhizin, and polyphenols. Polyphenol structures were adapted from [75] under a Creative Common CC BY license.
5.1. Bepridil
Bepridil (Figure 9), a calcium channel blocker with significant antianginal activity, was reported to be potent against SARS-CoV-2 in vitro [76]. The antiviral analysis of bepridil indicated that it has low micromolar EC50 values in inhibiting SARS-CoV-2 in two highly permissive mammalian cell lines, Vero E6 and A549/ACE2 cells [76]. The structure moiety N-phenyl-N-benzylamine may be responsible for the structure−activity relationship with the main protease and the potent effect against SARS-CoV-2 [76].
Bepridil has been reported as a class 1 according to the Biopharmaceutics Drug Disposition Classification System (BDDCS) [77], presenting high solubility and permeability. Therefore, according to the FDA, it can undergo biowaiver regulation. Moreover, due to its physicochemical properties, its complexation into CDs has not been reported, to the best of our knowledge.
5.2. Glycyrrhizin
Glycyrrhizic acid (GlyA) (Figure 9) is the major triterpene glycoside contained in licorice root (Figure 9) [78].
GlyA has been accepted as a treatment for chronic viral hepatitis C for over 20 years in Japan. Due to the availability of human safety data from GlyA intravenous administration in treating hepatitis C, GlyA has been proposed for COVID-19-infected patients using dose escalation studies under a compassionate use exception [79].
Interestingly, the use of GlyA nanoparticles against COVID-19 has been recently reported [80]. The formation of ICs of GlyA with CDs has revealed that the binding affinity of GlyA to γ-CD is about 300 times higher than that to β-CD [81]. The conjugation of GlyA complexed to β-CD has demonstrated beneficial results for treating influenza virus agents (Figure 10) [82]. However, the participation of this combination addressing COVID-19 treatment has not been exploited yet, to the best of our knowledge.
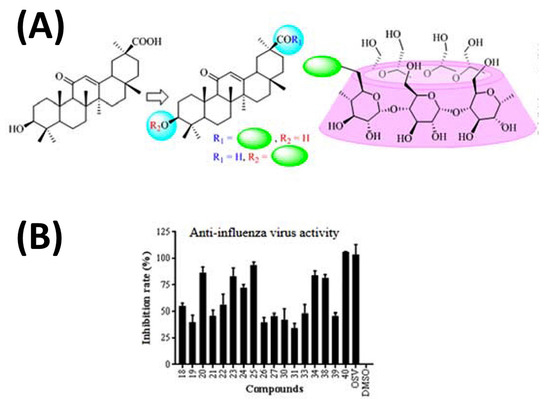
Figure 10.
Schematic representation of the synthesis approach to conjugate glycyrrhetinic (GlyA) acid (A) to β-cyclodextrin and (B) their efficacy in inhibiting influenza virus infection. Accordingly, the conjugation of GlyA complexed to β-CD has demonstrated beneficial results for treating influenza virus agents. Adapted from [82], Copyright (2021), with permission from Elsevier.
5.3. Plitidepsin
Plitidepsin (Figure 9) is a marine cyclic depsipeptide extracted from the ascidian Aplidium albicans. This compound has been actively studied due to its anticancer properties [83]. Indeed, in 2003, plitidepsin received orphan designation by the EMA (EU/3/03/151). Lately, plitidepsin has been repurposed for treating multiple myeloma because it targets cofactor Eukaryotic translation elongation factor 1 alpha (eEF1A) [84].
More recently, with the emergence of COVID-19, plitidepsin has been pointed out as a possible antiviral candidate against SARS-CoV-2 [84,85]. In fact, plitidepsin has shown antiviral activity against SARS-CoV-2 by inhibiting the activity of eEF1A. eEF1A participates in mRNA translation in RNA virus replication, being involved in the enzymatic delivery of aminoacyl tRNAs to the ribosome and the aminoacylation-dependent tRNA export pathway [86]. Plitidepsin can also inhibit the translation of the open reading frames (ORF) 1a and 1b, reducing the production of polyproteins (PP) and decreasing the amount of RNA-dependent RNA polymerase. Moreover, this drug is also responsible for inhibiting the translation of different subgenomic mRNAs, leading to a deficient production of viral structures and accessory proteins [86].
Preclinical data have revealed that plitidepsin presents potent antiviral effects against SARS-CoV-2, namely in infected Vero E6 and hACE2-293T cells, by reducing the expression of the viral structural protein N [85]. Moreover, plitidepsin underwent interventional clinical trials for COVID-19. The proof-of-concept phase 1 clinical trial, APLICOV-PC (NCT04382066), demonstrated the safety profile of plitidepsin. However, there were some limitations, namely the limited number of participants [87]. A phase 3 clinical trial, NEPTUNO (NCT04784559), is recruiting for hospitalized patients with COVID-19 of moderate severity. The therapeutic protocol includes the treatment groups that receive 1.5 or 2.5 mg/day of plitidepsin and DEX by intravenous administration and the control arms that only receive DEX [87].
Due to its hydrophobicity, with a LogP > 5, plitidepsin is nearly insoluble in aqueous media requiring an adjuvant to allow intravenous administration. Therefore, plitidepsin has been formulated using Cremophor® (CRE) and Tween 80 to increase drug solubility, although hypersensitive reactions have been reported. Taking it into consideration, other strategies have been proposed, namely the use of block copolymers, such as poly(ethylene glycol)-block-poly(γ-benzyl-L-glutamate) (PEG-b-PBLG) copolymer and poly(trimethylene carbonate)-block-poly(glutamic acid) (PTMC-b-PGA) [88]. To the best of our knowledge, the use of CDs to complex plitidepsin has not yet been addressed.
5.4. Thapsigargin
Thapsigargin (TG) (Figure 9) is a sesquiterpene lactone found in the roots and fruits of the Thapsia L. species. TG works as a non-competitive inhibitor of the sarcoplasmic/endoplasmic reticulum Ca2+ ATPase pump (SERCA) with potential applications in anticancer therapy [89].
Recently, TG has been proven to present potent antiviral properties against influenza A virus replication by the ER stress unfolded protein response (UPR) [90]. More recently, TG has been proposed to be an acid-stable inhibitor of SARS-CoV-2 [91] and other respiratory viruses, efficient in separate infections as well as in co-infections. The ER stress response seems to be the main underlying antiviral action mechanism, although it remains to be fully explored [92].
TG encapsulation into poly(lactic-co-glycolic acid) (PLGA) nanoparticles for a target release has been exploited [93,94]. As far as we know, no study has addressed the application of CDs to carrier TG. However, an interesting experiment reports the modulation of TG store-dependent Ca2+ entry in macrophages by methyl-β-CD [95].
5.5. Polyphenols
Polyphenols are secondary plant metabolites that protect them against diseases, infections, and damage [96]. Polyphenols include, but are not limited to, phenolic acids, coumarins, flavonoids, stilbenes, and lignans (Figure 9) [75].
These molecules have been reported to present a range of health benefits [97,98], including in treating infectious diseases [99,100,101]. Therefore, the use of polyphenols as SARS-CoV-2 antiviral agents has been explored. For example, an in silico study testing green tea polyphenols, e.g., epigallocatechin gallate (EGCG), epicatechin gallate, and gallocatechin-3-gallate against COVID-19 have revealed that these three components can strongly interact with the catalytic residues of the SARS-CoV-2 main protease (Mpro), constituting potential drug candidates for COVID-19 treatment [102]. Similarly, Ghosh et al. [103], using docking and molecular dynamics simulation approaches, have shown that the six polyphenols present in Broussonetia papyrifera can inhibit the catalytic activity of Mpro. Therefore, due to the promising applications of polyphenols in treating COVID-19, it is of interest to expand the research to other registered polyphenols [104]. Based on this, Wu et al. [105] have conducted a large virtual screening for more than 400 polyphenols with the potential to bind to SARS-CoV-2 Mpro or papain-like protease (PLpro), which are central proteases to the viral life cycle. Their results revealed that several polyphenols, such as petunidin 3-O-(6″-p-coumaroyl-glucoside), present promising binding interactions with SARS-CoV-2 Mpro and PLpro [105].
The translation of polyphenols from the bench to the bedside has already occurred for the treatment of COVID-19. Actually, resveratrol has been studied in a phase 2 clinical trial (NCT04400890) to evaluate its safety and explore its effectiveness for COVID-19. Moreover, the use of Caesalpinia spinosa extract is also being studied in patients with symptomatic COVID-19 (NCT04410510). Moreover, quadrate therapy of chicoric acid, 13-Cis retinoic acid (aerosolized), minocycline, and vitamin D has been explored for patients with multidrug-resistant COVID-19 (NCT05077813). Interestingly, glucoside- and rutinoside-rich crude has been investigated for vaccine-adverse reactions (NCT05387252).
