Physiological and Transcriptional Responses of Apocynum venetum to Salt Stress at the Seed Germination Stage
Abstract
:1. Introduction
2. Results
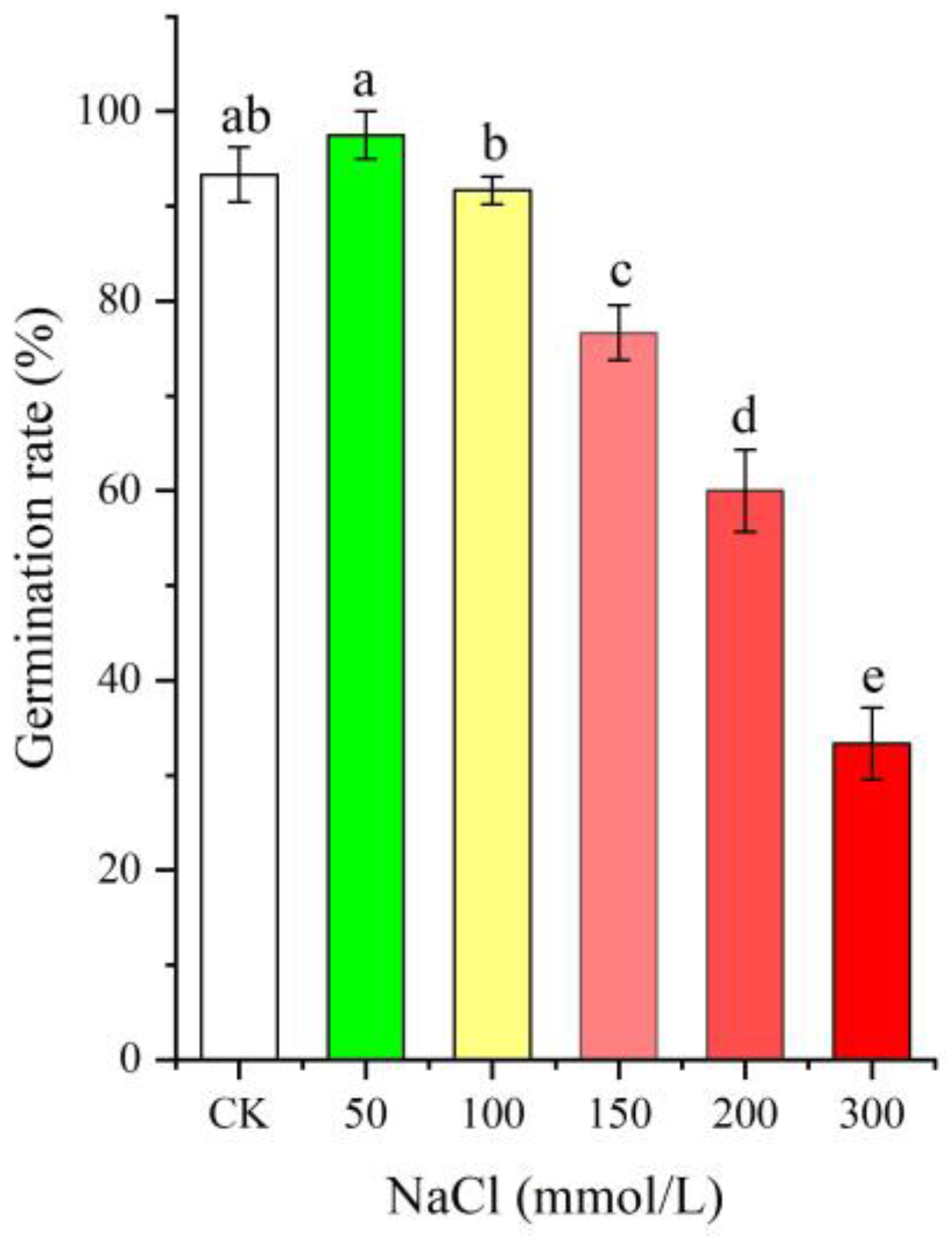
2.1. Seed Germination Rate at Different NaCl Treatments
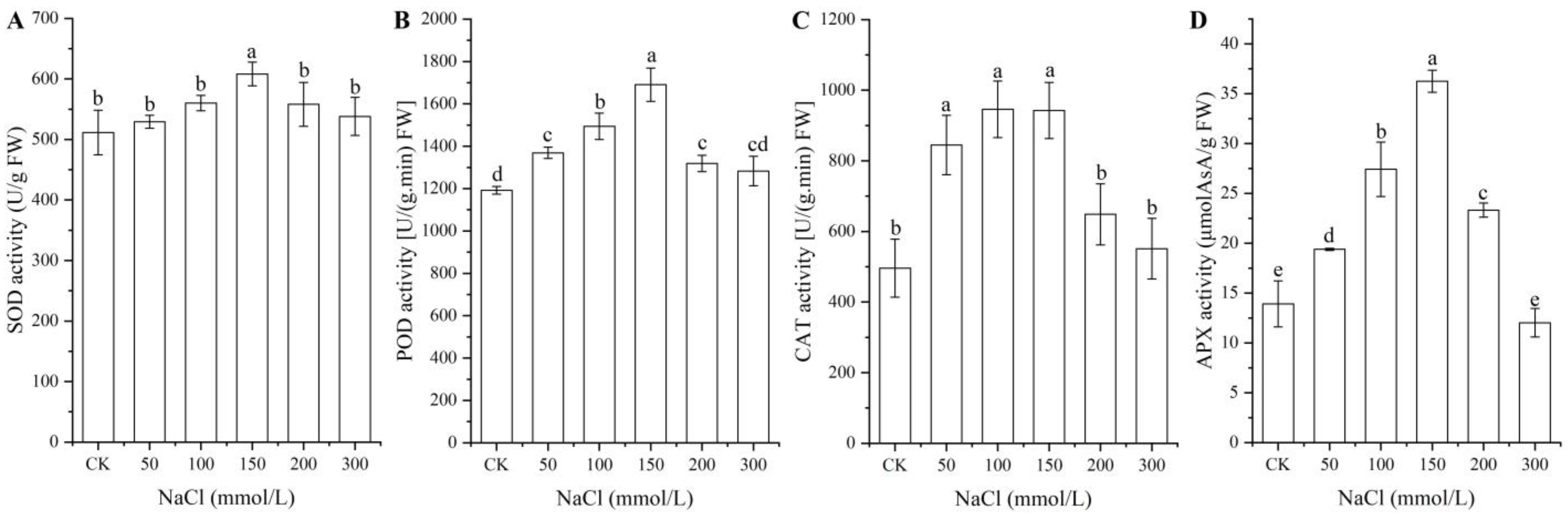
2.2. Antioxidant Enzyme Activities at Different NaCl Treatments
2.3. Osmolyte Contents under Different NaCl Treatments
2.4. Transcriptomic Analysis
2.4.1. Global Gene Analysis
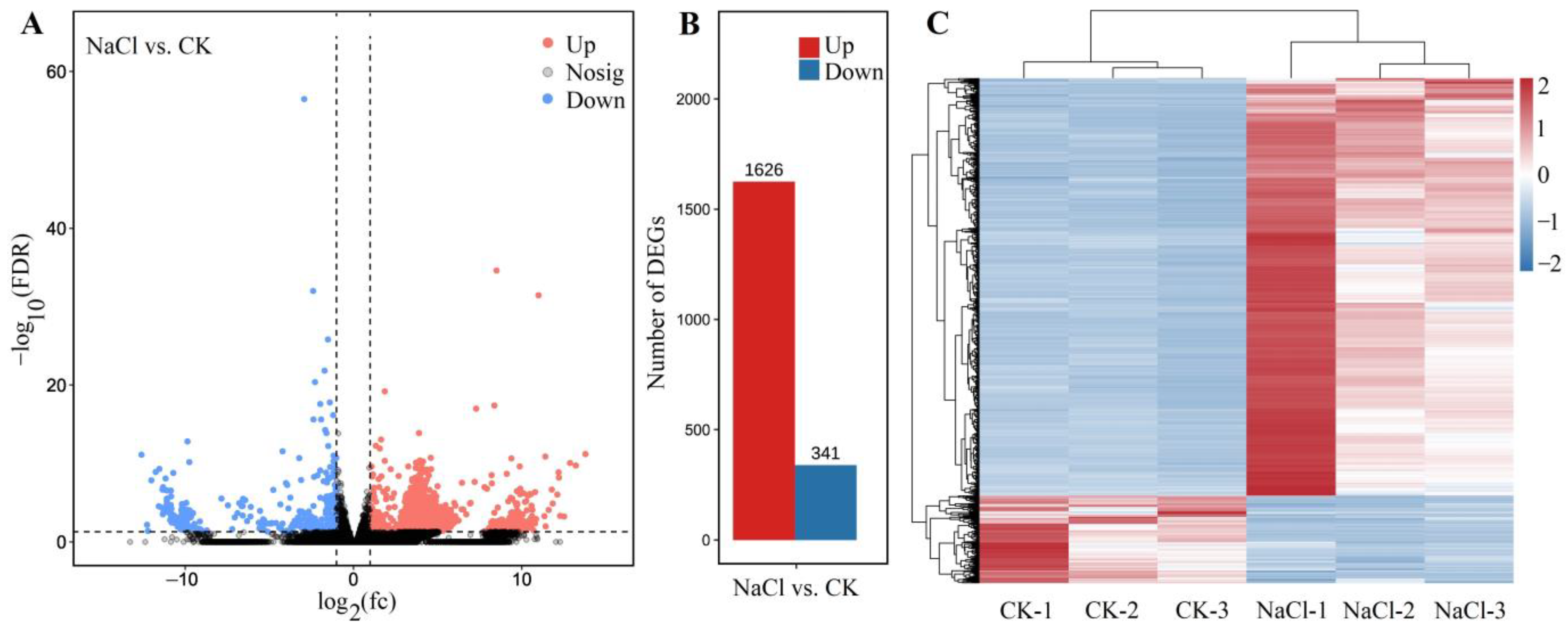
2.4.2. Identification of DEGs
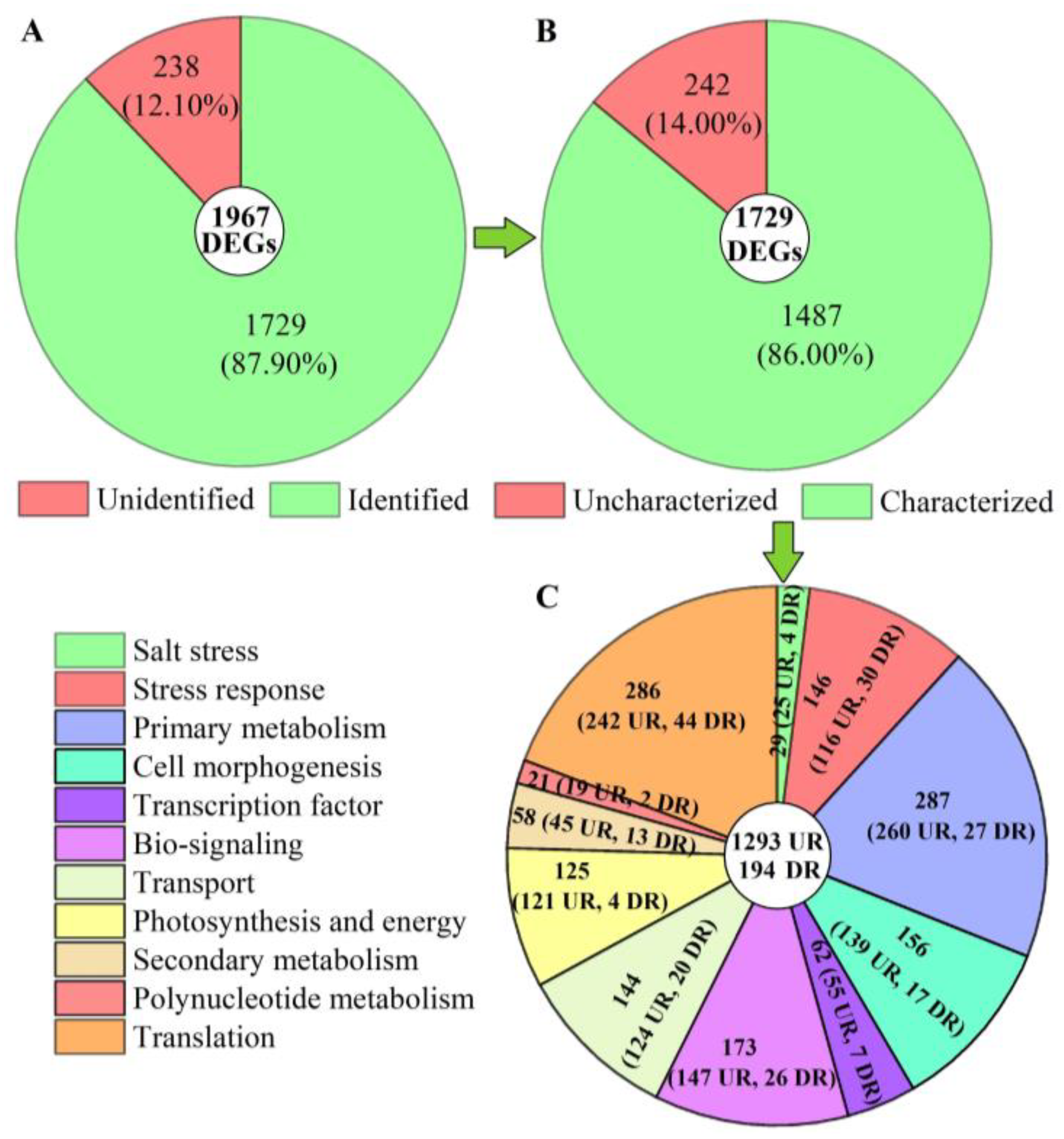
2.4.3. Distribution and Classification of DEGs
2.5. Specific Classification of DEGs and Validation of Expression Levels
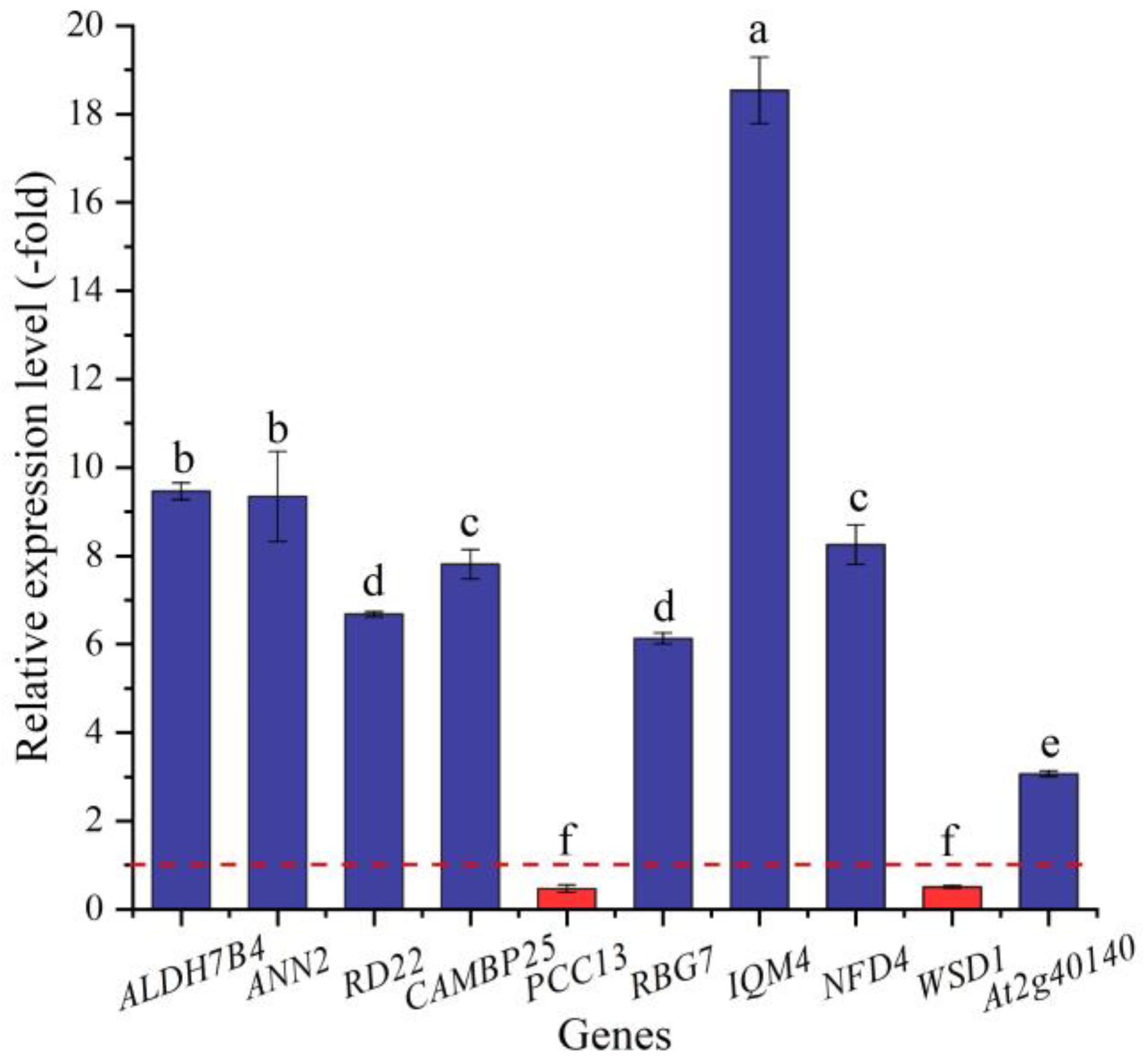
2.5.1. DEGs Directly Associated with Salt Stress
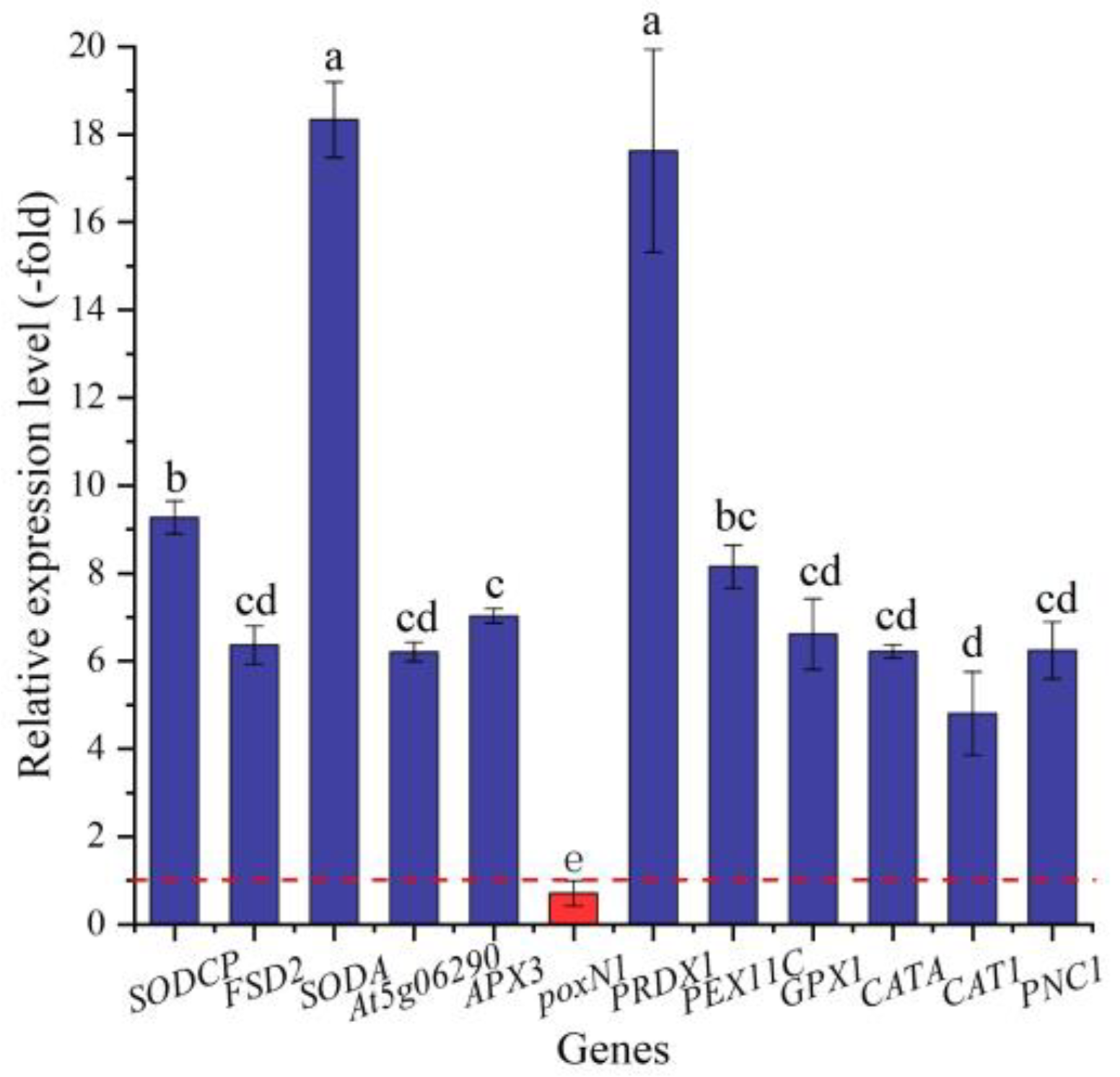
2.5.2. DEGs Directly Associated with Antioxidant Enzymes
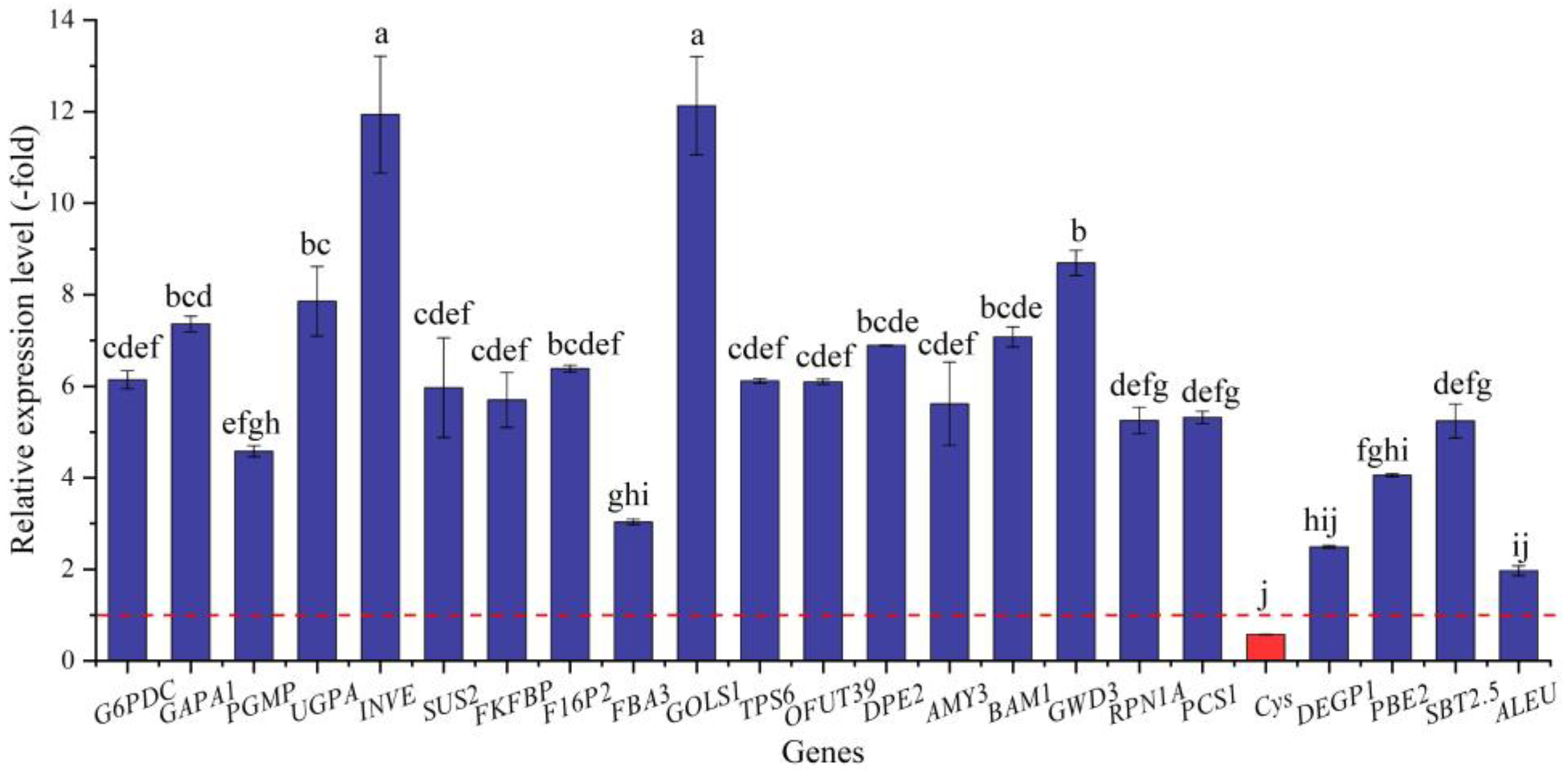
2.5.3. DEGs Directly Associated with Soluble Sugar and Protein Metabolism
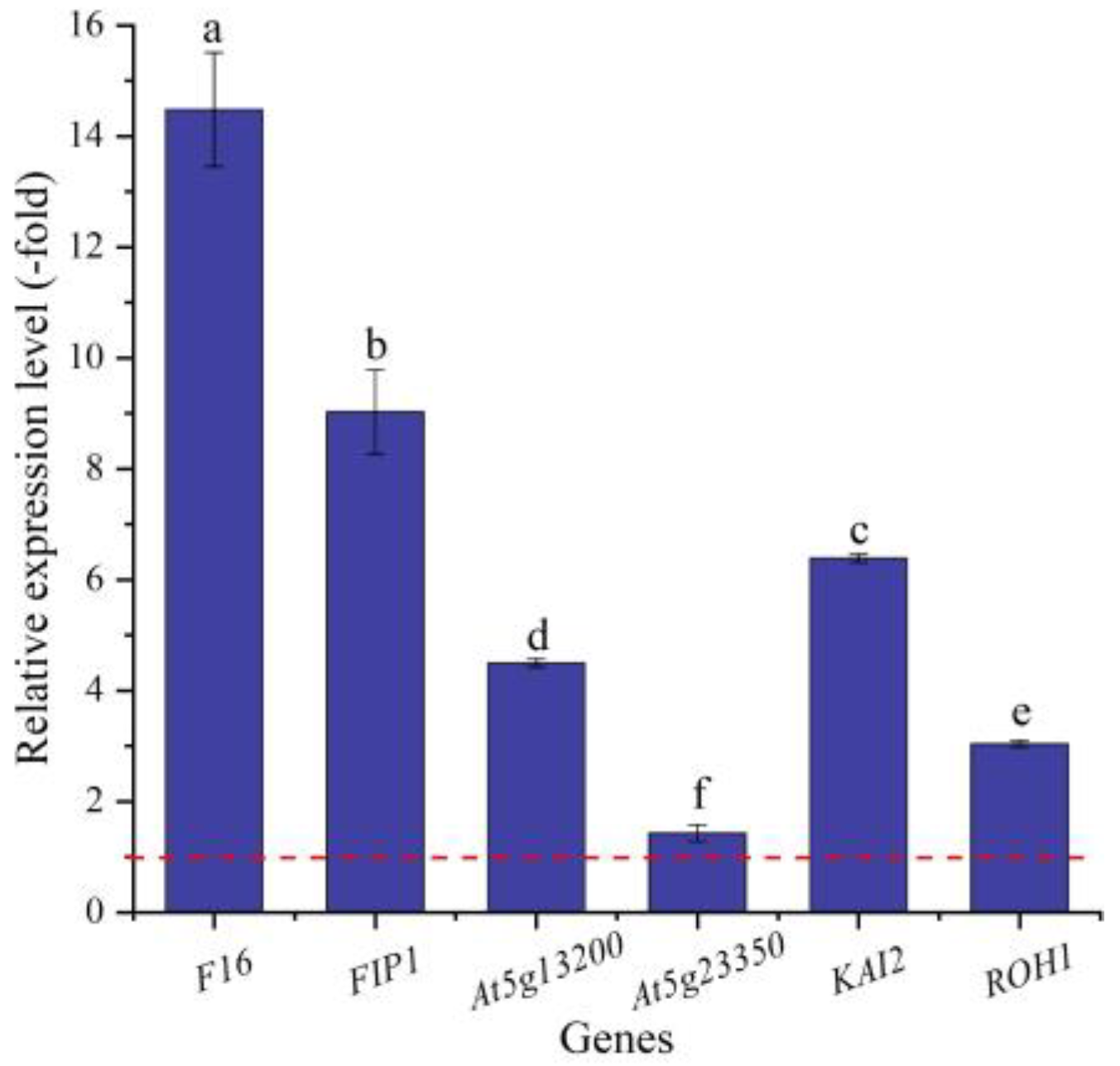
2.5.4. DEGs Directly Associated with Cell Morphogenesis for Seed Germination
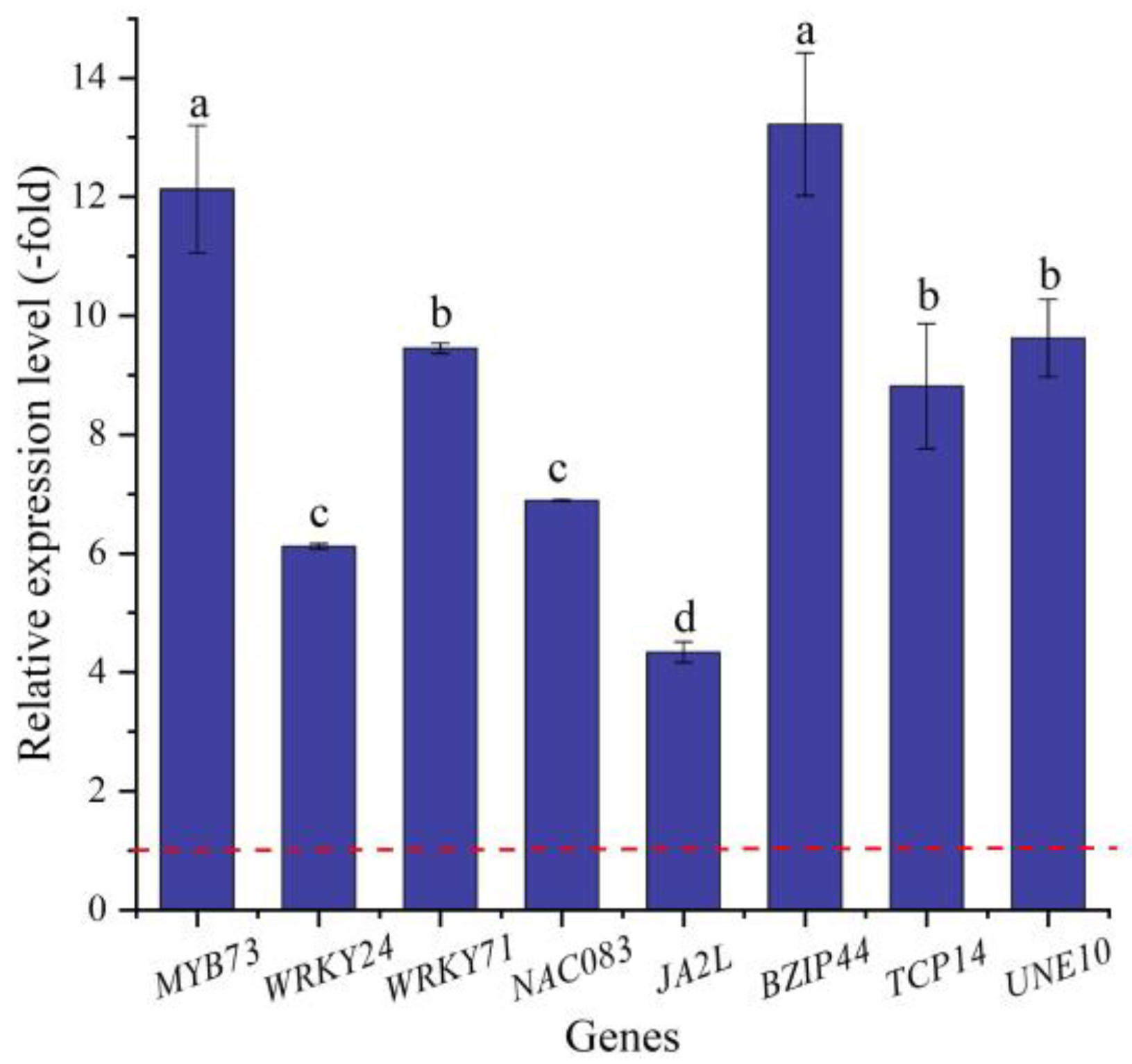
2.5.5. TFs Directly Associated with Stress Response and Seed Germination
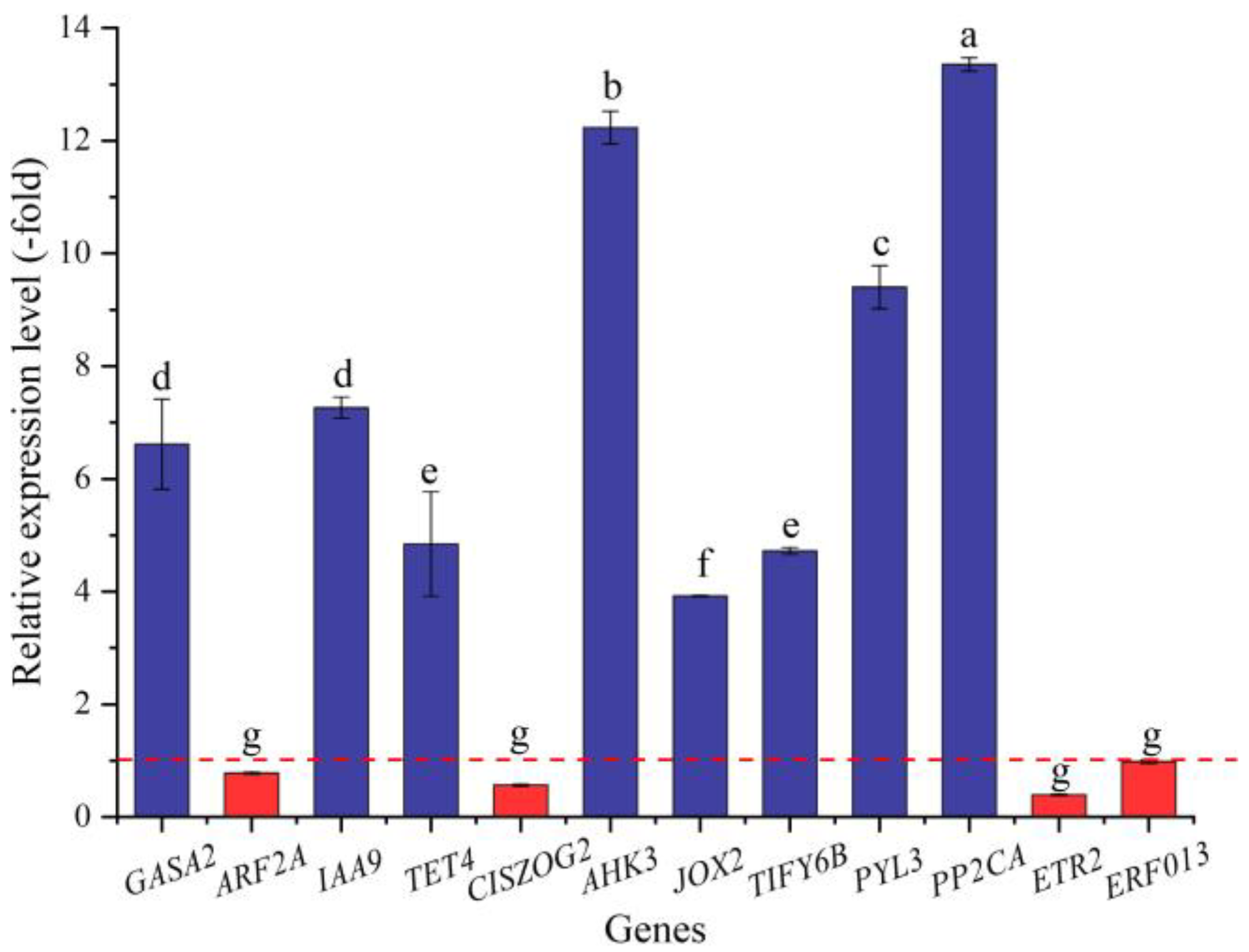
2.5.6. DEGs Directly Associated with Hormone Response
2.5.7. DEGs Directly Associated with Ion Transport
2.5.8. DEGs Associated with Other Biological Functions
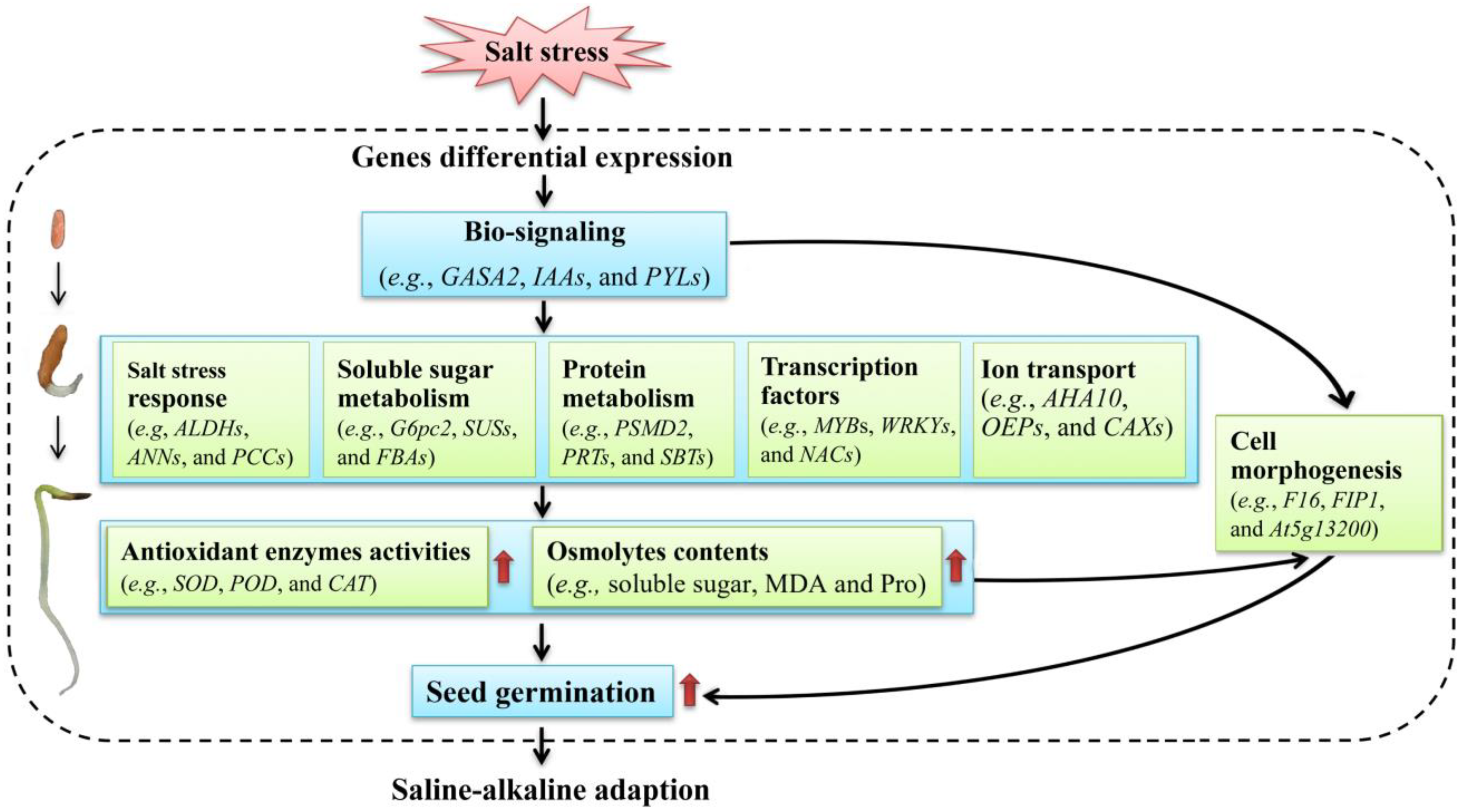
3. Discussion
4. Materials and Methods
4.1. Plant Materials
4.2. Measurement of Germination Rate
4.3. Determination of Antioxidant Enzyme Activities
4.4. Determination of Osmolytes Content
4.5. Transcriptomic Analysis
4.5.1. RNA Extraction, cDNA Library Construction, and Illumina Sequencing
4.5.2. Reads Filtration, Assembly, Unigene Expression Analysis, and Basic Annotation
4.5.3. qRT-PCR Validation
4.6. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Editorial Committee of the Flora of China of Chinese Academy of Science. Flora of China; Science Press: Beijing, China, 1977; p. 157. (In Chinese) [Google Scholar]
- Gao, G.; Chen, P.; Chen, J.; Chen, K.; Wang, X.; Abubakar, A.S.; Liu, N.; Yu, C.; Zhu, A. Genomic survey, transcriptome, and metabolome analysis of Apocynum venetum and Apocynum hendersonii to reveal major flavonoid biosynthesis pathways. Metabolites 2019, 9, 296. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, C.; Zhang, Z.; Wang, J.; Wu, G.; Li, S. Comprehensive separation and identification of chemical constituents from Apocynum venetum leaves by high performance counter-current chromatography and high performance liquid chromatography coupled with mass spectrometry. J. Chromatogr. 2010, 878, 3149–3155. [Google Scholar] [CrossRef] [PubMed]
