Species-Specific Response of Corals to Imbalanced Ratios of Inorganic Nutrients
Abstract
:1. Introduction
2. Results
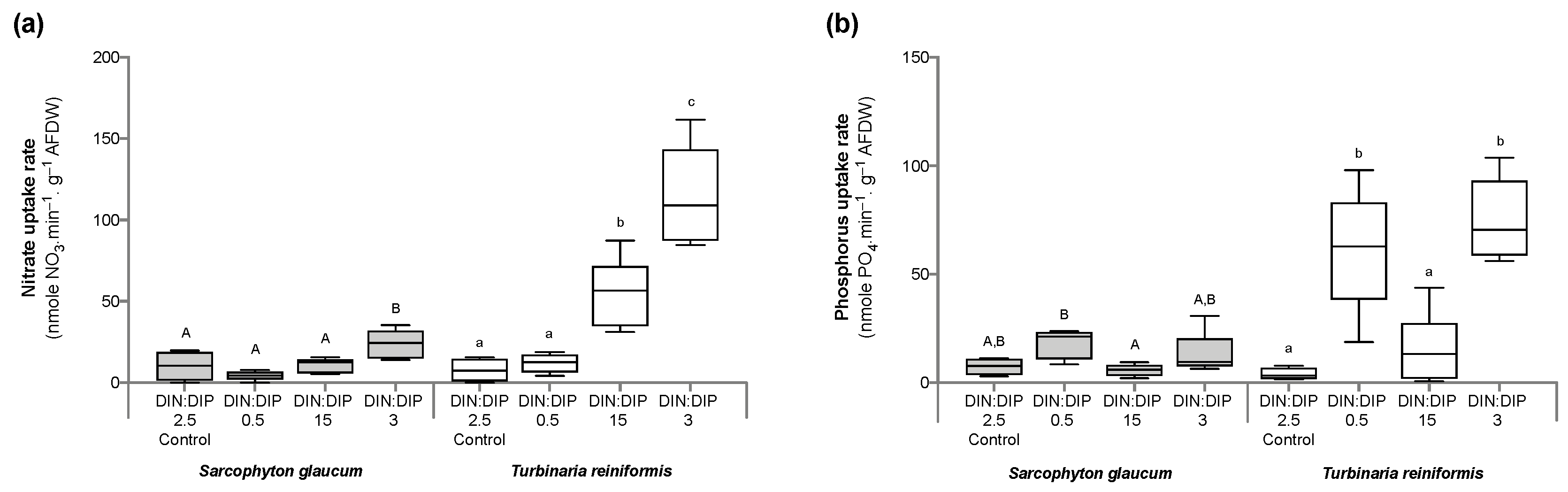
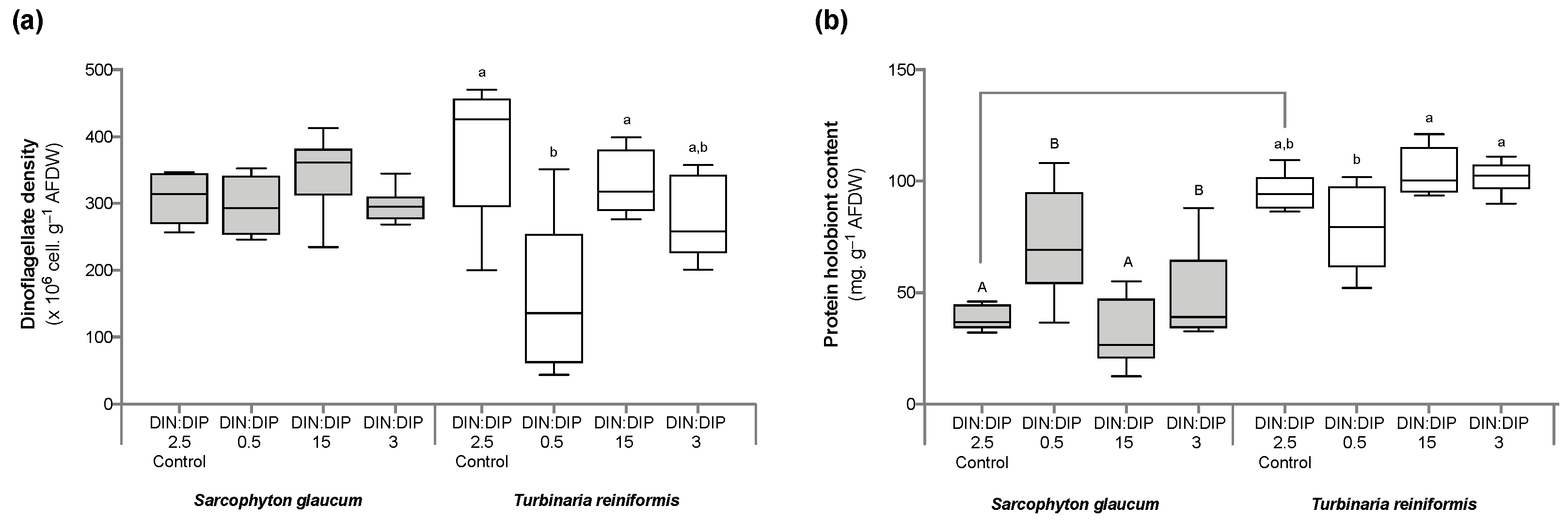
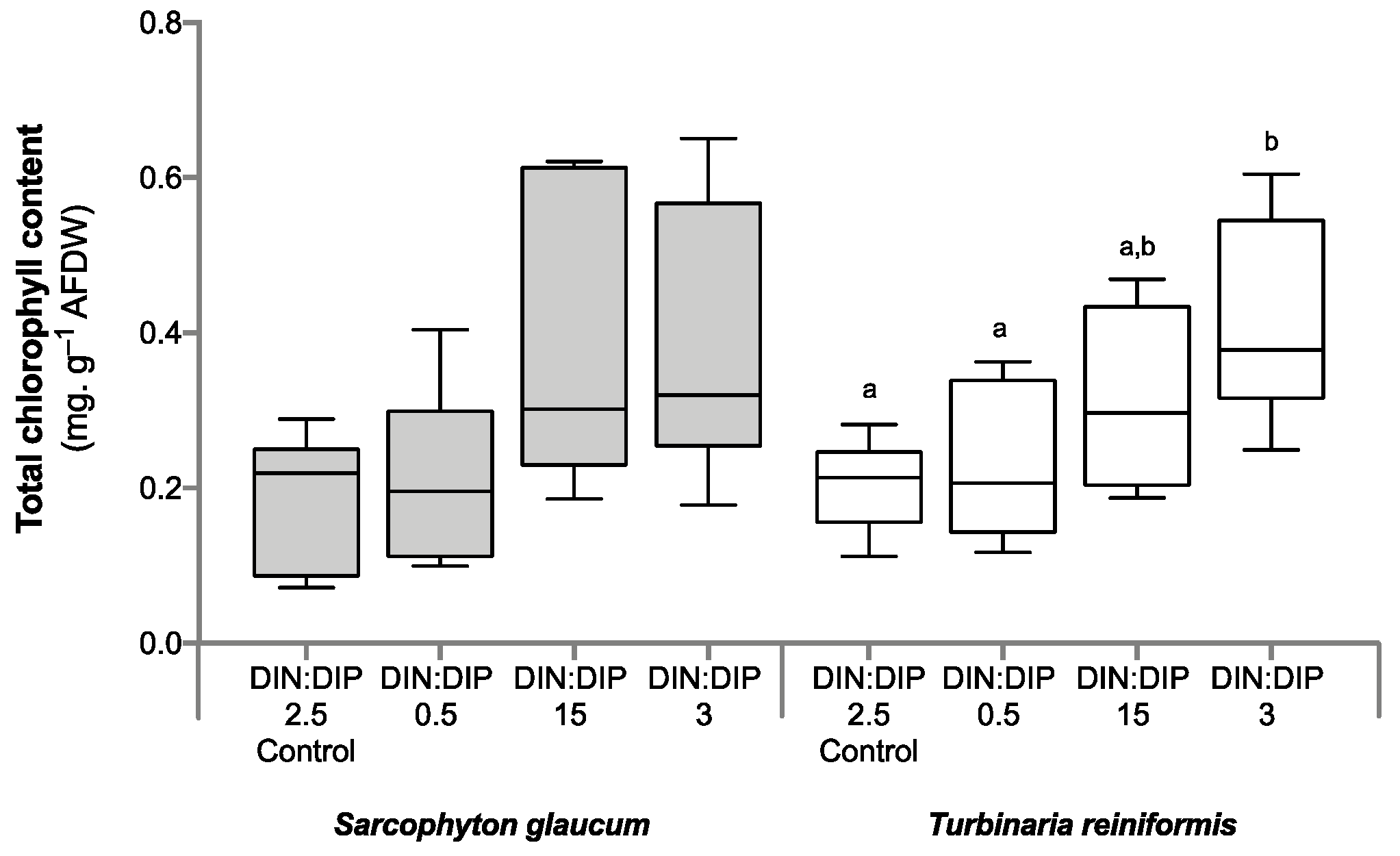
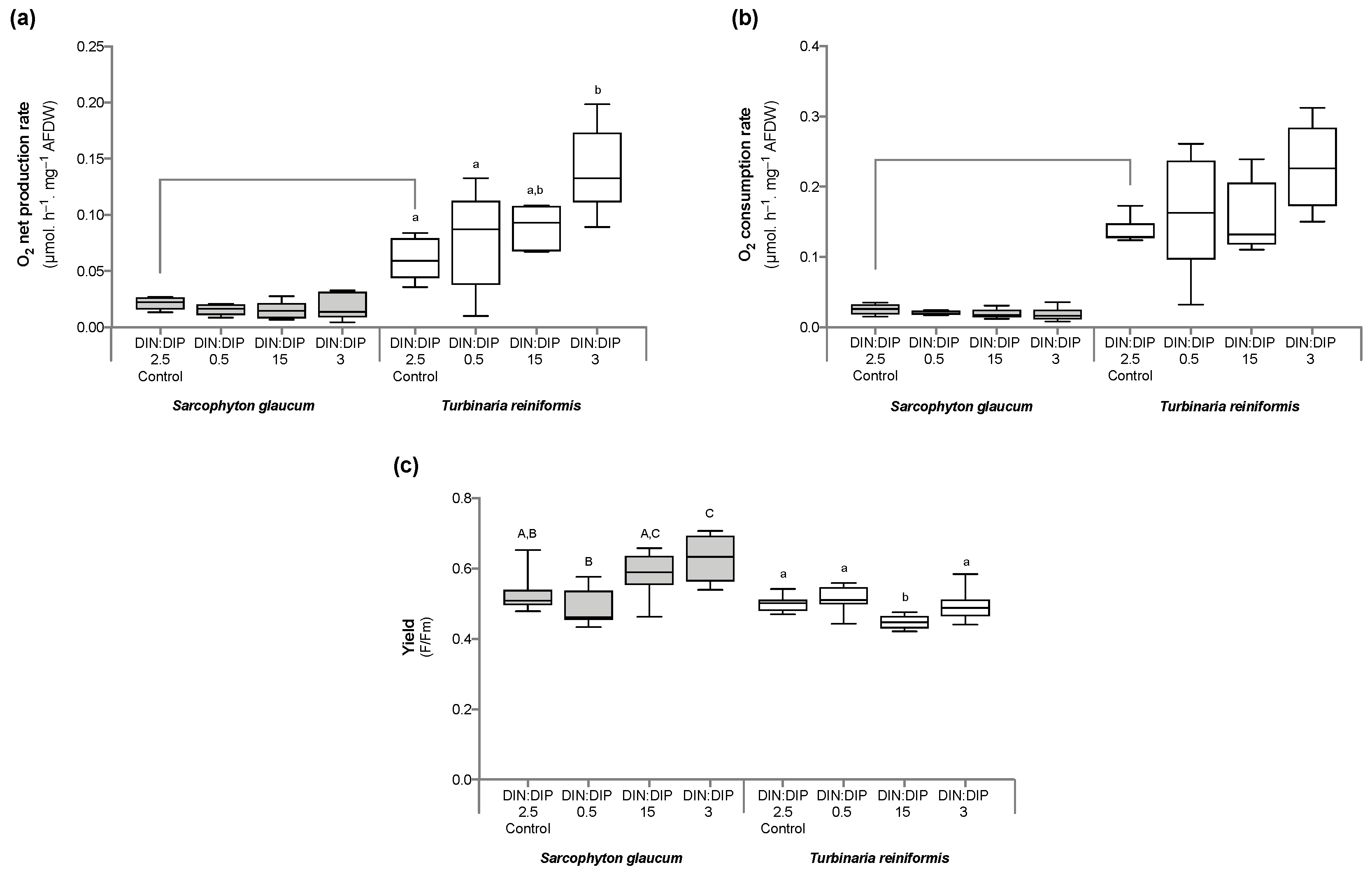
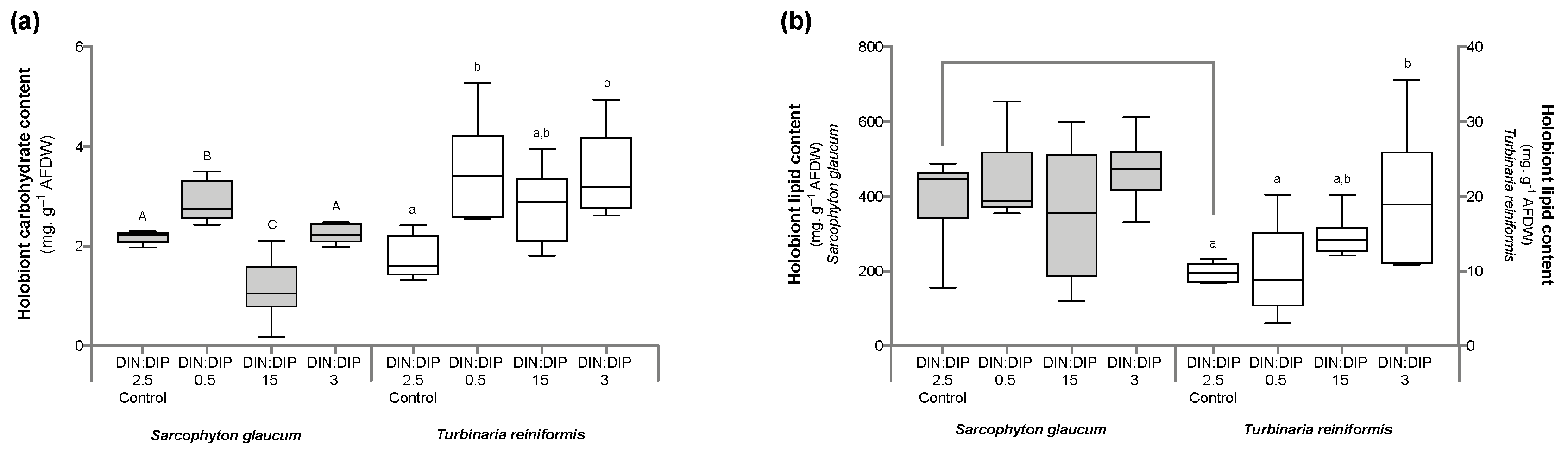
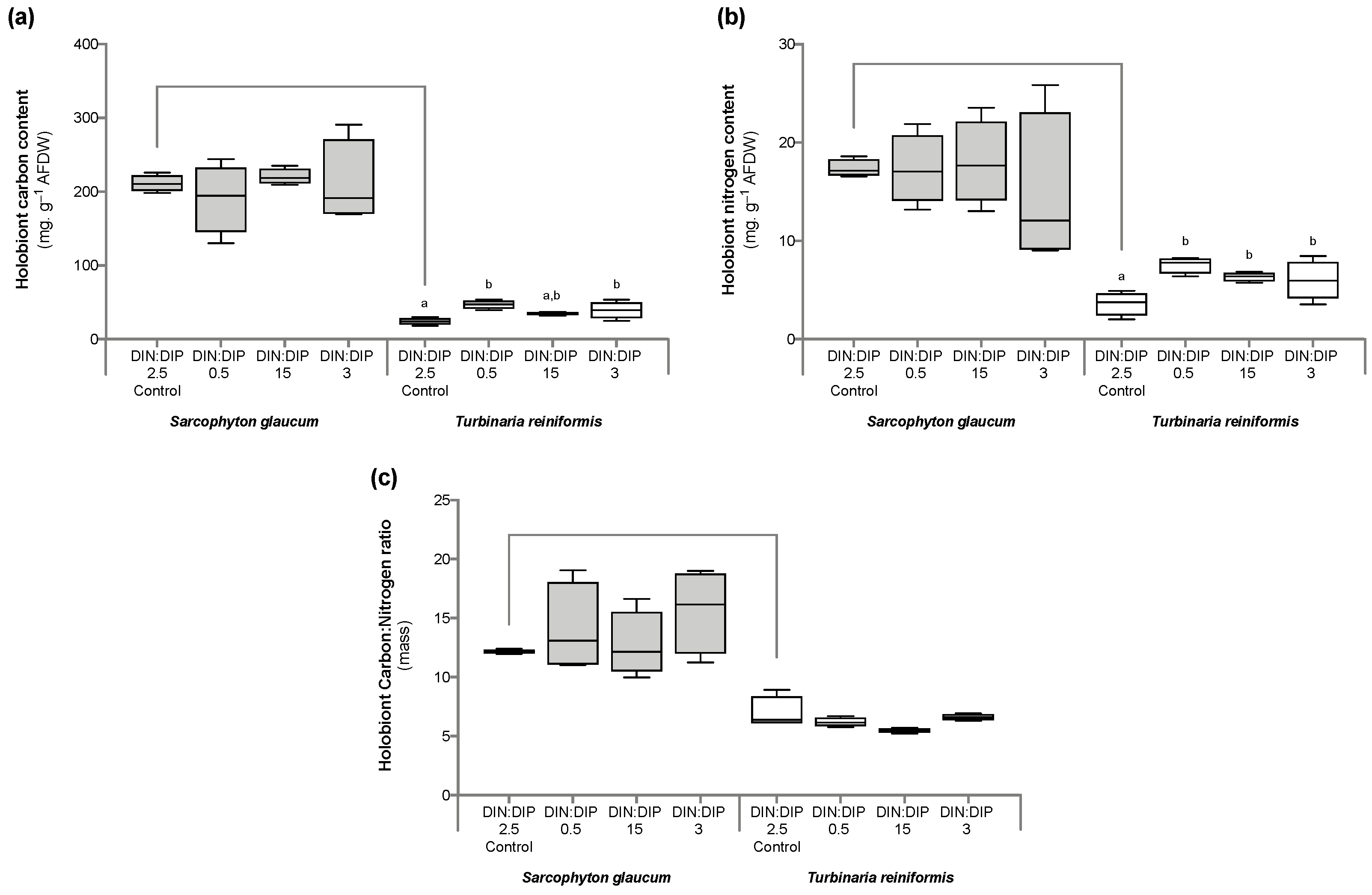
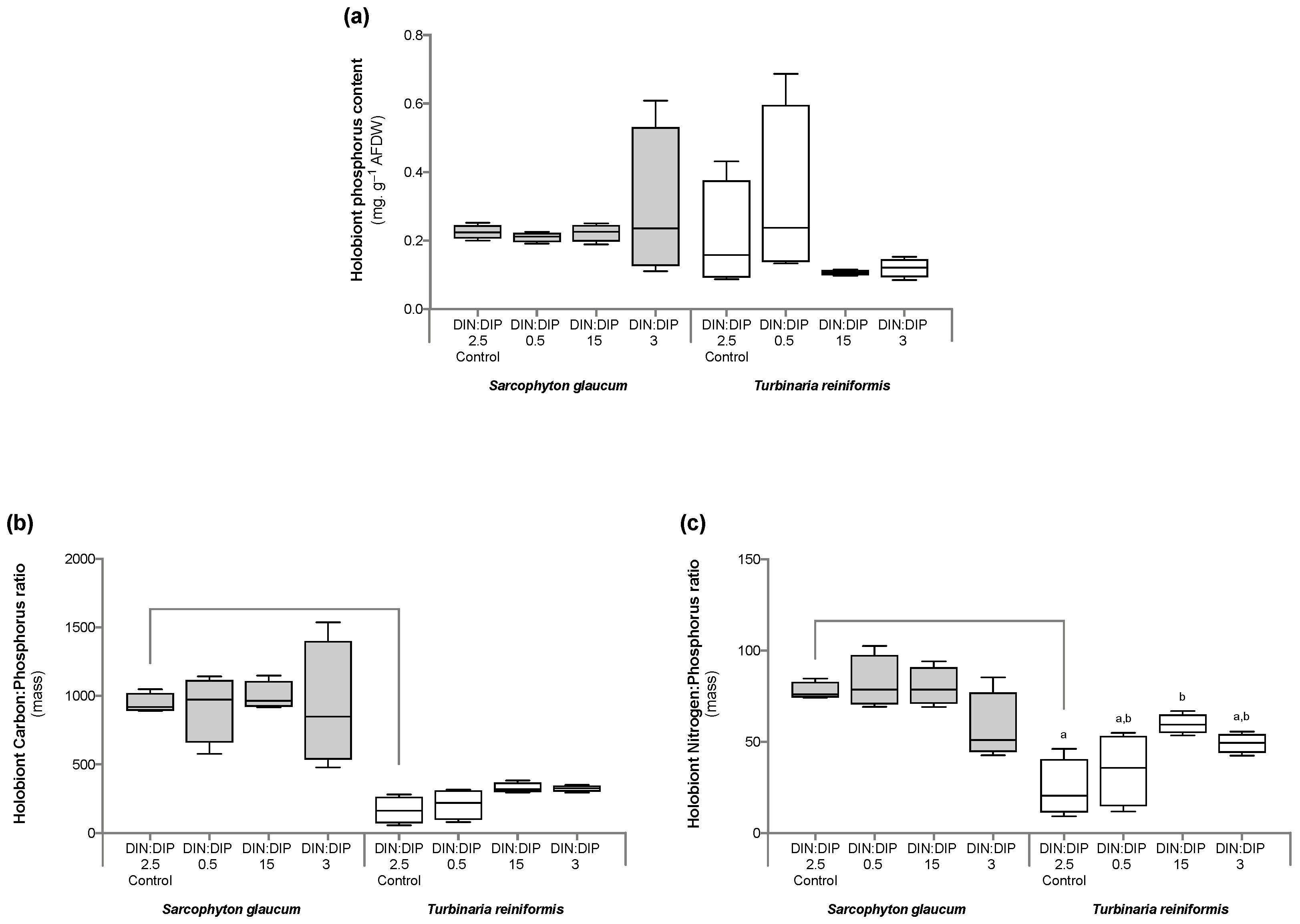
2.1. Effect of DIN:DIP Ratios on the Physiology and Tissue Composition of Turbinaria Reniformis
2.2. Effect of DIN:DIP Ratios on the Physiology and Tissue Composition of Sarcophyton Glaucum
2.3. Comparison in the Physiological Parameters between Turbinaria Reniformis and Sarcophyton Glaucum under a Control Balanced DIN:DIP2.5 without Enrichment
3. Discussion
3.1. Effect of DIN:DIP Ratio on the Physiology of T. Reniformis
3.2. Effect of DIN:DIP Ratio on the Physiology of S. Glaucum
4. Materials and Methods
4.1. Experimental Design
4.2. Dissolved Inorganic Nutrients Uptake Rate
4.3. Measurement of Photosynthetic Parameters
4.4. Tissue Parameters
4.4.1. Symbiodinium Density, Total Protein and Chlorophyll Content
4.4.2. Lipid and Carbohydrate Content
4.5. Oxidative Status
4.5.1. Non-Enzymatic Total Antioxidant Capacity (NETAC)
4.5.2. Lipid Peroxidation (LPO)
4.6. Carbon, Nitrogen, Phosphorus Content
4.6.1. Phosphorus Content
4.6.2. Carbon and Nitrogen Content
4.7. Statistical Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Darwin, C. The Structure and Distribution of Coral Reefs; University of California Press: Berkeley, CA, USA, 1842; ISBN 978-0-520-32733-7. [Google Scholar]
- Muscatine, L.; Porter, J.W. Reef Corals: Mutualistic Symbioses Adapted to Nutrient-Poor Environments. Bioscience 1977, 27, 454–460. [Google Scholar] [CrossRef]
- Rädecker, N.; Pogoreutz, C.; Voolstra, C.R.; Wiedenmann, J.; Wild, C. Nitrogen Cycling in Corals: The Key to Understanding Holobiont Functioning? Trends Microbiol. 2015, 23, 490–497. [Google Scholar] [CrossRef]
- Ferrier-Pagès, C.; Godinot, C.; D’angelo, C.; Wiedenmann, J.; Grover, R. Phosphorus Metabolism of Reef Organisms with Algal Symbionts. Ecol. Monogr. 2016, 86, 262–277. [Google Scholar] [CrossRef]
- Tremblay, P.; Grover, R.; Maguer, J.-F.; Hoogenboom, M.; Ferrier-Pagès, C. Carbon Translocation from Symbiont to Host Depends on Irradiance and Food Availability in the Tropical Coral Stylophora Pistillata. Coral Reefs 2014, 33, 1–13. [Google Scholar] [CrossRef]
- Larned, S.T. Nitrogen- versus Phosphorus-Limited Growth and Sources of Nutrients for Coral Reef Macroalgae. Mar. Biol. 1998, 132, 409–421. [Google Scholar] [CrossRef]
- D’Angelo, C.; Wiedenmann, J. Impacts of Nutrient Enrichment on Coral Reefs: New Perspectives and Implications for Coastal Management and Reef Survival. Curr. Opin. Environ. Sustain. 2014, 7, 82–93. [Google Scholar] [CrossRef]
