Plasma Sphingoid Base Profiles of Patients Diagnosed with Intrinsic or Idiosyncratic Drug-induced Liver Injury
Abstract
:1. Introduction
2. Results
2.1. Clinical Outcome
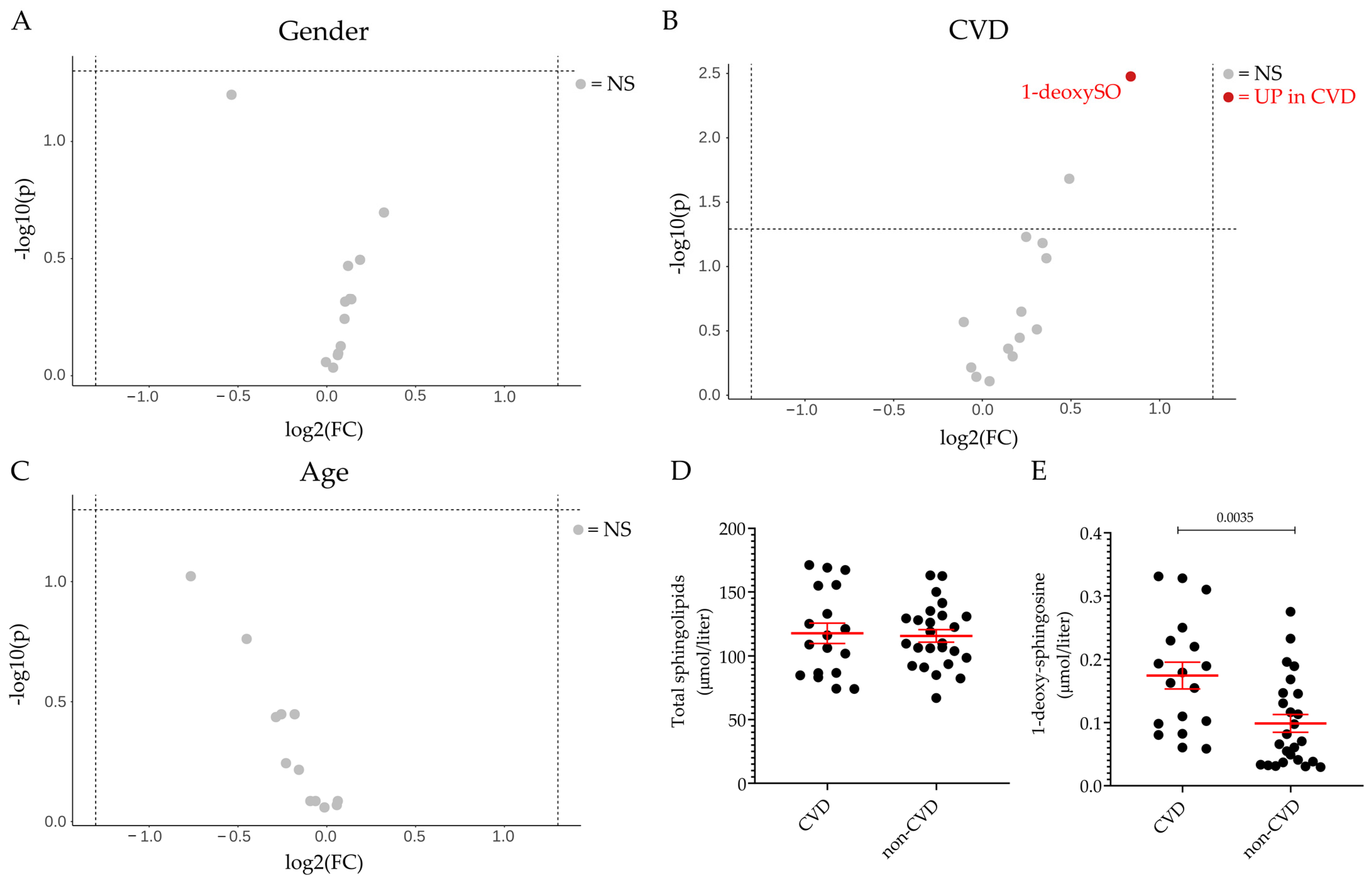
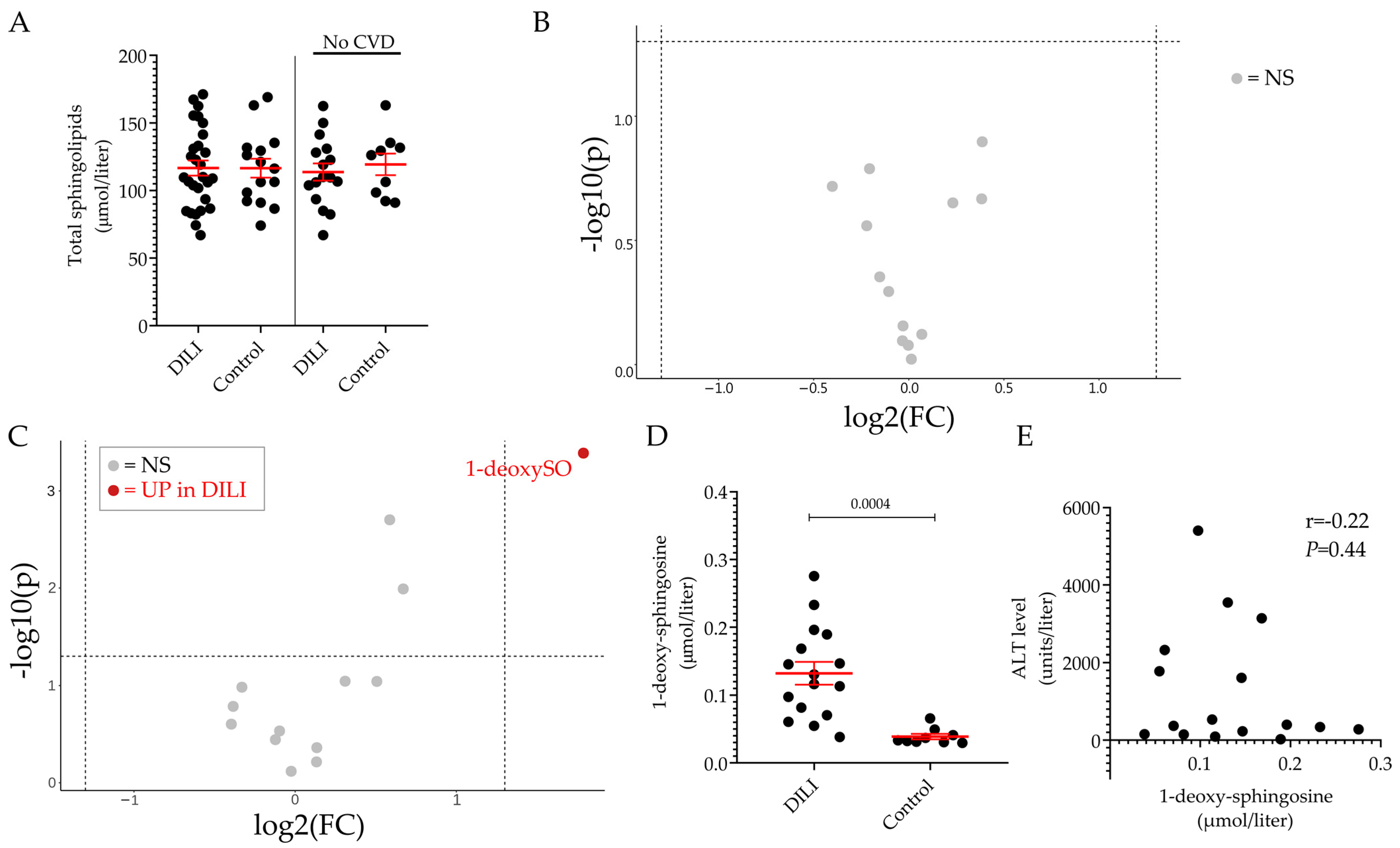
2.2. Plasma Sphingolipid Levels in Healthy Volunteers and Patients Diagnosed with DILI
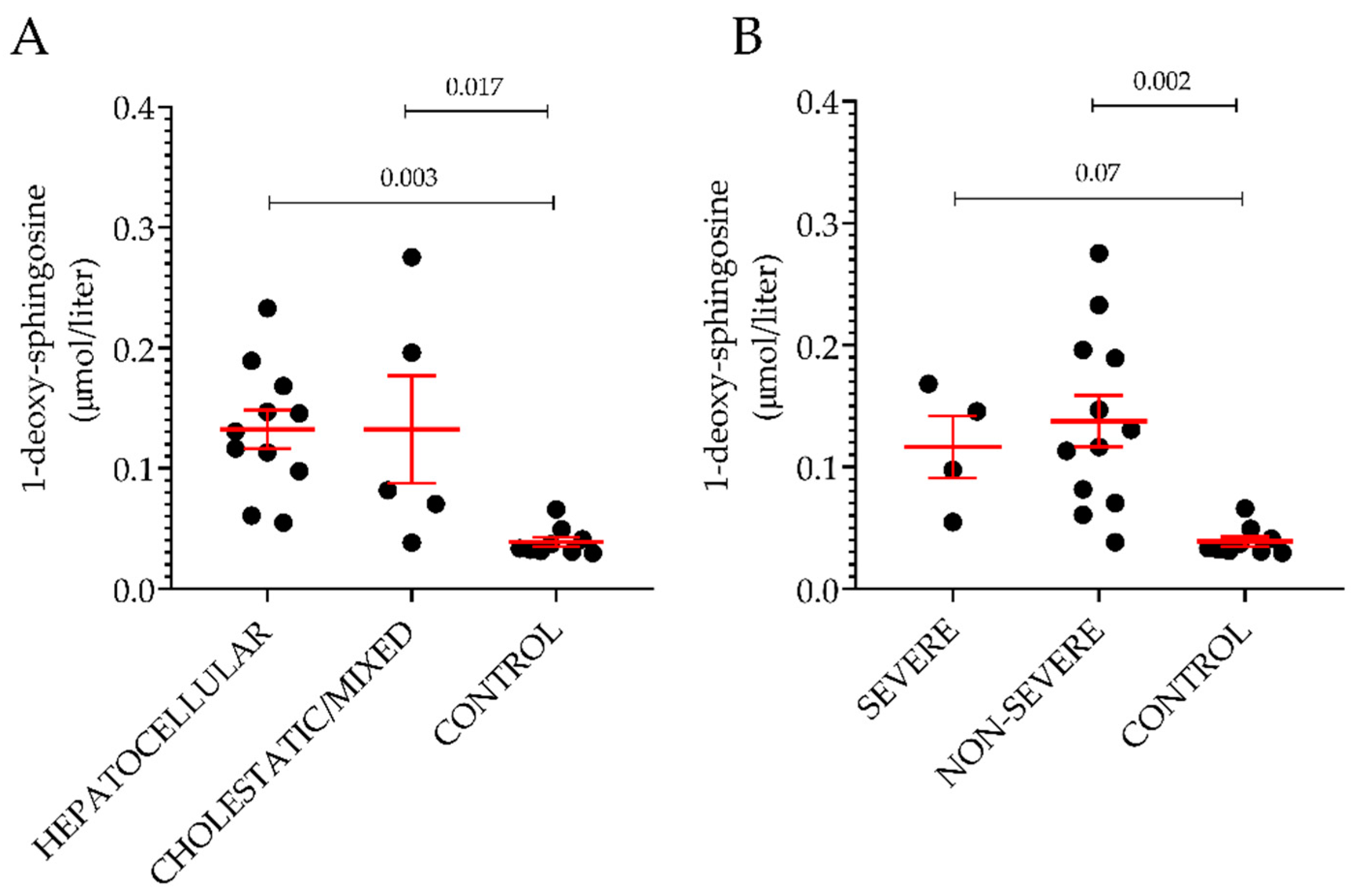
2.3. Sphingolipid Profile in DILI Subgroups
3. Discussion
4. Materials and Methods
4.1. Patient Selection
4.2. Classification According to Type, Severity and Mechanism of Injury
4.3. Sphingolipid Analysis
4.4. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kotronen, A.; Seppanen-Laakso, T.; Westerbacka, J.; Kiviluoto, T.; Arola, J.; Ruskeepaa, A.L.; Yki-Jarvinen, H.; Oresic, M. Comparison of lipid and fatty acid composition of the liver, subcutaneous and intra-abdominal adipose tissue, and serum. Obesity 2010, 18, 937–944. [Google Scholar] [CrossRef]
- Nojima, H.; Freeman, C.M.; Gulbins, E.; Lentsch, A.B. Sphingolipids in liver injury, repair and regeneration. Biol. Chem. 2015, 396, 633–643. [Google Scholar] [CrossRef]
- Patterson, A.D.; Maurhofer, O.; Beyoglu, D.; Lanz, C.; Krausz, K.W.; Pabst, T.; Gonzalez, F.J.; Dufour, J.F.; Idle, J.R. Aberrant lipid metabolism in hepatocellular carcinoma revealed by plasma metabolomics and lipid profiling. Cancer Res. 2011, 71, 6590–6600. [Google Scholar] [CrossRef]
- Chen, S.; Yin, P.; Zhao, X.; Xing, W.; Hu, C.; Zhou, L.; Xu, G. Serum lipid profiling of patients with chronic hepatitis B, cirrhosis, and hepatocellular carcinoma by ultra fast LC/IT-TOF MS. Electrophoresis 2013, 34, 2848–2856. [Google Scholar] [CrossRef] [PubMed]
- Puri, P.; Wiest, M.M.; Cheung, O.; Mirshahi, F.; Sargeant, C.; Min, H.K.; Contos, M.J.; Sterling, R.K.; Fuchs, M.; Zhou, H.; et al. The plasma lipidomic signature of nonalcoholic steatohepatitis. Hepatology 2009, 50, 1827–1838. [Google Scholar] [CrossRef]
- Sztolsztener, K.; Konstantynowicz-Nowicka, K.; Harasim-Symbor, E.; Chabowski, A. Time-Dependent Changes in Hepatic Sphingolipid Accumulation and PI3K/Akt/mTOR Signaling Pathway in a Rat Model of NAFLD. Int. J. Mol. Sci. 2021, 22, 12478. [Google Scholar] [CrossRef] [PubMed]