Despite the promising applications of polyphenols in the treatment of COVID-19, they are susceptible to the negative impact of light, oxygen, and pH, which may hamper their extraction process and applications [106]. Therefore, the encapsulation of polyphenols into nanocarriers has been reported to circumvent these limitations [106].
Indeed, the use of CDs to complex polyphenols has already been reported with encouraging repercussions [107,108,109,110,111,112]. Moreover, taking advantage of sustainable green chemistry, CDs have been demonstrated to be promising contributors to the extraction of polyphenols [113,114,115].
6. Regulatory Issues and Toxicity
CDs have been used for a variety of purposes globally. Therefore, the need to regulate their applications has been promoted in Western countries.
In Japan, natural CDs are presented in the Japanese Pharmacopoeia and are considered food additives [116].
Furthermore, the JECFA, an international expert committee composed of members of the Food and Agriculture Organization of the United Nations (FAO) and the World Health Organization (WHO) have worked on the regulation of native CDs in food and additives, being the pharmaceutical application of CDs under the responsibility of the EMA or the FDA [117].
From 2000 to 2004, the use of native CDs as food additives was declared “Generally Recognized As Safe” (GRAS) by the FDA [117]. Moreover, the chemically modified SBE-β-CD and HP-β-CD were listed as inactive pharmaceutical ingredients and can be used in oral formulations [118]. Due to their physicochemical properties, namely their high molecular weight and hydrophilic nature, low octanol-water partition coefficients, and the presence of several hydrogen bond donors and acceptors, CDs do not promptly permeate biological membranes through passive diffusion [116]. The oral availability of CDs is minimal, without significant absorption in the gastrointestinal tract. The total daily dose for α-CDs can reach 6000 mg and for γ-CDs 10,000 mg [118]. However, the acceptable daily intake of β-CD is restricted to 5 mg/kg [118]. Moreover, at high doses greater than 1000 mg/kg/day, the oral intake of CDs leads to reversible diarrhea and cecal enlargement (EMA/CHMP/495747/2013) [119]. CDs are poorly absorbed via mucosal membranes. However, at high doses, CDs can increase drug permeability by direct action on mucosal membranes, enhancing drug absorption and/or bioavailability by topical administration. These may be due to the solubilization of membrane components with mild and reversible perturbations on the cell membrane compared to surfactants that generally induce irreversible membrane damage [118]. For instance, nasal and pulmonary formulations containing 10% HP-β-CD, RM-β-CD, or less than 1.5% of β-CD have not shown tissue damage [119]. The use of CDs in rectal products has also been addressed, and the results revealed that in humans, the use of 230 mg of β-CD in suppositories did not induce irritation in rectal mucosa. In rabbits, the use of 12% of HP-β-CD did not cause rectal mucosal irritation. Alpha-CD can damage the epithelial cell layer [118], but no rectal product on the market reports its use [119]. Applying absorption-promoting agents can enhance the dermal absorption of CDs. The use of DM-β-CDs advantageously sustains a scent for a prolonged time compared to the use of surfactants. Alpha-, β-, and γ-CDs are safe for dermal applications at up to 0.1% [118]. CDs used in eye formulations have been reported to increase drug penetration and are not toxic or irritant for the eye of the rabbits when presented in a solution up to 10% (SBE-β-CD) or 12.5% HP-β-CD [119]. The parenteral administration of CDs has been reported to be favorable, but some safety and toxicity considerations should be addressed [120]. In fact, β-CDs have demonstrated pronounced hemolytic activity compared to α- and γ-CDs [116]. However, the use of α-CD, β-CD, and ME-β-CD has not been recommended for IV administration as they present nephrotoxicity at relatively low doses. The list of CDs not recommended for parenteral administration also includes RM-β-CD, which may only be used in topical formulations because of its high hemolytic activity and nephrotoxicity. On the other hand, the parenteral administration of HP-β-CD and SBE-β-CD have been considered safe at a concentration of ca. 250 mg/kg/day in humans older than two years when given for 21 days or six months, respectively [119].
Most of the regulators agree that CDs are excipients and not integral to the drug. However, this topic can be division- and product-specific and further studies to fully assess the toxicity associated with CDs are required [117,119,121].
7. Clinical Trials
Three clinical studies have stated the application of CDs in COVID-19 (Table 8).

Table 8.
Summary of clinical trials involving cyclodextrins for the treatment of COVID-19.
Briefly, the CTRI/2021/05/033744 clinical trial aims to address the lack of information on the pharmacokinetics of REM and its vehicle SBE-β-CD in patients with renal disease, taking into account that both are excreted through the kidneys. Inclusion criteria include people between the ages of 18 and 90 of either sex with severe COVID-19 and renal disease who have an indication for treatment with REM [50].
The following clinical study, EUCTR2020-003486-19-GB, aims to compare the efficacy of Sulforadex or Sulforaphane/α-Cyclodextrin complex (SFX-01) versus placebo in treating patients with a suspected COVID-19 respiratory tract infection. Moreover, it also intends to evaluate the safety of SFX-01 and explore its underlying action mechanisms [122].
The last study, EUCTR2020-001803-17-GB, plans to evaluate the safety, tolerability, pharmacokinetics, and efficacy of REM by determining its antiviral activity and exposure to SBE-β-CD in patients up to 18 years with laboratory-confirmed COVID-19 [51].
8. Final Remarks and Future Perspectives
Despite the rapid development of vaccines and therapies that helped control COVID-19, an open path remains to eradicate the disease [123]. In this regard, this review article was intended to explore the potential of using cyclodextrins (CDs) as drug delivery systems for the treatment of viral infections, specifically for COVID-19. Resulting from the formation of inclusion complexes (ICs) between CDs and antiviral drugs, which improve the physicochemical properties of the drugs, CDs can improve the solubility, stability, and absorption of antiviral drugs as well as increase bioactivity and reduce toxicity. These complexes protect drugs from degradation, increase their solubility, and interfere with drug pharmacokinetics, improving their bioavailability and biological activity. In addition, the use of CDs can also allow for oral, inhalation, or topical drug administration, which is useful for avoiding side effects or administration problems associated with direct systemic administration. Chemical modification of CDs can also improve their inclusion properties and capacity. Considering the information gathered, it appears that β-CD is the most suitable and effective for encapsulating antiviral molecules due to its ability to increase the solubility, stability, and absorption of the molecules as well as reduce toxicity. In addition, β-CD is the most common form of cyclodextrin used due to its simple production, complexation efficiency, and low cost. However, it is important to note that other forms of cyclodextrins, such as α-CD and γ-CD, have also been studied and used to encapsulate antiviral molecules. Several anti-SARS-COVID-19 molecules are presented, including remdesivir, dexamethasone, ivermectin, interferon-beta, lopinavir/ritonavir, oseltamivir, fenofibrate, and cetylpyridinium chloride, but also new candidates such as bepridil, glycyrrhizin, plitidepsin, thapsigargin, and polyphenols which have been studied in combination with CDs to increase their efficacy in treating COVID-19. Although there are promising pre-clinical reports on CDs as drug nanocarriers for the treatment of COVID-19, their applications in clinical trials remain scarce. Some of the clinical trials mentioned are still in the early stages and have not yet been completed. Therefore, more research is needed to evaluate the safety and efficacy of these drugs combined with CDs in the treatment of COVID-19, as well as the long-term safety of all these associations.
In addition, some results indicate that CDs may also work as potential active pharmaceutical ingredients by themselves, which may influence the current regulatory landscape in using CDs.
In the future, the use of computational approaches, namely molecular dynamics, may constitute an important tool to anticipate the solubility and interaction of some drugs with CDs [124,125,126].
Author Contributions
Conceptualization, A.F. and B.A.; Formal analysis, C.D. and F.M.-M.; Funding acquisition, F.V. and A.F.; Investigation, C.D. and B.A.; Supervision, A.F.; Writing—original draft, C.D. and B.A.; Figures, C.D. and B.A.; Tables, C.D., B.A. and I.S.; Writing—review and editing, all authors. All authors have read and agreed to the published version of the manuscript.
Funding
“Fundação para a Ciência e Tecnologia” (FCT-Portugal) through the research project PTDC/NAN-MAT/1431/2021 and the Ph.D. grant 2021.08095.BD.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Sivakumar, B.; Deepthi, B. Complexity of COVID-19 dynamics. Entropy 2022, 24, 50. [Google Scholar] [CrossRef] [PubMed]
- Rehman, S.U.; Rehman, S.U.; Yoo, H.H. COVID-19 challenges and its therapeutics. Biomed. Pharmacother. 2021, 142, 112015. [Google Scholar] [CrossRef] [PubMed]
- Jicsinszky, L.; Martina, K.; Cravotto, G. Cyclodextrins in the Antiviral Therapy. J. Drug Deliv. Sci. Technol. 2021, 64, 102589. [Google Scholar] [CrossRef] [PubMed]
- Lachowicz, M.; Stańczak, A.; Kołodziejczyk, M. Characteristic of Cyclodextrins: Their Role and Use in the Pharmaceutical Technology. Curr. Drug Targets 2020, 21, 1495–1510. [Google Scholar] [CrossRef]
- Global Market Insights Global Cyclodextrin Market Size, Share and Industry Analysis Report by Type (Alpha, Beta, Gamma) and Application (Pharmaceutical, Food & Beverage, Chemicals, Cosmetics & Personal Care), Regional Outlook, Competitive Market Share & Forecast, 2021–2027. Available online: https://www.gminsights.com/industry-analysis/global-cyclodextrin-market (accessed on 5 December 2022).