- Ezzat, L.; Maguer, J.-F.; Grover, R.; Ferrier-Pagès, C. Limited Phosphorus Availability Is the Achilles Heel of Tropical Reef Corals in a Warming Ocean. Sci. Rep. 2016, 6, 31768. [Google Scholar] [CrossRef]
- Ezzat, L.; Maguer, J.-F.; Grover, R.; Rottier, C.; Tremblay, P.; Ferrier-Pagès, C. Nutrient Starvation Impairs the Trophic Plasticity of Reef-building Corals under Ocean Warming. Funct. Ecol. 2019, 33, 643–653. [Google Scholar] [CrossRef]
- Rosset, S.; Wiedenmann, J.; Reed, A.J.; D’Angelo, C. Phosphate Deficiency Promotes Coral Bleaching and Is Reflected by the Ultrastructure of Symbiotic Dinoflagellates. Mar. Pollut. Bull. 2017, 118, 180–187. [Google Scholar] [CrossRef]
- Fabricius, K.E. Effects of Terrestrial Runoff on the Ecology of Corals and Coral Reefs: Review and Synthesis. Mar. Pollut. Bull. 2005, 50, 125–146. [Google Scholar] [CrossRef]
- Fabricius, K.E. Factors Determining the Resilience of Coral Reefs to Eutrophication: A Review and Conceptual Model. In Coral Reefs: An Ecosystem in Transition; Springer: Berlin/Heidelberg, Germany, 2011; pp. 493–505. [Google Scholar]
- Marubini, F.; Davies, P.S. Nitrate Increases Zooxanthellae Population Density and Reduces Skeletogenesis in Corals. Mar. Biol. 1996, 127, 319–328. [Google Scholar] [CrossRef]
- Nordemar, I.; Nyström, M.; Dizon, R. Effects of Elevated Seawater Temperature and Nitrate Enrichment on the Branching Coral Porites Cylindrica in the Absence of Particulate Food. Mar. Biol. 2003, 142, 669–677. [Google Scholar] [CrossRef]
- Renegar, D.A.; Riegl, B.M. Effect of Nutrient Enrichment and Elevated CO2 Partial Pressure on Growth Rate of Atlantic Scleractinian Coral Acropora Cervicornis. Mar. Ecol. Prog. Ser. 2005, 293, 69–76. [Google Scholar] [CrossRef]
- Wiedenmann, J.; D’Angelo, C.; Smith, E.G.; Hunt, A.N.; Legiret, F.-E.; Postle, A.D.; Achterberg, E.P. Nutrient Enrichment Can Increase the Susceptibility of Reef Corals to Bleaching. Nat. Clim. Change 2013, 3, 160–164. [Google Scholar] [CrossRef]
- Ezzat, L.; Maguer, J.-F.; Grover, R.; Ferrier-Pagès, C. New Insights into Carbon Acquisition and Exchanges within the Coral–Dinoflagellate Symbiosis under NH4+ and NO3− Supply. Proc. R. Soc. Lond. Ser. B 2015, 282, 20150610. [Google Scholar] [CrossRef]
- Liu, C.-Y.; Zhang, F.; Sun, Y.-F.; Yu, X.-L.; Huang, H. Effects of Nitrate Enrichment on Respiration, Photosynthesis, and Fatty Acid Composition of Reef Coral Pocillopora Damicornis Larvae. Front. Mar. Sci. 2020, 7, 531. [Google Scholar] [CrossRef]
- Marangoni, L.; Ferrier-Pagès, C.; Rottier, C.; Bianchini, A.; Grover, R. Unravelling the Different Causes of Nitrate and Ammonium Effects on Coral Bleaching. Sci. Rep. 2020, 10, 11975. [Google Scholar] [CrossRef]
- Ferrier-Pagès, C.; Gattuso, J.-P.; Dallot, S.; Jaubert, J. Effect of Nutrient Enrichment on Growth and Photosynthesis of the Zooxanthellate Coral Stylophora Pistillata. Coral Reefs 2000, 19, 103–113. [Google Scholar] [CrossRef]
- Harrison, P.; Ward, S. Elevated Levels of Nitrogen and Phosphorus Reduce Fertilisation Success of Gametes from Scleractinian Reef Corals. Mar. Biol. 2001, 139, 1057–1068. [Google Scholar] [CrossRef]
- Koop, K.; Booth, D.; Broadbent, A.; Brodie, J.; Bucher, D.; Capone, D.; Coll, J.; Dennison, W.; Erdmann, M.; Harrison, P. ENCORE: The Effect of Nutrient Enrichment on Coral Reefs. Synthesis of Results and Conclusions. Mar. Pollut. Bull. 2001, 42, 91–120. [Google Scholar] [CrossRef]
- Lirman, D.; Fong, P. Is Proximity to Land-Based Sources of Coral Stressors an Appropriate Measure of Risk to Coral Reefs? An Example from the Florida Reef Tract. Mar. Pollut. Bull. 2007, 54, 779–791. [Google Scholar] [CrossRef]
- Sawall, Y.; Teichberg, M.C.; Seemann, J.; Litaay, M.; Jompa, J.; Richter, C. Nutritional Status and Metabolism of the Coral Stylophora Subseriata along a Eutrophication Gradient in Spermonde Archipelago (Indonesia). Coral Reefs 2011, 30, 841–853. [Google Scholar] [CrossRef]
- Ezzat, L.; Towle, E.; Irisson, J.-O.; Langdon, C.; Ferrier-Pagès, C. The Relationship between Heterotrophic Feeding and Inorganic Nutrient Availability in the Scleractinian Coral T. Reniformis under a Short-Term Temperature Increase. Limnol. Oceanogr. 2016, 61, 89–102. [Google Scholar] [CrossRef]
- Meyer, J.L.; Schultz, E.T. Migrating Haemulid Fishes as a Source of Nutrients and Organic Matter on Coral Reefs 1. Limnol. Oceanogr. 1985, 30, 146–156. [Google Scholar] [CrossRef]
- Atkinson, M.J.; Carlson, B.; Crow, G.L. Coral Growth in High-Nutrient, Low-PH Seawater: A Case Study of Corals Cultured at the Waikiki Aquarium, Honolulu, Hawaii. Coral Reefs 1995, 14, 215–223. [Google Scholar] [CrossRef]
- Tanaka, Y.; Miyajima, T.; Koike, I.; Hayashibara, T.; Ogawa, H. Imbalanced Coral Growth between Organic Tissue and Carbonate Skeleton Caused by Nutrient Enrichment. Limnol. Oceanogr. 2007, 52, 1139–1146. [Google Scholar] [CrossRef]
- Tanaka, Y.; Inoue, M.; Nakamura, T.; Suzuki, A.; Sakai, K. Loss of Zooxanthellae in a Coral under High Seawater Temperature and Nutrient Enrichment. J. Exp. Mar. Biol. Ecol. 2014, 457, 220–225. [Google Scholar] [CrossRef]
- Chauvin, A.; Denis, V.; Cuet, P. Is the Response of Coral Calcification to Seawater Acidification Related to Nutrient Loading? Coral Reefs 2011, 30, 911–923. [Google Scholar] [CrossRef]
- Hoadley, K.D.; Pettay, D.T.; Grottoli, A.G.; Cai, W.-J.; Melman, T.F.; Levas, S.; Schoepf, V.; Ding, Q.; Yuan, X.; Wang, Y.; et al. High-Temperature Acclimation Strategies within the Thermally Tolerant Endosymbiont Symbiodinium Trenchii and Its Coral Host, Turbinaria Reniformis, Differ with Changing PCO2 and Nutrients. Mar. Biol. 2016, 163, 134. [Google Scholar] [CrossRef]
- Furnas, M.J.; Mitchell, A.W.; Skuza, M. Nitrogen and Phosphorus Budgets for the Central Great Barrier Reef Shelf; Great Barrier Reef Marine Park Authority: Townsville, Australia, 1995. [Google Scholar]