- Quehenberger, O.; Armando, A.M.; Brown, A.H.; Milne, S.B.; Myers, D.S.; Merrill, A.H.; Bandyopadhyay, S.; Jones, K.N.; Kelly, S.; Shaner, R.L.; et al. Lipidomics reveals a remarkable diversity of lipids in human plasma. J. Lipid Res. 2010, 51, 3299–3305. [Google Scholar] [CrossRef] [PubMed]
- Fahy, E.; Subramaniam, S.; Brown, H.A.; Glass, C.K.; Merrill, A.H., Jr.; Murphy, R.C.; Raetz, C.R.; Russell, D.W.; Seyama, Y.; Shaw, W.; et al. A comprehensive classification system for lipids. J. Lipid Res. 2005, 46, 839–861. [Google Scholar] [CrossRef]
- Pruett, S.T.; Bushnev, A.; Hagedorn, K.; Adiga, M.; Haynes, C.A.; Sullards, M.C.; Liotta, D.C.; Merrill, A.H., Jr. Biodiversity of sphingoid bases (“sphingosines”) and related amino alcohols. J. Lipid Res. 2008, 49, 1621–1639. [Google Scholar] [CrossRef] [PubMed]
- Lone, M.A.; Hulsmeier, A.J.; Saied, E.M.; Karsai, G.; Arenz, C.; von Eckardstein, A.; Hornemann, T. Subunit composition of the mammalian serine-palmitoyltransferase defines the spectrum of straight and methyl-branched long-chain bases. Proc. Natl. Acad. Sci. USA 2020, 117, 15591–15598. [Google Scholar] [CrossRef] [PubMed]
- Poss, A.M.; Maschek, J.A.; Cox, J.E.; Hauner, B.J.; Hopkins, P.N.; Hunt, S.C.; Holland, W.L.; Summers, S.A.; Playdon, M.C. Machine learning reveals serum sphingolipids as cholesterol-independent biomarkers of coronary artery disease. J. Clin. Investig. 2020, 130, 1363–1376. [Google Scholar] [CrossRef] [PubMed]
- Othman, A.; Rutti, M.F.; Ernst, D.; Saely, C.H.; Rein, P.; Drexel, H.; Porretta-Serapiglia, C.; Lauria, G.; Bianchi, R.; von Eckardstein, A.; et al. Plasma deoxysphingolipids: A novel class of biomarkers for the metabolic syndrome? Diabetologia 2012, 55, 421–431. [Google Scholar] [CrossRef] [PubMed]
- Chew, W.S.; Torta, F.; Ji, S.; Choi, H.; Begum, H.; Sim, X.; Khoo, C.M.; Khoo, E.Y.H.; Ong, W.Y.; Van Dam, R.M.; et al. Large-scale lipidomics identifies associations between plasma sphingolipids and T2DM incidence. JCI Insight 2019, 5, e126925. [Google Scholar] [CrossRef] [PubMed]
- Gai, Z.; Gui, T.; Alecu, I.; Lone, M.A.; Hornemann, T.; Chen, Q.; Visentin, M.; Hiller, C.; Hausler, S.; Kullak-Ublick, G.A. Farnesoid X receptor activation induces the degradation of hepatotoxic 1-deoxysphingolipids in non-alcoholic fatty liver disease. Liver Int. Off. J. Int. Assoc. Study Liver 2020, 40, 844–859. [Google Scholar] [CrossRef]
