Plant Metabolites Affect Fusarium proliferatum Metabolism and In Vitro Fumonisin Biosynthesis
Abstract
:1. Introduction
2. Results
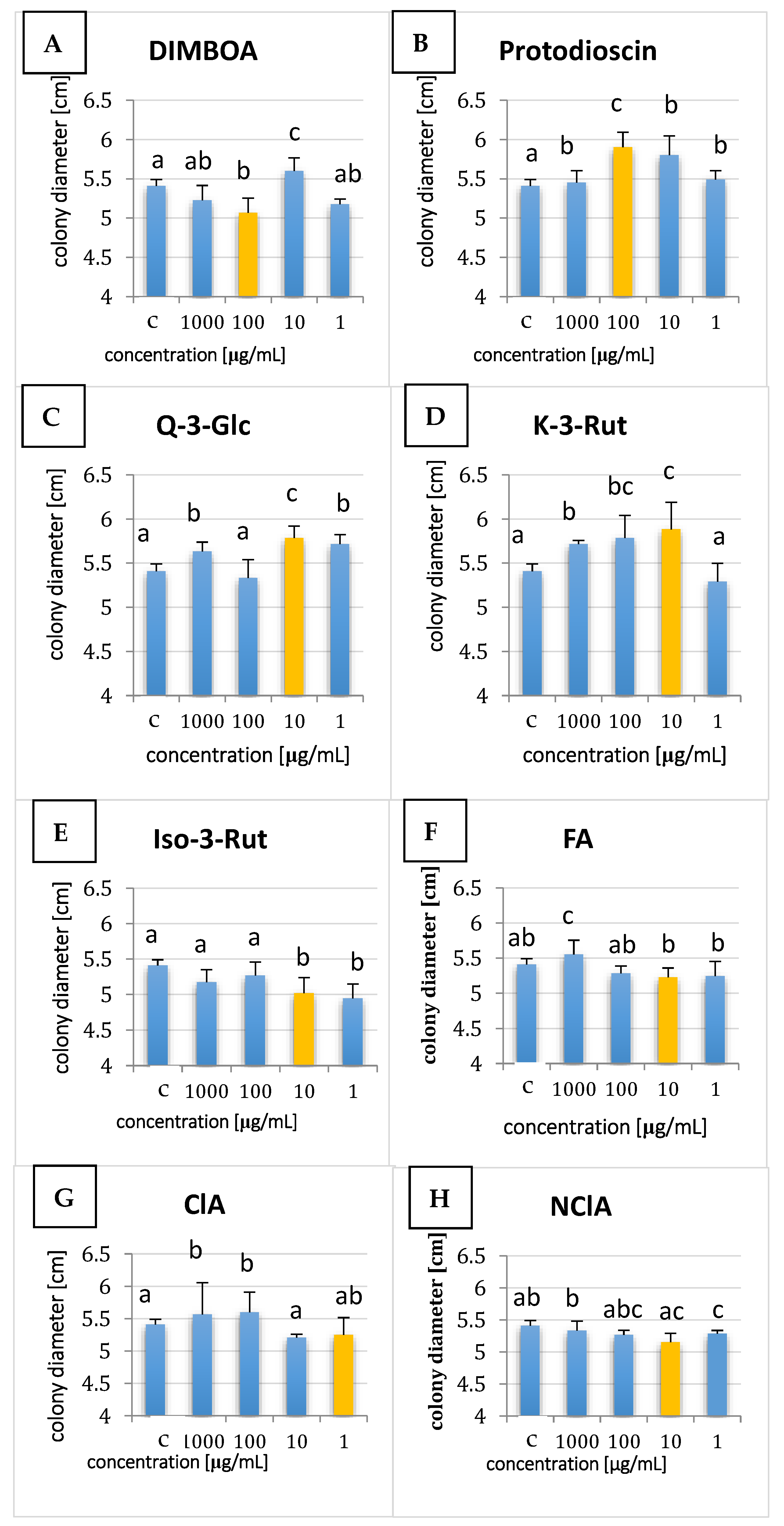
2.1. Effect of Plant Metabolites on Fungal Growth
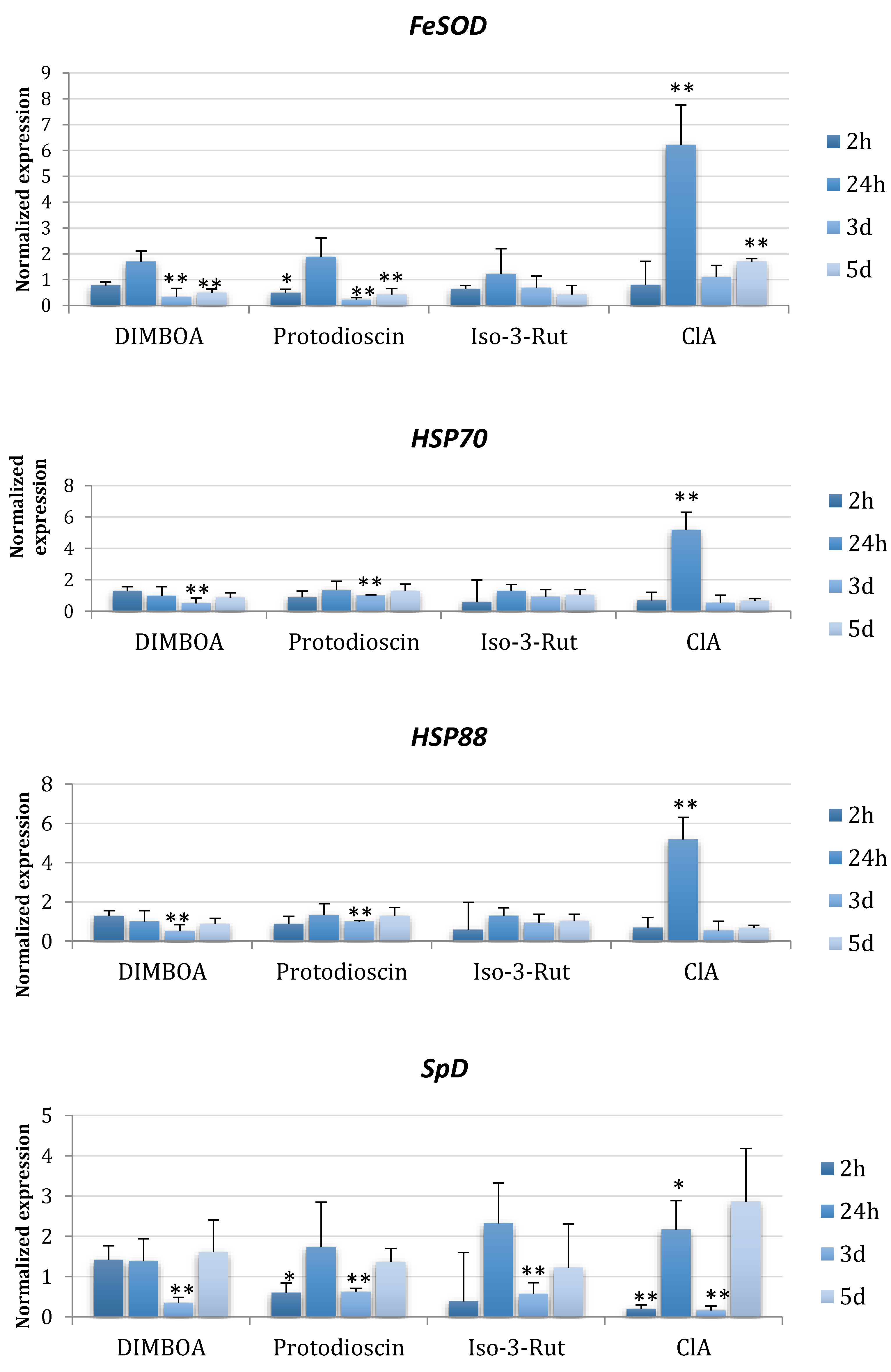
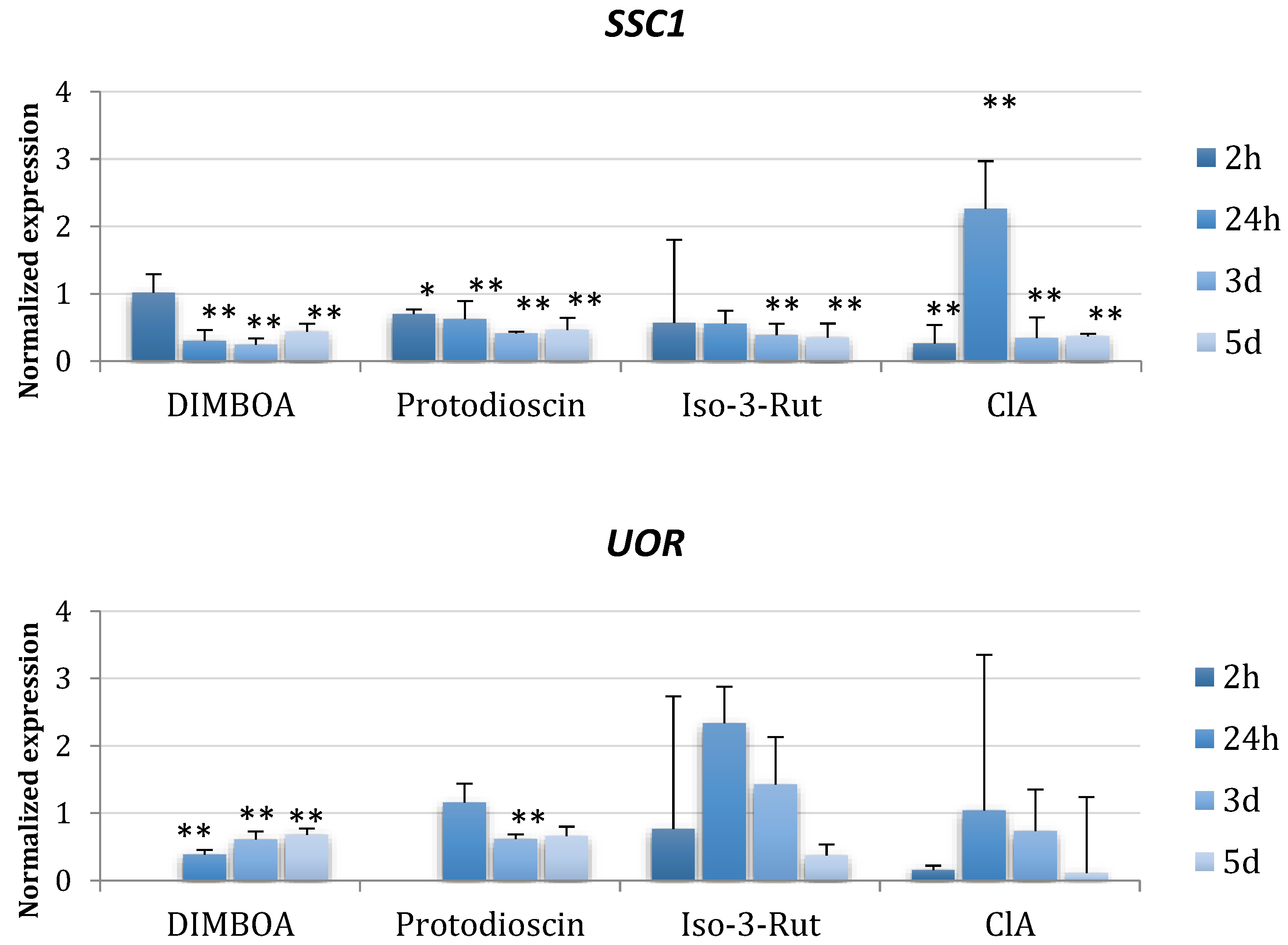
2.2. Expression Changes of Primary Metabolic Genes in the Presence of Plant Metabolites
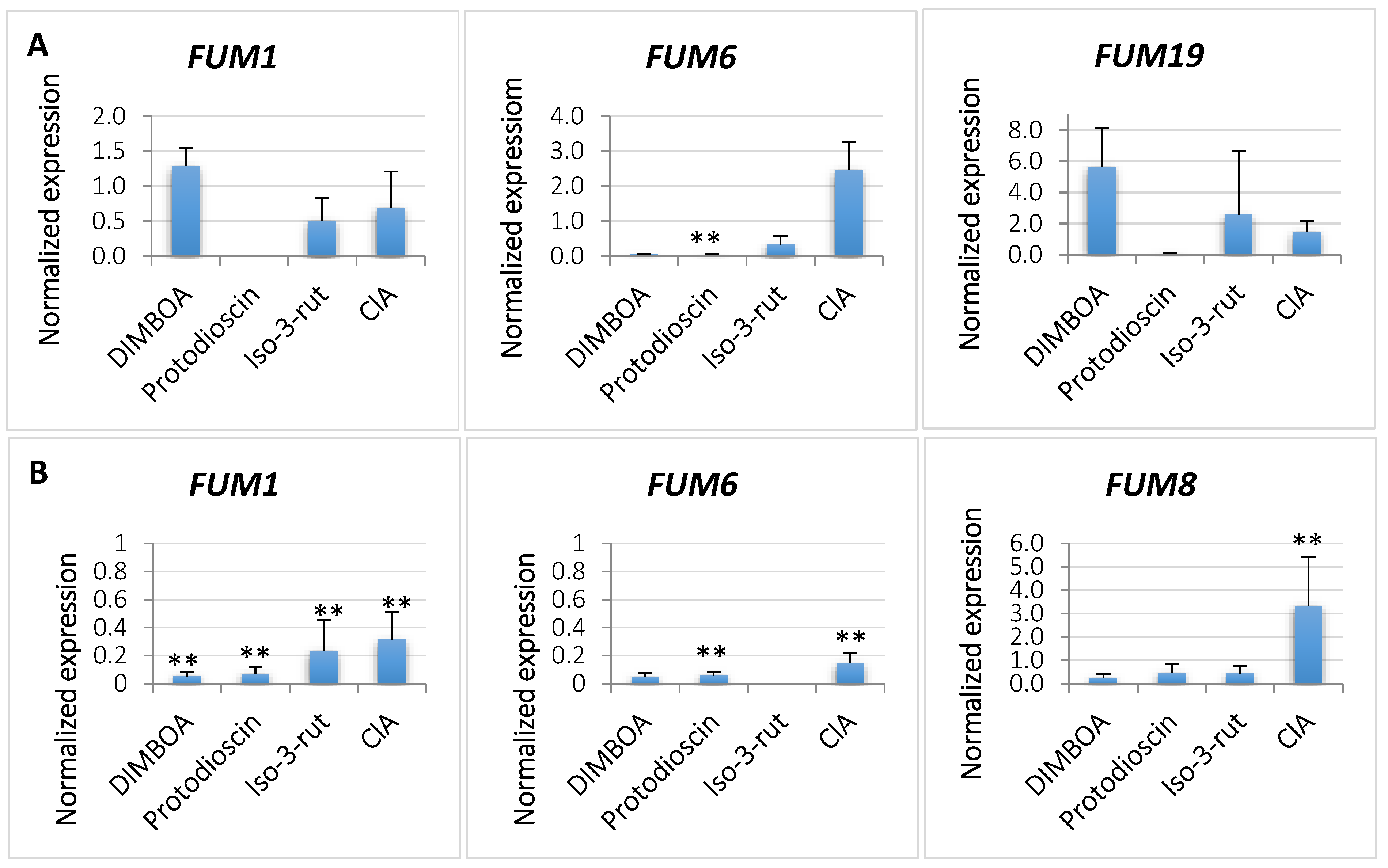
2.3. Expression of FUM Genes Governing Fumonisin Biosynthesis
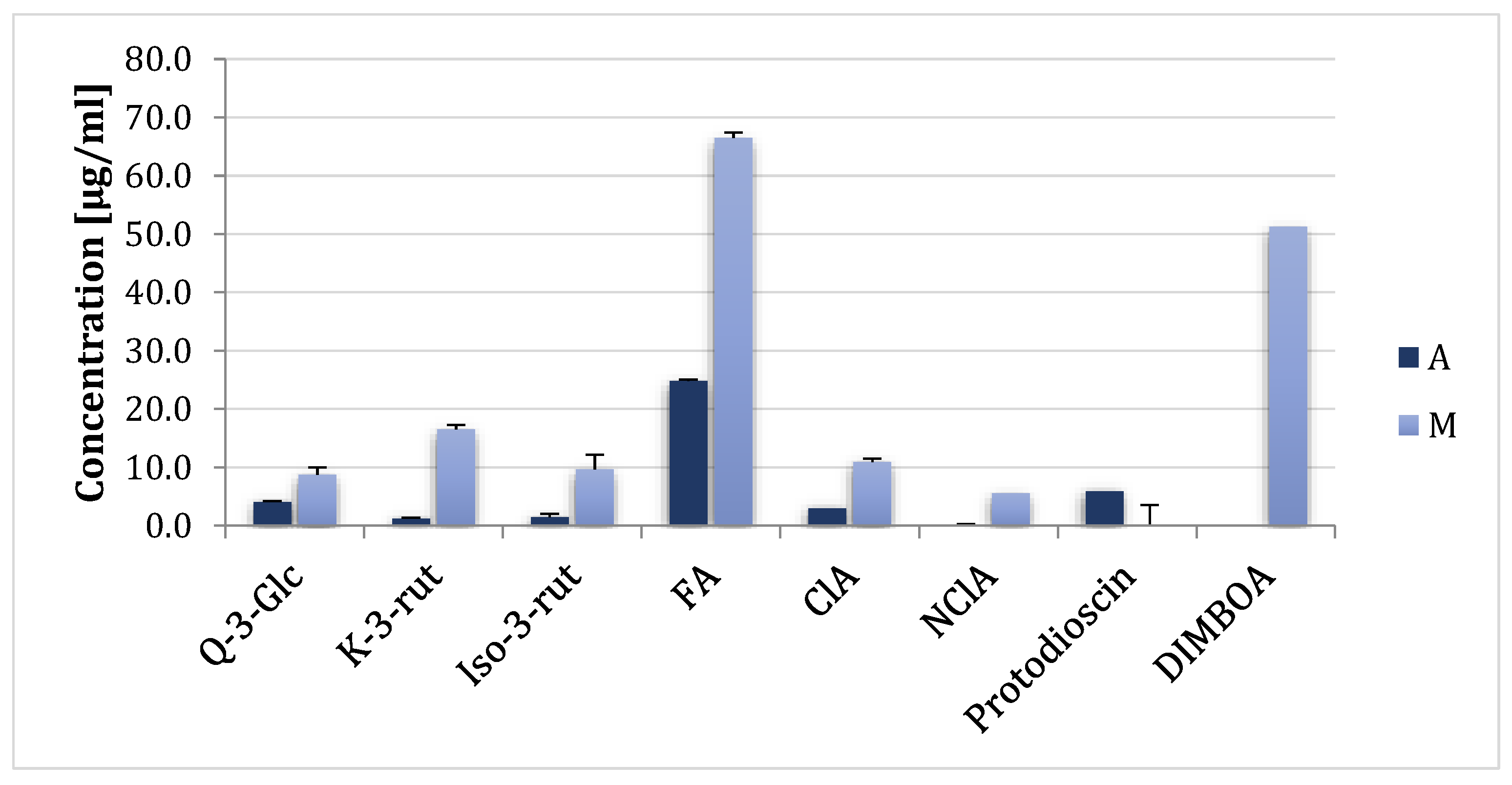
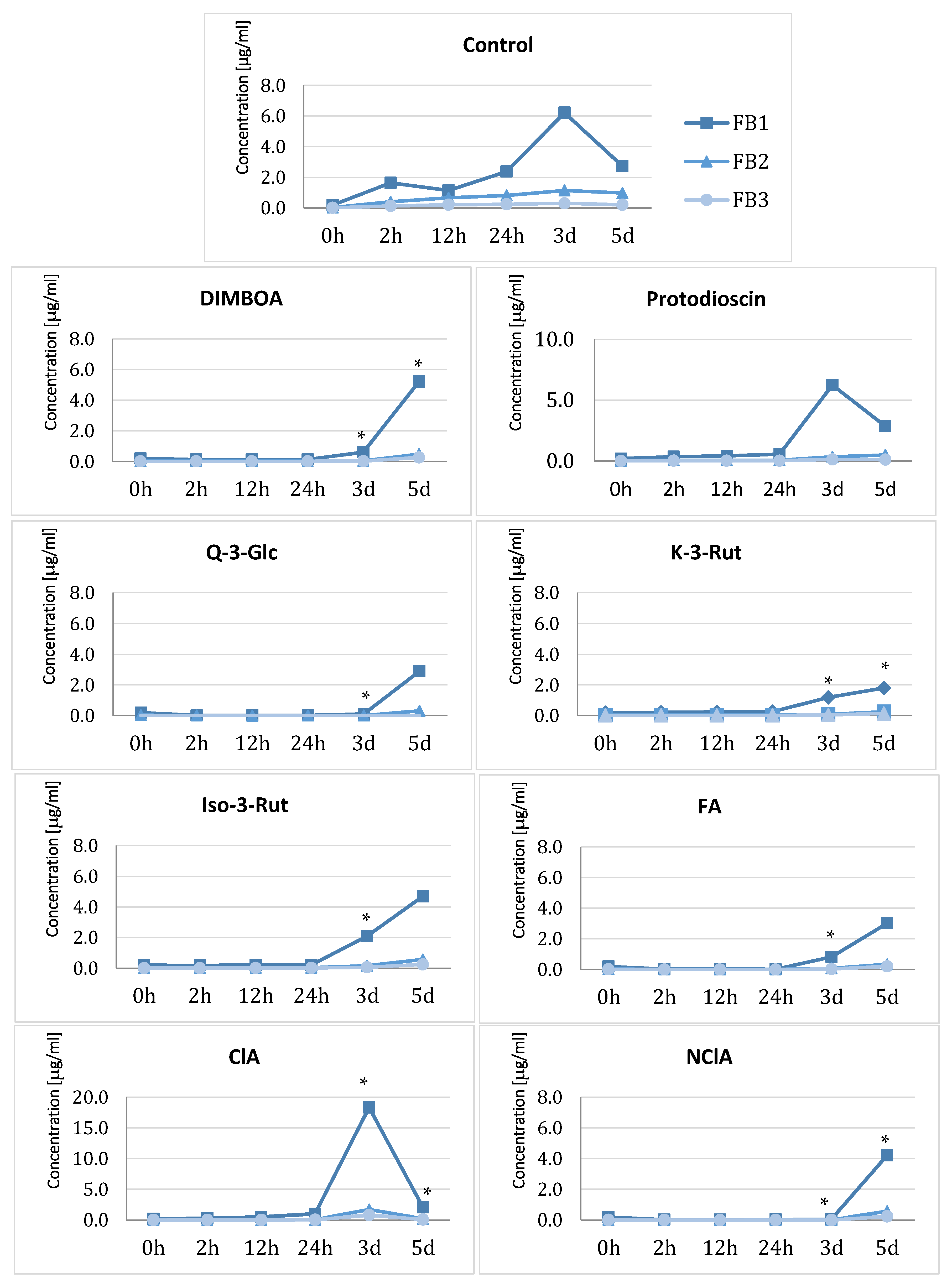
2.4. Effect of Plant Metabolites on Fumonisins Biosynthesis
3. Discussion
4. Materials and Methods
4.1. Fungal Strain and Culture Conditions
4.2. Plant Extract Preparation
4.3. Growth Rate Measurement
4.4. Liquid Cultures with Plant Metabolite Standards
4.5. Mass Spectrometry Analysis
4.6. Plant Metabolite Identification in Asparagus and Maize Extracts
4.7. Gene Expression Analyses
4.8. Fumonisin Quantification
4.9. Statistical Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Stępień, Ł.; Waśkiewicz, A.; Wilman, K. Host extract modulates metabolism and fumonisin biosynthesis by the plant-pathogenic fungus Fusarium proliferatum. Int. J. Food Microbiol. 2015, 193, 74–81. [Google Scholar] [CrossRef] [PubMed]
- Marín, S.; Homedes, V.; Sanchis, V.; Ramos, A.J.; Magan, N. Impact of Fusarium moniliforme and F. proliferatum colonisation of maize on calorific losses and fumonisin production under different environmental conditions. J. Stored Prod. Res. 1998, 35, 15–26. [Google Scholar] [CrossRef]
- Proctor, R.H.; Desjardins, A.E.; Moretti, A. Biological and chemical complexity of Fusarium proliferartum. In The Role of Plant Pathology in Food Safety and Food Security. Plant Pathology in the 21st Century; Stange, R., Gullino, M., Eds.; Springer: Dordrecht, The Netherlands, 2009. [Google Scholar] [CrossRef]
- Ma, L.-J.; Geiser, D.M.; Proctor, R.H.; Rooney, A.P.; O’Donnell, K.; Trail, F.; Gardiner, D.M.; Manners, J.M.; Kazan, K. Fusarium pathogenomics. Annu. Rev. Microbiol. 2013, 67, 399–416. [Google Scholar] [CrossRef]
- Munkvold, G.P. Epidemiology of Fusarium diseases and their mycotoxins ears. Eur. J. Plant Pathol. 2003, 109, 705–713. [Google Scholar] [CrossRef]