- Anka, A.U.; Tahir, M.I.; Abubakar, S.D.; Alsabbagh, M.; Zian, Z.; Hamedifar, H.; Sabzevari, A.; Azizi, G. Coronavirus disease 2019 (COVID-19): An overview of the immunopathology, serological diagnosis and management. Scand. J. Immunol. 2021, 93, e12998. [Google Scholar] [CrossRef]
- Forchette, L.; Sebastian, W.; Liu, T. A Comprehensive Review of COVID-19 Virology, Vaccines, Variants, and Therapeutics. Curr. Med. Sci. 2021, 41, 1037–1051. [Google Scholar] [CrossRef]
- Salian, V.S.; Wright, J.A.; Vedell, P.T.; Nair, S.; Li, C.; Kandimalla, M.; Tang, X.; Carmona Porquera, E.M.; Kalari, K.R.; Kandimalla, K.K. COVID-19 Transmission, Current Treatment, and Future Therapeutic Strategies. Mol. Pharm. 2021, 18, 754–771. [Google Scholar] [CrossRef]
- Harrison, A.G.; Lin, T.; Wang, P. Mechanisms of SARS-CoV-2 Transmission and Pathogenesis. Trends Immunol. 2020, 41, 1100–1115. [Google Scholar] [CrossRef]
- Salasc, F.; Lahlali, T.; Laurent, E.; Rosa-Calatrava, M.; Pizzorno, A. Treatments for COVID-19: Lessons from 2020 and new therapeutic options. Curr. Opin. Pharmacol. 2022, 62, 43–59. [Google Scholar] [CrossRef]
- Harvey, W.T.; Carabelli, A.M.; Jackson, B.; Gupta, R.K.; Thomson, E.C.; Harrison, E.M.; Ludden, C.; Reeve, R.; Rambaut, A.; Peacock, S.J.; et al. SARS-CoV-2 variants, spike mutations and immune escape. Nat. Rev. Microbiol. 2021, 19, 409–424. [Google Scholar] [CrossRef]
- Kotta, S.; Aldawsari, H.M.; Badr-Eldin, S.M.; Alhakamy, N.A.; Md, S.; Nair, A.B.; Deb, P.K. Combating the Pandemic COVID-19: Clinical Trials, Therapies and Perspectives. Front. Mol. Biosci. 2020, 7, 336. [Google Scholar] [CrossRef] [PubMed]
- Alam, S.; Kamal, T.B.; Sarker, M.M.R.; Zhou, J.R.; Rahman, S.M.A.; Mohamed, I.N. Therapeutic Effectiveness and Safety of Repurposing Drugs for the Treatment of COVID-19: Position Standing in 2021. Front. Pharmacol. 2021, 12, 659577. [Google Scholar] [CrossRef] [PubMed]
- Bhandari, R.; Khanna, G.; Kuhad, A. Pharmacological insight into potential therapeutic agents for the deadly COVID-19 pandemic. Eur. J. Pharmacol. 2021, 890, 173643. [Google Scholar] [CrossRef]
- Santos, J.C.; Ribeiro, M.L.; Gambero, A. The Impact of Polyphenols-Based Diet on the Inflammatory Profile in COVID-19 Elderly and Obese Patients. Front. Physiol. 2021, 11, 1783. [Google Scholar] [CrossRef] [PubMed]
- Alanagreh, L.; Alzoughool, F.; Atoum, M. The Human Coronavirus Disease COVID-19: Its Origin, Characteristics, and Insights into Potential Drugs and Its Mechanisms. Pathogens 2020, 9, 331. [Google Scholar] [CrossRef]
- Khani, E.; Khiali, S.; Entezari-Maleki, T. Potential COVID-19 Therapeutic Agents and Vaccines: An Evidence-Based Review. J. Clin. Pharmacol. 2021, 61, 429–460. [Google Scholar] [CrossRef]
- Angel, M. Compounds with Therapeutic Potential against Novel Respiratory 2019 Coronavirus Miguel. Antimicrob. Agents Chemother. 2020, 64, e00399-20. [Google Scholar]
- Fang, E.; Liu, X.; Li, M.; Zhang, Z.; Song, L.; Zhu, B.; Wu, X.; Liu, J.; Zhao, D.; Li, Y. Advances in COVID-19 mRNA vaccine development. Signal Transduct. Target. Ther. 2022, 7, 94. [Google Scholar] [CrossRef]
- Barbier, A.J.; Jiang, A.Y.; Zhang, P.; Wooster, R.; Anderson, D.G. The clinical progress of mRNA vaccines and immunotherapies. Nat. Biotechnol. 2022, 40, 840–854. [Google Scholar] [CrossRef]
- Hidayati, H.B.; Octavia, E.; Srisetyaningrum, C.T. Antiviral therapy for COVID-2019. Anaesth. Pain Intensive Care 2021, 25, 387–392. [Google Scholar] [CrossRef]
- Negrut, N.; Codrean, A.; Hodisan, I.; Bungau, S.; Tit, D.; Marin, R.; Behl, T.; Banica, F.; Diaconu, C.; Nistor-Cseppento, D. Efficiency of antiviral treatment in COVID-19. Exp. Ther. Med. 2021, 21, 648. [Google Scholar] [CrossRef] [PubMed]
- Gavriatopoulou, M.; Ntanasis-Stathopoulos, I.; Korompoki, E.; Fotiou, D.; Migkou, M.; Tzanninis, I.G.; Psaltopoulou, T.; Kastritis, E.; Terpos, E.; Dimopoulos, M.A. Emerging treatment strategies for COVID-19 infection. Clin. Exp. Med. 2021, 21, 167–179. [Google Scholar] [CrossRef] [PubMed]
- Cao, H.; Ming, L.; Chen, L.; Zhu, X.; Shi, Y. The Effectiveness of Convalescent Plasma for the Treatment of Novel Corona Virus Disease 2019: A Systematic Review and Meta-Analysis. Front. Med. 2021, 8, 1618. [Google Scholar] [CrossRef]
- Tao, K.; Jagannathan, P.; Shafer, W. SARS-CoV-2 Antiviral Therapy. Clin. Microbiol. Rev. 2021, 34, e00109-21. [Google Scholar] [CrossRef]
- Wu, Y.; Wang, F.; Shen, C.; Peng, W.; Li, D.; Zhao, C.; Li, Z.; Li, S.; Bi, Y.; Yang, Y.; et al. A noncompeting pair of human neutralizing antibodies block COVID-19 virus binding to its receptor ACE2. Science 2020, 368, 1274–1278. [Google Scholar] [CrossRef] [PubMed]
- Zost, S.J.; Gilchuk, P.; Case, J.B.; Binshtein, E.; Chen, R.E.; Nkolola, J.P.; Schäfer, A.; Reidy, J.X.; Trivette, A.; Nargi, R.S.; et al. Potently neutralizing and protective human antibodies against SARS-CoV-2. Nature 2020, 584, 443–449. [Google Scholar] [CrossRef] [PubMed]
- Chi, X.; Yan, R.; Zhang, J.; Zhang, G.; Zhang, Y.; Hao, M.; Zhang, Z.; Fan, P.; Dong, Y.; Yang, Y.; et al. A neutralizing human antibody binds to the N-terminal domain of the Spike protein of SARS-CoV-2. Science 2020, 369, 650–655. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Wang, P.; Nair, M.S.; Yu, J.; Rapp, M.; Wang, Q.; Luo, Y.; Chan, J.F.W.; Sahi, V.; Figueroa, A.; et al. Potent neutralizing antibodies against multiple epitopes on SARS-CoV-2 spike. Nature 2020, 584, 450–456. [Google Scholar] [CrossRef]