- Berkowitz, L.; Salazar, C.; Ryff, C.D.; Coe, C.L.; Rigotti, A. Serum sphingolipid profiling as a novel biomarker for metabolic syndrome characterization. Front. Cardiovasc. Med. 2022, 9, 1092331. [Google Scholar] [CrossRef] [PubMed]
- Balram, A.; Thapa, S.; Chatterjee, S. Glycosphingolipids in Diabetes, Oxidative Stress, and Cardiovascular Disease: Prevention in Experimental Animal Models. Int. J. Mol. Sci. 2022, 23, 15442. [Google Scholar] [CrossRef]
- Saito, K.; Maekawa, K.; Ishikawa, M.; Senoo, Y.; Urata, M.; Murayama, M.; Nakatsu, N.; Yamada, H.; Saito, Y. Glucosylceramide and lysophosphatidylcholines as potential blood biomarkers for drug-induced hepatic phospholipidosis. Toxicol. Sci. Off. J. Soc. Toxicol. 2014, 141, 377–386. [Google Scholar] [CrossRef] [PubMed]
- Qu, L.; Qu, F.; Jia, Z.; Wang, C.; Wu, C.; Zhang, J. Integrated targeted sphingolipidomics and transcriptomics reveal abnormal sphingolipid metabolism as a novel mechanism of the hepatotoxicity and nephrotoxicity of triptolide. J. Ethnopharmacol. 2015, 170, 28–38. [Google Scholar] [CrossRef] [PubMed]
- Tsunoda, M.; Sharma, R.P.; Riley, R.T. Early fumonisin B1 toxicity in relation to disrupted sphingolipid metabolism in male BALB/c mice. J. Biochem. Mol. Toxicol. 1998, 12, 281–289. [Google Scholar] [CrossRef]
- Riley, R.T.; Enongene, E.; Voss, K.A.; Norred, W.P.; Meredith, F.I.; Sharma, R.P.; Spitsbergen, J.; Williams, D.E.; Carlson, D.B.; Merrill, A.H., Jr. Sphingolipid perturbations as mechanisms for fumonisin carcinogenesis. Environ. Health Perspect. 2001, 109 (Suppl. S2), 301–308. [Google Scholar] [PubMed] [Green Version]
- Zitomer, N.C.; Mitchell, T.; Voss, K.A.; Bondy, G.S.; Pruett, S.T.; Garnier-Amblard, E.C.; Liebeskind, L.S.; Park, H.; Wang, E.; Sullards, M.C.; et al. Ceramide synthase inhibition by fumonisin B1 causes accumulation of 1-deoxysphinganine: A novel category of bioactive 1-deoxysphingoid bases and 1-deoxydihydroceramides biosynthesized by mammalian cell lines and animals. J. Biol. Chem. 2009, 284, 4786–4795. [Google Scholar]
- Li, L.; Wang, H.; Jones, J.W. Sphingolipid metabolism as a marker of hepatotoxicity in drug-induced liver injury. Prostaglandins Other Lipid Mediat. 2020, 151, 106484. [Google Scholar] [CrossRef] [PubMed]