- Xie, W.; Zhang, X.; Wang, T.; Hu, J. Botany, traditional uses, phytochemistry and pharmacology of Apocynum venetum L. (Luobuma): A review. J. Ethnopharmacol. 2012, 141, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Huang, G.; Tan, F.; Zhou, X.; Mu, J.; Zhao, X. In vitro analysis of antioxidant, anticancer, and bioactive components of Apocynum venetum tea extracts. J. Food Qual. 2019, 2019, 2465341. [Google Scholar] [CrossRef]
- Xu, Z.; Zhou, J.; Zhang, C.; Li, Y. Review of current research and utilization status of Apocynum venetum germplasm in China. Chin. Bull. Bot. 2018, 53, 382–390. [Google Scholar]
- Ning, J.F.; Zheng, Q.S.; Yang, S.H.; Zou, X.Z.; Sun, L.L.; Chen, Y. Impact of high salt stress on Apocynum venetum growth and ionic homeostasis. Chin. J. Appl. Ecol. 2010, 21, 325–330. [Google Scholar]
- Jiang, L.; She, C.W.; Tian, C.Y.; Tanveer, M.; Wang, L. Storage period and different abiotic factors regulate seed germination of two Apocynum species-cash crops in arid saline regions in the northwestern China. Front. Plant Sci. 2021, 12, 671157. [Google Scholar] [CrossRef]
- Ren, J.; Yu, F.K.; Tao, L. Research advances on the germination of desert plants under stress. Bull Bot. Res. 2011, 31, 121–128. [Google Scholar]
- Campobenedetto, C.; Grange, E.; Mannino, G.; van Arkel, J.; Beekwilder, J.; Karlova, R.; Garabello, C.; Contartese, V.; Bertea1, C.M. A biostimulant seed treatment improved heat stress tolerance during cucumber seed germination by acting on the antioxidant system and glyoxylate cycle. Front. Plant Sci. 2020, 11, 836. [Google Scholar] [CrossRef]
- Zhang, X.; Li, R.; Shi, F. Effects of salt stress on the seed germination of Apocynum venetum. Acta Sci. Univ. Nankaiensis 2007, 40, 13–18. [Google Scholar]
- Yu, D.H.; Xu, H.L.; Chang, S.L. Effect of NaCl stress on seed germination and seedling growth of Luobuma. Hubei Agric. Sci. 2008, 47, 772–775. [Google Scholar]
- Xu, Z.; Zhou, J.; Ren, T.; Du, H.; Liu, H.; Li, Y.; Zhang, C. Salt stress decreases seedling growth and development but increases quercetin and kaempferol content in Apocynum venetum. Plant Biol. 2020, 22, 813–821. [Google Scholar] [CrossRef]
- Wu, Z.B.; Chang, P.X.; Zhao, J.; Wang, W.S.; Cui, X.W.; Li, M.F. Physiological and transcriptional responses of seed germination to moderate drought in Apocynum venetum. Front. Ecol. Evol. 2022, 10, 975771. [Google Scholar] [CrossRef]
- Liu, Z.H.; Wang, N.; Chen, L.Y.; Geng, L. Measurement and analysis of physiological and biochemical indexes during natural aging on Apocynum seed. Seed 2015, 34, 43–46. [Google Scholar]