- Morris, L.A.; Voolstra, C.R.; Quigley, K.M.; Bourne, D.G.; Bay, L.K. Nutrient availability and metabolism affect the stability of coral–Symbiodiniaceae symbioses. Trends Microbiol. 2019, 27, 678–689. [Google Scholar] [CrossRef] [Green Version]
- Zhou, Y.; Yang, X.; Wang, Y.; Li, F.; Wang, J.; Tan, L. Exogenous Nutrient Inputs Restructure Phytoplankton Community and Ecological Stoichiometry of Eastern Indian Ocean. Ecol. Indic. 2021, 127, 107801. [Google Scholar] [CrossRef]
- Sauterey, B.; Ward, B.A. Environmental Control of Marine Phytoplankton Stoichiometry in the North Atlantic Ocean. Proc. Natl. Acad. Sci. USA 2022, 119, e2114602118. [Google Scholar] [CrossRef] [PubMed]
- Blanckaert, A.C.A.; Reef, R.; Pandolfi, J.M.; Lovelock, C.E. Variation in the Elemental Stoichiometry of the Coral–Zooxanthellae Symbiosis. Coral Reefs 2020, 39, 1071–1079. [Google Scholar] [CrossRef]
- Lovelock, C.E.; Reef, R.; Pandolfi, J.M. Variation in Elemental Stoichiometry and RNA: DNA in Four Phyla of Benthic Organisms from Coral Reefs. Funct. Ecol. 2014, 28, 1299–1309. [Google Scholar] [CrossRef]
- Muscatine, L.; Falkowski, P.G.; Dubinsky, Z.; Cook, P.A.; McCloskey, L.R. The Effect of External Nutrient Resources on the Population Dynamics of Zooxanthellae in a Reef Coral. Proc. R. Soc. Lond. Ser. B 1989, 236, 311–324. [Google Scholar] [CrossRef]
- Snidvongs, A.; Kinzie, R.A. Effects of Nitrogen and Phosphorus Enrichment on in Vivo Symbiotic Zooxanthellae of Pocillopora Damicornis. Mar. Biol. 1994, 118, 705–711. [Google Scholar] [CrossRef]
- McCauley, M.; Goulet, T.L. Caribbean Gorgonian Octocorals Cope with Nutrient Enrichment. Mar. Pollut. Bull. 2019, 141, 621–628. [Google Scholar] [CrossRef]
- Tanaka, Y.; Grottoli, A.G.; Matsui, Y.; Suzuki, A.; Sakai, K. Effects of Nitrate and Phosphate Availability on the Tissues and Carbonate Skeleton of Scleractinian Corals. Mar. Ecol. Prog. Ser. 2017, 570, 101–112. [Google Scholar] [CrossRef]
- Tanaka, Y.; Ogawa, H.; Miyajima, T. Effects of Nutrient Enrichment on the Release of Dissolved Organic Carbon and Nitrogen by the Scleractinian Coral Montipora Digitata. Coral Reefs 2010, 29, 675–682. [Google Scholar] [CrossRef]
- Anthony, K.R. Enhanced energy status of corals on coastal, high-turbidity reefs. Mar. Ecol. Prog. Ser. 2006, 319, 111–116. [Google Scholar] [CrossRef]
- Baum, G.; Januar, I.; Ferse, S.C.A.; Wild, C.; Kunzmann, A. Abundance and Physiology of Dominant Soft Corals Linked to Water Quality in Jakarta Bay, Indonesia. PeerJ 2016, 4, e2625. [Google Scholar] [CrossRef]
- De’ath, G.; Fabricius, K. Water quality as a regional driver of coral biodiversity and macroalgae on the Great Barrier Reef. Ecol. Appl. 2010, 20, 840–850. [Google Scholar] [CrossRef] [PubMed]