- Kullak-Ublick, G.A.; Andrade, R.J.; Merz, M.; End, P.; Benesic, A.; Gerbes, A.L.; Aithal, G.P. Drug-induced liver injury: Recent advances in diagnosis and risk assessment. Gut 2017, 66, 1154–1164. [Google Scholar]
- Andrade, R.J.; Chalasani, N.; Bjornsson, E.S.; Suzuki, A.; Kullak-Ublick, G.A.; Watkins, P.B.; Devarbhavi, H.; Merz, M.; Lucena, M.I.; Kaplowitz, N.; et al. Drug-induced liver injury. Nat. Rev. Dis. Prim. 2019, 5, 58. [Google Scholar] [CrossRef] [PubMed]
- Ramachandran, A.; Jaeschke, H. Acetaminophen Hepatotoxicity. Semin. Liver Dis. 2019, 39, 221–234. [Google Scholar] [CrossRef]
- Bjornsson, E.S.; Bergmann, O.M.; Bjornsson, H.K.; Kvaran, R.B.; Olafsson, S. Incidence, presentation, and outcomes in patients with drug-induced liver injury in the general population of Iceland. Gastroenterology 2013, 144, 1419–1425.e3. [Google Scholar] [CrossRef]
- Lucena, M.I.; Molokhia, M.; Shen, Y.; Urban, T.J.; Aithal, G.P.; Andrade, R.J.; Day, C.P.; Ruiz-Cabello, F.; Donaldson, P.T.; Stephens, C.; et al. Susceptibility to amoxicillin-clavulanate-induced liver injury is influenced by multiple HLA class I and II alleles. Gastroenterology 2011, 141, 338–347. [Google Scholar] [CrossRef] [PubMed]
- Hautekeete, M.L.; Horsmans, Y.; Van Waeyenberge, C.; Demanet, C.; Henrion, J.; Verbist, L.; Brenard, R.; Sempoux, C.; Michielsen, P.P.; Yap, P.S.; et al. HLA association of amoxicillin-clavulanate--induced hepatitis. Gastroenterology 1999, 117, 1181–1186. [Google Scholar] [CrossRef] [PubMed]
- Daly, A.K.; Donaldson, P.T.; Bhatnagar, P.; Shen, Y.; Pe’er, I.; Floratos, A.; Daly, M.J.; Goldstein, D.B.; John, S.; Nelson, M.R.; et al. HLA-B*5701 genotype is a major determinant of drug-induced liver injury due to flucloxacillin. Nat. Genet. 2009, 41, 816–819. [Google Scholar] [CrossRef] [PubMed]
- Krebs, K.; Bovijn, J.; Zheng, N.; Lepamets, M.; Censin, J.C.; Jurgenson, T.; Sarg, D.; Abner, E.; Laisk, T.; Luo, Y.; et al. Genome-wide Study Identifies Association between HLA-B(*)55:01 and Self-Reported Penicillin Allergy. Am. J. Hum. Genet. 2020, 107, 612–621. [Google Scholar] [CrossRef] [PubMed]