- Ning, J.F.; Zheng, Q.S.; Zou, X.Z.; Sun, L.L.; Yao, Y.; Chen, Y.; Wu, J.L.; Wei, L. PhysiologicaI responses of Apocynum venetum to different levels of salt stress. Chin. Bull. Bot. 2010, 45, 889–897. [Google Scholar]
- Fang, J.W.; Wu, Y.; Liu, Z.H. Effects of salt stress on seed germination and physiological characteristics of Apocynum venetum. Crops 2018, 4, 167–174. [Google Scholar]
- Yan, M.X.; Bai, R.; Cui, J.X.; Zhang, Y.Q.; Wang, C.F.; Wu, P.; Bing, Z.; Jin, B. Effects of mixed saline alkali stress on physiological characteristics of Apocynum venetum. Bull. Agric. Sci. Technol. 2021, 8, 153–157. [Google Scholar]
- Xu, Z.C.; Wang, M.; Ren, T.T.; Li, K.Y.; Li, Y.Q.; Marowa, P.; Zhang, C.S. Comparative transcriptome analysis reveals the molecular mechanism of salt tolerance in Apocynum venetum. Plant Physiol. Bioch. 2021, 167, 816–830. [Google Scholar] [CrossRef]
- Zhang, Z.J.; Xu, H.L.; Shi, S.B.; Li, J.M. Effect of water supply, salinity and buried depth on the seed germination of Halimodendron halodendron and Alhagi sparsifolia. Arid Land Geogr. 2008, 31, 687–692. [Google Scholar]
- Khan, M.A.; Gul, B.; Weber, D.J. Effect of germination regulating chemicals on seed germination of Halogeton glomeratus for alleviation of salinity stress. Pak. J. Bot. 2009, 41, 1205–1212. [Google Scholar]
- Hu, R.H.; Liu, H.; Liu, F.H. Seed germination of hemp (Cannabis sativa L.) cultivars responds differently to the stress of salt type and concentration. Ind. Crops Prod. 2018, 123, 254–261. [Google Scholar] [CrossRef]
- Rasool, S.; Ahmad, A.; Siddiqi, T.O.; Ahmad, P. Changes in growth, lipid peroxidation and some key antioxidant enzymes in chickpea genotypes under salt stress. Acta Physiol. Plant. 2013, 35, 1039–1050. [Google Scholar] [CrossRef]
- Zhao, S.S.; Zhang, Q.K.; Liu, M.Y.; Zhou, H.P.; Ma, C.L.; Wang, P.P. Regulation of plant responses to salt stress. Int. J. Mol. Sci. 2021, 22, 4609. [Google Scholar] [CrossRef]
- Li, R.J.; Zhang, M.; Jiang, Z.P.; Huang, L.B.; Wang, B.S.; Ji, Y.H.; Fang, Y.M. Effects of NaCl stress on antioxidant enzyme activities and isoenzyme pattern of Broyssonetia papyrifera Plantlets. Sci. Silvae Sin. 2009, 45, 40–47. [Google Scholar]
- Guo, Q.S.; Wu, Y.G.; Lin, Y.F.; Zheng, H.Q. Effect of NaCl stress on growth and antioxidant systems of Pogostemon cablin. China J. Chin. Mater. Med. 2009, 34, 530–534. [Google Scholar]
- Chan, Z.; Loescher, W.; Grumet, R. Transcriptional variation in response to salt stress in commonly used Arabidopsis thaliana accessions. Plant Physiol. Biochem. 2013, 73, 189–201. [Google Scholar] [CrossRef]