- Taylor, P.C.; Adams, A.C.; Hufford, M.M.; de la Torre, I.; Winthrop, K.; Gottlieb, R.L. Neutralizing monoclonal antibodies for treatment of COVID-19. Nat. Rev. Immunol. 2021, 21, 382–393. [Google Scholar] [CrossRef]
- Li, Z.; Li, S.; Zhang, G.; Peng, W.; Chang, Z.; Zhang, X.; Fan, Z.; Chai, Y.; Wang, F.; Zhao, X.; et al. An engineered bispecific human monoclonal antibody against SARS-CoV-2. Nat. Immunol. 2022, 23, 423–430. [Google Scholar] [CrossRef]
- Abraham, J. Monoclonal Antibodies with Extended Half-Life to Prevent COVID-19. N. Engl. J. Med. 2022, 386, 2236–2238. [Google Scholar] [CrossRef] [PubMed]
- Sodeifian, F.; Nikfarjam, M.; Kian, N.; Mohamed, K.; Rezaei, N. The role of type I interferon in the treatment of COVID-19. J. Med. Virol. 2022, 94, 63–81. [Google Scholar] [CrossRef] [PubMed]
- Spagnuolo, V.; Guffanti, M.; Galli, L.; Poli, A.; Querini, P.R.; Ripa, M.; Clementi, M.; Scarpellini, P.; Lazzarin, A.; Tresoldi, M.; et al. Viral clearance after early corticosteroid treatment in patients with moderate or severe COVID-19. Sci. Rep. 2020, 10, 21291. [Google Scholar] [CrossRef] [PubMed]
- Sterne, J.A.C.; Murthy, S.; Diaz, J.V.; Slutsky, A.S.; Villar, J.; Angus, D.C.; Annane, D.; Azevedo, L.C.P.; Berwanger, O.; Cavalcanti, A.B.; et al. Association between Administration of Systemic Corticosteroids and Mortality among Critically Ill Patients with COVID-19: A Meta-analysis. JAMA-J. Am. Med. Assoc. 2020, 324, 1330–1341. [Google Scholar] [CrossRef]
- van de Veerdonk, F.L.; Giamarellos-Bourboulis, E.; Pickkers, P.; Derde, L.; Leavis, H.; van Crevel, R.; Engel, J.J.; Wiersinga, W.J.; Vlaar, A.P.J.; Shankar-Hari, M.; et al. A guide to immunotherapy for COVID-19. Nat. Med. 2022, 28, 39–50. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.U.; Lee, S.S.; Lee, S.; Oh, H. Bin Noncovalent complexes of cyclodextrin with small organic molecules: Applications and insights into host–guest interactions in the gas phase and condensed phase. Molecules 2020, 25, 4048. [Google Scholar] [CrossRef]
- Soni, S.S.; Alsasa, A.; Rodell, C.B. Applications of Macrocyclic Host Molecules in Immune Modulation and Therapeutic Delivery. Front. Chem. 2021, 9, 190. [Google Scholar] [CrossRef]
- Garrido, P.F.; Calvelo, M.; Blanco-González, A.; Veleiro, U.; Suárez, F.; Conde, D.; Cabezón, A.; Piñeiro, Á.; Garcia-Fandino, R. The Lord of the NanoRings: Cyclodextrins and the battle against SARS-CoV-2. Int. J. Pharm. 2020, 588, 119689. [Google Scholar] [CrossRef]
- Chaves, O.A.; Sacramento, C.Q.; Ferreira, A.C.; Mattos, M.; Fintelman-Rodrigues, N.; Temerozo, J.R.; Vazquez, L.; Pinto, D.P.; da Silveira, G.P.E.; da Fonseca, L.B.; et al. Atazanavir Is a Competitive Inhibitor of SARS-CoV-2 Mpro, Impairing Variants Replication In Vitro and In Vivo. Pharmaceuticals 2021, 15, 21. [Google Scholar] [CrossRef]
- Owen, D.R.; Allerton, C.M.N.; Anderson, A.S.; Aschenbrenner, L.; Avery, M.; Berritt, S.; Boras, B.; Cardin, R.D.; Carlo, A.; Coffman, K.J.; et al. An oral SARS-CoV-2 Mpro inhibitor clinical candidate for the treatment of COVID-19. Science 2021, 374, 1586–1593. [Google Scholar] [CrossRef]
- Hau, R.K.; Wright, S.H.; Cherrington, N.J. PF-07321332 (Nirmatrelvir) does not interact with human ENT1 or ENT2: Implications for COVID-19 patients. Clin. Transl. Sci. 2022, 15, 1599–1605. [Google Scholar] [CrossRef]
- Joshi, S.; Parkar, J.; Ansari, A.; Vora, A.; Talwar, D. Role of favipiravir in the treatment of COVID-19. Int. J. Infect. Dis. 2021, 102, 501–508. [Google Scholar] [CrossRef] [PubMed]
- Łagocka, R.; Dziedziejko, V.; Kłos, P.; Pawlik, A. Favipiravir in therapy of viral infections. J. Clin. Med. 2021, 10, 273. [Google Scholar] [CrossRef] [PubMed]
- Braga, S.S.; Barbosa, J.S.; Santos, N.E.; El-Saleh, F.; Paz, F.A.A. Cyclodextrins in antiviral therapeutics and vaccines. Pharmaceutics 2021, 13, 409. [Google Scholar] [CrossRef] [PubMed]
- Várnai, B.; Malanga, M.; Sohajda, T.; Béni, S. Molecular interactions in remdesivir-cyclodextrin systems. J. Pharm. Biomed. Anal. 2022, 209, 114482. [Google Scholar] [CrossRef] [PubMed]
- Szente, L.; Puskás, I.; Sohajda, T.; Varga, E.; Vass, P.; Nagy, Z.K.; Farkas, A.; Várnai, B.; Béni, S.; Hazai, E. Sulfobutylether-beta-cyclodextrin-enabled antiviral remdesivir: Characterization of electrospun- and lyophilized formulations. Carbohydr. Polym. 2021, 264, 118011. [Google Scholar] [CrossRef] [PubMed]
- Piñeiro, Á.; Pipkin, J.; Antle, V.; Garcia-Fandino, R. Remdesivir interactions with sulphobutylether-β-cyclodextrins: A case study using selected substitution patterns. J. Mol. Liq. 2022, 346, 117157. [Google Scholar] [CrossRef]
- Piñeiro, Á.; Pipkin, J.; Antle, V.; Garcia-Fandino, R. Aggregation versus inclusion complexes to solubilize drugs with cyclodextrins. A case study using sulphobutylether-β-cyclodextrins and remdesivir. J. Mol. Liq. 2021, 343, 117588. [Google Scholar] [CrossRef]
- Adamsick, M.L.; Gandhi, R.G.; Bidell, M.R.; Elshaboury, R.H.; Bhattacharyya, R.P.; Kim, A.Y.; Nigwekar, S.; Rhee, E.P. Remdesivir in patients with acute or chronic kidney disease and COVID-19. J. Am. Soc. Nephrol. 2020, 31, 1384–1386. [Google Scholar] [CrossRef]
- Gilead Sciences Inc. A Phase 2/3 Single-Arm, Open-Label Study to Evaluate the Safety, Tolerability, Pharmacokinetics, and Efficacy of Remdesivir (GS-5734TM) in Participants from Birth to <18 Years of Age with COVID-19. Available online: https://www.clinicaltrials.gov/ct2/show/NCT04431453 (accessed on 23 January 2023).