- Nicoletti, P.; Aithal, G.P.; Bjornsson, E.S.; Andrade, R.J.; Sawle, A.; Arrese, M.; Barnhart, H.X.; Bondon-Guitton, E.; Hayashi, P.H.; Bessone, F.; et al. Association of Liver Injury From Specific Drugs, or Groups of Drugs, With Polymorphisms in HLA and Other Genes in a Genome-Wide Association Study. Gastroenterology 2017, 152, 1078–1089. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.J.; Phillips, E.J.; Dellinger, A.; Nicoletti, P.; Schutte, R.; Li, D.; Ostrov, D.A.; Fontana, R.J.; Watkins, P.B.; Stolz, A.; et al. Human Leukocyte Antigen B*14:01 and B*35:01 Are Associated With Trimethoprim-Sulfamethoxazole Induced Liver Injury. Hepatology 2021, 73, 268–281. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, P.H.; Rockey, D.C.; Fontana, R.J.; Tillmann, H.L.; Kaplowitz, N.; Barnhart, H.X.; Gu, J.; Chalasani, N.P.; Reddy, K.R.; Sherker, A.H.; et al. Death and liver transplantation within 2 years of onset of drug-induced liver injury. Hepatology 2017, 66, 1275–1285. [Google Scholar] [CrossRef]
- Roussel Uclaf Causality Assessment Method (RUCAM) in Drug Induced Liver Injury. In LiverTox: Clinical and Research Information on Drug-Induced Liver Injury; National Institute of Diabetes and Digestive and Kidney Diseases: Bethesda, MD, USA, 2012.
- Bertea, M.; Rutti, M.F.; Othman, A.; Marti-Jaun, J.; Hersberger, M.; von Eckardstein, A.; Hornemann, T. Deoxysphingoid bases as plasma markers in diabetes mellitus. Lipids Health Dis. 2010, 9, 84. [Google Scholar] [CrossRef] [PubMed]
- Knapp, M.; Baranowski, M.; Lisowska, A.; Musial, W. Decreased free sphingoid base concentration in the plasma of patients with chronic systolic heart failure. Adv. Med. Sci. 2012, 57, 100–105. [Google Scholar] [CrossRef] [PubMed]
- Mwinyi, J.; Bostrom, A.; Fehrer, I.; Othman, A.; Waeber, G.; Marti-Soler, H.; Vollenweider, P.; Marques-Vidal, P.; Schioth, H.B.; von Eckardstein, A.; et al. Plasma 1-deoxysphingolipids are early predictors of incident type 2 diabetes mellitus. PLoS ONE 2017, 12, e0175776. [Google Scholar]
- Morano, C.; Zulueta, A.; Caretti, A.; Roda, G.; Paroni, R.; Dei Cas, M. An Update on Sphingolipidomics: Is Something Still Missing? Some Considerations on the Analysis of Complex Sphingolipids and Free-Sphingoid Bases in Plasma and Red Blood Cells. Metabolites 2022, 12, 450. [Google Scholar] [CrossRef]
- Zimmermann, H.J. Drug-Induced Liver Disease. In Hepatotoxicity, The Adverse Effects of Drugs and Other Chemicals on the Liver, 2nd ed.; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 1978; pp. 428–433. [Google Scholar]