- Vogel, J.T.; Zarka, D.G.; Van Buskirk, H.A.; Fowler, S.G.; Thomashow, M.F. Roles of the CBF2 and ZAT12 transcription factors in configuring the low temperature transcriptome of Arabidopsis. Plant J. 2005, 41, 195–211. [Google Scholar] [CrossRef]
- Van Buskirk, H.A.; Thomashow, M.F. Arabidopsis transcription factors regulating cold acclimation. Physiol. Plant. 2006, 126, 72–80. [Google Scholar] [CrossRef]
- Jiang, Y.; Deyholos, M.K. Comprehensive transcriptional profiling of NaCl-stressed Arabidopsis roots reveals novel classes of responsive genes. BMC Plant Biol. 2006, 6, 25. [Google Scholar] [CrossRef]
- Taiz, L.; Zeiger, E. Plant Physiology. In Responses and Adaptations to Abiotic Stress; Radin, J., Bressan, R., Drew, M., Hasegawa, P., Locy, R., Mickelbart, M., Salt, D., Eds.; Sinauer Associates Inc.: Sunderland, MA, USA, 2010; pp. 620–621. [Google Scholar]
- Quan, R.; Lin, H.; Mendoza, I.; Zhang, Y.; Cao, W.; Yang, Y.; Shang, M.; Chen, S.; Pardo, J.M.; Guo, Y. SCABP8/CBL10, a putative calcium sensor, interacts with the protein kinase SOS2 to protect Arabidopsis shoots from salt stress. Plant Cell 2007, 19, 1415–1431. [Google Scholar] [CrossRef]
- Park, H.J.; Kim, W.Y.; Yun, D.J. A role for GIGANTEA. Plant Signal. Behav. 2013, 8, e24820. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Hoehenwarter, W. Changes in the phosphosproteome and metabolome link early signaling events to rearrangement of photosynthesis and central metabolism in salinity and oxidative stress response in Arabidopsis. Plant Physiol. 2015, 69, 3021–3033. [Google Scholar] [CrossRef]
- Liu, D.; Ma, Y.; Rui, M.M.; Lv, X.C.; Chen, R.J.; Chen, X.Y.; Wang, Y.Z. Is high pH the key factor of alkali stress on plant growth and physiology? A case study with Wheat (Triticum aestivum L.) seedlings. Agronomy 2022, 12, 1820. [Google Scholar] [CrossRef]
- Van Zelm, E.; Zhang, Y.; Testerink, C. Salt tolerance mechanisms of plants. Annu. Rev. Plant Biol. 2020, 71, 403–433. [Google Scholar] [CrossRef] [PubMed]
- Gong, Z. Plant abiotic stress: New insights into the factors that activate and modulate plant responses. J. Integr. Plant Biol. 2021, 63, 429–430. [Google Scholar] [CrossRef] [PubMed]
- Shao, Y.; Gao, J.; Wu, X.; Li, Q.; Wang, J.; Ding, P.; Lai, X. Effect of salt treatment on growth, isoenzymes and metabolites of Andrographis paniculata (Burm. f.) Nees. Acta Physiol. Plant. 2015, 37, 35. [Google Scholar] [CrossRef]
- Apel, K.; Hirt, H. Reactive oxygen species: Metabolism, oxidative stress, and signal transduction. Ann. Rev. Plant Biol. 2004, 55, 373–399. [Google Scholar] [CrossRef]
- Tuteja, N.; Ahmad, P.; Panda, B.B.; Tuteja, R. Genotoxic stress in plants: Shedding light on DNA damage, repair and DNA repair helicases. Mutat. Res. 2009, 681, 134–149. [Google Scholar] [CrossRef]
- Zheng, Y.H.; Xu, X.B.; Wang, M.Y.; Zheng, X.H.; Li, Z.J.; Jiang, G.M. Responses of salt-tolerant and intolerant wheat genotypes to sodium chloride: Photosynthesis, antioxidants activities, and yield. Photosynthetica 2009, 47, 87–94. [Google Scholar] [CrossRef]
- Ahmad, P.; Prasad, M.N.V. Environmental Adaptations and Stress Tolerance in Plants in the Era of Climate Change; Springer Science Business Media, LLC: New York, NY, USA, 2012. [Google Scholar]
- Ahmad, P.; Prasad, M.N.V. Abiotic Stress Responses in Plants: Metabolism, Productivity and Sustainability; Springer Science Business Media, LLC: New York, NY, USA, 2012. [Google Scholar]
- Koca, H.; Ozdemir, F.; Turkan, I. Effect of salt stress on lipid peroxidation and superoxide dismutase and peroxidase activities of Lycopersicon esculentum and L. pennellii. Biol. Plant 2006, 50, 745–748. [Google Scholar] [CrossRef]
- Yang, Y.; Guo, Y. Elucidating the molecular mechanisms mediating plant salt-stress responses. New Phytol. 2017, 217, 523–539. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Zhang, H.; Song, C.; Zhu, J.K.; Shabala, S. Mechanisms of plant responses and adaptation to soil salinity. Innovation 2020, 1, 100017. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.M.; Zhu, J.H.; Gong, Z.Z.; Zhu, J.K. Abiotic stress responses in plants. Nat. Rev. Genet. 2022, 23, 104–119. [Google Scholar] [CrossRef]
- Kirch, H.H.; Schlingensiepen, S.; Kotchoni, S.; Sunkar, R.; Bartels, D. Detailed expression analysis of selected genes of the aldehyde dehydrogenase (ALDH) gene superfamily in Arabidopsis thaliana. Plant Mol. Biol. 2005, 57, 315–332. [Google Scholar] [CrossRef] [PubMed]
- Cantero, A.; Barthakur, S.; Bushart, T.J.; Chou, S.; Morgan, R.O.; Fernandez, M.P.; Clark, G.B.; Roux, S.J. Expression profiling of the Arabidopsis annexin gene family during germination, de-etiolation and abiotic stress. Plant Physiol. Biochem. 2006, 44, 13–24. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi-Shinozaki, K.; Shinozaki, K. The plant hormone abscisic acid mediates the drought-induced expression but not the seed-specific expression of rd22, a gene responsive to dehydration stress in Arabidopsis thaliana. Mol. Gen. Genet. 1993, 238, 17–25. [Google Scholar] [CrossRef]
- Perruc, E.; Charpenteau, M.; Ramirez, B.C.; Jauneau, A.; Galaud, J.P.; Ranjeva, R.; Ranty, B. A novel calmodulin-binding protein functions as a negative regulator of osmotic stress tolerance in Arabidopsis thaliana seedlings. Plant J. 2004, 38, 410–420. [Google Scholar] [CrossRef]
- Piatkowski, D.; Schneider, K.; Salamini, F.; Bartels, D. Characterization of five abscisic acid-responsive cDNA clones isolated from the desiccation-tolerant plant Craterostigma plantagineum and their relationship to other water-stress genes. Plant Physiol. 1990, 94, 1682–1688. [Google Scholar] [CrossRef]
- Kwak, K.J.; Kim, Y.O.; Kang, H. Characterization of transgenic Arabidopsis plants overexpressing GR-RBP4 under high salinity, dehydration, or cold stress. J. Exp. Bot. 2005, 56, 3007–3016. [Google Scholar] [CrossRef]
- Zhou, Y.P.; Wu, J.H.; Xiao, W.H.; Chen, W.; Chen, Q.H.; Fan, T.; Xie, C.P.; Tian, C.E. Arabidopsis IQM4, a novel calmodulin-binding protein, is involved with seed dormancy and germination in Arabidopsis. Front. Plant Sci. 2018, 9, 721. [Google Scholar] [CrossRef]
- Sottosanto, J.B.; Saranga, Y.; Blumwald, E. Impact of AtNHX1, a vacuolar Na+/H+ antiporter, upon gene expression during short- and long-term salt stress in Arabidopsis thaliana. BMC Plant Biol. 2007, 7, 18. [Google Scholar] [CrossRef] [PubMed]
- Patwari, P.; Salewski, V.; Gutbrod, K.; Kreszies, T.; Dresen-Scholz, B.; Peisker, H.; Steiner, U.; Meyer, A.J.; Schreiber, L.; Doermann, P. Surface wax esters contribute to drought tolerance in Arabidopsis. Plant J. 2019, 98, 727–744. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Jiang, H.; Xu, Y.; Li, H.; Wu, X.; Xie, Q.; Li, C. The CCCH-type zinc finger proteins AtSZF1 and AtSZF2 regulate salt stress responses in Arabidopsis. Plant Cell Physiol. 2007, 48, 1148–1158. [Google Scholar] [CrossRef]
- Ahmad, P.; Jhon, R.; Sarwat, M.; Umar, S. Responses of proline, lipid peroxidation and antioxidative enzymes in two varieties of Pisum sativum L. under salt stress. Int. J. Plant Prod. 2008, 2, 353–366. [Google Scholar]
- Ahmad, P.; Sarwat, M.; Sharma, S. Reactive oxygen species, antioxidants and signaling in plants. J. Plant Biol. 2008, 51, 167–173. [Google Scholar] [CrossRef]
- Ahmad, P.; Jaleel, C.A.; Salem, M.A.; Nabi, G.; Sharma, S. Roles of enzymatic and non-enzymatic antioxidants in plants during abiotic stress. Crit. Rev. Biotechnol. 2010, 30, 161–175. [Google Scholar] [CrossRef] [PubMed]
- Azooz, M.M.; Youssef, A.M.; Ahmad, P. Evaluation of salicylic acid (SA) application on growth, osmotic solutes and antioxidant enzyme activities on broad bean seedlings grown under diluted seawater. Int. J. Plant. Physiol. Biochem. 2011, 3, 253–264. [Google Scholar]
- Broin, M.; Cuine, S.; Eymery, F.; Rey, P. The plastidic 2-cysteine peroxiredoxin is a target for a thioredoxin involved in the protection of the photosynthetic apparatus against oxidative damage. Plant Cell 2002, 14, 1417–1432. [Google Scholar] [CrossRef]
- Foyer, C.H.; Descourvieres, P.; Kunert, K.J. Protection against oxygen radicals: An important defense mechanism studied in transgenic plants. Plant Cell Environ. 1994, 17, 507–523. [Google Scholar] [CrossRef]
- Yuan, G.; Liu, J.; An, G.; Li, W.; Si, W.; Sun, D.; Zhu, Y. Genome-wide identification and characterization of the trehalose-6-phosphate synthetase (TPS) gene family in Watermelon (Citrullus lanatus) and their transcriptional responses to salt stress. Int. J. Mol. Sci. 2022, 23, 276. [Google Scholar] [CrossRef]
- Kong, W.; Gong, Z.; Zhong, H.; Zhang, Y.; Zhao, G.; Gautam, M.; Deng, X.; Liu, C.; Zhang, C.; Li, Y. Expansion and evolutionary patterns of glycosyltransferase family 8 in gramineae crop genomes and their expression under salt and cold stresses in Oryza sativa ssp. japonica. Biomolecules 2019, 9, 188. [Google Scholar] [CrossRef]
- Duan, X.; Wang, Z.; Zhang, Y.; Li, H.; Yang, M.; Yin, H.; Cui, J.; Chai, H.; Gao, Y.; Hu, G.; et al. Overexpression of a thioredoxin-protein-encoding gene, MsTRX, from Medicago sativa enhances salt tolerance to transgenic tobacco. Agronomy 2022, 12, 1467. [Google Scholar] [CrossRef]
- Wenderoth, I.; Scheibe, R.; von Schaewen, A. Identification of the cysteine residues involved in redox modification of plant plastidic glucose-6-phosphate dehydrogenase. J. Biol. Chem. 1997, 272, 26985–26990. [Google Scholar] [CrossRef] [PubMed]
- Holtgrefe, S.; Gohlke, J.; Starmann, J.; Druce, S.; Klocke, S.; Altmann, B.; Wojtera, J.; Lindermayr, C.; Scheibe, R. Regulation of plant cytosolic glyceraldehyde 3-phosphate dehydrogenase isoforms by thiol modifications. Physiol. Plant. 2008, 133, 211–228. [Google Scholar] [CrossRef] [PubMed]
- Gaudet, P.; Livstone, M.S.; Lewis, S.E.; Thomas, P.D. Phylogenetic-based propagation of functional annotations within the Gene Ontology consortium. Brief. Bioinform. 2011, 12, 449–462. [Google Scholar] [CrossRef] [PubMed]
- Tamoi, M.; Tabuchi, T.; Demuratani, M.; Otori, K.; Tanabe, N.; Maruta, T.; Shigeoka, S. Point mutation of a plastidic invertase inhibits development of the photosynthetic apparatus and enhances nitrate assimilation in sugar-treated Arabidopsis seedlings. J. Biol. Chem. 2010, 285, 15399–15407. [Google Scholar] [CrossRef]
- Angeles-Nunez, J.G.; Tiessen, A. Arabidopsis sucrose synthase 2 and 3 modulate metabolic homeostasis and direct carbon towards starch synthesis in developing seeds. Planta 2010, 232, 701–718. [Google Scholar] [CrossRef]
- Villadsen, D.; Rung, J.H.; Draborg, H.; Nielsen, T.H. Structure and heterologous expression of a gene encoding fructose-6-phosphate,2-kinase/fructose-2,6-bisphosphatase from Arabidopsis thaliana. Biochim. Biophys. Acta. 2000, 1492, 406–413. [Google Scholar] [CrossRef]
- Nishizawa, A.; Yabuta, Y.; Shigeoka, S. Galactinol and raffinose constitute a novel function to protect plants from oxidative damage. Plant Physiol. 2008, 147, 1251–1263. [Google Scholar] [CrossRef]
- Chary, S.N.; Hicks, G.R.; Choi, Y.G.; Carter, D.; Raikhel, N.V. Trehalose-6-phosphate synthase/phosphatase regulates cell shape and plant architecture in Arabidopsis. Plant Physiol. 2008, 146, 97–107. [Google Scholar] [CrossRef]
- Shi, S.; Stanley, P. Protein O-fucosyltransferase 1 is an essential component of Notch signaling pathways. Proc. Natl. Acad. Sci. USA 2003, 100, 5234–5239. [Google Scholar] [CrossRef] [PubMed]
- Smith, A.M.; Zeeman, S.C.; Smith, S.M. Starch degradation. Annu. Rev. Plant Biol. 2005, 56, 73–98. [Google Scholar] [CrossRef] [PubMed]
- Thalmann, M.; Pazmino, D.; Seung, D.; Horrer, D.; Nigro, A.; Meier, T.; Koelling, K.; Pfeifhofer, H.W.; Zeeman, S.C.; Santelia, D. Regulation of leaf starch degradation by abscisic acid is important for osmotic stress tolerance in plants. Plant Cell 2016, 28, 1860–1878. [Google Scholar] [CrossRef]
- Edner, C.; Li, J.; Albrecht, T.; Mahlow, S.; Hejazi, M.; Hussain, H.; Kaplan, F.; Guy, C.; Smith, S.M.; Steup, M.; et al. Glucan, water dikinase activity stimulates breakdown of starch granules by plastidial beta-amylases. Plant Physiol. 2007, 145, 17–28. [Google Scholar] [CrossRef] [PubMed]
- Baunsgaard, L.; Luetken, H.; Mikkelsen, R.; Glaring, M.A.; Pham, T.T.; Blennow, A. A novel isoform of glucan, water dikinase phosphorylates pre-phosphorylated alpha-glucans and is involved in starch degradation in Arabidopsis. Plant J. 2005, 41, 595–605. [Google Scholar] [CrossRef]
- Fu, H.; Reis, N.; Lee, Y.; Glickman, M.H.; Vierstra, R. Subunit interaction maps for the regulatory particle of the 26S proteasome and the COP9 signalosome. EMBO J. 2001, 20, 7096–7107. [Google Scholar] [CrossRef]
- Ge, X.; Dietrich, C.; Matsuno, M.; Li, G.; Berg, H.; Xia, Y. An Arabidopsis aspartic protease functions as an anti-cell-death component in reproduction and embryogenesis. EMBO Rep. 2005, 6, 282–288. [Google Scholar] [CrossRef]
- Aoki, H.; Ahsan, M.N.; Watabe, S. Molecular cloning and functional characterization of crustapain: A distinct cysteine proteinase with unique substrate specificity from northern shrimp Pandalus borealis. J. Biochem. 2003, 133, 799–810. [Google Scholar] [CrossRef]
- