Abstract
Sertoli cells within the testis are instrumental in providing an environment for spermatogenesis and protecting the developing germ cells from detrimental immune responses which could affect fertility. Though these immune responses consist of many immune processes, this review focuses on the understudied complement system. Complement consists of 50+ proteins including regulatory proteins, immune receptors, and a cascade of proteolytic cleavages resulting in target cell destruction. In the testis, Sertoli cells protect the germ cells from autoimmune destruction by creating an immunoregulatory environment. Most studies on Sertoli cells and complement have been conducted in transplantation models, which are effective in studying immune regulation during robust rejection responses. In grafts, Sertoli cells survive activated complement, have decreased deposition of complement fragments, and express many complement inhibitors. Moreover, the grafts have delayed infiltration of immune cells and contain increased infiltration of immunosuppressive regulatory T cells as compared to rejecting grafts. Additionally, anti-sperm antibodies and lymphocyte infiltration have been detected in up to 50% and 30% of infertile testes, respectively. This review seeks to provide an updated overview of the complement system, describe its relationship with immune cells, and explain how Sertoli cells may regulate complement in immunoprotection. Identifying the mechanism Sertoli cells use to protect themselves and germ cells against complement and immune destruction is relevant for male reproduction, autoimmunity, and transplantation.
1. Introduction
The testis is unique as it is one of only a few immune-privileged sites within the body. Since advanced male germ cells (spermatocytes, spermatids, and spermatozoa) do not emerge until puberty, these cells express immunogenic antigens and are at risk of autoimmune destruction. Sertoli cells are important contributors to germ cell protection from autoimmune destruction, and since infertility affects about 2.5–12% of males worldwide [1], the majority of men have viable germ cells due to the development and maintenance of an immune-privileged environment in the testis.
Understanding the mechanisms behind immune responses and immune protection in reproduction is a relevant issue. The immune response is complicated and consists of many components including serum proteins, complement, antibodies, and immune cells. Of these, the complement system has been particularly understudied and underappreciated. As various complement-related proteins are expressed in the testis and have important roles in fertilization and reproduction, this review strives to provide a thorough review of the complement system, complement modulation of lymphocytes, and Sertoli cell regulation of complement in the immune-privileged environment.
2. Sertoli Cell Immune Regulation in the Testis
Sertoli cells are immunoregulatory cells that line the seminiferous tubules of the testis, which physiologically function to nurture spermatogenesis and protect maturing germ cells from an immune response (Figure 1) [2,3]. This immunoprotection is required due to the immunogenicity of the developing sperm since the male immune system develops central self-tolerance before the germ cells enter meiosis [4]. Sertoli cells regulate the immune response to create this immune protective environment by maintaining the blood–testis barrier and through the expression of immunoregulatory factors [2,5].
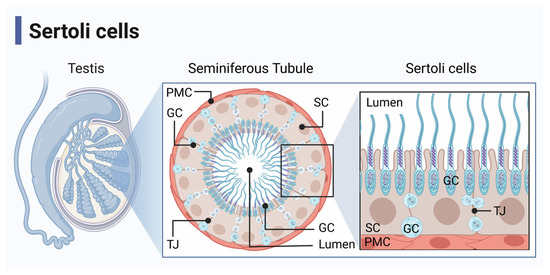
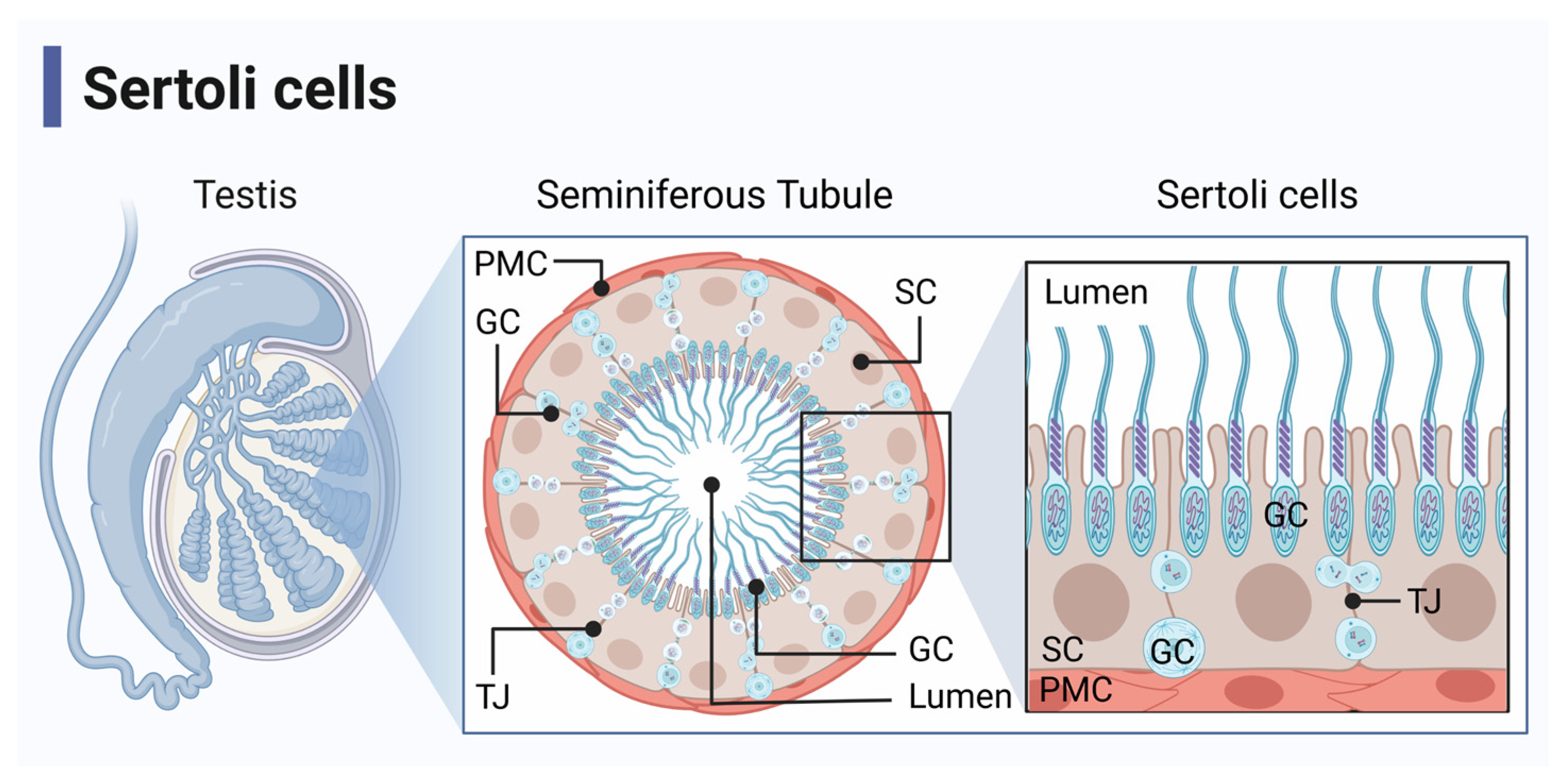
Figure 1.
Seminiferous tubule anatomy. Sertoli cells (SC) and germ cells (GC) are located in the seminiferous tubules of the testis, which are surrounded by peritubular myoid cells (PMC). SC function to nurture spermatogenesis and protect GC from immune responses. They make up the blood–testis barrier by forming tight junctions (TJ) with adjacent SC. Figure created with BioRender accessed on 1 December 2022.
The blood–testis barrier is a physical barrier formed by tight junctions between adjacent Sertoli cells that sequester the meiotic and haploid germ cells from the immune system [2,6]. In this manner, the blood–testis barrier acts as a physical shield, directly protecting maturing sperm from destruction. However, this sequestration is incomplete since immune cells can encounter germ cell antigens as the germ cell egress into the interstitium, which influences the development of systemic tolerance [7,8]. Furthermore, since allografts and xenografts transplanted into the interstitial space, outside of the blood–testis barrier enjoy prolonged graft survival, the blood–testis barrier is not the sole factor responsible for testicular immune protection [2]. Additionally, Sertoli cells survive long-term as allografts and xenografts without the use of immune suppressants and Sertoli cell allografts have been shown to protect ectopically transplanted pancreatic islet grafts, also indicating there is more to Sertoli cell immune regulation [3,9,10,11,12].
Sertoli cells produce and secrete many different factors that can regulate immune response such as transforming growth factor beta (TGF-β), activin A, fas ligand, programmed death-ligand 1 (PD-L1), galectin-1, thrombospondin-1 (THBS1), and indoleamine-2, 3-dioxygenase (IDO) ([13,14,15,16,17,18,19,20], extensively reviewed in [21]). These factors can suppress cytotoxic immune functions to protect germ cells and have been implicated in reducing effector immune cell proliferation, inhibiting inflammatory responses, and generating regulatory immune cells [7,9,14,16,22]. Sertoli cells have also been shown to express many membrane-bound and soluble complement inhibitors that may aid in the protection of germ cells from complement activation, amplification, and destruction [23,24].
These immunoregulatory factors are most likely responsible for the extended survival of Sertoli cell allografts and xenografts, which risk immune destruction, similar to immunogenic germ cells. We have previously shown that pig Sertoli cell xenografts transplanted into immunocompetent Lewis rats survived throughout the 90-day experiment while pig islet xenografts were rejected around day six post-transplantation [9,16,25]. These Sertoli cell grafts contain an increased ratio of infiltrating regulatory T cells (Tregs) and decreased complement activation [16,25], indicating the establishment of an immunoregulatory environment within the graft that may be similar to the immune-privileged environment seen in the testis.
When testicular immune protection is disrupted, as in the case of autoimmune orchitis, immune responses are generated against the immunogenic germ cells. A hallmark of autoimmune orchitis and testicular inflammatory lesions in humans is immune cell infiltrate governed by T cells [3]. Subsequently, a humoral response can be induced, although the appearance of autoantibodies against germ cells (anti-sperm antibodies, ASA) is likely confined to epididymal immune pathologies [26,27,28]. This highlights the importance of proper complement regulation within the delicate immune privileged environment of the testis and this review will focus on complement, particularly in male reproduction.
3. The Complement System
As complement is understudied, this review will provide an in-depth overview of complement, complement regulation, and complement modulation of immune cells and how it affects/may affect reproduction.
3.1. The Cascade of Complement Activation
The complement system is a series of proteins that, when activated, undergo a cascade of proteolytic cleavages eventually leading to cell lysis, opsonization and phagocytosis, inflammation and leukocyte recruitment, and further modulation of immune responses (Figure 2) [29]. The complement cascade can be divided into three overarching events: activation, response expansion, and termination.
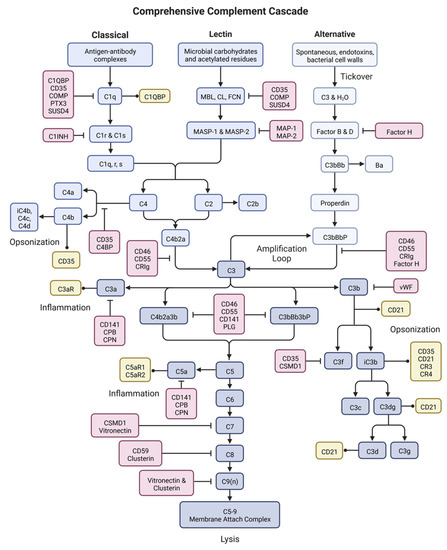
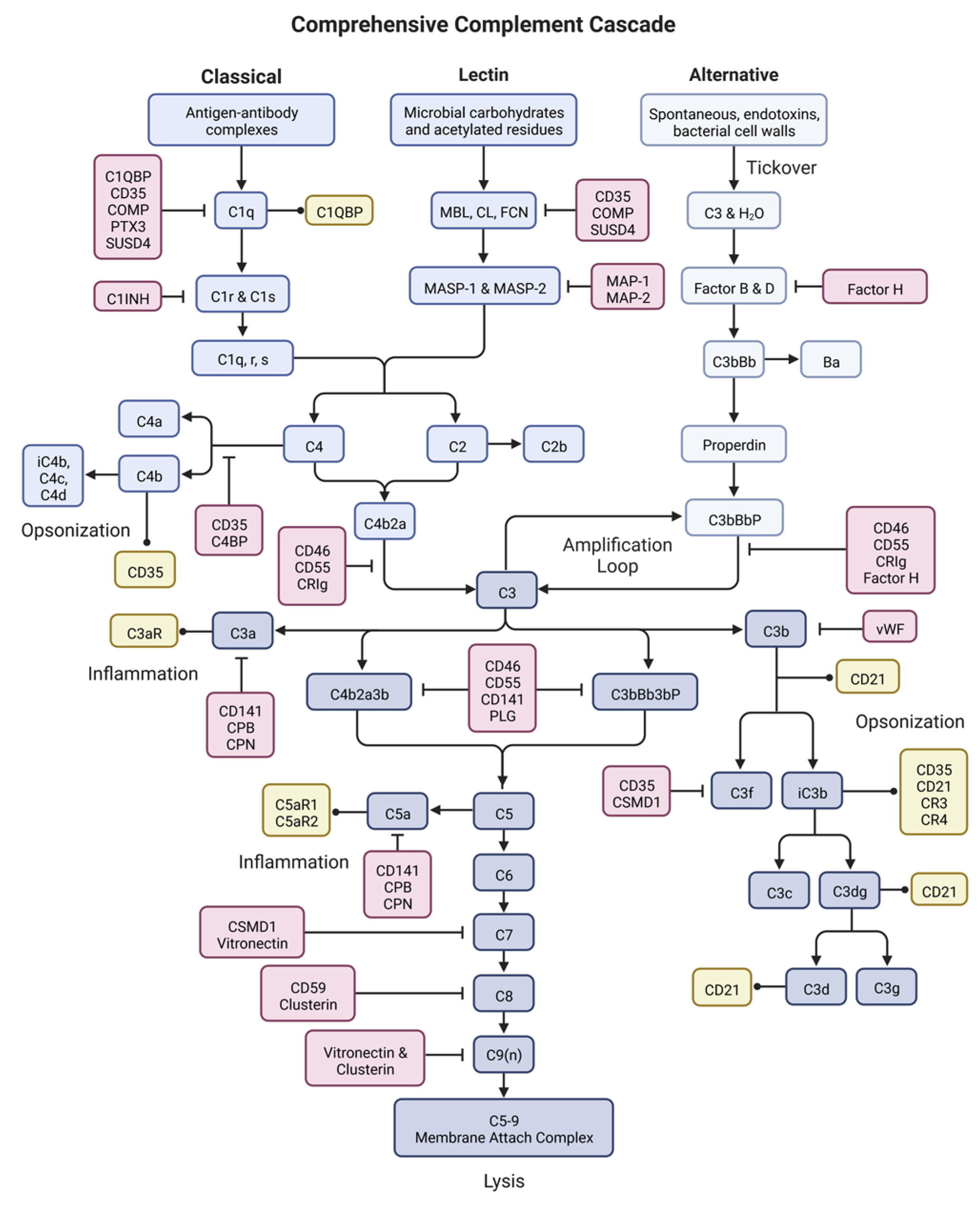
Figure 2.
The complement system. Complement is an enzymatic cascade consisting of over 50 proteins including complement components (blues), inhibitors (reds), and receptors (yellows). Complement functions to destroy pathogens or other target cells through cell lysis, inflammation, and immune activation. This figure was created with BioRender accessed on 1 December 2022.
3.1.1. Activation
Complement is activated through three primary pathways: the classical pathway, the lectin pathway, and the alternative pathway. Classical pathway activation is predominantly triggered by antigen-antibody complexes, usually involving IgG or IgM antibodies [29]. Complement protein C1q, the recognition portion of the C1 complex, binds to the antigen-antibody complex [29,30]. This binding causes a conformational shift in C1q structure, uncovering the binding site for three C1r components [31,32]. Upon C1r binding, a further conformational shift occurs allowing for binding of three C1s components, which is the enzymatic portion of the C1 complex [32]. Binding of C1s to the completed C1 complex exposes the enzymatic site and allows for cleavage of C2 and C4 to C2a/C2b and C4a/C4b, respectively [32,33]. C4a and C2b are both soluble proteins whose functions are poorly understood [34]. C4b and C2a covalently bind to each other and to the target cell to form the C3 convertase, an enzymatic complex that is the convergence point of the classical and lectin pathways [29]. C4b and C2a also act as opsonization molecules to encourage phagocytosis by cells such as macrophages and dendritic cells [35].