- Noreen, S.; Maqbool, I.; Madni, A. Dexamethasone: Therapeutic potential, risks, and future projection during COVID-19 pandemic. Eur. J. Pharmacol. 2020, 894, 173854. [Google Scholar] [CrossRef]
- Belhocine, Y.; Rahali, S.; Allal, H.; Assaba, I.M.; Ghoniem, M.G.; Ali, F.A.M. A dispersion corrected DFT investigation of the inclusion complexation of dexamethasone with β-cyclodextrin and molecular docking study of its potential activity against COVID-19. Molecules 2021, 26, 7622. [Google Scholar] [CrossRef] [PubMed]
- Canga, A.G.; Prieto, A.M.S.; Diez Liébana, M.J.; Martínez, N.F.; Sierra Vega, M.; García Vieitez, J.J. The pharmacokinetics and interactions of ivermectin in humans—A mini-review. AAPS J. 2008, 10, 42–46. [Google Scholar] [CrossRef] [PubMed]
- Levin, A.; Armon-Omer, A.; Rosenbluh, J.; Melamed-Book, N.; Graessmann, A.; Waigmann, E.; Loyter, A. Inhibition of HIV-1 integrase nuclear import and replication by a peptide bearing integrase putative nuclear localization signal. Retrovirology 2009, 6, 112. [Google Scholar] [CrossRef] [PubMed]
- Wagstaff, K.M.; Headey, S.; Telwatte, S.; Tyssen, D.; Hearps, A.C.; Thomas, D.R.; Tachedjian, G.; Jans, D.A. Molecular dissection of an inhibitor targeting the HIV integrase dependent preintegration complex nuclear import. Cell. Microbiol. 2019, 21, e12953. [Google Scholar] [CrossRef] [PubMed]
- Cruciani, M.; Pati, I.; Masiello, F.; Malena, M.; Pupella, S.; De Angelis, V. Ivermectin for prophylaxis and treatment of COVID-19: A systematic review and meta-analysis. Diagnostics 2021, 11, 1645. [Google Scholar] [CrossRef] [PubMed]
- Khandelwal, A.; Singh, G.P.; Jamil, S. Ivermectin as a multifaceted drug in COVID-19: Current insights. Med. J. Armed Forces India 2021, 77, S254–S256. [Google Scholar] [CrossRef] [PubMed]
- Mansour, S.M.; Shamma, R.N.; Ahmed, K.A.; Sabry, N.A.; Esmat, G.; Mahmoud, A.A.; Maged, A. Safety of inhaled ivermectin as a repurposed direct drug for treatment of COVID-19: A preclinical tolerance study. Int. Immunopharmacol. 2021, 99, 108004. [Google Scholar] [CrossRef]
- Sosa, J.P.; Caceres, M.M.F.; Comptis, J.R.; Quiros, J.; Príncipe-Meneses, F.S.; Riva-Moscoso, A.; Belizaire, M.P.; Malanyaon, F.Q.; Agadi, K.; Jaffery, S.S.; et al. Effects of interferon beta in COVID-19 adult patients: Systematic review. Infect. Chemother. 2021, 53, 247–260. [Google Scholar] [CrossRef]
- Acu, E.; Arellano, G.; Morales, P.; Sotomayor, P.; Gonz, L.F.; Oyarzun-ampuero, F.; Naves, R. Intranasal delivery of interferon- β-loaded nanoparticles induces control of neuroinflammation in a preclinical model of multiple sclerosis: A promising simple, effective, non-invasive, and low-cost therapy. J. Control. Release 2021, 331, 443–459. [Google Scholar] [CrossRef]
- Adeoye, O.; Conceição, J.; Serra, P.A.; Bento da Silva, A.; Duarte, N.; Guedes, R.C.; Corvo, M.C.; Aguiar-Ricardo, A.; Jicsinszky, L.; Casimiro, T.; et al. Cyclodextrin solubilization and complexation of antiretroviral drug lopinavir: In silico prediction; Effects of derivatization, molar ratio and preparation method. Carbohydr. Polym. 2020, 227, 115287. [Google Scholar] [CrossRef]
- Cvetkovic, R.S.; Goa, K.L. Lopinavir/ritonavir: A review of its use in the management of HIV infection. Drugs 2003, 63, 769–802. [Google Scholar] [CrossRef] [PubMed]
- Patel, T.K.; Patel, P.B.; Barvaliya, M.; Saurabh, M.K.; Bhalla, H.L.; Khosla, P.P. Efficacy and safety of lopinavir-ritonavir in COVID-19: A systematic review of randomized controlled trials. J. Infect. Public Health 2021, 14, 740–748. [Google Scholar] [CrossRef]
- Adeoye, O.; Bártolo, I.; Conceição, J.; da Silva, A.B.; Duarte, N.; Francisco, A.P.; Taveira, N.; Cabral-Marques, H. Pyromellitic dianhydride crosslinked soluble cyclodextrin polymers: Synthesis, lopinavir release from sub-micron sized particles and anti-HIV-1 activity. Int. J. Pharm. 2020, 583, 119356. [Google Scholar] [CrossRef] [PubMed]
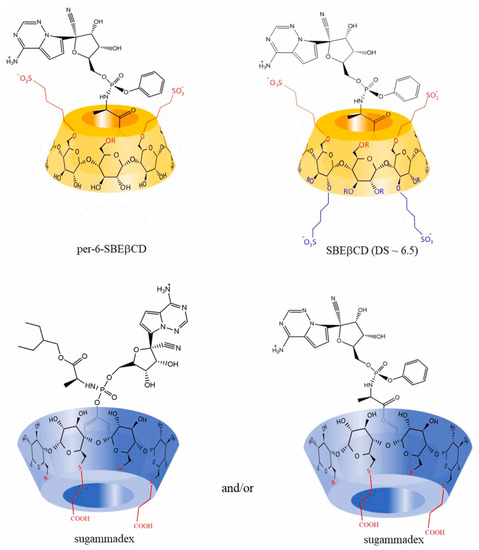
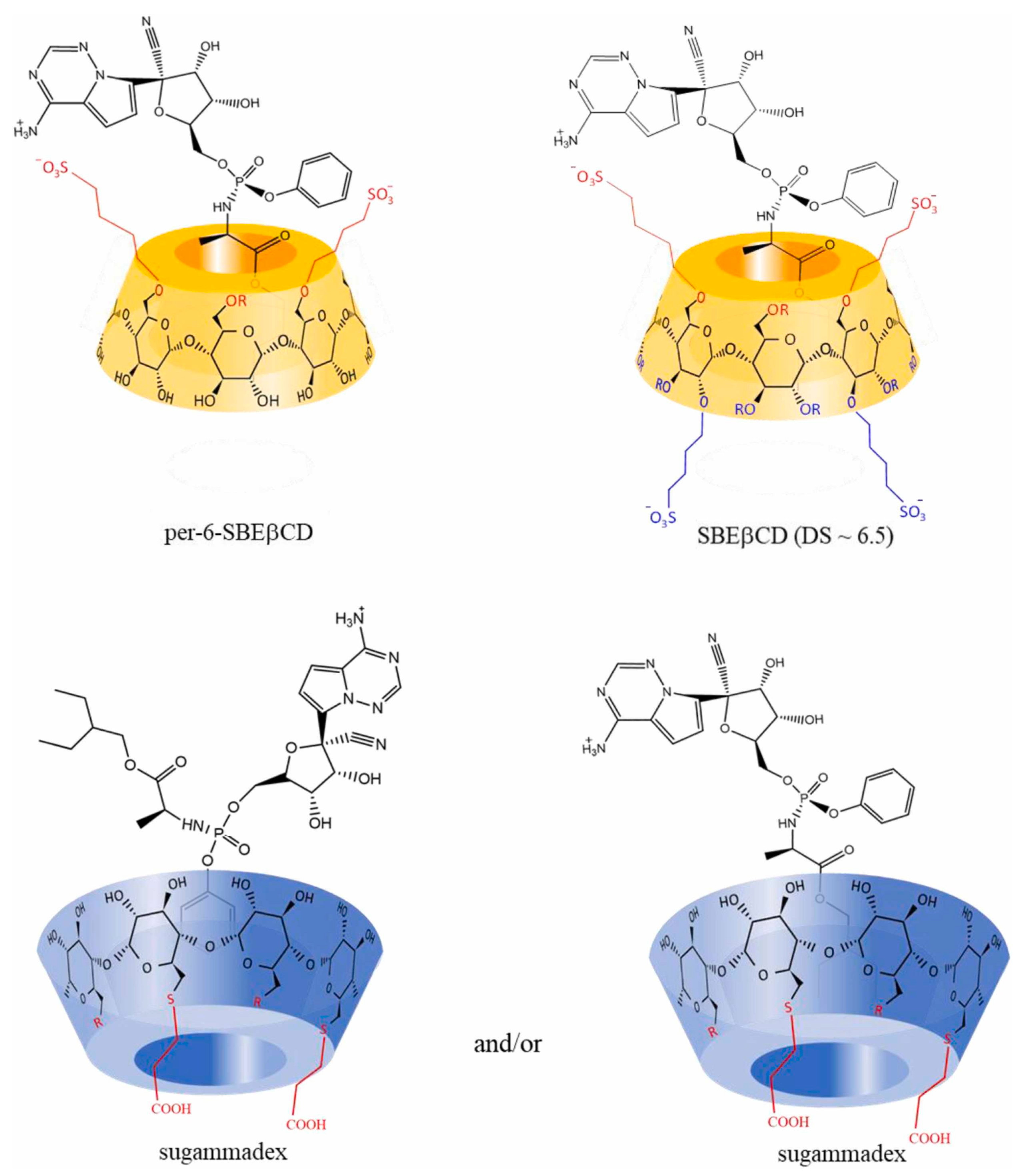
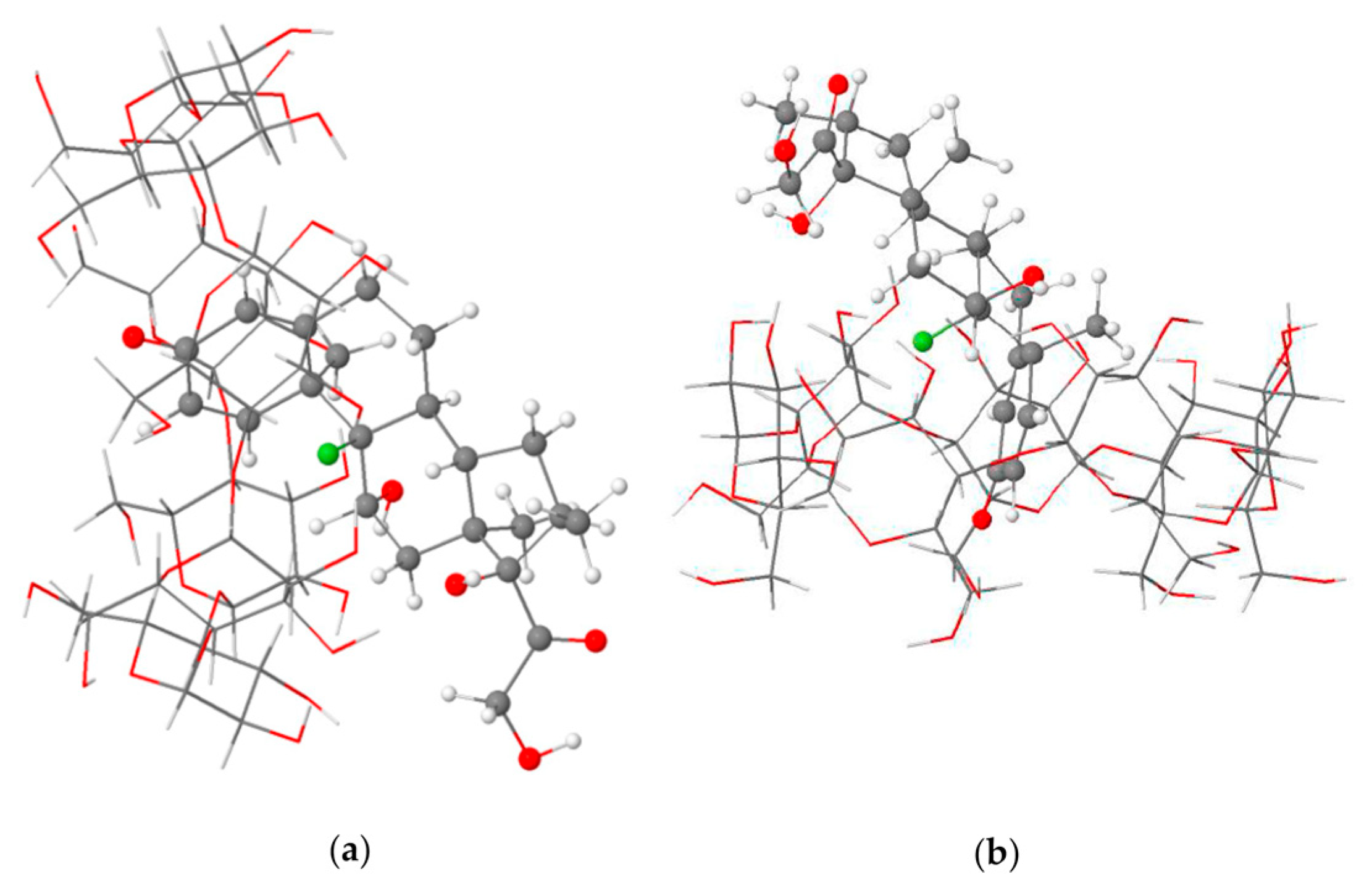
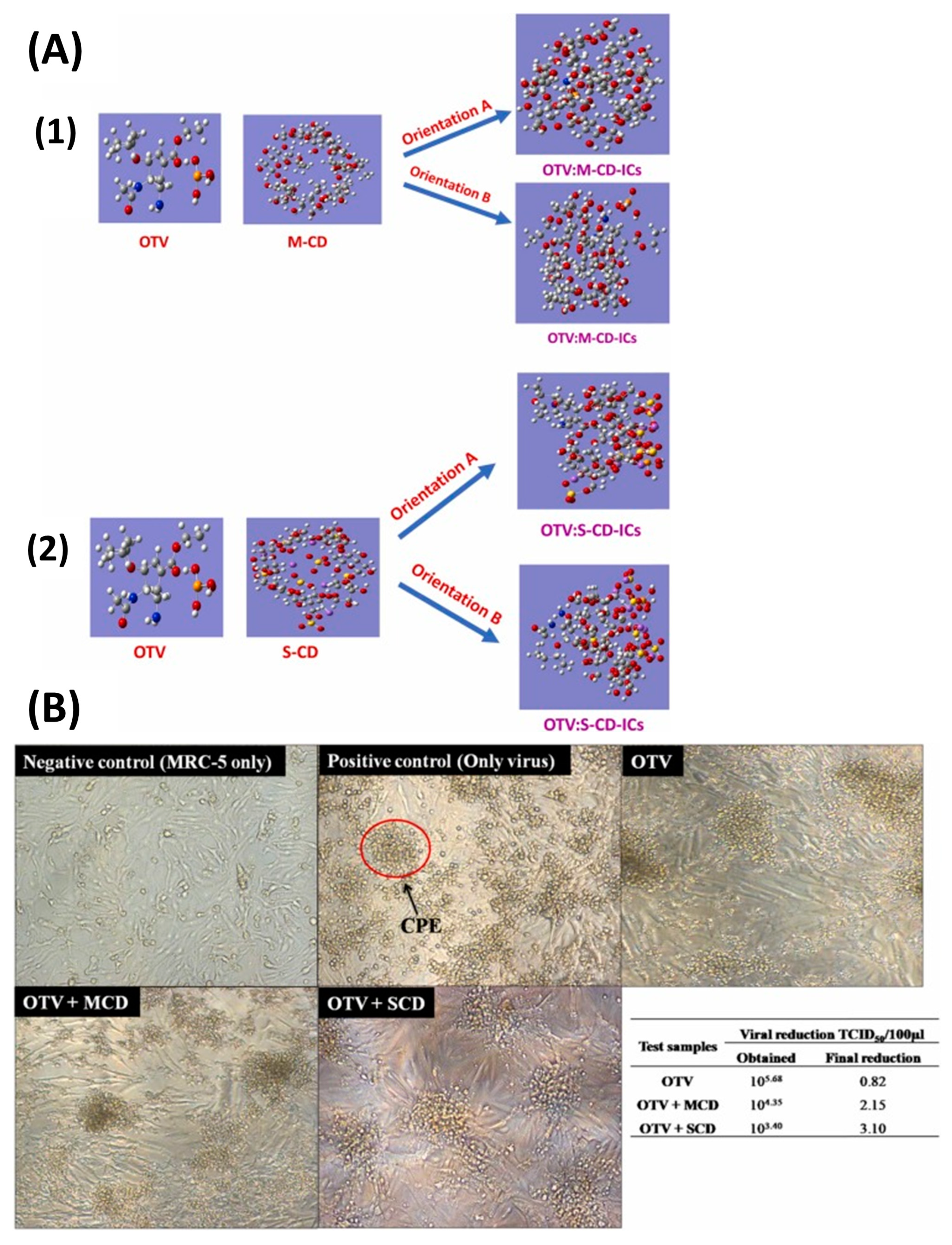
- Rajamohan, R.; Mohandoss, S.; Ashokkumar, S.; Madi, F.; Leila, N.; Murugavel, K.; Lee, Y.R. A novel and water-soluble material for coronavirus inactivation from oseltamivir in the cavity of methyl and sulfated-β-cyclodextrins through inclusion complexation. J. Pharm. Biomed. Anal. 2022, 221, 115057. [Google Scholar] [CrossRef] [PubMed]
- Hasson, K.J. Innovated formulation of oseltamivir powder for suspension with stability study after reconstitution using a developed ion-pair reversed phase high-performance liquid chromatography method. J. Adv. Pharm. Technol. Res. 2022, 13, 177–181. [Google Scholar] [CrossRef] [PubMed]
- Deepak Gunwal; Braham Dutt; Manjusha Choudhary; Vikas Budhwar A Comprehensive Review on the Drug: Fenofibrate. Int. J. Res. Pharm. Sci. 2021, 12, 2164–2172. [CrossRef]
- Pawar, A.; Pal, A.; Goswami, K.; Squitti, R.; Rongiolettie, M. Molecular basis of quercetin as a plausible common denominator of macrophage-cholesterol-fenofibrate dependent potential COVID-19 treatment axis. Results Chem. 2021, 3, 100148. [Google Scholar] [CrossRef]
- Chirinos, J.A.; Lopez-Jaramillo, P.; Giamarellos-Bourboulis, E.J.; Dávila-del-Carpio, G.H.; Bizri, A.R.; Andrade-Villanueva, J.F.; Salman, O.; Cure-Cure, C.; Rosado-Santander, N.R.; Cornejo Giraldo, M.P.; et al. A randomized clinical trial of lipid metabolism modulation with fenofibrate for acute coronavirus disease 2019. Nat. Metab. 2022, 4, 1847–1857. [Google Scholar] [CrossRef]
- Jagdale, S.K.; Dehghan, M.H.; Paul, N.S. Enhancement of dissolution of fenofibrate using complexation with hydroxy propyl β-cyclodextrin. Turk. J. Pharm. Sci. 2019, 16, 48–53. [Google Scholar] [CrossRef]
- Pérez-Errázuriz, S.; Velasco-Ortega, E.; Jiménez-Guerra, Á.; Aguilera-Navarro, E. Cetylpyridinium Chloride as a Tool Against COVID-19. Int. J. Odontostomatol. 2021, 15, 27–30. [Google Scholar] [CrossRef]
- Okamoto, N.; Saito, A.; Okabayashi, T.; Komine, A. Virucidal activity and mechanism of action of cetylpyridinium chloride against SARS-CoV-2. J. Oral Maxillofac. Surg. Med. Pathol. 2022, 34, 800–804. [Google Scholar] [CrossRef] [PubMed]
- Vilchez-Chavez, A.; Carruitero, M.J.; Chavez-Cruzado, E. Cetylpyridinium chloride mouthwashes: Potential role in COVID-19 control. J. Oral Maxillofac. Surg. Med. Pathol. 2022, 34, 213. [Google Scholar] [CrossRef] [PubMed]
- Hano, C.; Tungmunnithum, D. Plant Polyphenols, More than Just Simple Natural Antioxidants: Oxidative Stress, Aging and Age-Related Diseases. Medicines 2020, 7, 26. [Google Scholar] [CrossRef]
- Vatansever, E.C.; Yang, K.S.; Drelich, A.K.; Kratch, K.C.; Cho, C.C.; Kempaiah, K.R.; Hsu, J.C.; Mellott, D.M.; Xu, S.; Tseng, C.T.K.; et al. Bepridil is potent against SARS-CoV-2 in vitro. Proc. Natl. Acad. Sci. USA 2021, 118, e2012201118. [Google Scholar] [CrossRef] [PubMed]
- Benet, L.Z.; Broccatelli, F.; Oprea, T.I. BDDCS Applied to Over 900 Drugs. AAPS J. 2011, 13, 519–547. [Google Scholar] [CrossRef]
- Wahab, S.; Annadurai, S.; Abullais, S.S.; Das, G.; Ahmad, W.; Ahmad, M.F.; Kandasamy, G.; Vasudevan, R.; Ali, M.S.; Amir, M. Glycyrrhiza glabra (Licorice): A comprehensive review on its phytochemistry, biological activities, clinical evidence and toxicology. Plants 2021, 10, 2751. [Google Scholar] [CrossRef]
- Al-Kamel, H.; Grundmann, O. Glycyrrhizin as a Potential Treatment for the Novel Coronavirus (COVID-19). Mini-Rev. Med. Chem. 2021, 21, 2204–2208. [Google Scholar] [CrossRef]
- Zhao, Z.; Xiao, Y.; Xu, L.; Liu, Y.; Jiang, G.; Wang, W.; Li, B.; Zhu, T.; Tan, Q.; Tang, L.; et al. Glycyrrhizic Acid Nanoparticles as Antiviral and Anti-inflammatory Agents for COVID-19 Treatment. ACS Appl. Mater. Interfaces 2021, 13, 20995–21006. [Google Scholar] [CrossRef] [PubMed]
- Oda, M.; Kuroda, M. Molecular dynamics simulations of inclusion complexation of glycyrrhizic acid and cyclodextrins (1:1) in water. J. Incl. Phenom. Macrocycl. Chem. 2016, 85, 271–279. [Google Scholar] [CrossRef]
- Liang, S.; Li, M.; Yu, X.; Jin, H.; Zhang, Y.; Zhang, L.; Zhou, D.; Xiao, S. Synthesis and structure-activity relationship studies of water-soluble β-cyclodextrin-glycyrrhetinic acid conjugates as potential anti-influenza virus agents. Eur. J. Med. Chem. 2019, 166, 328–338. [Google Scholar] [CrossRef]
- Alonso-Álvarez, S.; Pardal, E.; Sánchez-Nieto, D.; Navarro, M.; Caballero, M.D.; Mateos, M.V.; Martin, A. Plitidepsin: Design, development, and potential place in therapy. Drug Des. Devel. Ther. 2017, 11, 253–264. [Google Scholar] [CrossRef] [PubMed]
- Martinez, M.A. Plitidepsin: A Repurposed Drug for the Treatment of COVID-19. Antimicrob. Agents Chemother. 2021, 65, e00200-21. [Google Scholar] [CrossRef] [PubMed]
- White, K.M.; Rosales, R.; Yildiz, S.; Kehrer, T.; Miorin, L.; Moreno, E.; Jangra, S.; Uccellini, M.B.; Rathnasinghe, R.; Coughlan, L.; et al. Plitidepsin has potent preclinical efficacy against SARS-CoV-2 by targeting the host protein eEF1A. Science 2021, 371, 926–931. [Google Scholar] [CrossRef]
- Papapanou, M.; Papoutsi, E.; Giannakas, T.; Katsaounou, P. Plitidepsin: Mechanisms and clinical profile of a promising antiviral agent against COVID-19. J. Pers. Med. 2021, 11, 668. [Google Scholar] [CrossRef] [PubMed]
- Varona, J.F.; Landete, P.; Lopez-Martin, J.A.; Estrada, V.; Paredes, R.; Guisado-Vasco, P.; de Orueta, L.F.; Torralba, M.; Fortun, J.; Vates, R.; et al. Preclinical and randomized phase I studies of plitidepsin in adults hospitalized with COVID-19. Life Sci. Alliance 2022, 5, e202101200. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, H.; Thevenot, J.; Garanger, E.; Ibarboure, E.; Calvo, P.; Aviles, P.; Guillen, M.J.; Lecommandoux, S. Nano-encapsulation of plitidepsin: In vivo pharmacokinetics, biodistribution, and efficacy in a renal xenograft tumor model. Pharm. Res. 2014, 31, 983–991. [Google Scholar] [CrossRef]