- Burkepile, D.E.; Shantz, A.A.; Adam, T.C.; Munsterman, K.S.; Speare, K.E.; Ladd, M.C.; Holbrook, S.J. Nitrogen identity drives differential impacts of nutrients on coral bleaching and mortality. Ecosystems 2020, 23, 798–811. [Google Scholar] [CrossRef]
- Pogoreutz, C.; Rädecker, N.; Cárdenas, A.; Gärdes, A.; Wild, C.; Voolstra, C.R. Dominance of Endozoicomonas bacteria throughout coral bleaching and mortality suggests structural inflexibility of the Pocillopora verrucosa microbiome. Ecol. Evol. 2018, 8, 2240–2252. [Google Scholar] [CrossRef]
- Boilard, A.; Dubé; C.E.; Gruet, C.; Mercière, A.; Hernandez-Agreda, A.; Derome, N. Defining coral bleaching as a microbial dysbiosis within the coral holobiont. Microorganisms 2020, 8, 1682. [Google Scholar] [CrossRef]
- Krediet, C.J.; Ritchie, K.B.; Paul, V.J.; Teplitski, M. Coral-associated micro-organisms and their roles in promoting coral health and thwarting diseases. Proc. R. Soc. Lond. Ser. B 2013, 280, 20122328. [Google Scholar] [CrossRef]
- Klinke, A.; Mezger, S.; Thobor, B.; Tilstra, A.; El-Khaled, Y.C.; Wild, C. Phosphate enrichment increases the resistance of the pulsating soft coral Xenia umbellata to warming. Front. Mar. Sci. 2022, 9, 1026321. [Google Scholar] [CrossRef]
- Perini, V.; Bracken, M.E.S. Nitrogen Availability Limits Phosphorus Uptake in an Intertidal Macroalga. Oecologia 2014, 175, 667–676. [Google Scholar] [CrossRef]
- Lubsch, A.; Timmermans, K.R. Phosphate and Nitrate Uptake Dynamics in Palmaria Palmata (Rhodophyceae): Ecological and Physiological Aspects of Nutrient Availability. J. Phycol. 2020, 56, 1184–1195. [Google Scholar] [CrossRef]
- Aubriot, L.; Bonilla, S. Regulation of Phosphate Uptake Reveals Cyanobacterial Bloom Resilience to Shifting N:P Ratios. Freshw. Biol. 2018, 63, 318–329. [Google Scholar] [CrossRef]
- Treseder, K.K.; Vitousek, P.M. Effects of Soil Nutrient Availability on Investment in Acquisition of N and P in Hawaiian Rain Forests. Ecology 2001, 82, 946–954. [Google Scholar] [CrossRef]
- Elser, J.J.; Bracken, M.E.S.; Cleland, E.E.; Gruner, D.S.; Harpole, W.S.; Hillebrand, H.; Ngai, J.T.; Seabloom, E.W.; Shurin, J.B.; Smith, J.E. Global Analysis of Nitrogen and Phosphorus Limitation of Primary Producers in Freshwater, Marine and Terrestrial Ecosystems. Ecol. Lett. 2007, 10, 1135–1142. [Google Scholar] [CrossRef]
- Rodriguez-Casariego, J.A.; Ladd, M.C.; Shantz, A.A.; Lopes, C.; Cheema, M.S.; Kim, B.; Roberts, S.B.; Fourqurean, J.W.; Ausio, J.; Burkepile, D.E.; et al. Coral Epigenetic Responses to Nutrient Stress: Histone H2A.X Phosphorylation Dynamics and DNA Methylation in the Staghorn Coral Acropora Cervicornis. Ecol. Evol. 2018, 8, 12193–12207. [Google Scholar] [CrossRef] [PubMed]
- Béraud, E.; Gevaert, F.; Rottier, C.; Ferrier-Pagès, C. The Response of the Scleractinian Coral Turbinaria Reniformis to Thermal Stress Depends on the Nitrogen Status of the Coral Holobiont. J. Exp. Biol. 2013, 216, 2665–2674. [Google Scholar] [CrossRef] [PubMed]