The lectin pathway is activated when acetylated residues and/or carbohydrate moieties on microbes, usually on bacterial and fungal surfaces, bind to the antigen-recognition molecules of the lectin pathway: mannose-binding lectins (MBL), collectins (CL), or ficolins (FCN) [29,32]. Once antigen-bound, these complexes bind to MBL-associated serine proteases 1 and 2 (MASP-1 and MASP-2), allowing them to enzymatically cleave C4 and C2 to form the C3 convertase C4bC2a [29,32]. This is the same C3 convertase that is formed by the classical pathway of activation, and the subsequent cascade steps are the same.
The alternative pathway is distinct from the lectin and classical pathways as it is constitutively active at low levels, a process called tickover [29,36]. During tickover, complement component C3 undergoes a conformation shift allowing for hydrolyzation of a thioester domain, forming C3(H2O), which can now bind Factor B [29,37]. The alternative pathway can also be activated by bacterial cell walls and endotoxins [29]. Once bound, Factor B is cleaved by Factor D releasing Ba and forming C3(H2O)Bb, a fluid-state initiation C3 convertase [29]. Factor D circulates in an inactive form called profactor D, which must be cleaved by MASP-3 to become enzymatically active [29]. The soluble C3 convertase cleaves C3 to C3a and C3b. C3a is an anaphylatoxin that is released into surrounding tissue and circulation, while the C3b product can covalently attach to a target surface and bind to Factor B [29]. Again, Factor B is cleaved by Factor D, but this time it is covalently attached to the target cell forming the alternative pathway C3 convertase, C3bBb [37]. The alternative pathway C3 convertase is relatively unstable, lasting only about 1.5 min [38]. However, when bound to the complement regulatory protein properdin, the complex becomes stable for about 15 min, which is the same length of time that the classical and lectin pathway convertase is stable [37,39].
3.1.2. Response Expansion
During response expansion, all three pathways of activation converge at the formation of a C3 convertase bound to the surface of the target cell. For the classical and lectin pathways, the C3 convertase is C4bC2a. For the alternative pathway, the C3 convertase is C3bBb complexed with the regulatory protein properdin (C3bBbP). C3 convertase binds C3 and cleaves it into two fragments: C3a and C3b [29,33,40].
Cleavage of C3 and successive binding of Factor B is continually repeated by cross-pathway formation of C3 convertases in a process called amplification [36]. Through amplification, target cells can become opsonized by complement fragments resulting in their effective phagocytosis by immune cells [29]. Additionally, amplification leads to complement cascade progression through response expansion and eventually insertion of the membrane attack complex (MAC) intermembrane pore.
After C3 cleavage, the anaphylatoxin C3a is released into surrounding tissue and circulation where it carries out immunomodulatory functions [41,42,43]. C3b can bind to the target cell where it acts as an opsonization signal for phagocytes, or it can bind to the C3 convertase to form the C5 convertase (C4bC2aC3b or C3bBbC3b) [35]. C5 convertase functions similarly to its C3 counterpart, but it binds and cleaves C5 into the anaphylatoxin C5a, which is released into the surrounding tissue, and C5b, which is also a soluble molecule [41,42,43].
In a study of 30 infertile men, IgG and C3 were detected on abnormal basement membrane structures of seminiferous tubules in immune complex orchitis indicative of classical pathway activation of the complement system [44]. In the case of idiopathic male fertility, which makes up about two-thirds of male infertility cases, IgG and C3 were detected in 20–70% percent of testicular tissue from biopsies (n = 20–50) [44,45,46] and ASA were present in up to 6% of men that are referred to receive treatment for infertility [47]. Additionally, in a rabbit model of allergic orchitis, IgG and IgM ASA along with C3 deposits were detected in the testis [48]. Thus, activation of complement through C3 involvement has been detected, however no further studies have been conducted to investigate other complement components, such as C5 or MAC, in orchitis nor in the testis.
3.1.3. Termination
C5b marks the beginning of MAC assembly. The C5b fragment remains soluble and binds C6, then C7 in solution [30,32,49]. Once C7 is bound, the C5b-7 complex can bind back to the target cell membrane and can also bind to C8. Binding to C8 can occur in solution or on the membrane, however it is only when C5b-7 is bound to the membrane that further MAC assembly can occur [41,42,43]. When C8 binds to C5b-7, the complex attaches with a much stronger affinity to the target cell and now can serve as an attachment point for C9, the pore fragment of MAC [50,51]. Anywhere from three to 18 (usually about 12) C9 molecules are recruited by the C5b-8 complex and inserted into the target cell membrane to form the MAC pore [29,51]. Insertion of multiple MACs into the target cell leads to cellular destruction by cytolysis and termination of the cascade. Unlike apoptosis, this is a highly inflammatory form of cell death that continues to attract inflammatory immune cells and further immune responses.
3.2. Functions of Complement
Complement has three overarching functions in immunity: opsonization and phagocytosis, inflammation and immune modulation, and cytolysis (Figure 3).
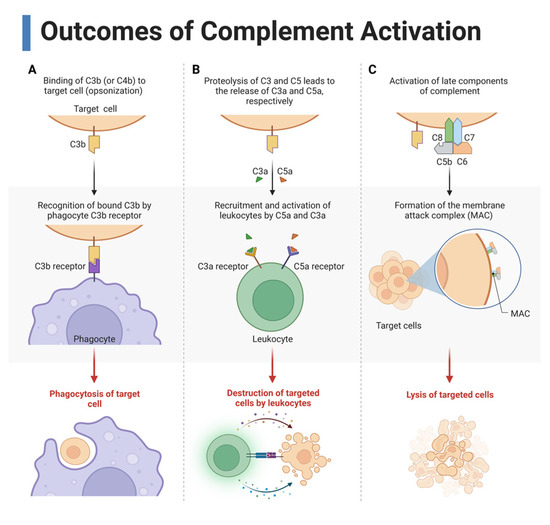
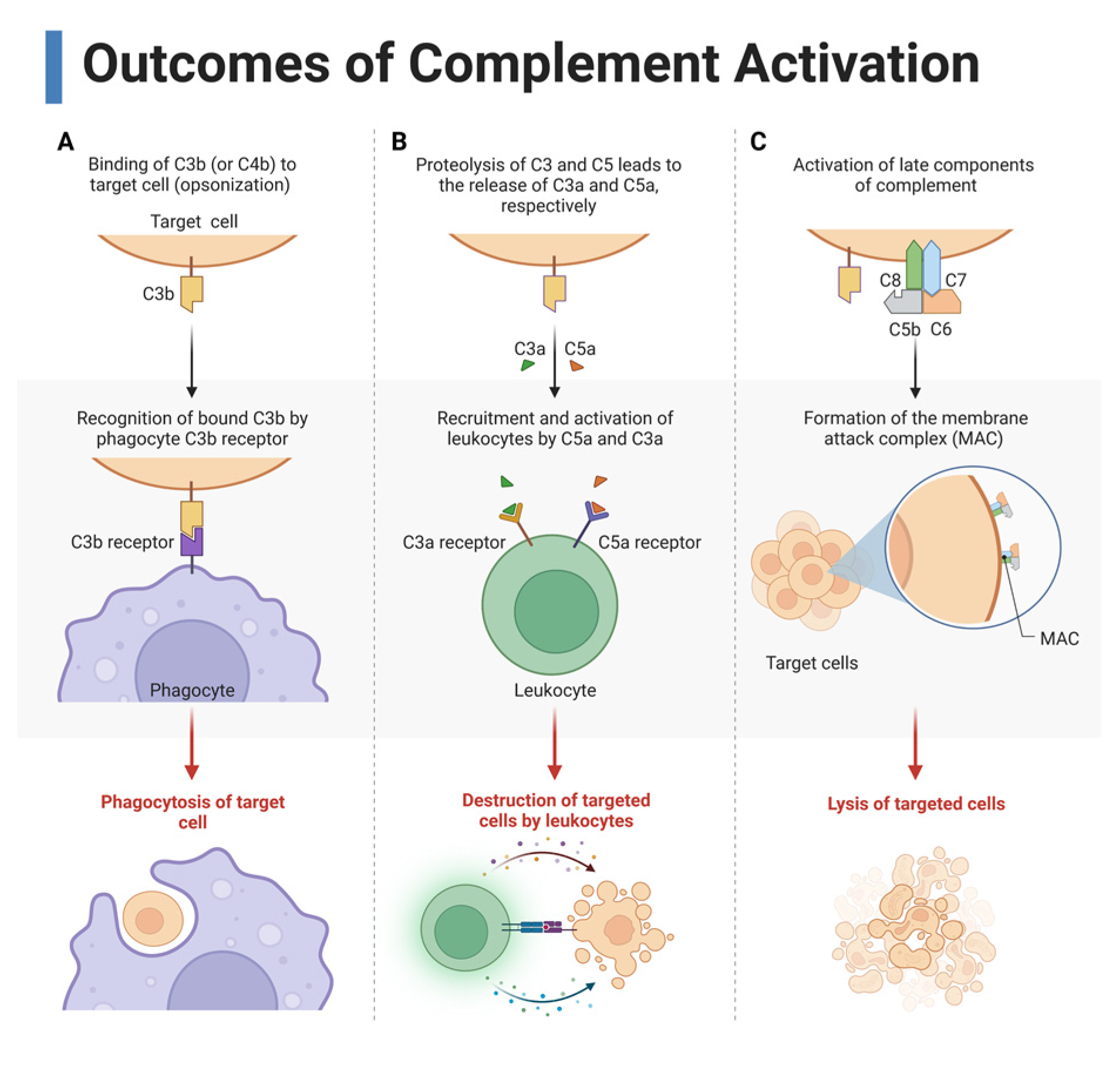
Figure 3.
Three main outcomes of complement activation. (A) Complement cleavage products such as C3b opsonize the target cells, which allows for recognition by phagocytes for phagocytosis. (B) Cleavage of C3 and C5 form the anaphylatoxins C3a and C5a, respectively, which recruit and activate immune cells by binding to their receptors on leukocytes. (C) Late complement components C5, C6, C7, C8, and C9 form the membrane attack complex (MAC), a pore that causes cytolysis. This figure was created with BioRender, accessed on 1 December 2022.
3.2.1. Three Main Outcomes of Complement Activation
As previously discussed, the complement system causes lysis of the target cell or pathogen by insertion of the MAC [35]. Many cleavage products are deposited on the target cell including C3b, C3c, C3d, C3f, C3dg, C4b, C4c, and C4d, which mark the cell for opsonization [29]. These fragments act as ligands to the complement receptors (CR) C1QBP, CD35, CD21, CR3, and CR4, which are expressed on immune cells including the phagocytes (Table 1) [52]. Ligation of CR allows for targeted and more efficient phagocytosis of the complemented cell and cellular debris [53]. Phagocytosed cell products are then presented for B and T cell activation.
Complement also modulates immune responses [49]. This occurs through immune cell interaction with anaphylatoxins, complement components, and complement inhibitory proteins, which will be discussed later. The anaphylatoxins are C3a and C5a, protein fragments formed from the cleavage of C3 and C5, respectively, that function to influence further immune response through induction of inflammation, recruitment of leukocytes, activation of immune cells, and modulation of the adaptive immune response [36,40]. Anaphylatoxins carry out these functions in an autocrine, paracrine, or systemic manner by binding to their respective receptors: C3a binds to C3a receptor (C3aR) and C5a binds to C5a receptor (C5aR1) or C5a receptor 2 (C5aR2) (Table 1) [49,54]. These receptors are expressed on leukocytes, endothelial cells, and epithelial cells [40,49]. Depending on which anaphylatoxin(s) are engaging which receptor(s) plays a large role in determining the type of response of the ligated cell.

Table 1.
Receptors of complement components.
Table 1.
Receptors of complement components.
| Complement Receptor | Ligands | Cellular Distribution | Role in Complement | Role in Male Reproduction | References |
|---|---|---|---|---|---|
| Type 1 complement receptor (CD35, CR1) | C3b, C4b, iC3b | T cells, B cells, macrophages, neutrophils, dendritic cells, eosinophils, erythrocytes | Cofactor for cleavage of C3b and C4b, opsonization, clears immune complexes | Associated with testicular interdigitating dendritic cell tumors; any role in reproduction is unknown | [52,55,56,57] |
| Type 2 complement receptor (CD21, CR2) | C3d, C3dg, iC3b | B cells, dendritic cells, epithelium | Coreceptor in B cell activation, sequesters antigens in lymphoid germinal centers | Unknown | [52,56] |
| Type 3 complement receptor (CR3, CD11b/CD18) | iC3b, ICAM-1 | Macrophages, dendritic cells, neutrophils, natural killer cells | Opsonization, adhesion of leukocytes to endothelium | Unknown | [52,58,59] |
| Type 4 complement receptor (CR4, CD11c/CD18) | iC3b | Macrophages, dendritic cells, neutrophils, natural killer cells | Opsonization, adhesion of leukocytes to endothelium | Unknown | [52,58,59] |
| C1q binding protein (C1QBP, gC1q receptor, gC1qR) | C1q | T cells, B cells, monocytes, dendritic cells, neutrophils, endothelium | Opsonization, mitochondrial fitness, T cell activation and proliferation, regulates cellular cytotoxicity of B cells, chemotaxis induction | Unknown | [31,60,61] |
| Complement receptor of immunoglobulin family (CRIg) | C3b, iC3b | T cells, macrophages | Stabilizes Tregs, enhances Treg response, opsonization | Unknown | [62,63,64,65] |
| C3a receptor (C3aR) | C3a, C3a-DesArg | T cells, B cells, macrophages, dendritic cells, myeloid cells | Leukocyte chemotaxis and extravasation, T cell activation and proliferation, regulates cellular cytotoxicity | Unknown | [49,66] |
| C5a receptor 1 (C5aR) | C5a, C5a-DesArg | T cells, B cells, macrophages, dendritic cells, myeloid cells | Leukocyte chemotaxis and extravasation, T cell activation and proliferation, regulates cellular cytotoxicity | Unknown | [49,66] |
| C5a receptor 2 (C5L2) | C5a, C5a-DesArg | T cells, neutrophils, macrophages, dendritic cells | Negative regulator of C5aR, decoy receptor, proinflammatory and anti-inflammatory functions | Unknown | [67,68] |
3.2.2. Anaphylatoxin Functions
Anaphylatoxins are known to influence the adaptive immune response in three ways. First, anaphylatoxins encourage leukocyte chemotaxis and migration since immune cells migrate toward the C3a/C5a gradient [40]. Anaphylatoxins in circulation and tissue act as a trail of breadcrumbs, leading leukocytes to the site of complement activation [29]. Leukocytes follow the increasing concentration gradient of anaphylatoxins, particularly with the binding of C5a to C5aR. Anaphylatoxins are especially powerful chemoattractants for macrophages and T cells [53,69].
Second, anaphylatoxins can activate a pro-inflammatory response by engaging their receptors on endothelial and epithelial cells [49]. This binding encourages increased expression of leukocyte recruitment and chemotaxis molecules such as P-selectin and intercellular adhesion molecule-1 (ICAM-1), which allow for the tethering of circulating immune cells to blood vessel endothelium [70,71]. Furthermore, anaphylatoxins induce leukocytes to produce inflammatory cytokines such as tumor necrosis factor-α (TNF-α) and interferon-γ (IFN-γ) [49,72]. Anaphylatoxin receptor engagement leads to decreased expression of tight junctions such as claudin-5 and zona occludin 1, making the endothelium more permeable and more conducive for leukocyte extravasation into the affected tissue [73,74].