- Hanada, K. Serine palmitoyltransferase, a key enzyme of sphingolipid metabolism. Biochim. Et Biophys. Acta 2003, 1632, 16–30. [Google Scholar] [CrossRef]
- Billi de Catabbi, S.C.; Setton-Advruj, C.P.; Sterin-Speziale, N.; San Martin de Viale, L.C.; Cochon, A.C. Hexachlorobenzene-induced alterations on neutral and acidic sphingomyelinases and serine palmitoyltransferase activities. A time course study in two strains of rats. Toxicology 2000, 149, 89–100. [Google Scholar] [CrossRef]
- Bejaoui, K.; Wu, C.; Scheffler, M.D.; Haan, G.; Ashby, P.; Wu, L.; de Jong, P.; Brown, R.H., Jr. SPTLC1 is mutated in hereditary sensory neuropathy, type 1. Nat. Genet. 2001, 27, 261–262. [Google Scholar] [CrossRef]
- Dawkins, J.L.; Hulme, D.J.; Brahmbhatt, S.B.; Auer-Grumbach, M.; Nicholson, G.A. Mutations in SPTLC1, encoding serine palmitoyltransferase, long chain base subunit-1, cause hereditary sensory neuropathy type I. Nat. Genet. 2001, 27, 309–312. [Google Scholar] [CrossRef]
- Othman, A.; Saely, C.H.; Muendlein, A.; Vonbank, A.; Drexel, H.; von Eckardstein, A.; Hornemann, T. Plasma 1-deoxysphingolipids are predictive biomarkers for type 2 diabetes mellitus. BMJ Open Diabetes Res. Care 2015, 3, e000073. [Google Scholar] [CrossRef] [PubMed]
- Alecu, I.; Tedeschi, A.; Behler, N.; Wunderling, K.; Lamberz, C.; Lauterbach, M.A.; Gaebler, A.; Ernst, D.; Van Veldhoven, P.P.; Al-Amoudi, A.; et al. Localization of 1-deoxysphingolipids to mitochondria induces mitochondrial dysfunction. J. Lipid Res. 2017, 58, 42–59. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bettigole, S.E.; Glimcher, L.H. Endoplasmic reticulum stress in immunity. Annu. Rev. Immunol. 2015, 33, 107–138. [Google Scholar] [CrossRef] [PubMed]
- Murphy, M.P. How mitochondria produce reactive oxygen species. Biochem. J. 2009, 417, 1–13. [Google Scholar] [CrossRef] [PubMed]
- European Association for the Study of the Liver; Clinical Practice Guideline Panel: Chair; Panel Members; EASL Governing Board Representative. EASL Clinical Practice Guidelines: Drug-induced liver injury. J. Hepatol. 2019, 70, 1222–1261. [Google Scholar] [CrossRef]
- Xiao, L.L.; Zhang, F.; Zhao, Y.L.; Zhang, L.J.; Xie, Z.Y.; Huang, K.Z.; Ouyang, X.X.; Wu, X.X.; Xu, X.W.; Li, L.J. Using advanced oxidation protein products and ischaemia-modified albumin to monitor oxidative stress levels in patients with drug-induced liver injury. Sci. Rep. 2020, 10, 18128. [Google Scholar] [CrossRef] [PubMed]