- Dobson, K.L.; Levas, S.; Schoepf, V.; Warner, M.E.; Cai, W.-J.; Hoadley, K.D.; Yuan, X.; Matsui, Y.; Melman, T.F.; Grottoli, A.G. Moderate Nutrient Concentrations Are Not Detrimental to Corals under Future Ocean Conditions. Mar. Biol. 2021, 168, 98. [Google Scholar] [CrossRef]
- Shantz, A.A.; Burkepile, D.E. Context-dependent Effects of Nutrient Loading on the Coral–Algal Mutualism. Ecology 2014, 95, 1995–2005. [Google Scholar] [CrossRef] [PubMed]
- Holcomb, M.; McCorkle, D.C.; Cohen, A.L. Long-Term Effects of Nutrient and CO2 Enrichment on the Temperate Coral Astrangia Poculata (Ellis and Solander, 1786). J. Exp. Mar. Biol. Ecol. 2010, 386, 27–33. [Google Scholar] [CrossRef]
- Dobson, K.L.; Ferrier-Pagès, C.; Saup, C.M.; Grottoli, A.G. The Effects of Temperature, Light, and Feeding on the Physiology of Pocillopora Damicornis, Stylophora Pistillata, and Turbinaria Reniformis Corals. Water 2021, 13, 2048. [Google Scholar] [CrossRef]
- Bongiorni, L.; Shafir, S.; Rinkevich, B. Effects of Particulate Matter Released by a Fish Farm (Eilat, Red Sea) on Survival and Growth of Stylophora Pistillata Coral Nubbins. Mar. Pollut. Bull. 2003, 46, 1120–1124. [Google Scholar] [CrossRef] [PubMed]
- Dizon, R.; Yap, H. Coral Responses in Single- and Mixed-Species Plots to Nutrient Disturbance. Mar. Ecol. Prog. Ser. 2005, 296, 165–172. [Google Scholar] [CrossRef] [Green Version]
- Ezzat, L.; Fine, M.; Maguer, J.-F.; Grover, R.; Ferrier-Pages, C. Carbon and Nitrogen Acquisition in Shallow and Deep Holobionts of the Scleractinian Coral S. Pistillata. Front. Mar. Sci. 2017, 4, 102. [Google Scholar] [CrossRef]
- Bednarz, V.N.; Van De Water, J.A.; Grover, R.; Maguer, J.-F.; Fine, M.; Ferrier-Pagès, C. Unravelling the Importance of Diazotrophy in Corals–Combined Assessment of Nitrogen Assimilation, Diazotrophic Community and Natural Stable Isotope Signatures. Front. Microbiol. 2021, 12, 1638. [Google Scholar] [CrossRef]
- Pupier, C.A.; Grover, R.; Fine, M.; Rottier, C.; van de Water, J.A.J.M.; Ferrier-Pagès, C. Dissolved Nitrogen Acquisition in the Symbioses of Soft and Hard Corals With Symbiodiniaceae: A Key to Understanding Their Different Nutritional Strategies? Front. Microbiol. 2021, 12, 657759. [Google Scholar] [CrossRef] [PubMed]
- Furla, P.; Bénazet-Tambutté, S.; Jaubert, J.; Allemand, D. Diffusional Permeability of Dissolved Inorganic Carbon through the Isolated Oral Epithelial Layers of the Sea Anemone, Anemonia Viridis. J. Exp. Mar. Biol. Ecol. 1998, 221, 71–88. [Google Scholar] [CrossRef]
- McConnaughey, T.A.; Whelan, J.F. Calcification Generates Protons for Nutrient and Bicarbonate Uptake. Earth-Sci. Rev. 1997, 42, 95–117. [Google Scholar] [CrossRef]
- Crossland, C.J.; Barnes, D.J. The Role of Metabolic Nitrogen in Coral Calcification. Mar. Biol. 1974, 28, 325–332. [Google Scholar] [CrossRef]