Third, when anaphylatoxins bind to their receptors on immune cells, they initiate a signaling cascade that leads to effector cell activation, survival, and proliferation [49]. In opposition, a decrease in anaphylatoxin receptor binding is a critical step in the generation of regulatory immune cells such as Tregs, which play an important role in suppressing the cytotoxic immune response [33,49,54,75,76]. Taken together, the role of anaphylatoxins in infection and graft rejection is to lead the immune cells to the site of complement activation and to create an environment where immune cells can more easily access the affected area. Nevertheless, anaphylatoxins and their effects have not yet been investigated in the testis. More information regarding the direct effect of anaphylatoxins on the immune cell responses will be provided later.
4. Complement Regulation
Due to the highly inflammatory nature of complement, tight regulation of the complement cascade by host cells is critical to prevent collateral damage, tissue injury, and autoimmunity. Complement can be inhibited at just about every step of the cascade through action of complement inhibitory proteins (Table 2). Complement inhibitors are either membrane-bound or soluble proteins that act to regulate the function of and prevent continued activation of complement. Membrane-bound complement inhibitory proteins inhibit complement components that directly interact with the cell expressing these inhibitors [50,77]. Soluble complement inhibitors can extend their regulatory effect further, affecting both the expressing cells and neighboring cells in a paracrine fashion [51]. Soluble complement inhibitors can also inhibit soluble complement components such as C5b, C5b-6, and C5b-7, thus thwarting insertion of the MAC [51].

Table 2.
Complement inhibitory proteins.
4.1. Activation Pathway Inhibitors
Multiple complement inhibitors have been identified that target the classical, lectin, and alternative activation pathways to inhibit complement activation. Classical pathway inhibition is focused on the C1 complex, lectin pathway inhibition tends to occur by inhibiting MASP-1 and MASP-2, and alternative pathway inhibition focuses on Factor B. C1 inhibitor (C1INH, SERPING1), C1q binding protein (C1QBP), pentraxin (PTX3), and cartilage oligomeric matrix protein (COMP) are plasma soluble proteins that inhibit action of the C1 complex through inhibiting C1q, dissociating C1r/C1s, or preventing association of the C1 complex components [77,79,84,85,91,92,96].
Regarding the reproductive system, C1INH deficiency is associated with the development of hereditary angioedema, which can include presentation of testicular swelling [82,146]. C4BP is synthesized in the epididymis through an androgen-dependent mechanism, but its role in male reproduction is unknown [88]. In males, PTX3 has been detected in the male reproductive tract where it binds immotile spermatozoa and is correlated with the number of normal spermatozoa [97]. Furthermore, PTX3 is upregulated in oocytes prior to ovulation in mice and is important in female fertility [98].
SUSD4 is both a plasma soluble and membrane-bound inhibitor that prevents assembly of the classical and lectin C3 convertase by inhibiting cleavage of C2 [102]. SUSD4 is primarily expressed in the testis, brain, eye, and spinal cord—immune privileged organs [103]. Moreover, COMP and SUSD4 have also been shown to prevent activation of the lectin pathway by binding MBLs to prevent further activation [91,92]. MAP-1 and MAP-2 competitively bind to MBL to prevent MASP interaction [99,100,101]. Lastly, type 1 complement receptor 1 (CR1, CD35) acts as a membrane-bound inhibitor, preventing assembly of the C1 complex and association of MASP proteins with MBL, CL, or FCN. Additionally, CD35 acts with Factor I to inhibit the convertases, which will be discussed later [55,57].
Factor H is the only well-characterized inhibitors of the alternative pathway identified so far. Factor H is a plasma soluble protein that accelerates dissociation of Bb from C3b, and when acting as a cofactor for Factor I will cleave C3b to inactive fragments [93]. Thus, Factor H not only inhibits alternative pathway activation, but also amplification of the complement response. Additionally, Factor H is detected in the seminal plasma of pigs and the outer acrosome of sperm where it has been shown to protect sperm from complement-mediated destruction through reproductive tracts [95].
4.2. C3 and C5 Convertase Inhibitors
All three pathways of complement activation converge on the assembly of the C3 convertase, which functions to assemble the C5 convertase. As these are critical steps in complement function, inhibition of the convertases by different complement inhibitors is critical to shutting down undesired complement activation. Some of these complement inhibitory proteins have already been discussed: CD35 and Factor H—both are Factor I cofactors. Factor I is a plasma protein that circulates in an inactive state and requires a cofactor for activation. When activated, Factor I and its cofactor inhibit both the classical/lectin and alternative convertases by cleaving C4b and C3b into inactive fragments [124]. The Factor I cofactors are CD35, Factor H, complement factor H-related proteins 1–5 (CFHR1-5), C4 binding protein (C4BP), CUB and sushi domains protein 1 (CSMD1), and CD46 [124]. The plasma proteins CFHR1-5 also dissociate Bb from C3b and interact with C3b, C3dg, and iC3b to enhance opsonization [77,93]. VWF is a large serum protein that inactivates C3b by cleaving it to iC3b [130,131]. CD141 and PLG are both membrane-bound and serum inhibitors that inhibit complement response expansion by decaying the C3 and C5 convertases [104,105,125,126]. Concerning reproduction, CD141 is a marker for adenocarcinoma in the rete testis [107]. As recurrent miscarriage can be caused by defects in coagulation, a decrease of placental CD141 has been associated with this condition [106]. PLG is detected on oolemma and decreases the amount of sperm that penetrate into the oocyte [127,128].
CSMD1 also inhibits the terminal pathway by preventing binding of C7 to C5b-6 [102,122]. CD46, also known as membrane cofactor protein (MCP), is a membrane bound inhibitor that also plays an important role in the regulation of T cells, which will be discussed later. Furthermore, CSMD1 and CD46 have been shown to have roles related to fertility [123,147]. CSMD1 has been detected at the Sertoli cell-Sertoli cell and Sertoli cell-spermatid interfaces, and CSMD1 knockout male mice have decreased fertility, increased testicular C3 deposition, and testicular degradation [123]. On sperm and spermatids, CD46 is located solely on the acrosomal membrane and is believed to be important in stabilizing the acrosome reaction during egg-sperm fusion [108,109,110].
The last two convertase inhibitors are membrane-bound complement receptor of the immunoglobulin superfamily (CRIg) and CD55. CRIg is a membrane bound inhibitor and the only complement receptor with immunoglobulin domains that converts C3b and iC3b and also acts as a negative regulator of T cell activation and proliferation [65,120]. The glycophosphatidylinositol (GPI)-anchored CD55, also known as decay-accelerating factor (DAF), accelerates the decay of the convertases by dissociating C2a and Bb from C4b and C3b, respectively, and plays a role in regulating T cell tolerance [117,118]. CD55 has been detected on the inner acrosomal membrane of sperm where it likely is important in protecting sperm from female complement-mediated destruction and may play other roles in reproduction [115], which should be investigated further.
4.3. Terminal Pathway Inhibitors
Even if complement activation proceeds through the convertases, the various components of the terminal pathway can be inhibited through other complement inhibitors. Clusterin is a membrane-bound and plasma soluble inhibitor expressed at significantly high levels in seminal plasma [137]. Clusterin is thought to influence maturity processes of sperm [135] and to prevent male stress proteins from aggregating allowing them to be endocytosed by dendritic cells [138]. In this manner, clusterin may be creating a tolerogenic environment within the semen to allow sperm to survive in the female reproductive tract. Regarding complement, clusterin inhibits MAC assembly by binding the terminal complement components to prevent insertion of the MAC [50,136,137]. Clusterin can act with plasma-soluble vitronectin to interact with free C5b-7, C5b-8, and C5b-9 to form the soluble terminal complement complex (TCC, or sC5b-9) [50,136,137]. On its own, vitronectin inhibits C8 binding, but also maintains vascular homeostasis and promotes cell adhesion and migration processes involved with tissue repair [139]. In reproduction, vitronectin plays a role in sperm aggregation and adherence to the oocyte [141]. Lastly, CD59 is a GPI-anchored membrane protein that acts as a suicide inhibitor of the terminal pathway by irreversibly binding to C8 to block C9 recruitment [133,148]. Like the other GPI-anchored complement inhibitor CD55, CD59 is also expressed on the inner acrosomal membrane where it may protect sperm from female complement [115] and has been shown to correlate positively with testicular androgen synthesis [134].
4.4. Anaphylatoxin Inhibitors
In addition to inhibitors of complement cascade components, anaphylatoxins can also be inhibited by complement inhibitory proteins called carboxypeptidases (CPX). The specific CPX that inhibit anaphylatoxins are CPA, CPB2, CPN1, CPN2, and CPR [142,143,144,145]. These are primarily synthesized by hepatocytes and are plasma soluble circulating proteins [142]. CPX proteins remove the carboxy terminus from C3a and C5a, converting them to C3a desArg and C5a desArg, respectively. Though C3a desArg loses its ability to bind C3aR, C5a desArg can still engage C5aR, but at 10–15-fold lower affinity than C3a and C5a [41,149]. Interestingly, C5a desArg can bind with high affinity to C5L2, which may lead to anti-inflammatory effects [67,68]. In addition to inhibiting the action of anaphylatoxins, CPX also reduce degradation of the extracellular matrix, inhibit cellular migration of leukocytes, and inhibit fibrinolysis [142,144].
Overall, the complement inhibitors function to keep complement activation in check to prevent detrimental inflammation and overactivation of the immune system. Some of the inhibitors may play important roles in reproduction and immune privilege. Furthermore, CD35, CD46, CD55, and CD59 even play roles in T cell activation and differentiation [117,118,150]. Along with CRs and anaphylatoxins, complement inhibitory proteins can shape the course of innate and adaptive immune cell responses.
5. Complement Anaphylatoxin Modulation of Immune Cells
Complement fragments and regulatory proteins influence the action of immune cells to modulate the course of the immune response to foreign antigens such as pathogens, immunogenic germ cells, and grafted cells [50,151]. Although complement interacts with all the immune cells, this review will focus on its interaction with antigen presenting cells (APC) and T cells.
5.1. Antigen Presenting Cells
APC are phagocytes that ingest pathogens, cells, and cellular debris, and then present the antigens on their cell surface for T cell recognition. T cells are lymphocytic immune cells that mediate cellular immune responses after recognition of antigens, either through activating other immune cells or by direct cytotoxic killing of infected cells. If T cells recognize the antigen as foreign, then they initiate further immune response by releasing cytokines to recruit and activate leukocytes to destroy any other cells which express that antigen [152].
The most abundant immune cell population in the testis are testicular macrophages [153]. Macrophages are innate, large mononuclear phagocytes that function to destroy pathogens and clear apoptotic cells [154]. Macrophages have two main phenotypes: M1 and M2. M1 macrophages produce and secrete proinflammatory cytokines to destroy microbes while M2 macrophages are associated with anti-inflammatory effects and tissue repair [155,156]. The M1 macrophage phenotype is most implicated in the case of graft rejection and inflammatory autoimmune diseases [155]. They are the primary cellular mediators of robust xenograft rejection, which can occur directly by the macrophage itself, or indirectly through macrophage activation of T cells [157,158,159,160]. On the other hand, anti-inflammatory M2 macrophages are associated with tolerogenic environments [155]. In fact, testicular macrophages, the primary immune cell type within the testis, have an M2 or M2-like phenotype conducive to immune-privilege [161,162,163].
Testicular macrophages are important in establishing immune tolerance to germ cell antigens, infection response, and control of inflammation [164]. Because of these roles, testicular macrophages contribute to immune regulation in the testis. However, in rats with experimental autoimmune orchitis, testicular macrophages participate in disease pathogenesis through production of proinflammatory cytokines and activation of T cells, so proper balance of M1 and M2 phenotypes is critical [165]. Since macrophages are so abundant in the testis, it makes sense that they are among the first APC to infiltrate the inflammatory testicular environment of autoimmune orchitis [166]. Interestingly, they are also among the first APC that infiltrate into grafts [166].
Another phagocytic APC, dendritic cells, are the most potent activators of T cells and the only known activators of naïve T cells. Dendritic cell presence in an autoimmune and rejecting graft can lead to a strong T cell response [160,167]. Conversely, under the right circumstances dendritic cells can act in a tolerogenic fashion and can generate an immune-protective Treg response [168]. Though dendritic cells make up a small percentage of testicular immune cells, it is these tolerogenic or immature dendritic cells that are the primary dendritic cell population in the testis [162,169].
Interestingly, the complement anaphylatoxin receptors C3aR, C5aR1, and C5aR2 have been shown to affect phagocyte response and function during episodes of complement activation. Ligation of anaphylatoxin receptors induces chemotaxis of phagocytes toward an increasing anaphylatoxin gradient. This signaling pathway causes immune cells such as macrophages and T cells to follow the increasing C3a/C5a gradient toward the site of complement activation [170]. Anaphylatoxin receptor interaction enhances phagocytosis and leads to the production and release of pro-inflammatory cytokines such as IL-6, TNF-α, and IL-1β [171]. Regarding high-inflammatory cells such as neutrophils, the anaphylatoxin signaling pathway activates neutrophils to degranulate and undergo respiratory burst, and C5aR1 inhibits their apoptosis, thus increasing their capacity to destroy grafts [171,172,173].
Ultimately, anaphylatoxin receptor ligation on APC leads to cell migration toward the graft, cell adhesion and extravasation into the graft, enhanced phagocytosis, release of proinflammatory factors, and overall increases efficiency of phagocytes to destroy grafted cells [170]. In the absence of C3aR or C5aR1 ligation however, the tolerogenic APC phenotype become more dominant, thus encouraging the induction of immune regulatory lymphocytes [169,174]. The role of CD46 and other complement regulators on APC outside of complement regulation has yet to be fully elucidated, but preliminary studies indicate that CD46 may play a role in the polarization of innate immune cells [175].
5.2. T Cells
The primary purpose of phagocytes acting as APC is to activate T cells [169]. T cells are most rigorously studied pertaining to their role in autoimmunity, cancer, and transplant survival outcomes. T cells are divided into the following subsets: helper T cells (Th, CD4+CD25loFoxp3lo), cytotoxic T cells (CTLs, CD8+CD25loFoxp3lo), and regulatory T cells (Tregs, CD4+CD25hiFoxp3hi and/or CD8+CD25hiFoxp3hi) [16,176,177,178,179,180].
Th function in the cellular adaptive immune response by priming immune cells including phagocytes, B cells (antibody-producing cells), and CTLs to target cells whose major histocompatibility complex (MHC) express specific antigens. Th1 cells release proinflammatory cytokines such as IL-1 and IL-17 to activate the cytotoxic immune functions of macrophages and CTLs [154,181,182]. IL-1 serves to recruit leukocytes, induce expression of cell adhesion molecules, and promote leukocyte extravasation; while IL-17 activates and mobilizes inflammatory immune cells [182].
After activation by Th, CTLs directly kill target cells and specialize in the destruction of viral-infected cells and tumor cells. CTLs are a critical component of the cellular immune response against MHC-mismatched allografts in acute rejection [177,180]. CTLs directly lyse target cells through granule exocytosis of perforin and granzyme B [180]. Perforin forms an intermembrane pore that allows granzyme B, a pro-apoptotic mediator, to enter the cell and trigger cell death [183]. CTLs also recruit immune cells to the area through the release of proinflammatory cytokines [178,180]. Overall, activation of CTLs against a cell leads to cytotoxicity.
In contrast to Th and CTLs, Tregs suppress and contract the cytotoxic immune response, and they accomplish this through multiple mechanisms. In particular, Tregs release anti-inflammatory mediators such as TGF-β and IL-10, which suppress function and proliferation of Th1 cells [184]. They also express the IL-2 receptor CD25, which has a high affinity for IL-2 binding and sequesters IL-2, a growth factor for T cell survival and proliferation [184]. Acting together, Treg expression of these factors prevent Th1 cell expansion, suppresses inflammation, and contracts the immune response [184].