- Stępień, Ł. The use of Fusarium secondary metabolite biosynthetic genes in chemotypic and phylogenetic studies. Crit. Rev. Microbiol. 2014, 40, 176–185. [Google Scholar] [CrossRef]
- Lumsangkul, C.; Chiang, H.-I.; Lo, N.-W.; Fan, Y.-K.; Ju, J.C. Developmental Toxicity of Mycotoxin Fumonisin B1 in Animal Embryogenesis: An Overview. Toxins 2019, 11, 114. [Google Scholar] [CrossRef]
- Berkey, R.; Bendigeri, D.; Xiao, S. Sphingolipids and Plant Defense/Disease: The “Death” Connection and Beyond. Front. Plant Sci. 2012, 3, 68. [Google Scholar] [CrossRef]
- Glazebrook, J. Contrasting Mechanisms of Defense Against Biotrophic and Necrotrophic Pathogens. Annu. Rev. Phytopathol. 2005, 43, 205–227. [Google Scholar] [CrossRef]
- Neelam Khatkar, A.; Sharma, K.K. Phenylpropanoids and Its Derivatives: Biological Activities and Its Role in Food, Pharmaceutical and Cosmetic Industries. Crit. Rev. Food Sci. Nutr. 2019, 60, 2655–2675. [Google Scholar] [CrossRef]
- Padmavati, M.; Sakthivel, N.; Thara, K.V.; Reddy, A.R. Differential Sensitivity of Rice Pathogens to Growth Inhibition by Flavonoids. Phytochemistry 1997, 46, 499–502. [Google Scholar] [CrossRef]
- Parvez, M.M.; Tomita-Yokotani, K.; Fujii, Y.; Konishi, T.; Iwashina, T. Effects of Quercetin and Its Seven Derivatives on the Growth of Arabidopsis thaliana and Neurospora crassa. Biochem. Syst. Ecol. 2004, 32, 631–635. [Google Scholar] [CrossRef]
- Bilska, K.; Stuper-Szablewska, K.; Kulik, T.; Buśko, M.; Załuski, D.; Jurczak, S.; Perkowski, J. Changes in Phenylpropanoid and Trichothecene Production by Fusarium culmorum and F. graminearum sensu stricto via Exposure to Flavonoids. Toxins 2018, 10, 110. [Google Scholar] [CrossRef] [PubMed]
- Krysenko, S.; Wohlleben, W. Polyamine and Ethanolamine Metabolism in Bacteria as an Important Component of Nitrogen Assimilation for Survival and Pathogenicity. Med. Sci. 2022, 10, 40. [Google Scholar] [CrossRef]
- Wojtasik, W.; Kulma, A.; Namysł, K.; Preisner, M.; Szopa, J. Polyamine metabolism in flax in response to treatment with pathogenic and non–pathogenic Fusarium strains. Front. Plant Sci. 2015, 6, 291. [Google Scholar] [CrossRef] [PubMed]
- Perincherry, L.; Witaszak, N.; Urbaniak, M.; Waśkiewicz, A.; Stępień, Ł. Effects of secondary metabolites from pea on Fusarium growth and mycotoxin biosynthesis. J. Fungi 2021, 7, 1004. [Google Scholar] [CrossRef] [PubMed]