- Biscéré, T.; Ferrier-Pagès, C.; Grover, R.; Gilbert, A.; Rottier, C.; Wright, A.; Payri, C.; Houlbrèque, F. Enhancement of Coral Calcification via the Interplay of Nickel and Urease. Aquat. Toxicol. 2018, 200, 247–256. [Google Scholar] [CrossRef] [PubMed]
- Grover, R.; Maguer, J.-F.; Reynaud-Vaganay, S.; Ferrier-Pages, C. Uptake of Ammonium by the Scleractinian Coral Stylophora Pistillata: Effect of Feeding, Light, and Ammonium Concentrations. Limnol. Oceanogr. 2002, 47, 782–790. [Google Scholar] [CrossRef]
- Schleyer, M.H.; Celliers, L. Coral Dominance at the Reef–Sediment Interface in Marginal Coral Communities at Sodwana Bay, South Africa. Mar. Freshw. Res. 2003, 54, 967–972. [Google Scholar] [CrossRef]
- Fabricius, K.E.; Benayahu, Y.; Genin, A. Herbivory in Asymbiotic Soft Corals. Science 1995, 268, 90–92. [Google Scholar] [CrossRef]
- Fabricius, K.; Yahel, G.; Genin, A. In Situ Depletion of Phytoplankton by an Azooxanthellate Soft Coral. Limnol. Oceanogr. 1998, 43, 354–356. [Google Scholar] [CrossRef]
- Fabricius, K.E.; Dommisse, M. Depletion of Suspended Particulate Matter over Coastal Reef Communities Dominated by Zooxanthellate Soft Corals. Mar. Ecol. Prog. Ser. 2000, 196, 157–167. [Google Scholar] [CrossRef]
- Wafar, M.; Qurban, M.A.; Ashraf, M.; Manikandan, K.P.; Flandez, A.V.; Balala, A.C. Patterns of distribution of inorganic nutrients in Red Sea and their implications to primary production. J. Mar. Syst. 2016, 156, 86–98. [Google Scholar] [CrossRef]
- Costa, O.S.; Leão, Z.M.D.A.N.; Nimmo, M.; Attrill, M.J. Nutrification impacts on coral reefs from northern Bahia, Brazil. In Island, Ocean and Deep-Sea Biology; Springer: Dordrecht, The Netherlands, 2000; pp. 307–315. [Google Scholar] [CrossRef]
- Aminot, A.; Kérouel, R.; Coverly, S.C. Nutrients in Seawater Using Segmented Flow Analysis. In Practical Guidelines for the Analysis of Seawater; CRC Press Taylor & Francis Group: Boca Raton, FL, USA, 2009; pp. 143–178. [Google Scholar]
- Schrameyer, V.; Wangpraseurt, D.; Hill, R.; Kühl, M.; Larkum, A.W.; Ralph, P.J. Light Respiratory Processes and Gross Photosynthesis in Two Scleractinian Corals. PLoS ONE 2014, 9, e110814. [Google Scholar] [CrossRef] [PubMed]
- Pupier, C.A.; Bednarz, V.N.; Ferrier-Pagès, C. Studies With Soft Corals–Recommendations on Sample Processing and Normalization Metrics. Front. Mar. Sci. 2018, 5, 348. [Google Scholar] [CrossRef]
- Jeffrey, S.; Humphrey, G.F. New Spectrophotometric Equations for Determining Chlorophylls a, b, C1 and C2 in Higher Plants, Algae and Natural Phytoplankton. Biochem. Physiol. Pflanz. 1975, 167, 191–194. [Google Scholar] [CrossRef]
- Smith, P.K.; Krohn, R.I.; Hermanson, G.T.; Mallia, A.K.; Gartner, F.H.; Provenzano, M.D.; Fujimoto, E.K.; Goeke, N.M.; Olson, B.J.; Klenk, D.C. Measurement of Protein Using Bicinchoninic Acid. Anal. Biochem. 1985, 150, 76–85. [Google Scholar] [CrossRef] [PubMed]
- Barnes, H.; Blackstock, J. Estimation of Lipids in Marine Animals and Tissues: Detailed Investigation of the Sulphophosphovanilun Method for ‘Total’ Lipids. J. Exp. Mar. Biol. Ecol. 1973, 12, 103–118. [Google Scholar] [CrossRef]
- Knight, J.A.; Anderson, S.; Rawle, J.M. Chemical Basis of the Sulfo-Phospho-Vanillin Reaction for Estimating Total Serum Lipids. Clin. Chem. 1972, 18, 199–202. [Google Scholar] [CrossRef]
- Oakes, K.D.; Van Der Kraak, G.J. Utility of the TBARS Assay in Detecting Oxidative Stress in White Sucker (Catostomus Commersoni) Populations Exposed to Pulp Mill Effluent. Aquat. Toxicol. 2003, 63, 447–463. [Google Scholar] [CrossRef] [PubMed]
- Menzel, D.W.; Corwin, N. The Measurement of Total Phosphorus in Seawater Based on the Liberation of Organically Bound Fractions by Persulfate Oxidation. Limnol. Oceanogr. 1965, 10, 280–282. [Google Scholar] [CrossRef]
- Levene, H. Robust Tests for the Equality of Variance. Contrib. Probab. Stat. 1960, 27, 278–292. [Google Scholar]
- Donovan, M.K.; Adam, T.C.; Shantz, A.A.; Speare, K.E.; Munsterman, K.S.; Rice, M.M.; Schmitt, R.J.; Holbrook, S.J.; Burkepile, D.E. Nitrogen Pollution Interacts with Heat Stress to Increase Coral Bleaching across the Seascape. Proc. Natl. Acad. Sci. USA 2020, 117, 5351–5357. [Google Scholar] [CrossRef] [PubMed]
- Stimson, J.; Larned, S.; Conklin, E. Effects of Herbivory, Nutrient Levels, and Introduced Algae on the Distribution and Abundance of the Invasive Macroalga Dictyosphaeria Cavernosa in Kaneohe Bay, Hawaii. Coral Reefs 2001, 19, 343–357. [Google Scholar] [CrossRef]
- Szmant, A.M. Nutrient Enrichment on Coral Reefs: Is It a Major Cause of Coral Reef Decline? Estuaries 2002, 25, 743–766. [Google Scholar] [CrossRef]
- Tanaka, Y.; Miyajima, T.; Watanabe, A.; Nadaoka, K.; Yamamoto, T.; Ogawa, H. Distribution of Dissolved Organic Carbon and Nitrogen in a Coral Reef. Coral Reefs 2011, 30, 533–541. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Blanckaert, A.C.A.; Biscéré, T.; Grover, R.; Ferrier-Pagès, C. Species-Specific Response of Corals to Imbalanced Ratios of Inorganic Nutrients. Int. J. Mol. Sci. 2023, 24, 3119. https://doi.org/10.3390/ijms24043119
Blanckaert ACA, Biscéré T, Grover R, Ferrier-Pagès C. Species-Specific Response of Corals to Imbalanced Ratios of Inorganic Nutrients. International Journal of Molecular Sciences. 2023; 24(4):3119. https://doi.org/10.3390/ijms24043119
Chicago/Turabian StyleBlanckaert, Alice C. A., Tom Biscéré, Renaud Grover, and Christine Ferrier-Pagès. 2023. "Species-Specific Response of Corals to Imbalanced Ratios of Inorganic Nutrients" International Journal of Molecular Sciences 24, no. 4: 3119. https://doi.org/10.3390/ijms24043119
APA StyleBlanckaert, A. C. A., Biscéré, T., Grover, R., & Ferrier-Pagès, C. (2023). Species-Specific Response of Corals to Imbalanced Ratios of Inorganic Nutrients. International Journal of Molecular Sciences, 24(4), 3119. https://doi.org/10.3390/ijms24043119