- Jaskulska, A.; Janecka, A.E.; Gach-Janczak, K. Thapsigargin—From traditional medicine to anticancer drug. Int. J. Mol. Sci. 2021, 22, 4. [Google Scholar] [CrossRef]
- Goulding, L.V.; Yang, J.; Jiang, Z.; Zhang, H.; Lea, D.; Emes, R.D.; Dottorini, T.; Pu, J.; Liu, J.; Chang, K.C. Thapsigargin at non-cytotoxic levels induces a potent host antiviral response that blocks influenza a virus replication. Viruses 2020, 12, 1093. [Google Scholar] [CrossRef]
- Shaban, M.S.; Mayr-Buro, C.; Meier-Soelch, J.; Albert, B.V.; Schmitz, M.L.; Ziebuhr, J.; Kracht, M. Thapsigargin: Key to new host-directed coronavirus antivirals? Trends Pharmacol. Sci. 2022, 43, 557–568. [Google Scholar] [CrossRef]
- Al-Beltagi, S.; Preda, C.A.; Goulding, L.V.; James, J.; Pu, J.; Skinner, P.; Jiang, Z.; Wang, B.L.; Yang, J.; Banyard, A.C.; et al. Thapsigargin is a broad-spectrum inhibitor of major human respiratory viruses: Coronavirus, respiratory syncytial virus and influenza a virus. Viruses 2021, 13, 234. [Google Scholar] [CrossRef]
- Cheng, F.Y.; Lee, Y.H.; Hsu, Y.H.; Chiu, I.J.; Chiu, Y.J.; Lin, Y.F.; Chiu, H.W. Promising therapeutic effect of thapsigargin nanoparticles on chronic kidney disease through the activation of Nrf2 and FoxO1. Aging 2019, 11, 9875–9892. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Cai, F.; Li, Y.; Chen, J.; Han, F.; Lin, W. A review of the application of nanoparticles in the diagnosis and treatment of chronic kidney disease. Bioact. Mater. 2020, 5, 732–743. [Google Scholar] [CrossRef] [PubMed]
- Krutetskaya, Z.I.; Milenina, L.S.; Naumova, A.A.; Butov, S.N.; Antonov, V.G.; Nozdrachev, A.D. Methyl-β-cyclodextrin modulates thapsigargin-induced store-dependent Ca2+ entry in macrophages. Dokl. Biochem. Biophys. 2017, 473, 88–90. [Google Scholar] [CrossRef] [PubMed]
- Amawi, H.; Ashby, C.R.; Samuel, T.; Peraman, R.; Tiwari, A.K. Polyphenolic nutrients in cancer chemoprevention and metastasis: Role of the epithelial-to-mesenchymal (EMT) pathway. Nutrients 2017, 9, 911. [Google Scholar] [CrossRef]
- Cory, H.; Passarelli, S.; Szeto, J.; Tamez, M.; Mattei, J. The Role of Polyphenols in Human Health and Food Systems: A Mini-Review. Front. Nutr. 2018, 5, 87. [Google Scholar] [CrossRef]
- Zhang, Z.; Li, X.; Sang, S.; McClements, D.J.; Chen, L.; Long, J.; Jiao, A.; Jin, Z.; Qiu, C. Polyphenols as Plant-Based Nutraceuticals: Health Effects, Encapsulation, Nano-Delivery, and Application. Foods 2022, 11, 2189. [Google Scholar] [CrossRef] [PubMed]
- Turuvekere Vittala Murthy, N.; Agrahari, V.; Chauhan, H. Polyphenols against infectious diseases: Controlled release nano-formulations. Eur. J. Pharm. Biopharm. 2021, 161, 66–79. [Google Scholar] [CrossRef]
- Montenegro-Landívar, M.F.; Tapia-Quirós, P.; Vecino, X.; Reig, M.; Valderrama, C.; Granados, M.; Cortina, J.L.; Saurina, J. Polyphenols and their potential role to fight viral diseases: An overview. Sci. Total Environ. 2021, 801, 149719. [Google Scholar] [CrossRef]
- Alzaabi, M.M.; Hamdy, R.; Ashmawy, N.S.; Hamoda, A.M.; Alkhayat, F.; Khademi, N.N.; Al Joud, S.M.A.; El-Keblawy, A.A.; Soliman, S.S.M. Flavonoids are promising safe therapy against COVID-19. Phytochem. Rev. 2022, 21, 291–312. [Google Scholar] [CrossRef]
- Ghosh, R.; Chakraborty, A.; Biswas, A.; Chowdhuri, S. Evaluation of green tea polyphenols as novel corona virus (SARS-CoV-2) main protease (Mpro) inhibitors–an in silico docking and molecular dynamics simulation study. J. Biomol. Struct. Dyn. 2021, 39, 4362–4374. [Google Scholar] [CrossRef]
- Ghosh, R.; Chakraborty, A.; Biswas, A.; Chowdhuri, S. Identification of polyphenols from Broussonetia papyrifera as SARS-CoV-2 main protease inhibitors using in silico docking and molecular dynamics simulation approaches. J. Biomol. Struct. Dyn. 2021, 39, 6747–6760. [Google Scholar] [CrossRef] [PubMed]
- Neveu, V.; Perez-Jimenez, J.; Vos, F.; Crespy, V.; du Chaffaut, L.; Mennen, L.; Knox, C.; Eisner, R.; Cruz, J.; Wishart, D.; et al. Phenol-Explorer: An online comprehensive database on polyphenol contents in foods. Database 2010, 2010, bap024. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Pegan, S.D.; Crich, D.; Desrochers, E.; Starling, E.B.; Hansen, M.C.; Booth, C.; Nicole Mullininx, L.; Lou, L.; Chang, K.Y.; et al. Polyphenols as alternative treatments of COVID-19. Comput. Struct. Biotechnol. J. 2021, 19, 5371–5380. [Google Scholar] [CrossRef] [PubMed]
- Milinčić, D.D.; Popović, D.A.; Lević, S.M.; Kostić, A.; Tešić, Ž.L.; Nedović, V.A.; Pešić, M.B. Application of polyphenol-loaded nanoparticles in food industry. Nanomaterials 2019, 9, 1629. [Google Scholar] [CrossRef] [PubMed]
- Roy, P.; Dinda, A.K.; Chaudhury, S.; Dasgupta, S. β-cyclodextrin encapsulated polyphenols as effective antioxidants. Biopolymers 2018, 109, e23084. [Google Scholar] [CrossRef]