- Schöneberg, T.; Kibler, K.; Sulyok, M.; Musa, T.; Bucheli, T.D.; Mascher, F.; Bertossa, M.; Voegele, R.T.; Vogelgsang, S. Can Plant Phenolic Compounds Reduce Fusarium Growth and Mycotoxin Production in Cereals? Food Addit. Contamin. A 2018, 3, 2455–2470. [Google Scholar] [CrossRef] [PubMed]
- Cho, W.K.; Lee, K.M.; Yu, J.; Son, M.; Kim, K.H. Insight into mycoviruses infecting Fusarium species. Adv. Virus Res. 2013, 86, 273–288. [Google Scholar] [CrossRef] [PubMed]
- Ferrochio, L.; Cendoya, E.; Farnochi, M.C.; Massad, W.; Ramirez, M.L. Evaluation of ability of ferulic acid to control growth and fumonisin production of Fusarium verticillioides and Fusarium proliferatum on maize based media. Int. J. Food Microbiol. 2013, 167, 215–220. [Google Scholar] [CrossRef]
- Reynoso, M.M.; Torres, A.M.; Ramirez, M.L.; Rodríguez, M.I.; Chulze, S.N.; Magan, N. Efficacy of antioxidant mixtures on growth, fumonisisn production and hydrolytic enzyme production by Fusarium verticillioides and F. proliferatum in vitro on maize-based media. Mycol. Res. 2002, 106, 1093–1099. [Google Scholar] [CrossRef]
- Gauthier, L.; Atanasova-Penichon, V.; Chéreau, S.; Richard-Forget, F. Metabolomics to Decipher the Chemical Defense of Cereals against Fusarium graminearum and Deoxynivalenol Accumulation. Int. J. Mol. Sci. 2015, 16, 24839–24872. [Google Scholar] [CrossRef]
- Assabgui, R.A.; Reid, L.M.; Hamilton, R.I.; Arnason, T. Correlation of kernel (E)-ferulic acid content of maize with resistance to Fusarium graminearum. Phytopatology 1993, 83, 949–953. [Google Scholar] [CrossRef]
- McKeehen, J.D.; Busch, R.H.; Fulcher, R.G. Evaluation of wheat (Triticum aesticum L.) phenolic acids during grain development and their contribution to Fusarium resistance. J. Agric. Food Chem. 1999, 47, 1476–1482. [Google Scholar] [CrossRef] [PubMed]
- Sampietro, D.A.; Fauguel, C.M.; Vattuone, M.A.; Presello, D.A.; Catalan, C.A.N. Phenylpropanoids from maize pericarp: Resistance factors to kernel infection and fumonisin accumulation by Fusarium verticillioides. Eur. J. Plant Pathol. 2012, 135, 105–113. [Google Scholar] [CrossRef]
- Górna, K.; Pawłowicz, I.; Waśkiewicz, A.; Stępień, Ł. Fusarium proliferatum strains change fumonisin biosynthesis and accumulation when exposed to host plant extracts. Fungal Biol. 2016, 120, 884–893. [Google Scholar] [CrossRef] [PubMed]
- Witaszak, N.; Lalak-Kańczugowska, J.; Waśkiewicz, A.; Stępień, Ł. The impacts of asparagus extract fractions on growth and fumonisins biosynthesis in Fusarium proliferatum. Toxins 2020, 12, 95. [Google Scholar] [CrossRef] [PubMed]
- Górna, K.; Perlikowski, D.; Kosmala, A.; Stępień, Ł. Host extracts induce changes in the proteome of plant pathogen Fusarium proliferatum. Fungal Biol. 2017, 121, 676–688. [Google Scholar] [CrossRef]
- Swatek, A.; Staszczak, M. Effect of Ferulic Acid, a Phenolic Inducer of Fungal Laccase, on 26S Proteasome Activities In Vitro. Int. J. Mol. Sci. 2020, 21, 2463. [Google Scholar] [CrossRef]
- Jiménez-Sánchez, C.; Lozano-Sánchez, J.; Rodríguez-Pérez, C.; Segura-Carretero, A.; Fernández-Gutiérrez, A. Comprehensive, untargeted, and qualitative RP-HPLC-ESI-QTOF/MS2 metabolite profiling of green asparagus (Asparagus officinalis). J. Food Compos. Anal. 2016, 46, 78–87. [Google Scholar] [CrossRef]
- Pegiou, E.; Mumm, R.; Acharya, P.; de Vos, R.C.H.; Hall, R.D. Green and White Asparagus (Asparagus officinalis): A Source of Developmental, Chemical and Urinary Intrigue. Metabolites 2020, 10, 17. [Google Scholar] [CrossRef]
- Váry, Z.; Mullins, E.; McElwain, J.C.; Doohan, F.M. The severity of wheat diseases increases when plants and pathogens are acclimatized to elevated carbon dioxide. Glob. Change Biol. 2015, 21, 2661–2669. [Google Scholar] [CrossRef]
- Timmusk, S.; Nevo, E.; Ayele, F.; Noe Niinemets, Ü. Fighting Fusarium pathogens in the era of climate change: A conceptual approach. Pathogens 2020, 9, 419. [Google Scholar] [CrossRef] [PubMed]
- Uwineza, P.A.; Urbaniak, M.; Bryła, M.; Stępień, Ł.; Modrzewska, M.; Waśkiewicz, A. In vitro Effects of Lemon Balm Extracts in Reducing the Growth and Mycotoxins Biosynthesis of Fusarium culmorum and F. proliferatum. Toxins 2022, 14, 355. [Google Scholar] [CrossRef] [PubMed]
- Oufensou, S.; Balmas, V.; Azara, E.; Fabbri, D.; Dettori, M.A.; Schüller, C.; Zehetbauer, F.; Strauss, J.; Delgou, G.; Migheli, Q. Naturally occuring phenols modulate vegetative growth and deoxynivalenol biosynthesis in Fusarium graminearum. ACS Omega 2020, 45, 29407–29415. [Google Scholar] [CrossRef] [PubMed]
- Proctor, R.H.; Plattner, R.D.; Desjardins, A.E.; Busman, M.; Butchko, R.A. Fumonisin production in the maize pathogen Fusarium verticillioides: Genetic basis of naturally occurring chemical variation. J. Agric. Food Chem. 2006, 54, 2424–2430. [Google Scholar] [CrossRef]
- Proctor, R.H.; Busman, M.; Seo, J.A.; Lee, Y.W.; Plattner, R.D. A fumonisin biosynthetic gene cluster in Fusarium oxysporum strain O-1890 and the genetic basis for B versus C fumonisin production. Fungal Genet. Biol. 2008, 45, 1016–1026. [Google Scholar] [CrossRef]
- Brown, D.W.; Cheung, F.; Proctor, R.H.; Butchko, R.A.; Zheng, L.; Lee, Y.; Utterback, T.; Smith, S.; Feldblyum, T.; Glenn, A.E.; et al. Comparative analysis of 87,000 expressed sequence tags from the fumonisin-producing fungus Fusarium verticillioides. Fungal Genet. Biol. 2005, 42, 848–861. [Google Scholar] [CrossRef]
- Sun, L.; Chen, X.; Gao, J.; Zhao, Y.; Liu, L.; Hou, Y.; Wang, L.; Huang, S. Effects of Disruption of Five FUM Genes on Fumonisin Biosynthesis and Pathogenicity in Fusarium proliferatum. Toxins 2019, 11, 327. [Google Scholar] [CrossRef]
- Massi, F.P.; Sartori, D.; de Souza Ferranti, L.; Iamanaka, B.T.; Taniwaki, M.H.; Vieira, M.L.C.; Fungaro, M.H.P. Prospecting for the incidence of genes involved in ochratoxin and fumonisin biosynthesis in Brazilian strains of Aspergillus niger and Aspergillus welwitschiae. Int. J. Food Microbiol. 2016, 221, 19–28. [Google Scholar] [CrossRef]
- Alseekh, S.; de Souza, L.P.; Benina, M.; Fernie, A.R. The style and substance of plant flavonoid decoration: Towards defining both structure and function. Phytochemistry 2020, 174, 112347. [Google Scholar] [CrossRef]
- Li, T.; Gong, L.; Jiang, G.; Wang, Y.; Gupta, V.W.; Qu, H.; Duan, X.; Wang, J.; Jiang, Y. Carbon sources influence fumonisin production in Fusarium proliferatum. Proteomics 2017, 17, 1700070. [Google Scholar] [CrossRef]
- Rocha, L.O.; Barroso, V.M.; Andrade, L.J.; Pereira, G.H.; Ferreira-Castro, F.L.; Duarte, A.P.; Michelotto, M.D.; Correa, B. FUM Gene Expression Profile and Fumonisin Production by Fusarium verticillioides Inoculated in Bt and Non-Bt Maize. Front. Microbiol. 2016, 6, 1503. [Google Scholar] [CrossRef] [PubMed]
- Jurado, M.; Marín, P.; Magan, N.; González-Jaén, M.T. Relationship between solute and matric potential stress, temperature, growth, and FUM1 gene expression in two Fusarium verticillioides strains from Spain. Appl. Environ. Microbiol. 2008, 74, 2032–2036. [Google Scholar] [CrossRef] [PubMed]
- Lazzaro, I.; Falavigna, C.; Galaverna, G.; Dall’Asta, C.; Battilani, P. Cornmeal and starch influence the dynamic fumonisin B, A, C production and masking in Fusarium verticillioides and F. proliferatum. Int. J. Food Microbiol. 2013, 166, 21–27. [Google Scholar] [CrossRef] [PubMed]
- Medina, A.; Rodriguez, A.; Magan, N. Effect of climate change on Aspergillus flavus and aflatoxin B1 production. Front. Microbiol. 2014, 5, 348. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Li, T.; Gong, L.; Wang, Y.; Jiang, Y. Effects of different carbon sources on fumonisin production and FUM gene expression by Fusarium proliferatum. Toxins 2019, 11, 289. [Google Scholar] [CrossRef]
- Dozolme, P.M.A.; Moukha, S.M. The in vitro production potentialities of secondary toxic metabolites by the fungal factory Fusarium verticillioides is, fortunately, largely underestimated in fields: Pioneering study on fumonisins. Front. Microbiol. 2020, 11, 562754. [Google Scholar] [CrossRef]
- Ponts, N.; Pinson-Gadais, L.; Barreau, C.; Richard-Forget, F.; Ouellet, T. Exogenous H2O2 and catalase treatments interfere with Tri genes expression in liquid cultures of Fusarium graminearum. FEBS Lett. 2007, 581, 443–447. [Google Scholar] [CrossRef]
- Atanasova-Penichon, V.; Bernillon, S.; Marchegay, G.; Lornac, A.; Pinson-Gadais, L.; Ponts, N.; Zehraoui, E.; Barreau, C.; Richard-Forget, F. Bioguided isolation, characterization and biotransformation of maize kenrels compounds that inhibit fumonisin production. Mol. Plant-Microbe Inter. 2014, 10, 1148–1158. [Google Scholar] [CrossRef]
- Ferruz, E.; Atanasova-Penichon, V.; Bonnin-Verdal, M.-N.; Marchegay, G.; Pinson-Gadais, L.; Ducos, C.; Lorán, S.; Ariño, A.; Barreau, C.; Richard-Forget, F. Effects of phenolic acids on the growth and production of T-2 and HT-2 toxins by Fusarium langsethiae and F. sporotrichioides. Molecules 2016, 21, 449. [Google Scholar] [CrossRef] [Green Version]
- Beekrum, S.; Govinden, R.; Padayachee, T.; Odhav, B. Naturally occuring phenols: A detoxification strategy for fumonisin B1. Food Addit. Contamin. 2003, 20, 490–493. [Google Scholar] [CrossRef]
- Niggeweg, R.; Michael, A.J.; Martin, C. Engineering plants with increased levels of the antioxidant chlorogenic acid. Nat. Biotechnol. 2004, 22, 746–754. [Google Scholar] [CrossRef] [PubMed]
- Atanasova-Perichon, V.; Pons, S.; Pinson-Gadais Picot, A.; Marchegay, G.; Bonnin-Verdal, M.N.; Ducos, C.; Barreau, C.; Roucolle, J.; Sehabiiague, P.; Carolo, P.; et al. Chlorogenic Acid and Maize Ear Rot Resistance: A Dynamic Study Investigating Fusarium graminearum Development, Deoxynivalenol Production, and Phenolic Acid Accumulation. Mol. Plant-Microbe Inter. 2012, 25, 1605–1616. [Google Scholar] [CrossRef] [PubMed]
- Papadopolou, K.; Melton, R.E.; Legget, M.; Daniels, M.J.; Osbourn, A.E. Compromised disease resistance in saponin-deficient plants. Proc. Natl. Acad. USA 1999, 96, 12923–12928. [Google Scholar] [CrossRef] [PubMed]
- Perincherry, L.; Urbaniak, M.; Pawłowicz, I.; Kotowska, K.; Waśkiewicz, A.; Stępień, Ł. Dynamics of Fusarium Mycotoxins and Lytic Enzymes during Pea Plants’ Infection. Int. J. Mol. Sci. 2021, 22, 9888. [Google Scholar] [CrossRef]
- Aboody, M.S.A.; Mickymaray, S. Anti-Fungal Efficacy and Mechanisms of Flavonoids. Antibiotics 2020, 9, 45. [Google Scholar] [CrossRef]
- Stępień, Ł.; Koczyk, G.; Waśkiewicz, A. Genetic and phenotypic variation of Fusarium proliferatum isolates from different host species. J. Appl. Genet. 2011, 52, 487–496. [Google Scholar] [CrossRef]
- Tsugawa, H.; Cajka, T.; Kind, T.; Ma, Y.; Higgins, B.; Ikeda, K.; Kanazawa, M.; VanderGheynst, J.; Fiehn, O.; Arita, M. MS-DIAL: Data-Independent MS/MS Deconvolution for Comprehensive Metabolome Analysis. Nat. Methods 2015, 12, 523–526. [Google Scholar] [CrossRef]
- Tyanova, S.; Temu, T.; Sinitcyn, P.; Carlson, A.; Hein, M.Y.; Geiger, T.; Mann, M.; Cox, J. The Perseus Computational Platform for Comprehensive Analysis of (Prote)Omics Data. Nat. Methods 2016, 13, 731–740. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]

| Plant Metabolite | Molecular Formula | Ionization Mode | Parent Ion [m/z] | Product Ions [m/z] |
|---|---|---|---|---|
| Quercetin-3-glucoside | C21H20O12 | [M-H]- | 463.1 | 301.0 |
| Kaempferol-3-rutinoside | C27H30O15 | [M-H]- | 593.1 | 285.0 |
| Isorhamnetin-3-O-rutinoside | C28H32016 | [M-H]- | 623.2 | 300.0 |
| Ferulic acid | C10H10O4 | [M-H]- | 193.1 | 177.4 |
| Chlorogenic acid | C16H18O9 | [M-H]- | 353.1 | 191.1 |
| Neochlorogenic acid | C16H18O9 | [M-H]- | 353.1 | 191.1; 179.0 |
| Protodioscin | C51H84O22 | [M + H]+ | 1050.2 | 741.9 |
| DIMBOA | C9H9NO5 | [M-H]- | 210.0 | 149.0 |
| Primer Name | Sequence 5′-3′ | Concentration [nM] | Annealing Temperature [°C] |
|---|---|---|---|
| FeSOD_F | TGCCAGAGTTCTTGTCCCTG | 300 | 64 |
| FeSOD_R | ATGAGGACTTTGGCTCCTTC | ||
| SSC1_F | AGGACGCAAGTTCAAGGATG | 300 | 64 |
| SSC1_R | AGGACGAAACCACCAATCTG | ||
| HSP88_F | TTCAGCTGTGCCATTCTCTC | 300 | 64 |
| HSP88_R | ACGTTGCCCTTGTTGAAGAC | ||
| HSP70_F | TGTCTTCGATGCCAAGTGA | 300 | 64 |
| HSP70_R | CAATCTTGAAGGGCCAAGAC | ||
| CPR6_F | AATGAGGCTAACACCCTTGC | 300 | 64 |
| CPR6_R | TGTAGGCAGCCTTTTCCTTG | ||
| UOR_F | CGATATTCGAGTCGAGGAGTG | 300 | 66 |
| UOR_R | CTATGATCTTCGAGAGGCTT | ||
| SpD_F | GTCGCCATCATGTCTGACTATC | 250 | 64 |
| SpD_R | CAGCATCAACGAGTGTCTTG | ||
| FUM1_F | TTCTCCCAGCCACTTGTATGC | 500 | 62 |
| FUM1_R | CCATCTCCCTTGTACTGTTGA | ||
| FUM6_F | ATGACCGTTCGCCCAGTAGG | 500 | 66.4 |
| FUM6_R | CGTGTCCTTGATGAAGTCCCA | ||
| FUM8_F | TCAACAAGCGAGCGACAACACA | 500 | 68 |
| FUM8_R | CCAGCCATACTTCCCATAAGTC | ||
| FUM19_F | TCTCCCAACGCCCTGCC | 500 | 62 |
| FUM19_R | GGTGAGAAACTGACCGA | ||
| H3_F | ACTAAGCAGACCGCCCGCAGG | 250 | 62 |
| H3_R | GCGGGCGAGCTGGATGTCTT |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lalak-Kańczugowska, J.; Witaszak, N.; Waśkiewicz, A.; Bocianowski, J.; Stępień, Ł. Plant Metabolites Affect Fusarium proliferatum Metabolism and In Vitro Fumonisin Biosynthesis. Int. J. Mol. Sci. 2023, 24, 3002. https://doi.org/10.3390/ijms24033002
Lalak-Kańczugowska J, Witaszak N, Waśkiewicz A, Bocianowski J, Stępień Ł. Plant Metabolites Affect Fusarium proliferatum Metabolism and In Vitro Fumonisin Biosynthesis. International Journal of Molecular Sciences. 2023; 24(3):3002. https://doi.org/10.3390/ijms24033002
Chicago/Turabian StyleLalak-Kańczugowska, Justyna, Natalia Witaszak, Agnieszka Waśkiewicz, Jan Bocianowski, and Łukasz Stępień. 2023. "Plant Metabolites Affect Fusarium proliferatum Metabolism and In Vitro Fumonisin Biosynthesis" International Journal of Molecular Sciences 24, no. 3: 3002. https://doi.org/10.3390/ijms24033002
APA StyleLalak-Kańczugowska, J., Witaszak, N., Waśkiewicz, A., Bocianowski, J., & Stępień, Ł. (2023). Plant Metabolites Affect Fusarium proliferatum Metabolism and In Vitro Fumonisin Biosynthesis. International Journal of Molecular Sciences, 24(3), 3002. https://doi.org/10.3390/ijms24033002