- Hornemann, T.; Penno, A.; Rutti, M.F.; Ernst, D.; Kivrak-Pfiffner, F.; Rohrer, L.; von Eckardstein, A. The SPTLC3 subunit of serine palmitoyltransferase generates short chain sphingoid bases. J. Biol. Chem. 2009, 284, 26322–26330. [Google Scholar] [CrossRef]
- Lone, M.A.; Bourquin, F.; Hornemann, T. Serine Palmitoyltransferase Subunit 3 and Metabolic Diseases. In Sphingolipid Metabolism and Metabolic Disease; Jiang, X.-C., Ed.; Springer: Berlin/Heidelberg, Germany, 2022; Volume 1372, pp. 47–56. [Google Scholar]
- Kim, G.T.; Devi, S.; Sharma, A.; Cho, K.H.; Kim, S.J.; Kim, B.R.; Kwon, S.H.; Park, T.S. Upregulation of the serine palmitoyltransferase subunit SPTLC2 by endoplasmic reticulum stress inhibits the hepatic insulin response. Exp. Mol. Med. 2022, 54, 573–584. [Google Scholar] [CrossRef]
- Memon, R.A.; Holleran, W.M.; Moser, A.H.; Seki, T.; Uchida, Y.; Fuller, J.; Shigenaga, J.K.; Grunfeld, C.; Feingold, K.R. Endotoxin and cytokines increase hepatic sphingolipid biosynthesis and produce lipoproteins enriched in ceramides and sphingomyelin. Arterioscler. Thromb. Vasc. Biol. 1998, 18, 1257–1265. [Google Scholar] [CrossRef] [PubMed]
- Hornemann, T.; Richard, S.; Rutti, M.F.; Wei, Y.; von Eckardstein, A. Cloning and initial characterization of a new subunit for mammalian serine-palmitoyltransferase. J. Biol. Chem. 2006, 281, 37275–37281. [Google Scholar] [CrossRef] [PubMed]
- Foufelle, F.; Fromenty, B. Role of endoplasmic reticulum stress in drug-induced toxicity. Pharm. Res. Perspect. 2016, 4, e00211. [Google Scholar] [CrossRef]
- Chen, S.; Melchior, W.B., Jr.; Guo, L. Endoplasmic reticulum stress in drug- and environmental toxicant-induced liver toxicity. J. Environ. Sci. Health. Part C Environ. Carcinog. Ecotoxicol. Rev. 2014, 32, 83–104. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chalasani, N.P.; Hayashi, P.H.; Bonkovsky, H.L.; Navarro, V.J.; Lee, W.M.; Fontana, R.J.; on behalf of the Practice Parameters Committee of the American College of Gastroenterology. ACG Clinical Guideline: The diagnosis and management of idiosyncratic drug-induced liver injury. Am. J. Gastroenterol. 2014, 109, 950–966. [Google Scholar] [CrossRef] [PubMed]