High levels of anaphylatoxins binding to their receptor on T cells activates a signaling cascade that encourages high-affinity binding of the MHC-TCR (T cell receptor) complex. This leads to the generation, activation, and expansion of Th1 cells and CTLs [49,185]. Specifically, anaphylatoxin-receptor engagement on T cells decreases expression of the pro-apoptotic Fas receptor and increases expression of the anti-apoptotic molecule B cell lymphoma-2 (Bcl-2) [49]. The anaphylatoxin signaling cascade suppresses production of IL-4, a cytokine that stimulates T helper 2 cells (Th2, Th subset associated with wound healing and tissue repair) differentiation, and inhibits production of TGF-β, which is important in Treg generation. Moreover, when anaphylatoxins bind their receptors on differentiated Tregs, their suppressive functions are downregulated [54]. This effect was confirmed when C3aR/C5aR1 receptors were knocked down and Treg suppression was enhanced in the presence of anaphylatoxins [54]. Thus, C3aR/C5aR1 ligation on CD4+ T cells promotes differentiation of Th1 cells and inhibits generation of anti-inflammatory Tregs. In the case of pathogen infiltration, this is a desired result, as these proinflammatory CD4+ T cells develop an immune response that is necessary to clear the infection, but in autoimmunity and transplantation this response leads to the destruction of host cells and grafts.
Ligation of C5aR1 on T helper 1 cells (Th1, Th subset associated with mounting pro-inflammatory response) induces the production of reactive oxygen species (ROS) and development of the inflammasome, which is important in the expression of IL-1β [171,186]. C5aR1 signaling on CTLs induces a stronger proliferative response and increases expression of perforin and proinflammatory cytokines [187]. Overall, anaphylatoxin receptor signaling leads to enhancement of pro-inflammatory Th1 cell and CTL responses. Conversely, C5aR2 tends to act antagonistically to C5aR1 by sequestering C5a and preventing further signaling through C5aR1, and other functions of this receptor are currently under investigation [67,68].
6. Sertoli Cell Regulation of Complement
Activation of the complement cascade inevitably leads to destruction of the target cell [49,188,189], which is why Sertoli cell survival of complement activation is so unique. In vitro complement cytotoxicity assay experiments have shown that control cells (pig endothelial cells and pig islets) are killed by robust human and rabbit complement, while mouse and pig Sertoli cells survive [25,190,191]. Pig Sertoli cells and control cells (pig endothelial cells) cultured on chamber slides were exposed to human serum, and human serum + rabbit complement and immunostained for deposition of the complement proteins C4b, Bb, C3b, and MAC [190]. C4b and C3b were deposited on both the controls and the Sertoli cells, while Bb and MAC were only observed significantly deposited on the controls [190]. These results coincide with cell death (endothelial cells) and cell survival (Sertoli cells) of complement and imply that Sertoli cells are inhibiting complement before MAC deposition. In vivo data agreed with the in vitro results as basically no complement deposition of C4, C3, or MAC was observed on mouse Sertoli cell allografts or pig Sertoli cell xenografts through day 20 post-transplantation [25,191]. One mechanism Sertoli cells could be using to inhibit the complement cascade is expression of complement inhibitory proteins.
We previously have shown through bioinformatic analyses that mouse Sertoli cells express 14 complement inhibitor genes [191] and pig Sertoli cells express 21 complement inhibitor genes [192] that inhibit all throughout the complement cascade (Figure 4). qPCR analyses have shown that pig Sertoli cells express C1INH, CD35, CD46, CD55, COMP, CPN2, CSMD1, and PTX3 genes at significantly elevated levels as compared to pig islet controls, which are killed by complement [25,192]. ELISA of Sertoli cell conditioned media confirmed mouse Sertoli cell secretion of C1INH, C1QBP, and COMP [191] and confirmed pig Sertoli cell secretion of CPN2 and PTX3, also significantly elevated above pig islet controls [192]. Protein analyses of pig Sertoli cell complement inhibitors by Western blot revealed no change in CLU, but significantly higher expression of CD46 by pig Sertoli cells as compared to pig islets, and that CD55 expression was only detectable in Sertoli cells [25]. These studies demonstrate that Sertoli cells express many complement inhibitory proteins that inhibit critical points throughout the complement cytolytic cascade, some of which are involved in T cell activation and differentiation. Future study is needed to determine the importance of these inhibitors individually and together in Sertoli cell survival and germ cell protection.
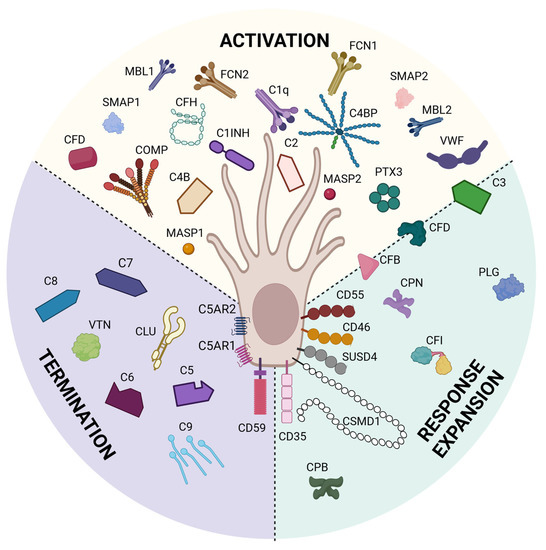
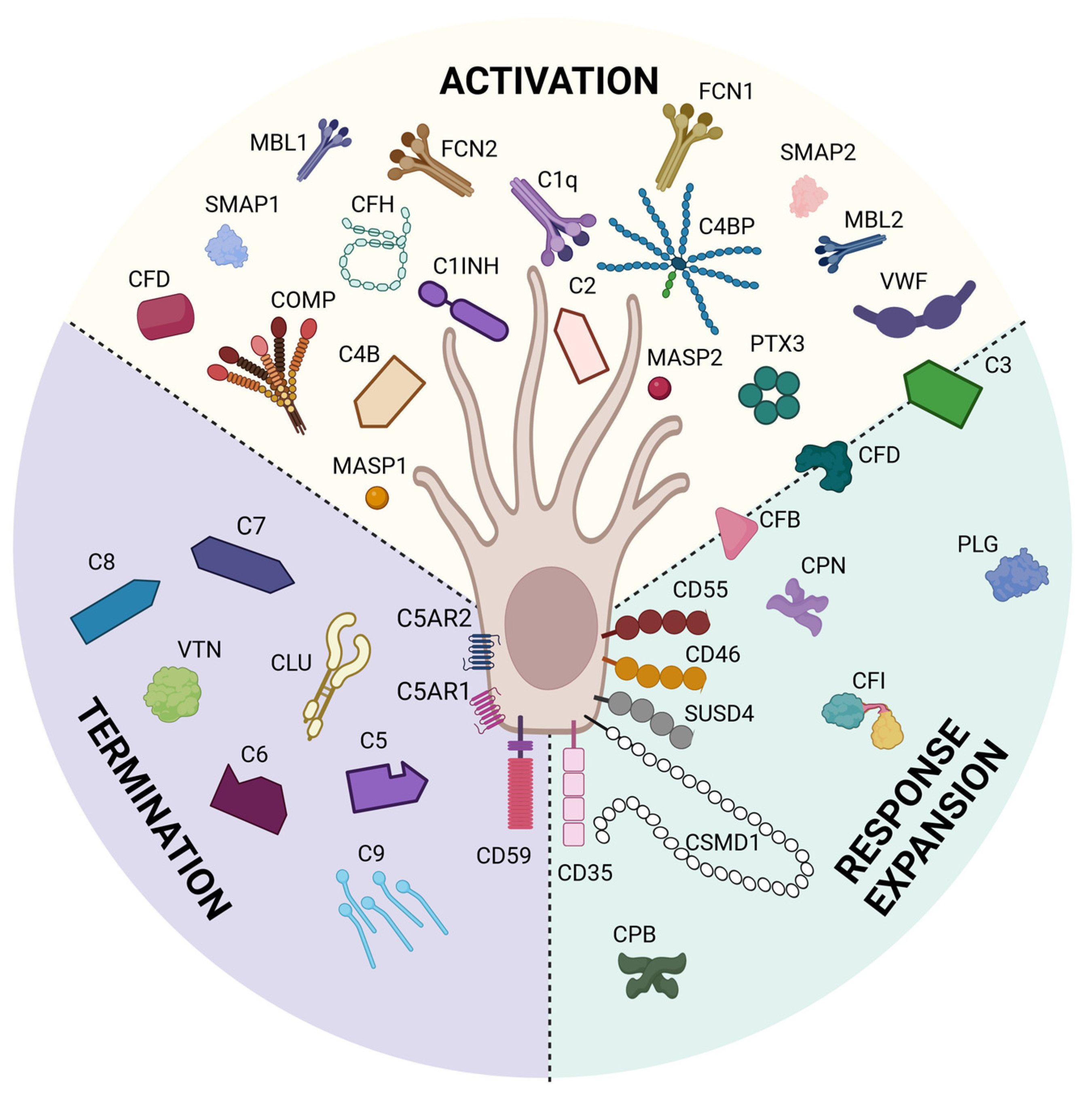
Figure 4.
Sertoli cells express complement proteins. Bioinformatic analyses have identified 21 complement inhibitors, 25 complement factors, and 3 complement receptors expressed by Sertoli cells. These inhibitors block complement throughout the whole cascade from activation (yellow), response expansion (green), and termination (purple). This figure was created with BioRender, accessed on 1 December 2022.
Additional RNA sequencing analysis determined that pig Sertoli cells express nearly all complement cascade genes (only C1r was not detected) and three complement receptor genes (CD35, C5aR1, and C5aR2) [192]. The reason behind this complement expression remains understudied, however it is possible that Sertoli cells express complement cascade components to provide an antimicrobial response in the seminiferous tubules if infected. However, as complement components increasingly have been shown to regulate part of immune function (discussed previously), this role may be more complicated and is worthy of future study.
As previously discussed, complement can affect immune cell recruitment and activation. Interestingly, in the aforementioned pig Sertoli cell xenografts, a delay of macrophage migration into the surviving Sertoli cell grafts was observed as compared to the rejecting pig islet xenografts at days four and six post-transplantation [16]. Since the presence of early macrophage infiltrate within the control xenografts is indicative of transplant rejection, this indicates that Sertoli cells are impeding macrophage recruitment.
CD4+ and CD8+ T cell infiltrate was also analyzed in these xenografts. After flow cytometry and immunohistochemical (IHC) analyses of CD4+ T cells, no significant difference in migration between the two types of grafts was measured at early timepoints [16]. CD4+ Treg infiltrate within the xenografts was further analyzed to determine if any of the CD4+ T cells were Tregs, which are associated with graft survival. Both IHC (CD4+Foxp3+ markers) and flow cytometry (CD4+CD25+Foxp3+ markers) for Tregs indicated that 30% of the CD4+ T cell infiltrate in Sertoli cells grafts was Tregs and that this number was significantly higher at day four post-transplantation in Sertoli cell grafts [16]. In fact, the Treg/Th ratio at days four and six were 0.85 and 0.48 in Sertoli cell xenografts, while the Treg/Th ratio in control islet xenografts at the same time points were significantly lower, at 0 and 0.39 [16]. In effect, Th cells were the only measurable CD4+ T cells in the control xenografts at day four, as no Tregs were detectable in the control grafts until day six post-transplantation [16].
CD8+ T cell infiltrate was also measured in these xenografts. Sertoli cell xenografts had significantly more CD8+ T cells at day four post-transplantation than control grafts, however at day six post-transplantation there was no significant difference in CD8+ T cell numbers between controls and Sertoli cell grafts [16]. The grafts were also analyzed for CD8+ Tregs by flow cytometry (CD8+CD25+Foxp3+ markers)and IHC (CD8+Foxp3+ markers) [16]. At day four post-transplantation, CD8+ Tregs were only observed in Sertoli cell grafts and, at day six post-transplantation, CD8+ Treg infiltrate in Sertoli cell grafts with significantly higher numbers compared to control islet grafts [16]. The presence of so many Tregs at day four and the continued presence of Tregs at day six post-transplantation in the Sertoli cell grafts implies that their survival as xenografts is associated with an immune suppressive environment [16].
Overall, this study demonstrates a delayed infiltration of immune effector cells and increased infiltration of Tregs into Sertoli cell xenografts by day six post-transplantation [16]. As complement anaphylatoxins are important in effector immune cell activation and recruitment, it is possible that the expression of so many different complement inhibitors by Sertoli cells are restraining the formation of anaphylatoxins, which in turn is decreasing effector infiltrate and encouraging Treg development. The information discussed clearly shows that Sertoli cells regulate immune responses and complement in transplantation models, and we speculate that Sertoli cells also perform this for germ cell protection. Complement has not really been studied in the testes, except briefly in the context of autoimmune orchitis, where anti-sperm antibodies bind to germ cells and have the potential to activate complement. Thus, one role Sertoli cells could be performing is to regulate complement to protect germ cells from an undesired immune response [26,27].
7. Discussion
Sertoli cells create an immunoregulatory environment within the testis to protect immunogenic germ cells. Sertoli cells are also able to accomplish this within immunogenic grafts, and the mechanisms behind this are currently under investigation. The focus of this review was Sertoli cell inhibition and modulation of complement in immunoregulatory environments. Complement components, anaphylatoxins, receptors, and inhibitory proteins can modulate phagocytes, T cells, and B cells to generate either a damaging pro-inflammatory cytotoxic response, or a tolerant anti-inflammatory immune suppressive response [75,90,118,193]. When complement becomes dysregulated, it leads to destruction of host cells, germ cells, and transplants [194]. Thus, the proper regulation of complement is imperative in immune privilege.
In summary, Sertoli cells have been shown to survive robust xenogeneic human complement and rabbit complement in vitro, and the complement cascade termination component MAC was not detected on Sertoli cell allografts or xenografts [25,190,191]. Gene expression of 21 different complement inhibitors has been identified so far in Sertoli cells, eight have elevated gene expression (C1INH, CD35, CD46, CD55, COMP, CPN2, CSMD1, and PTX3) and four of these (CD46, CD55, CPN2, and PTX3) had elevated protein expression as compared to islets, which are killed by complement [25,191,192]. Furthermore, Sertoli cell xenografts have significantly elevated Treg infiltrate and decreased macrophage, Th, and CTL presence at days four and six post-transplantation as compared to control xenografts [16,49]. This could potentially be due to decreased anaphylatoxin levels resultant from Sertoli cell inhibition of complement and should be investigated. As Tregs are associated with graft survival, while Th cells, CTLs, and macrophages are associated with graft destruction, this is indicative of an immune protective environment.
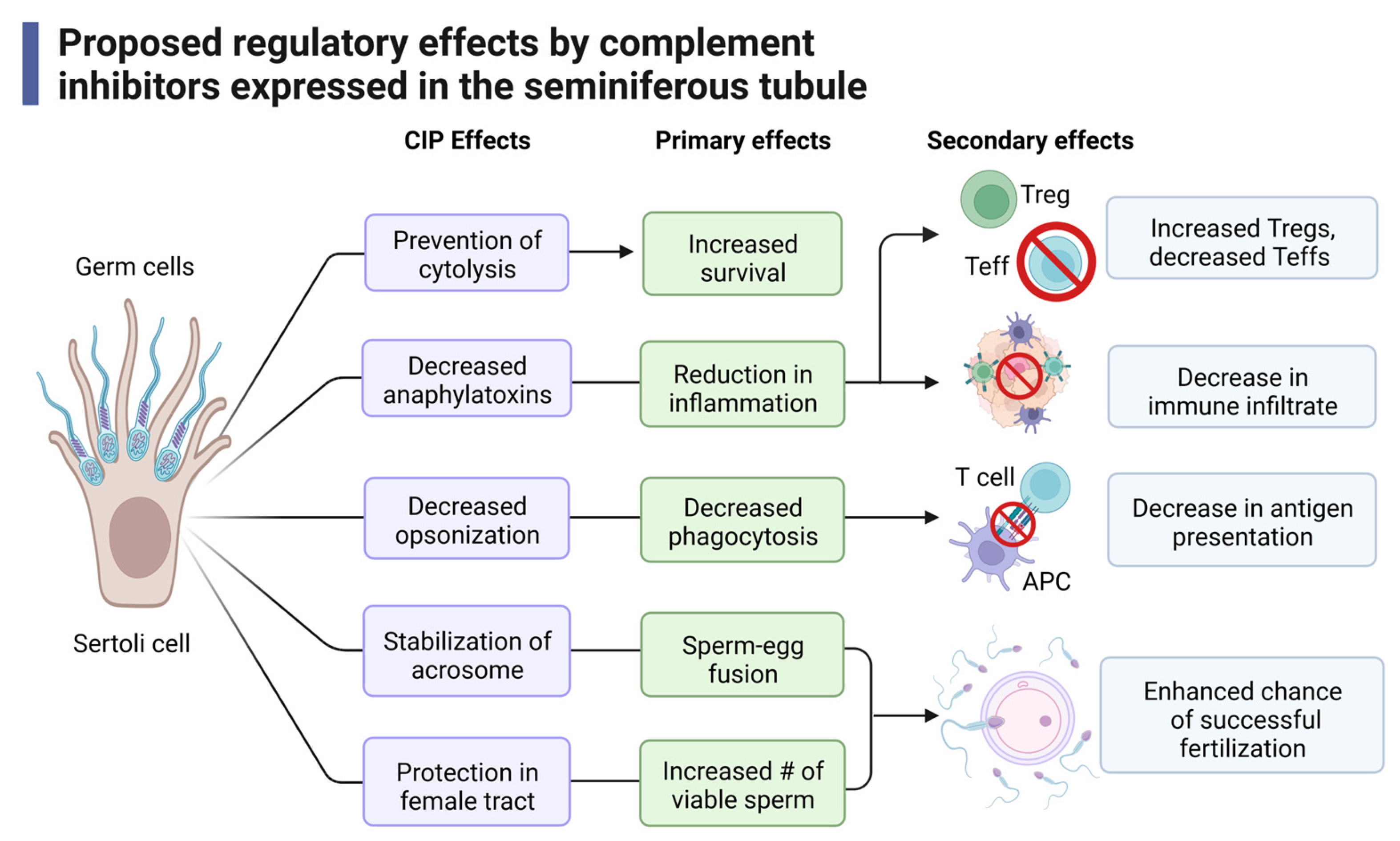
Taken together, we propose that Sertoli cells express complement inhibitors to regulate the immune response in establishing immune protective environments and that this may be accomplished through the following mechanisms (Figure 5). Complement inhibitors prevent progression through the complement cascade, preventing cytolysis and increasing survival of the cells [195]. This inhibition decreases levels of anaphylatoxins produced to directly reduce inflammation. Furthermore, a reduction in anaphylatoxins also reduces signaling through C3aR and C5aR, which decreases effector T cell (Teff, Th, and CTL) activation while allowing for the induction of Tregs that help to sustain an immune suppressed environment [49,75,196]. Along these lines, immune cell recruitment is also decreased [42,53,170]. Opsonization markers are also diminished, which reduces phagocytosis and antigen presentation.
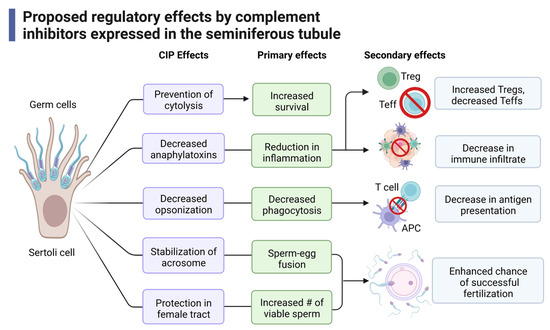
Figure 5.
Proposed mechanisms of immune regulation by complement inhibitors expressed in the seminiferous tubule. Expression of complement inhibitors by Sertoli cells and germ cells play many different and distinct roles including direct inhibition of the complement system, modulation of immune cell activation, fertility, and fertilization. CIP: complement inhibitory proteins. This figure was made with BioRender, accessed on 1 December 2022.
The acrosome of sperm is a vesicle that is secreted during the acrosome reaction. The acrosome reaction, which is critical in sperm–egg fusion, is when the outer membrane of the acrosome fuses with the plasma membrane of the oocyte [197]. The complement inhibitor CD46 has been shown to stabilize the acrosome to prevent premature and spontaneous acrosomal reactions, thus allowing for more effective sperm–egg fusion [197,198]. Other complement inhibitors (CD55, CD59, CLU) provide protection of complement within the female reproductive tract, increasing the number of viable sperm (Figure 5) [109,135,147]. Together, these functions allow for an enhanced chance of successful fertilization.
Though these studies have identified many novel and important complement regulatory functions of Sertoli cells in immune privilege, most of this work has been performed in transplantation studies. Heretofore, there is a knowledge gap in the understanding of the impact and importance of complement regulation in the testis and in reproduction. Elucidating the function(s) of complement in immune protection and reproduction may shed some much-needed light for males struggling with infertility.
8. Conclusions
Sertoli cells express a variety of complement inhibitory proteins that block just about every point of the complement cascade thus preventing MAC-mediated cytolysis, opsonization, and anaphylatoxin production. Not only does the expression of complement inhibitors protect Sertoli cells, but it also prevents the development of inflammation which reduces effector immune cell infiltrate while potentially allowing for the generation of regulatory cells such as Tregs, which aid in survival. As more studies are conducted in Sertoli cell regulation of complement, expression of more complement inhibitors, complement receptors, and even complement components will be revealed that may continue to shed light on the development of an immune-protective environment both within the testes and within grafts. Overall, understanding Sertoli cell regulation of the complement system has many applications in reproduction, autoimmunity, inflammation, and transplantation.
Author Contributions
Conceptualization, R.L.W. and J.M.D. writing—original draft preparation, R.L.W.; writing—review and editing, R.L.W. and J.M.D. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by The CH Foundation (J.M.D.).
Conflicts of Interest
J.M.D. has stock in Sernova, Corp. Sernova, Corp. has not seen the manuscript. They had no role in writing the manuscripts, nor in the decision to publish the results. R.L.W. declares no conflict of interest.
Abbreviations
APC: Antigen presenting cell. C1INH: C1 inhibitor, SERPING1. C1QBP: C1q binding protein, C1q inhibitor, C1qI. C3aR: C3a receptor. C4BP: C4 binding protein. C5aR: C5a receptor 1. C5aR2: C5a receptor 2.CD21: Type 2 complement receptor, CR2. CD35: Type 1 complement receptor, CR1. CD46: Membrane cofactor protein, MCP. CD55: Decay-accelerating factor, DAF. CFHR: Complement factor H-related protein. CIP: Complement inhibitory protein. CL: Collectin. COMP: Cartilage oligomeric matrix protein. CPB: Carboxypeptidase B. CPN: Carboxypeptidase N. CR: Complement receptor. CRIg: Complement receptor of the immunoglobulin family. CSMD1: CUB and sushi domain protein 1. CTL: CD8+ cytotoxic T cell. FCN: Ficolin. GPI: Glycophosphatidylinositol. IDO: Indoleamine-2, 3-dioxygenase. IFN: Interferon. IHC: Immunohistochemistry. IL: Interleukin. MAC: Membrane attack complex. MASP: MBL-associated serine proteases. MBL: Mannose-binding lectin. MHC: Major histocompatibility complex. PD-L: Programmed death ligand. PLG: Plasminogen. PTX3: Pentraxin 3. SMAP1: Small MBL-associated protein 1. SMAP2: Small MBL-associated protein 2. SUSD4: Sushi-domain containing protein 4. TCR: T cell receptor. Teff: Effector T cell (Th and CTL). Th: CD4+ helper T cell. TGF-β: Transforming growth factor beta. THBS: Thrombospondin. TNF: Tumor necrosis factor. Treg: Regulatory T cell. VTN: Vitronectin. VWF: von Willebrand factor.
References
- Agarwal, A.; Mulgund, A.; Hamada, A.; Chyatte, M.R. A unique view on male infertility around the globe. Reprod. Biol. Endocrinol. 2015, 13, 37. [Google Scholar] [CrossRef]
- Kaur, G.; Mital, P.; Dufour, J.M. Testisimmune privilege—Assumptions versus facts. Anim. Reprod. 2013, 10, 3–15. [Google Scholar]
- Kaur, G.; Thompson, L.A.; Dufour, J.M. Sertoli cells—Immunological sentinels of spermatogenesis. Semin. Cell Dev. Biol. 2014, 30, 36–44. [Google Scholar] [CrossRef]
- Iliadou, P.K.; Tsametis, C.; Kaprara, A.; Papadimas, I.; Goulis, D.G. The Sertoli cell: Novel clinical potentiality. Hormones 2015, 14, 504–514. [Google Scholar] [CrossRef]
- Washburn, R.L.; Hibler, T.; Thompson, L.A.; Kaur, G.; Dufour, J.M. Therapeutic application of Sertoli cells for treatment of various diseases. Semin. Cell Dev. Biol. 2021, 121, 10–23. [Google Scholar] [CrossRef]
- Mruk, D.D.; Cheng, C.Y. The Mammalian Blood-Testis Barrier: Its Biology and Regulation. Endocr. Rev. 2015, 36, 564–591. [Google Scholar] [CrossRef] [PubMed]
- Tung, K.S.K.; Harakal, J.; Qiao, H.; Rival, C.; Li, J.C.H.; Paul, A.G.A.; Wheeler, K.; Pramoonjago, P.; Grafer, C.M.; Sun, W.; et al. Egress of sperm autoantigen from seminiferous tubules maintains systemic tolerance. J. Clin. Investig. 2017, 127, 1046–1060. [Google Scholar] [CrossRef] [PubMed]
- O’Donnell, L.; Rebourcet, D.; Dagley, L.F.; Sgaier, R.; Infusini, G.; O’Shaughnessy, P.J.; Chalmel, F.; Fietz, D.; Weidner, W.; Legrand, J.M.D.; et al. Sperm proteins and cancer-testis antigens are released by the seminiferous tubules in mice and men. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2021, 35, e21397. [Google Scholar] [CrossRef]
- Dufour, J.M.; Rajotte, R.V.; Seeberger, K.; Kin, T.; Korbutt, G.S. Long-term survival of neonatal porcine Sertoli cells in non-immunosuppressed rats. Xenotransplantation 2003, 10, 577–586. [Google Scholar] [CrossRef]
- Selawry, H.P.; Cameron, D.F. Sertoli cell-enriched fractions in successful islet cell transplantation. Cell Transplant. 1993, 2, 123–129. [Google Scholar] [CrossRef]
- Korbutt, G.S.; Elliott, J.F.; Rajotte, R.V. Cotransplantation of allogeneic islets with allogeneic testicular cell aggregates allows long-term graft survival without systemic immunosuppression. Diabetes 1997, 46, 317–322. [Google Scholar] [CrossRef]
- Dufour, J.M.; Hemendinger, R.; Halberstadt, C.R.; Gores, P.; Emerich, D.F.; Korbutt, G.S.; Rajotte, R.V. Genetically engineered Sertoli cells are able to survive allogeneic transplantation. Gene Ther. 2004, 11, 694–700. [Google Scholar] [CrossRef] [PubMed]
- Skinner, M.K.; Moses, H.L. Transforming growth factor beta gene expression and action in the seminiferous tubule: Peritubular cell-Sertoli cell interactions. Mol. Endocrinol. 1989, 3, 625–634. [Google Scholar] [CrossRef]
- Gualdoni, G.S.; Jacobo, P.V.; Sobarzo, C.M.; Pérez, C.V.; Matzkin, M.E.; Höcht, C.; Frungieri, M.B.; Hill, M.; Anegon, I.; Lustig, L.; et al. Role of indoleamine 2,3-dioxygenase in testicular immune-privilege. Sci. Rep. 2019, 9, 15919. [Google Scholar] [CrossRef]
- Wang, F.; Han, D. Sertoli Cell Phagocytosis: An Essential Event for Spermatogenesis. In Male Reproductive Health; IntechOpen: London, UK, 2020. [Google Scholar]
- Kaur, G.; Wright, K.; Mital, P.; Hibler, T.; Miranda, J.M.; Thompson, L.A.; Halley, K.; Dufour, J.M. Neonatal Pig Sertoli Cells Survive Xenotransplantation by Creating an Immune Modulatory Environment Involving CD4 and CD8 Regulatory T Cells. Cell Transplant. 2020, 29, 963689720947102. [Google Scholar] [CrossRef]
- Hedger, M.P.; Winnall, W.R.; Phillips, D.J.; de Kretser, D.M. The regulation and functions of activin and follistatin in inflammation and immunity. Vitam. Horm. 2011, 85, 255–297. [Google Scholar] [CrossRef]
- Morianos, I.; Papadopoulou, G.; Semitekolou, M.; Xanthou, G. Activin-A in the regulation of immunity in health and disease. J. Autoimmun. 2019, 104, 102314. [Google Scholar] [CrossRef] [PubMed]
- Lustig, L.; Guazzone, V.A.; Theas, M.S.; Pleuger, C.; Jacobo, P.; Pérez, C.V.; Meinhardt, A.; Fijak, M. Pathomechanisms of Autoimmune Based Testicular Inflammation. Front. Immunol. 2020, 11, 583135. [Google Scholar] [CrossRef]
- Fallarino, F.; Luca, G.; Calvitti, M.; Mancuso, F.; Nastruzzi, C.; Fioretti, M.C.; Grohmann, U.; Becchetti, E.; Burgevin, A.; Kratzer, R.; et al. Therapy of experimental type 1 diabetes by isolated Sertoli cell xenografts alone. J. Exp. Med. 2009, 206, 2511–2526. [Google Scholar] [CrossRef]
- Washburn, R.L.; Hibler, T.; Kaur, G.; Dufour, J.M. Sertoli Cell Immune Regulation: A Double-Edged Sword. Front. Immunol. 2022, 13, 913502. [Google Scholar] [CrossRef]
- Sipione, S.; Simmen, K.C.; Lord, S.J.; Motyka, B.; Ewen, C.; Shostak, I.; Rayat, G.R.; Dufour, J.M.; Korbutt, G.S.; Rajotte, R.V.; et al. Identification of a novel human granzyme B inhibitor secreted by cultured sertoli cells. J. Immunol 2006, 177, 5051–5058. [Google Scholar] [CrossRef]
- Doyle, T.J.; Kaur, G.; Putrevu, S.M.; Dyson, E.L.; Dyson, M.; McCunniff, W.T.; Pasham, M.R.; Kim, K.H.; Dufour, J.M. Immunoprotective properties of primary Sertoli cells in mice: Potential functional pathways that confer immune privilege. Biol. Reprod. 2012, 86, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Washburn, R.L.; KG, D.J. Sertoli Cells and Complement Inhibitors: A Possible Mechanism to Increase Pancreatic Islet Viability. Ann. Diabetes Res. 2020, 4, 1013. [Google Scholar]
- Wright, K.; Dziuk, R.; Mital, P.; Kaur, G.; Dufour, J.M. Xenotransplanted Pig Sertoli Cells Inhibit Both the Alternative and Classical Pathways of Complement-Mediated Cell Lysis While Pig Islets Are Killed. Cell Transplant. 2016, 25, 2027–2040. [Google Scholar] [CrossRef]
- Itoh, M. Testicular Autoimmunity: A Cause of Male Infertility; Springer: Berlin/Heidelberg, Germany, 2017. [Google Scholar]
- Qu, N.; Ogawa, Y.; Kuramasu, M.; Nagahori, K.; Sakabe, K.; Itoh, M. Immunological microenvironment in the testis. Reprod. Med. Biol. 2020, 19, 24–31. [Google Scholar] [CrossRef]
- Hedger, M.P. The Immunophysiology of Male Reproduction. Knobil Neill’s Physiol. Reprod. 2015, 805–892. [Google Scholar] [CrossRef]
- Merle, N.S.; Church, S.E.; Fremeaux-Bacchi, V.; Roumenina, L.T. Complement System Part I-Molecular Mechanisms of Activation and Regulation. Front. Immunol. 2015, 6, 262. [Google Scholar] [CrossRef] [PubMed]
- Morgan, B.P.; Harris, C.L. Chapter 1—The complement system: A brief overview. In Complement Regulatory Proteins; Morgan, B.P., Harris, C.L., Eds.; Academic Press: London, UK, 1999; pp. 1–31. [Google Scholar]
- Chen, A.; Gaddipati, S.; Hong, Y.; Volkman, D.J.; Peerschke, E.I.; Ghebrehiwet, B. Human T cells express specific binding sites for C1q. Role in T cell activation and proliferation. J. Immunol. 1994, 153, 1430–1440. [Google Scholar] [CrossRef]
- Barnum, S.; Schein, T. Chapter 2—The complement system. In The Complement FactsBook, 2nd ed.; Elsevier Ltd.: Amsterdam, The Netherlands, 2018; pp. 7–17. [Google Scholar]
- Sarma, J.V.; Ward, P.A. The complement system. Cell Tissue Res. 2011, 343, 227–235. [Google Scholar] [CrossRef]
- Barnum, S.R. C4a: An Anaphylatoxin in Name Only. J. Innate Immun. 2015, 7, 333–339. [Google Scholar] [CrossRef]
- Dunkelberger, J.R.; Song, W.-C. Complement and its role in innate and adaptive immune responses. Cell Res. 2010, 20, 34–50. [Google Scholar] [CrossRef]
- Varela, J.C.; Tomlinson, S. Complement: An overview for the clinician. Hematol. Oncol. Clin. North Am. 2015, 29, 409–427. [Google Scholar] [CrossRef]
- Zewde, N.; Gorham, R.D., Jr.; Dorado, A.; Morikis, D. Quantitative Modeling of the Alternative Pathway of the Complement System. PloS ONE 2016, 11, e0152337. [Google Scholar] [CrossRef] [PubMed]
- Kölln, J.; Spillner, E.; Andrä, J.; Klensang, K.; Bredehorst, R. Complement inactivation by recombinant human C3 derivatives. J. Immunol. 2004, 173, 5540–5545. [Google Scholar] [CrossRef]
- Gigli, I.; Sorvillo, J.; Halbwachs-Mecarelli, L. Regulation and deregulation of the fluid-phase classical pathway C3 convertase. J. Immunol. 1985, 135, 440–444. [Google Scholar] [CrossRef]
- Klos, A.; Tenner, A.J.; Johswich, K.O.; Ager, R.R.; Reis, E.S.; Köhl, J. The role of the anaphylatoxins in health and disease. Mol. Immunol. 2009, 46, 2753–2766. [Google Scholar] [CrossRef]
- Gao, S.; Cui, Z.; Zhao, M.H. The Complement C3a and C3a Receptor Pathway in Kidney Diseases. Front. Immunol. 2020, 11, 1875. [Google Scholar] [CrossRef]
- Jagels, M.A.; Daffern, P.J.; Hugli, T.E. C3a and C5a enhance granulocyte adhesion to endothelial and epithelial cell monolayers: Epithelial and endothelial priming is required for C3a-induced eosinophil adhesion. Immunopharmacology 2000, 46, 209–222. [Google Scholar] [CrossRef] [PubMed]
- Kwan, W.H.; van der Touw, W.; Paz-Artal, E.; Li, M.O.; Heeger, P.S. Signaling through C5a receptor and C3a receptor diminishes function of murine natural regulatory T cells. J. Exp. Med. 2013, 210, 257–268. [Google Scholar] [CrossRef]
- Salomon, F.; Saremaslani, P.; Jakob, M.; Hedinger, C.E. Immune complex orchitis in infertile men. Immunoelectron microscopy of abnormal basement membrane structures. Lab. Investig. A J. Tech. Methods Pathol. 1982, 47, 555–567. [Google Scholar]
- Naito, M.; Terayama, H.; Hirai, S.; Qu, N.; Lustig, L.; Itoh, M. Experimental autoimmune orchitis as a model of immunological male infertility. Med. Mol. Morphol. 2012, 45, 185–189. [Google Scholar] [CrossRef] [PubMed]
- Jadot-Van De Casseye, M.; De Bled, G.; Gepts, W.; Schoysman, R. An immunohistochemical study for testicular biopsies in cases of male infertility. Andrologia 1980, 12, 122–129. [Google Scholar] [CrossRef] [PubMed]
- Fijak, M.; Pilatz, A.; Hedger, M.P.; Nicolas, N.; Bhushan, S.; Michel, V.; Tung, K.S.K.; Schuppe, H.C.; Meinhardt, A. Infectious, inflammatory and ‘autoimmune’ male factor infertility: How do rodent models inform clinical practice? Hum. Reprod. Update 2018, 24, 416–441. [Google Scholar] [CrossRef]
- Tung, K.S.; Woodroffe, A.J. Immunopathology of experimental allergic orchitis in the rabbit. J. Immunol. 1978, 120, 320–328. [Google Scholar] [CrossRef]
- Merle, N.S.; Noe, R.; Halbwachs-Mecarelli, L.; Fremeaux-Bacchi, V.; Roumenina, L.T. Complement System Part II: Role in Immunity. Front. Immunol. 2015, 6, 257. [Google Scholar] [CrossRef]
- Morgan, B.P.; Harris, C.L. Chapter 2—Regulation in the complement system. In Complement Regulatory Proteins; Morgan, B.P., Harris, C.L., Eds.; Academic Press: London, UK, 1999; pp. 32–40. [Google Scholar]
- Morgan, B.P.; Harris, C.L. Chapter 4—Regulation in the terminal pathway. In Complement Regulatory Proteins; Morgan, B.P., Harris, C.L., Eds.; Academic Press: London, UK, 1999; pp. 137–170. [Google Scholar]
- Erdei, A.; Kovács, K.G.; Nagy-Baló, Z.; Lukácsi, S.; Mácsik-Valent, B.; Kurucz, I.; Bajtay, Z. New aspects in the regulation of human B cell functions by complement receptors CR1, CR2, CR3 and CR4. Immunol. Lett. 2021, 237, 42–57. [Google Scholar] [CrossRef]
- Guo, R.F.; Ward, P.A. Role of C5a in inflammatory responses. Annu. Rev. Immunol. 2005, 23, 821–852. [Google Scholar] [CrossRef]
- Strainic, M.G.; Shevach, E.M.; An, F.; Lin, F.; Medof, M.E. Absence of signaling into CD4(+) cells via C3aR and C5aR enables autoinductive TGF-beta1 signaling and induction of Foxp3(+) regulatory T cells. Nat. Immunol. 2013, 14, 162–171. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, C.H.; Matthiesen, S.H.; Lyng, I.; Leslie, R.G. The role of complement receptor type 1 (CR1, CD35) in determining the cellular distribution of opsonized immune complexes between whole blood cells: Kinetic analysis of the buffering capacity of erythrocytes. Immunology 1997, 90, 129–137. [Google Scholar] [CrossRef]
- Kazatchkine, M.D.; Fearon, D.T. Deficiencies of human C3 complement receptors type 1 (CR1, CD35) and type 2 (CR2, CD21). Immunodefic. Rev. 1990, 2, 17–41. [Google Scholar]
- Ghiran, I.N.-W.A. CR1. In The Complement Factsbook, 2nd ed.; Barnum, S., Schein, T.N., Eds.; Elsevier Ltd.: Amsterdam, The Netherlands, 2018; pp. 295–308. [Google Scholar]
- Lukácsi, S.; Gerecsei, T.; Balázs, K.; Francz, B.; Szabó, B.; Erdei, A.; Bajtay, Z. The differential role of CR3 (CD11b/CD18) and CR4 (CD11c/CD18) in the adherence, migration and podosome formation of human macrophages and dendritic cells under inflammatory conditions. PLoS ONE 2020, 15, e0232432. [Google Scholar] [CrossRef]
- Lukácsi, S.; Nagy-Baló, Z.; Erdei, A.; Sándor, N.; Bajtay, Z. The role of CR3 (CD11b/CD18) and CR4 (CD11c/CD18) in complement-mediated phagocytosis and podosome formation by human phagocytes. Immunol. Lett. 2017, 189, 64–72. [Google Scholar] [CrossRef]
- Ghebrehiwet, B. Functions associated with the C1q receptor. Behring Inst. Mitt. 1989, 84, 204–215. [Google Scholar]
- Fonseca, M.I.; Carpenter, P.M.; Park, M.; Palmarini, G.; Nelson, E.L.; Tenner, A.J. C1qR(P), a myeloid cell receptor in blood, is predominantly expressed on endothelial cells in human tissue. J. Leukoc. Biol. 2001, 70, 793–800. [Google Scholar] [CrossRef]
- Yuan, X.; Yang, B.H.; Dong, Y.; Yamamura, A.; Fu, W. CRIg, a tissue-resident macrophage specific immune checkpoint molecule, promotes immunological tolerance in NOD mice, via a dual role in effector and regulatory T cells. eLife 2017, 6, e29540. [Google Scholar] [CrossRef]
- Liu, G.; Fu, Y.; Yosri, M.; Chen, Y.; Sun, P.; Xu, J.; Zhang, M.; Sun, D.; Strickland, A.B.; Mackey, Z.B.; et al. CRIg plays an essential role in intravascular clearance of bloodborne parasites by interacting with complement. Proc. Natl. Acad. Sci. USA 2019, 116, 24214–24220. [Google Scholar] [CrossRef]
- Helmy, K.Y.; Katschke, K.J., Jr.; Gorgani, N.N.; Kljavin, N.M.; Elliott, J.M.; Diehl, L.; Scales, S.J.; Ghilardi, N.; van Lookeren Campagne, M. CRIg: A macrophage complement receptor required for phagocytosis of circulating pathogens. Cell 2006, 124, 915–927. [Google Scholar] [CrossRef]
- Vogt, L.; Schmitz, N.; Kurrer, M.O.; Bauer, M.; Hinton, H.I.; Behnke, S.; Gatto, D.; Sebbel, P.; Beerli, R.R.; Sonderegger, I.; et al. VSIG4, a B7 family-related protein, is a negative regulator of T cell activation. J. Clin. Investig. 2006, 116, 2817–2826. [Google Scholar] [CrossRef]
- Boshra, H.; Li, J.; Peters, R.; Hansen, J.; Matlapudi, A.; Sunyer, J.O. Cloning, expression, cellular distribution, and role in chemotaxis of a C5a receptor in rainbow trout: The first identification of a C5a receptor in a nonmammalian species. J. Immunol. 2004, 172, 4381–4390. [Google Scholar] [CrossRef]
- Bamberg, C.E.; Mackay, C.R.; Lee, H.; Zahra, D.; Jackson, J.; Lim, Y.S.; Whitfeld, P.L.; Craig, S.; Corsini, E.; Lu, B.; et al. The C5a receptor (C5aR) C5L2 is a modulator of C5aR-mediated signal transduction. J. Biol. Chem. 2010, 285, 7633–7644. [Google Scholar] [CrossRef]
- Li, R.; Coulthard, L.G.; Wu, M.C.; Taylor, S.M.; Woodruff, T.M. C5L2: A controversial receptor of complement anaphylatoxin, C5a. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2013, 27, 855–864. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.A.; Assiri, A.M.; Broering, D.C. Complement and macrophage crosstalk during process of angiogenesis in tumor progression. J. Biomed. Sci. 2015, 22, 58. [Google Scholar] [CrossRef] [PubMed]
- Floreani, A.A.; Wyatt, T.A.; Stoner, J.; Sanderson, S.D.; Thompson, E.G.; Allen-Gipson, D.; Heires, A.J. Smoke and C5a induce airway epithelial intercellular adhesion molecule-1 and cell adhesion. Am. J. Respir. Cell Mol. Biol. 2003, 29, 472–482. [Google Scholar] [CrossRef] [PubMed]
- DiScipio, R.G.; Schraufstatter, I.U. The role of the complement anaphylatoxins in the recruitment of eosinophils. Int. Immunopharmacol. 2007, 7, 1909–1923. [Google Scholar] [CrossRef]
- Moulton, R.A.; Mashruwala, M.A.; Smith, A.K.; Lindsey, D.R.; Wetsel, R.A.; Haviland, D.L.; Hunter, R.L.; Jagannath, C. Complement C5a anaphylatoxin is an innate determinant of dendritic cell-induced Th1 immunity to Mycobacterium bovis BCG infection in mice. J. Leukoc. Biol. 2007, 82, 956–967. [Google Scholar] [CrossRef]
- Mahajan, S.D.; Parikh, N.U.; Woodruff, T.M.; Jarvis, J.N.; Lopez, M.; Hennon, T.; Cunningham, P.; Quigg, R.J.; Schwartz, S.A.; Alexander, J.J. C5a alters blood-brain barrier integrity in a human in vitro model of systemic lupus erythematosus. Immunology 2015, 146, 130–143. [Google Scholar] [CrossRef]
- Jacob, A.; Hack, B.; Chen, P.; Quigg, R.J.; Alexander, J.J. C5a/CD88 signaling alters blood-brain barrier integrity in lupus through nuclear factor-κB. J. Neurochem. 2011, 119, 1041–1051. [Google Scholar] [CrossRef]
- Le Friec, G.; Kohl, J.; Kemper, C. A complement a day keeps the Fox(p3) away. Nat. Immunol. 2013, 14, 110–112. [Google Scholar] [CrossRef]
- Pekkarinen, P.T.; Vaali, K.; Junnikkala, S.; Rossi, L.H.; Tuovinen, H.; Meri, S.; Vaarala, O.; Arstila, T.P. A functional complement system is required for normal T helper cell differentiation. Immunobiology 2011, 216, 737–743. [Google Scholar] [CrossRef]
- Morgan, B.P.; Harris, C.L. (Eds.) Chapter 3—Regulation in the activation pathways. In Complement Regulatory Proteins; Academic Press: London, UK, 1999; pp. 41–136. [Google Scholar]
- Drouet, C.; Ponard, D.; Ghannam, A. Chapter 23—C1 Inhibitor. In The Complement Factsbook, 2nd ed.; Barnum, S., Schein, T.N., Eds.; Elsevier Ltd.: Amsterdam, The Netherlands, 2018; pp. 241–249. [Google Scholar]
- Davis, A.E., 3rd. Biological effects of C1 inhibitor. Drug News Perspect. 2004, 17, 439–446. [Google Scholar] [CrossRef]
- Han, E.D.; MacFarlane, R.C.; Mulligan, A.N.; Scafidi, J.; Davis, A.E., 3rd. Increased vascular permeability in C1 inhibitor-deficient mice mediated by the bradykinin type 2 receptor. J. Clin. Investig. 2002, 109, 1057–1063. [Google Scholar] [CrossRef]
- Ren, Z.; Zhao, S.; Li, T.; Wedner, H.J.; Atkinson, J.P. Insights into the pathogenesis of hereditary angioedema using genetic sequencing and recombinant protein expression analyses. J. Allergy Clin. Immunol 2022. [Google Scholar] [CrossRef]
- Dhairyawan, R.; Harrison, R.; Buckland, M.; Hourihan, M. Hereditary angioedema: An unusual cause of genital swelling presenting to a genitourinary medicine clinic. Int. J. STD AIDS 2011, 22, 356–357. [Google Scholar] [CrossRef]
- Galanakis, D.K.; Ghebrehiwet, B. A unique property of a plasma proteoglycan, the C1q inhibitor. An anticoagulant state resulting from its binding to fibrinogen. J. Clin. Investig. 1994, 93, 303–310. [Google Scholar] [CrossRef]
- Silvestri, L.; Baker, J.R.; Roden, L.; Stroud, R.M. The C1q inhibitor in serum is a chondroitin 4-sulfate proteoglycan. J. Biol. Chem. 1981, 256, 7383–7387. [Google Scholar] [CrossRef]
- Ghebrehiwet, B. C1q inhibitor (C1qINH): Functional properties and possible relationship to a lymphocyte membrane-associated C1q precipitin. J. Immunol. 1981, 126, 1837–1842. [Google Scholar] [CrossRef]
- Conradie, J.D.; Volanakis, J.E.; Stroud, R.M. Evidence for a serum inhibitor of Clq. Immunochemistry 1975, 12, 967–971. [Google Scholar] [CrossRef] [PubMed]
- Okroj, M.; Blom, A.M. Chapter 24—C4b-Binding Protein. In The Complement Factsbook, 2nd ed.; Barnum, S., Schein, T.N., Eds.; Elsevier Ltd.: Amsterdam, The Netherlands, 2018; pp. 251–259. [Google Scholar]
- Nonaka, M.I.; Zsigmond, E.; Kudo, A.; Kawakami, H.; Yoshida, K.; Yoshida, M.; Kawano, N.; Miyado, K.; Nonaka, M.; Wetsel, R.A. Epididymal C4b-binding protein is processed and degraded during transit through the duct and is not essential for fertility. Immunobiology 2015, 220, 467–475. [Google Scholar] [CrossRef] [PubMed]
- Török, K.; Dezső, B.; Bencsik, A.; Uzonyi, B.; Erdei, A. Complement receptor type 1 (CR1/CD35) expressed on activated human CD4+ T cells contributes to generation of regulatory T cells. Immunol. Lett. 2015, 164, 117–124. [Google Scholar] [CrossRef] [PubMed]
- Blom, A.M. The role of complement inhibitors beyond controlling inflammation. J. Intern. Med. 2017, 282, 116–128. [Google Scholar] [CrossRef]
- Vilim, V.; Olejarova, M.; Machacek, S.; Gatterova, J.; Kraus, V.B.; Pavelka, K. Serum levels of cartilage oligomeric matrix protein (COMP) correlate with radiographic progression of knee osteoarthritis. Osteoarthr. Cartil. 2002, 10, 707–713. [Google Scholar] [CrossRef]
- Mundermann, A.; Dyrby, C.O.; Andriacchi, T.P.; King, K.B. Serum concentration of cartilage oligomeric matrix protein (COMP) is sensitive to physiological cyclic loading in healthy adults. Osteoarthr. Cartil. 2005, 13, 34–38. [Google Scholar] [CrossRef]
- Barlow, P.N. Chapter 31—Factor H and Factor H-like Protein 1. In The Complement Factsbook, 2nd ed.; Barnum, S., Schein, T.N., Eds.; Elsevier Ltd.: Amsterdam, The Netherlands, 2018; pp. 317–327. [Google Scholar]
- Blatt, A.Z.; Pathan, S.; Ferreira, V.P. Properdin: A tightly regulated critical inflammatory modulator. Immunol. Rev. 2016, 274, 172–190. [Google Scholar] [CrossRef]
- Sakaue, T.; Takeuchi, K.; Maeda, T.; Yamamoto, Y.; Nishi, K.; Ohkubo, I. Factor H in porcine seminal plasma protects sperm against complement attack in genital tracts. J. Biol. Chem. 2010, 285, 2184–2192. [Google Scholar] [CrossRef]
- Doni, A.; Stravalaci, M.; Inforzato, A.; Magrini, E.; Mantovani, A.; Garlanda, C.; Bottazzi, B. The Long Pentraxin PTX3 as a Link between Innate Immunity, Tissue Remodeling, and Cancer. Front. Immunol. 2019, 10, 712. [Google Scholar] [CrossRef]
- Doni, A.; Paffoni, A.; Nebuloni, M.; Ragni, G.; Pasqualini, F.; Valentino, S.; Bonetti, S.; Mantovani, A.; Somigliana, E.; Garlanda, C. The long pentraxin 3 is a soluble and cell-associated component of the human semen. Int. J. 2009, 32, 255–264. [Google Scholar] [CrossRef]
- Camaioni, A.; Klinger, F.G.; Campagnolo, L.; Salustri, A. The Influence of Pentraxin 3 on the Ovarian Function and Its Impact on Fertility. Front. Immunol. 2018, 9, 2808. [Google Scholar] [CrossRef]
- Iwaki, D.; Kanno, K.; Takahashi, M.; Endo, Y.; Lynch, N.J.; Schwaeble, W.J.; Matsushita, M.; Okabe, M.; Fujita, T. Small mannose-binding lectin-associated protein plays a regulatory role in the lectin complement pathway. J. Immunol. 2006, 177, 8626–8632. [Google Scholar] [CrossRef] [PubMed]
- Smedbråten, J.; Mjøen, G.; Hartmann, A.; Åsberg, A.; Rollag, H.; Mollnes, T.E.; Sandvik, L.; Fagerland, M.W.; Thiel, S.; Sagedal, S. Low level of MAp44, an inhibitor of the lectin complement pathway, and long-term graft and patient survival; a cohort study of 382 kidney recipients. BMC Nephrol. 2016, 17, 148. [Google Scholar] [CrossRef]
- Degn, S.E.; Hansen, A.G.; Steffensen, R.; Jacobsen, C.; Jensenius, J.C.; Thiel, S. MAp44, a human protein associated with pattern recognition molecules of the complement system and regulating the lectin pathway of complement activation. J. Immunol. 2009, 183, 7371–7378. [Google Scholar] [CrossRef]
- Holmquist, E.; Okroj, M.; Nodin, B.; Jirstrom, K.; Blom, A.M. Sushi domain-containing protein 4 (SUSD4) inhibits complement by disrupting the formation of the classical C3 convertase. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2013, 27, 2355–2366. [Google Scholar] [CrossRef]
- Tu, Z.; Cohen, M.; Bu, H.; Lin, F. Tissue distribution and functional analysis of Sushi domain-containing protein 4. Am. J. Pathol. 2010, 176, 2378–2384. [Google Scholar] [CrossRef] [PubMed]
- Barratt-Due, A.; Pischke, S.E.; Nilsson, P.H.; Espevik, T.; Mollnes, T.E. Dual inhibition of complement and Toll-like receptors as a novel approach to treat inflammatory diseases-C3 or C5 emerge together with CD14 as promising targets. J. Leukoc. Biol. 2017, 101, 193–204. [Google Scholar] [CrossRef] [PubMed]
- Califano, F.; Giovanniello, T.; Pantone, P.; Campana, E.; Parlapiano, C.; Alegiani, F.; Vincentelli, G.M.; Turchetti, P. Clinical importance of thrombomodulin serum levels. Eur. Rev. Med. Pharm. Sci. 2000, 4, 59–66. [Google Scholar]
- Stortoni, P.; Cecati, M.; Giannubilo, S.R.; Sartini, D.; Turi, A.; Emanuelli, M.; Tranquilli, A.L. Placental thrombomodulin expression in recurrent miscarriage. Reprod. Biol. Endocrinol. 2010, 8, 1. [Google Scholar] [CrossRef]
- Skailes, G.E.; Menasce, L.; Banerjee, S.S.; Shanks, J.H.; Logue, J.P. Adenocarcinoma of the rete testis. Clin. Oncol. 1998, 10, 401–403. [Google Scholar] [CrossRef]
- Mizuno, M.; Harris, C.L.; Johnson, P.M.; Morgan, B.P. Rat membrane cofactor protein (MCP; CD46) is expressed only in the acrosome of developing and mature spermatozoa and mediates binding to immobilized activated C3. Biol. Reprod. 2004, 71, 1374–1383. [Google Scholar] [CrossRef]
- Johnson, P.M.; Clift, L.E.; Andrlikova, P.; Jursova, M.; Flanagan, B.F.; Cummerson, J.A.; Stopka, P.; Dvorakova-Hortova, K. Rapid sperm acrosome reaction in the absence of acrosomal CD46 expression in promiscuous field mice (Apodemus). Reproduction 2007, 134, 739–747. [Google Scholar] [CrossRef]
- Inoue, N.; Ikawa, M.; Nakanishi, T.; Matsumoto, M.; Nomura, M.; Seya, T.; Okabe, M. Disruption of mouse CD46 causes an accelerated spontaneous acrosome reaction in sperm. Mol. Cell Biol. 2003, 23, 2614–2622. [Google Scholar] [CrossRef]
- Liszewski, M.K.; Atkinson, J.P. Membrane Cofactor Protein. In The Complement Factsbook, 2nd ed.; Barnum, S., Schein, T.N., Eds.; Elsevier Ltd.: Amsterdam, The Netherlands, 2018; pp. 271–281. [Google Scholar]
- Arbore, G.; West, E.E.; Rahman, J.; Le Friec, G.; Niyonzima, N.; Pirooznia, M.; Tunc, I.; Pavlidis, P.; Powell, N.; Li, Y.; et al. Complement receptor CD46 co-stimulates optimal human CD8(+) T cell effector function via fatty acid metabolism. Nat. Commun. 2018, 9, 4186. [Google Scholar] [CrossRef]
- Ghannam, A.; Fauquert, J.L.; Thomas, C.; Kemper, C.; Drouet, C. Human complement C3 deficiency: Th1 induction requires T cell-derived complement C3a and CD46 activation. Mol. Immunol. 2014, 58, 98–107. [Google Scholar] [CrossRef] [PubMed]
- Liszewski, M.K.; Kemper, C. Complement in Motion: The Evolution of CD46 from a Complement Regulator to an Orchestrator of Normal Cell Physiology. J. Immunol. 2019, 203, 3–5. [Google Scholar] [CrossRef] [PubMed]
- Cummerson, J.A.; Flanagan, B.F.; Spiller, D.G.; Johnson, P.M. The complement regulatory proteins CD55 (decay accelerating factor) and CD59 are expressed on the inner acrosomal membrane of human spermatozoa as well as CD46 (membrane cofactor protein). Immunology 2006, 118, 333–342. [Google Scholar] [CrossRef] [PubMed]
- Christy, J.M.; Toomey, C.B.; Cauvi, D.M.; Pollard, K.M. Decay-Accelerating Factor. In The Complement Factsbook, 2nd ed.; Barnum, S., Schein, T.N., Eds.; Elsevier Ltd.: Amsterdam, The Netherlands, 2018; pp. 261–270. [Google Scholar]
- Heeger, P.S.; Lalli, P.N.; Lin, F.; Valujskikh, A.; Liu, J.; Muqim, N.; Xu, Y.; Medof, M.E. Decay-accelerating factor modulates induction of T cell immunity. J. Exp. Med. 2005, 201, 1523–1530. [Google Scholar] [CrossRef] [PubMed]
- Longhi, M.P.; Harris, C.L.; Morgan, B.P.; Gallimore, A. Holding T cells in check—A new role for complement regulators? Trends Immunol. 2006, 27, 102–108. [Google Scholar] [CrossRef]
- Barnum, S.; Barlow, P.N. Factor H-Related Proteins 1-5. In The Complement Factsbook, 2nd ed.; Barnum, S., Schein, T.N., Eds.; Elsevier Ltd.: Amsterdam, The Netherlands, 2018; pp. 329–340. [Google Scholar]
- van Lookeren Champagne, M.; Orozco, L.D. Chapter 29— CRIg. In The Complement Factsbook, 2nd ed.; Barnum, S., Schein, T.N., Eds.; Elsevier Ltd.: Amsterdam, The Netherlands, 2018; pp. 309–316. [Google Scholar]
- Barnum, S.; Barlow, P.N. CSMD1. In The Complement Factsbook, 2nd ed.; Barnum, S., Schein, T.N., Eds.; Elsevier Ltd.: Amsterdam, The Netherlands, 2018; pp. 369–381. [Google Scholar]
- Escudero-Esparza, A.; Kalchishkova, N.; Kurbasic, E.; Jiang, W.G.; Blom, A.M. The novel complement inhibitor human CUB and Sushi multiple domains 1 (CSMD1) protein promotes factor I-mediated degradation of C4b and C3b and inhibits the membrane attack complex assembly. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2013, 27, 5083–5093. [Google Scholar] [CrossRef]
- Lee, A.S.; Rusch, J.; Lima, A.C.; Usmani, A.; Huang, N.; Lepamets, M.; Vigh-Conrad, K.A.; Worthington, R.E.; Mägi, R.; Wu, X.; et al. Rare mutations in the complement regulatory gene CSMD1 are associated with male and female infertility. Nat. Commun. 2019, 10, 4626. [Google Scholar] [CrossRef]
- Okroj, M.B.A. Chapter 15—Factor I. In The Complement Factsbook, 2nd ed.; Barnum, S., Schein, T.N., Eds.; Elsevier Ltd.: Amsterdam, The Netherlands, 2018; pp. 147–154. [Google Scholar]
- Barthel, D.; Schindler, S.; Zipfel, P.F. Plasminogen is a complement inhibitor. J. Biol. Chem. 2012, 287, 18831–18842. [Google Scholar] [CrossRef]
- Austen, D.E.G. 29-Clinical Biochemistry of Blood Coagulation. In Scientific Foundations of Biochemistry in Clinical Practice, 2nd ed.; Williams, D.L., Marks, V., Eds.; Butterworth-Heinemann: Oxford, UK, 1994; pp. 495–513. [Google Scholar]
- Grullón, L.A.; Gadea, J.; Mondéjar, I.; Matás, C.; Romar, R.; Coy, P. How is plasminogen/plasmin system contributing to regulate sperm entry into the oocyte? Reprod. Sci. 2013, 20, 1075–1082. [Google Scholar] [CrossRef]
- Coy, P.; Jiménez-Movilla, M.; García-Vázquez, F.A.; Mondéjar, I.; Grullón, L.; Romar, R. Oocytes use the plasminogen-plasmin system to remove supernumerary spermatozoa. Hum. Reprod. 2012, 27, 1985–1993. [Google Scholar] [CrossRef]
- Odet, F.; Guyot, R.; Leduque, P.; Le Magueresse-Battistoni, B. Evidence for Similar Expression of Protein C Inhibitor and the Urokinase-Type Plasminogen Activator System during Mouse Testis Development. Endocrinology 2004, 145, 1481–1489. [Google Scholar] [CrossRef]
- Feng, S.; Liang, X.; Kroll, M.H.; Chung, D.W.; Afshar-Kharghan, V. von Willebrand factor is a cofactor in complement regulation. Blood 2015, 125, 1034–1037. [Google Scholar] [CrossRef] [PubMed]
- Yawn, B.; Nichols, W.L.; Rick, M.E. Diagnosis and management of von Willebrand disease: Guidelines for primary care. Am. Fam. Physician 2009, 80, 1261–1268. [Google Scholar] [PubMed]
- Möller, R.; Kaiser, K.; Baulain, U.; Petersen, B.; Detering, C.; Ekhlasi-Hundrieser, M.; Pfarrer, C.; von Depka Prondzinski, M.; Lehner, S. Influence of Von Willebrand Disease (VWD) and pregnancy on the expression of angiogenic factors in the porcine female reproductive tract. Reprod. Biol. 2022, 22, 100700. [Google Scholar] [CrossRef] [PubMed]
- Morgan, B. CD59. In The Complement Factsbook, 2nd ed.; Barnum, S., Schein, T.N., Eds.; Elsevier Ltd.: Amsterdam, The Netherlands, 2018; pp. 361–367. [Google Scholar]
- Li, Y.; Mi, P.; Wu, J.; Tang, Y.; Liu, X.; Cheng, J.; Huang, Y.; Qin, W.; Cheng, C.Y.; Sun, F. High Throughput scRNA-Seq Provides Insights Into Leydig Cell Senescence Induced by Experimental Autoimmune Orchitis: A Prominent Role of Interstitial Fibrosis and Complement Activation. Front. Immunol. 2021, 12, 771373. [Google Scholar] [CrossRef]
- Sylvester, S.R.; Morales, C.; Oko, R.; Griswold, M.D. Localization of sulfated glycoprotein-2 (clusterin) on spermatozoa and in the reproductive tract of the male rat. Biol. Reprod. 1991, 45, 195–207. [Google Scholar] [CrossRef]
- Tschopp, J.; Chonn, A.; Hertig, S.; French, L.E. Clusterin, the human apolipoprotein and complement inhibitor, binds to complement C7, C8 beta, and the b domain of C9. J. Immunol. 1993, 151, 2159–2165. [Google Scholar] [CrossRef]
- Naponelli, V.; Bettuzzi, S. Chapter 32—Clusterin. In The Complement Factsbook, 2nd ed.; Barnum, S., Schein, T.N., Eds.; Elsevier Ltd.: Amsterdam, The Netherlands, 2018; pp. 341–349. [Google Scholar]
- Merlotti, A.; Dantas, E.; Remes Lenicov, F.; Ceballos, A.; Jancic, C.; Varese, A.; Rubione, J.; Stover, S.; Geffner, J.; Sabatté, J. Fucosylated clusterin in semen promotes the uptake of stress-damaged proteins by dendritic cells via DC-SIGN. Hum. Reprod. 2015, 30, 1545–1556. [Google Scholar] [CrossRef]
- Milis, L.; Morris, C.A.; Sheehan, M.C.; Charlesworth, J.A.; Pussell, B.A. Vitronectin-mediated inhibition of complement: Evidence for different binding sites for C5b-7 and C9. Clin. Exp. Immunol. 1993, 92, 114–119. [Google Scholar] [CrossRef]
- Bronson, R.; Peresleni, T.; Golightly, M.; Preissner, K. Vitronectin is sequestered within human spermatozoa and liberated following the acrosome reaction. Mol. Hum. Reprod. 2000, 6, 977–982. [Google Scholar] [CrossRef]
- Fusi, F.M.; Bernocchi, N.; Ferrari, A.; Bronson, R.A. Integrins and adhesion molecules: Is vitronectin the velcro that binds the gametes together? Mol. Hum. Reprod. 1996, 2, 859–866. [Google Scholar] [CrossRef]
- Skidgel, R.A.; Erdos, E.G. Structure and function of human plasma carboxypeptidase N, the anaphylatoxin inactivator. Int. Immunopharmacol. 2007, 7, 1888–1899. [Google Scholar] [CrossRef]
- Campbell, W.; Okada, N.; Okada, H. Carboxypeptidase R is an inactivator of complement-derived inflammatory peptides and an inhibitor of fibrinolysis. Immunol. Rev. 2001, 180, 162–167. [Google Scholar] [CrossRef]
- Campbell, W.D.; Lazoura, E.; Okada, N.; Okada, H. Inactivation of C3a and C5a octapeptides by carboxypeptidase R and carboxypeptidase N. Microbiol. Immunol. 2002, 46, 131–134. [Google Scholar] [CrossRef]
- Morser, J.; Shao, Z.; Nishimura, T.; Zhou, Q.; Zhao, L.; Higgins, J.; Leung, L.L.K. Carboxypeptidase B2 and N play different roles in regulation of activated complements C3a and C5a in mice. J. Thromb. Haemost. JTH 2018, 16, 991–1002. [Google Scholar] [CrossRef]
- Abuzakouk, M.; AlMahmeed, N.; Memisoglu, E.; McManus, M.; Alrakawi, A. Hereditary Angioedema Type II: First Presentation in Adulthood with Recurrent Severe Abdominal Pain. Case Rep. Immunol. 2018, 2018, 7435870. [Google Scholar] [CrossRef]
- Frolíková, M.; Stopková, R.; Antalíková, J.; Johnson, P.M.; Stopka, P.; Dvořáková-Hortová, K. Role of complement regulatory proteins CD46, CD55 and CD59 in reproduction. Folia Zool. 2012, 61, 84–94. [Google Scholar] [CrossRef]
- Lee, H.M.; Oh, B.C.; Lim, D.P.; Lee, D.S.; Cho, J.; Lee, G.; Lee, J.R. Role of complement regulatory proteins in the survival of murine allo-transplanted Sertoli cells. J. Korean Med. Sci. 2007, 22, 277–282. [Google Scholar] [CrossRef]
- Yancey, K.B.; Lawley, T.J.; Dersookian, M.; Harvath, L. Analysis of the interaction of human C5a and C5a des Arg with human monocytes and neutrophils: Flow cytometric and chemotaxis studies. J. Investig. Dermatol. 1989, 92, 184–189. [Google Scholar] [CrossRef]
- Cardone, J.; Le Friec, G.; Kemper, C. CD46 in innate and adaptive immunity: An update. Clin. Exp. Immunol. 2011, 164, 301–311. [Google Scholar] [CrossRef]
- Sacks, S.H.; Chowdhury, P.; Zhou, W. Role of the complement system in rejection. Curr. Opin. Immunol. 2003, 15, 487–492. [Google Scholar] [CrossRef]
- Gaudino, S.J.; Kumar, P. Cross-Talk Between Antigen Presenting Cells and T Cells Impacts Intestinal Homeostasis, Bacterial Infections, and Tumorigenesis. Front. Immunol. 2019, 10, 360. [Google Scholar] [CrossRef] [PubMed]
- Bhushan, S.; Meinhardt, A. The macrophages in testis function. J. Reprod. Immunol. 2017, 119, 107–112. [Google Scholar] [CrossRef] [PubMed]
- Hirayama, D.; Iida, T.; Nakase, H. The Phagocytic Function of Macrophage-Enforcing Innate Immunity and Tissue Homeostasis. Int. J. Mol. Sci. 2017, 19, 92. [Google Scholar] [CrossRef] [PubMed]
- Mills, C.D. M1 and M2 Macrophages: Oracles of Health and Disease. Crit. Rev. Immunol 2012, 32, 463–488. [Google Scholar] [CrossRef]
- Chávez-Galán, L.; Olleros, M.L.; Vesin, D.; Garcia, I. Much More than M1 and M2 Macrophages, There are also CD169(+) and TCR(+) Macrophages. Front. Immunol. 2015, 6, 263. [Google Scholar] [CrossRef]
- Wang, H.; Yang, Y.G. Innate cellular immunity and xenotransplantation. Curr. Opin. Organ Transplant. 2012, 17, 162–167. [Google Scholar] [CrossRef]
- Cadili, A.; Kneteman, N. The role of macrophages in xenograft rejection. Transplant. Proc. 2008, 40, 3289–3293. [Google Scholar] [CrossRef]
- Navarro-Alvarez, N.; Yang, Y.G. CD47: A new player in phagocytosis and xenograft rejection. Cell. Mol. Immunol. 2011, 8, 285–288. [Google Scholar] [CrossRef]
- Zhuang, Q.; Liu, Q.; Divito, S.J.; Zeng, Q.; Yatim, K.M.; Hughes, A.D.; Rojas-Canales, D.M.; Nakao, A.; Shufesky, W.J.; Williams, A.L.; et al. Graft-infiltrating host dendritic cells play a key role in organ transplant rejection. Nat. Commun. 2016, 7, 12623. [Google Scholar] [CrossRef]
- Mossadegh-Keller, N.; Sieweke, M.H. Testicular macrophages: Guardians of fertility. Cell Immunol. 2018, 330, 120–125. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Fijak, M.; Hossain, H.; Markmann, M.; Nüsing, R.M.; Lochnit, G.; Hartmann, M.F.; Wudy, S.A.; Zhang, L.; Gu, H.; et al. Characterization of the Micro-Environment of the Testis that Shapes the Phenotype and Function of Testicular Macrophages. J. Immunol. 2017, 198, 4327–4340. [Google Scholar] [CrossRef] [PubMed]
- Hedger, M.P. Testicular macrophages and inflammation. Andrologia 1999, 31, 308–310. [Google Scholar]
- Winnall, W.R.; Hedger, M.P. Phenotypic and functional heterogeneity of the testicular macrophage population: A new regulatory model. J. Reprod. Immunol. 2013, 97, 147–158. [Google Scholar] [CrossRef] [PubMed]
- Rival, C.; Theas, M.S.; Suescun, M.O.; Jacobo, P.; Guazzone, V.; van Rooijen, N.; Lustig, L. Functional and phenotypic characteristics of testicular macrophages in experimental autoimmune orchitis. J. Pathol. 2008, 215, 108–117. [Google Scholar] [CrossRef]
- Samdani, T.C.E.; Greenstein, S.M.; Atray, N.; Vachharajani, T.J. Xenotranplantation: Overview, Choosing the Donor Species, Immunologic Barriers to Xenotransplantation; Medscape: New York, NY, USA, 2018. [Google Scholar]
- Wang, H.; Arp, J.; Huang, X.; Liu, W.; Ramcharran, S.; Jiang, J.; Garcia, B.; Kanai, N.; Min, W.; O’Connell, P.J.; et al. Distinct subsets of dendritic cells regulate the pattern of acute xenograft rejection and susceptibility to cyclosporine therapy. J. Immunol. 2006, 176, 3525–3535. [Google Scholar] [CrossRef]
- Lechler, R.; Ng, W.F.; Steinman, R.M. Dendritic cells in transplantation—Friend or foe? Immunity 2001, 14, 357–368. [Google Scholar] [CrossRef]
- Bhushan, S.; Theas, M.S.; Guazzone, V.A.; Jacobo, P.; Wang, M.; Fijak, M.; Meinhardt, A.; Lustig, L. Immune Cell Subtypes and Their Function in the Testis. Front. Immunol. 2020, 11, 583304. [Google Scholar] [CrossRef]
- Vandendriessche, S.; Cambier, S.; Proost, P.; Marques, P.E. Complement Receptors and Their Role in Leukocyte Recruitment and Phagocytosis. Front. Cell Dev. Biol. 2021, 9, 624025. [Google Scholar] [CrossRef]
- Coulthard, L.G.; Hawksworth, O.A.; Woodruff, T.M. Chapter 36-38—Anaphylatoxin and Leukocyte Receptors C3aR, C5aR1, C5aR2. In The Complement FactsBook, 2nd ed.; Academic Press: Cambridge, MA, USA, 2017; pp. 385–414. [Google Scholar]
- Schreiber, A.; Xiao, H.; Jennette, J.C.; Schneider, W.; Luft, F.C.; Kettritz, R. C5a receptor mediates neutrophil activation and ANCA-induced glomerulonephritis. J. Am. Soc. Nephrol. 2009, 20, 289–298. [Google Scholar] [CrossRef]
- Ehrnthaller, C.; Braumüller, S.; Kellermann, S.; Gebhard, F.; Perl, M.; Huber-Lang, M. Complement Factor C5a Inhibits Apoptosis of Neutrophils—A Mechanism in Polytrauma? J. Clin. Med. 2021, 10, 3157. [Google Scholar] [CrossRef]
- Zhu, Y.; Wang, X.; Xu, Y.; Chen, L.; Ding, P.; Chen, J.; Hu, W. An Integrated Analysis of C5AR2 Related to Malignant Properties and Immune Infiltration of Breast Cancer. Front. Oncol 2021, 11, 736725. [Google Scholar] [CrossRef]
- Russell, S. CD46: A complement regulator and pathogen receptor that mediates links between innate and acquired immune function. Tissue Antigens 2004, 64, 111–118. [Google Scholar] [CrossRef]
- Fischbein, M.P.; Yun, J.; Laks, H.; Irie, Y.; Fishbein, M.C.; Bonavida, B.; Ardehali, A. Role of CD8+ lymphocytes in chronic rejection of transplanted hearts. J. Thorac. Cardiovasc. Surg. 2002, 123, 803–809. [Google Scholar] [CrossRef]
- Harper, S.J.F.; Ali, J.M.; Wlodek, E.; Negus, M.C.; Harper, I.G.; Chhabra, M.; Qureshi, M.S.; Mallik, M.; Bolton, E.; Bradley, J.A.; et al. CD8 T-cell recognition of acquired alloantigen promotes acute allograft rejection. Proc. Natl. Acad. Sci. USA 2015, 112, 12788–12793. [Google Scholar] [CrossRef]
- Kenway-Lynch, C.S.; Das, A.; Lackner, A.A.; Pahar, B. Cytokine/Chemokine responses in activated CD4+ and CD8+ T cells isolated from peripheral blood, bone marrow, and axillary lymph nodes during acute simian immunodeficiency virus infection. J. Virol. 2014, 88, 9442–9457. [Google Scholar] [CrossRef]
- Vieyra, M.; Leisman, S.; Raedler, H.; Kwan, W.H.; Yang, M.; Strainic, M.G.; Medof, M.E.; Heeger, P.S. Complement regulates CD4 T-cell help to CD8 T cells required for murine allograft rejection. Am. J. Pathol. 2011, 179, 766–774. [Google Scholar] [CrossRef]
- Wong, P.; Pamer, E.G. CD8 T Cell Responses to Infectious Pathogens. Annu. Rev. Immunol. 2003, 21, 29–70. [Google Scholar] [CrossRef]
- Janeway, C.A., Jr.; Travers, p.; Walport, M.; Shlomchik, M.J. General properties of armed effector T cells. In Immunobiology: The Immune System in Health and Disease, 5th ed.; Garland Science: New York, NY, USA, 2001. [Google Scholar]
- Dinarello, C.A. Overview of the IL-1 family in innate inflammation and acquired immunity. Immunol. Rev. 2018, 281, 8–27. [Google Scholar] [CrossRef]
- Voskoboinik, I.; Whisstock, J.C.; Trapani, J.A. Perforin and granzymes: Function, dysfunction and human pathology. Nat. Rev. Immunol. 2015, 15, 388–400. [Google Scholar] [CrossRef]
- Corthay, A. How do regulatory T cells work? Scand J. Immunol. 2009, 70, 326–336. [Google Scholar] [CrossRef] [PubMed]
- Suresh, M.; Molina, H.; Salvato, M.S.; Mastellos, D.; Lambris, J.D.; Sandor, M. Complement component 3 is required for optimal expansion of CD8 T cells during a systemic viral infection. J. Immunol. 2003, 170, 788–794. [Google Scholar] [CrossRef] [PubMed]
- de Rivero Vaccari, J.P.; Dietrich, W.D.; Keane, R.W. Activation and regulation of cellular inflammasomes: Gaps in our knowledge for central nervous system injury. J. Cereb. Blood Flow Metab. Off. J. Int. Soc. Cereb. Blood Flow Metab. 2014, 34, 369–375. [Google Scholar] [CrossRef] [PubMed]
- Raedler, H.; Yang, M.; Lalli, P.N.; Medof, M.E.; Heeger, P.S. Primed CD8(+) T-cell responses to allogeneic endothelial cells are controlled by local complement activation. Am. J. Transplant. Off. J. Am. Soc. Transplant. Am. Soc. Transpl. Surg. 2009, 9, 1784–1795. [Google Scholar] [CrossRef]
- McKenzie, I.F.; Koulmanda, M.; Mandel, T.E.; Sandrin, M.S. Pig islet xenografts are susceptible to “anti-pig” but not Gal alpha(1,3)Gal antibody plus complement in Gal o/o mice. J. Immunol. 1998, 161, 5116–5119. [Google Scholar] [CrossRef]
- Ekser, B.; Li, P.; Cooper, D.K.C. Xenotransplantation: Past, present, and future. Curr. Opin. Organ Transplant. 2017, 22, 513–521. [Google Scholar] [CrossRef]
- Dufour, J.M.; Hamilton, M.; Rajotte, R.V.; Korbutt, G.S. Neonatal porcine Sertoli cells inhibit human natural antibody-mediated lysis. Biol. Reprod. 2005, 72, 1224–1231. [Google Scholar] [CrossRef]
- Washburn, R.L.; Kaur, G.; Dufour, J.M. Mouse Sertoli Cells Inhibit Humoral-Based Immunity. Int. J. Mol. Sci. 2022, 23, 12760. [Google Scholar] [CrossRef]
- Washburn, R.L.; Martinez-Marin, D.; Korać, K.; Sniegowski, T.; Rodriguez, A.R.; Chilton, B.S.; Hibler, T.; Pruitt, K.; Bhutia, Y.D.; Dufour, J.M. The Sertoli cell complement signature: A suspected mechanism in xenograft survival. Int. J. Mol. Sci. 2023, 24, 1890. [Google Scholar] [CrossRef]
- Kemper, C.; Atkinson, J.P. T-cell regulation: With complements from innate immunity. Nat. Rev. Immunol. 2007, 7, 9–18. [Google Scholar] [CrossRef]
- Grafals, M.; Thurman, J.M. The Role of Complement in Organ Transplantation. Front. Immunol. 2019, 10, 2380. [Google Scholar] [CrossRef]
- Zipfel, P.F.; Skerka, C. Complement regulators and inhibitory proteins. Nat. Rev. Immunol. 2009, 9, 729–740. [Google Scholar] [CrossRef]
- Verghese, D.A.; Demir, M.; Chun, N.; Fribourg, M.; Cravedi, P.; Llaudo, I.; Woodruff, T.M.; Yadav, P.; Lira, S.A.; Medof, M.E.; et al. T Cell Expression of C5a Receptor 2 Augments Murine Regulatory T Cell (T(REG)) Generation and T(REG)-Dependent Cardiac Allograft Survival. J. Immunol. 2018, 200, 2186–2198. [Google Scholar] [CrossRef]
- Klinovska, K.; Sebkova, N.; Dvorakova-Hortova, K. Sperm-egg fusion: A molecular enigma of mammalian reproduction. Int. J. Mol. Sci. 2014, 15, 10652–10668. [Google Scholar] [CrossRef]
- Anderson, D.J.; Abbott, A.F.; Jack, R.M. The role of complement component C3b and its receptors in sperm-oocyte interaction. Proc. Natl. Acad. Sci. USA 1993, 90, 10051–10055. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).