- Gautam, S.; Karmakar, S.; Batra, R.; Sharma, P.; Pradhan, P.; Singh, J.; Kundu, B.; Chowdhury, P.K. Polyphenols in combination with β-cyclodextrin can inhibit and disaggregate α-synuclein amyloids under cell mimicking conditions: A promising therapeutic alternative. Biochim. Biophys. Acta-Proteins Proteom. 2017, 1865, 589–603. [Google Scholar] [CrossRef]
- Krstić, L.; Jarho, P.; Ruponen, M.; Urtti, A.; González-García, M.J.; Diebold, Y. Improved ocular delivery of quercetin and resveratrol: A comparative study between binary and ternary cyclodextrin complexes. Int. J. Pharm. 2022, 624, 122028. [Google Scholar] [CrossRef]
- Fernández-Romero, A.M.; Maestrelli, F.; García-Gil, S.; Talero, E.; Mura, P.; Rabasco, A.M.; González-Rodríguez, M.L. Preparation, characterization and evaluation of the anti-inflammatory activity of epichlorohydrin-β-cyclodextrin/curcumin binary systems embedded in a pluronic®/hyaluronate hydrogel. Int. J. Mol. Sci. 2021, 22, 13566. [Google Scholar] [CrossRef]
- Jeandet, P.; Sobarzo-Sánchez, E.; Uddin, M.S.; Bru, R.; Clément, C.; Jacquard, C.; Nabavi, S.F.; Khayatkashani, M.; Batiha, G.E.S.; Khan, H.; et al. Resveratrol and cyclodextrins, an easy alliance: Applications in nanomedicine, green chemistry and biotechnology. Biotechnol. Adv. 2021, 53, 107844. [Google Scholar] [CrossRef]
- Gligorijević, N.; Stanić-Vučinić, D.; Radomirović, M.; Stojadinović, M.; Khulal, U.; Nedić, O.; Ćirković Veličković, T. Role of resveratrol in prevention and control of cardiovascular disorders and cardiovascular complications related to COVID-19 disease: Mode of action and approaches explored to increase its bioavailability. Molecules 2021, 26, 2834. [Google Scholar] [CrossRef]
- Rajha, H.N.; Chacar, S.; Afif, C.; Vorobiev, E.; Louka, N.; Maroun, R.G. β-Cyclodextrin-Assisted Extraction of Polyphenols from Vine Shoot Cultivars. J. Agric. Food Chem. 2015, 63, 3387–3393. [Google Scholar] [CrossRef] [PubMed]
- Bozinou, E.; Lakka, A.; Poulianiti, K.; Lalas, S.; Makris, D.P. Cyclodextrins as high-performance green co-solvents in the aqueous extraction of polyphenols and anthocyanin pigments from solid onion waste. Eur. Food Res. Technol. 2021, 247, 2831–2845. [Google Scholar] [CrossRef]
- Athanasiadis, V.; Palaiogiannis, D.; Bozinou, E.; Lalas, S.I.; Makris, D.P. β-Cyclodextrin-Aided Aqueous Extraction of Antioxidant Polyphenols from Peppermint (Mentha × piperita L.). Oxygen 2022, 2, 424–436. [Google Scholar] [CrossRef]
- Conceicao, J.; Adeoye, O.; Cabral-Marques, H.M.; Lobo, J.M.S. Cyclodextrins as Drug Carriers in Pharmaceutical Technology: The State of the Art. Curr. Pharm. Des. 2018, 24, 1405–1433. [Google Scholar] [CrossRef] [PubMed]
- Braga, S.S. Cyclodextrins: Emerging medicines of the new millennium. Biomolecules 2019, 9, 801. [Google Scholar] [CrossRef] [PubMed]
- Committee for Human Medicinal Products (CHMP). Background Review for Cyclodextrins Used as Excipients; European Medicines Agency: Amsterdam, The Netherlands, 2007. [Google Scholar]
- Committee for Human Medicinal Products (CHMP). Questions and Answers on Cyclodextrins Used as Excipients in Medicinal Products for Human Use; European Medicines Agency: Amsterdam, The Netherlands, 2007. [Google Scholar]
- Ferreira, L.; Campos, J.; Veiga, F.; Cardoso, C.; Paiva-Santos, A.C. Cyclodextrin-based delivery systems in parenteral formulations: A critical update review. Eur. J. Pharm. Biopharm. 2022, 178, 35–52. [Google Scholar] [CrossRef]
- Matencio, A.; Navarro-Orcajada, S.; García-Carmona, F.; López-Nicolás, J.M. Applications of cyclodextrins in food science. A review. Trends Food Sci. Technol. 2020, 104, 132–143. [Google Scholar] [CrossRef]
- Chalmers, J. A Randomised, Double-Blind, Placebo-Controlled Trial of SFX-01 or Placebo on a Backbone of Best Standard Care, to Improve Outcomes in Patients with Community Acquired Pneumonia and Suspected or Confirmed SARS-CoV-2 Infection. Available online: https://www.clinicaltrialsregister.eu/ctr-search/trial/2020-003486-19/GB (accessed on 23 January 2023).
- Raman, R.; Patel, K.J.; Ranjan, K. COVID-19: Unmasking emerging SARS-CoV-2 variants, vaccines and therapeutic strategies. Biomolecules 2021, 11, 993. [Google Scholar] [CrossRef]
- Boroushaki, T.; Dekamin, M.G. Interactions between β-cyclodextrin as a carrier for anti-cancer drug delivery: A molecular dynamics simulation study. J. Biomol. Struct. Dyn. 2022. [Google Scholar] [CrossRef]
- Cao, C.; Deng, C.; Hu, J.; Zhou, Y. Formation and molecular dynamics simulation of inclusion complex of large-ring cyclodextrin and 4-terpineol. J. Food Sci. 2022, 87, 4609–4621. [Google Scholar] [CrossRef]
- Raffaini, G.; Elli, S.; Ganazzoli, F.; Catauro, M. Inclusion Complexes Between β-cyclodextrin and the Anticancer Drug 5-Fluorouracil for its Solubilization: A Molecular Dynamics Study at Different Stoichiometries. Macromol. Symp. 2022, 404, 2100305. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).