- NIH. LiverTox. Available online: https://www.ncbi.nlm.nih.gov/books/NBK547852/ (accessed on 3 October 2022).
- Church, R.J.; Kullak-Ublick, G.A.; Aubrecht, J.; Bonkovsky, H.L.; Chalasani, N.; Fontana, R.J.; Goepfert, J.C.; Hackman, F.; King, N.M.P.; Kirby, S.; et al. Candidate biomarkers for the diagnosis and prognosis of drug-induced liver injury: An international collaborative effort. Hepatology 2019, 69, 760–773. [Google Scholar] [CrossRef] [PubMed]
- Aithal, G.P.; Watkins, P.B.; Andrade, R.J.; Larrey, D.; Molokhia, M.; Takikawa, H.; Hunt, C.M.; Wilke, R.A.; Avigan, M.; Kaplowitz, N.; et al. Case definition and phenotype standardization in drug-induced liver injury. Clin. Pharmacol. Ther. 2011, 89, 806–815. [Google Scholar] [CrossRef] [PubMed]


| DILI (n = 28) | non-DILI (n = 15) | ||
|---|---|---|---|
| Parameters | Category | N (%) | |
| Age | ≤55 >55 | 15 (54) 13 (47) | 10 (67) 5 (33) |
| Gender | Male Female | 17 (59) 12 (41) | 7 (47) 8 (53) |
| Type of DILI | Hepatocellular Cholestatic Mixed | 20 (69) 6 (21) 3 (10) | - - - |
| Severity of injury * | Severe Non-severe | 8 (28) 21 (72) | - - |
| Steatosis | Yes No | 4 (14) 25 (86) | - - |
| T2D | Yes No NA | 4 (14) 19 (68) 5 (18) | 1 (7) 14 (93) - |
| CVD | Yes No | 12 (43) 16 (57) | 6 (40) 9 (60) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gai, Z.; Samodelov, S.L.; Alecu, I.; Hornemann, T.; Grove, J.I.; Aithal, G.P.; Visentin, M.; Kullak-Ublick, G.A. Plasma Sphingoid Base Profiles of Patients Diagnosed with Intrinsic or Idiosyncratic Drug-induced Liver Injury. Int. J. Mol. Sci. 2023, 24, 3013. https://doi.org/10.3390/ijms24033013
Gai Z, Samodelov SL, Alecu I, Hornemann T, Grove JI, Aithal GP, Visentin M, Kullak-Ublick GA. Plasma Sphingoid Base Profiles of Patients Diagnosed with Intrinsic or Idiosyncratic Drug-induced Liver Injury. International Journal of Molecular Sciences. 2023; 24(3):3013. https://doi.org/10.3390/ijms24033013
Chicago/Turabian StyleGai, Zhibo, Sophia L. Samodelov, Irina Alecu, Thorsten Hornemann, Jane I. Grove, Guruprasad P. Aithal, Michele Visentin, and Gerd A. Kullak-Ublick. 2023. "Plasma Sphingoid Base Profiles of Patients Diagnosed with Intrinsic or Idiosyncratic Drug-induced Liver Injury" International Journal of Molecular Sciences 24, no. 3: 3013. https://doi.org/10.3390/ijms24033013





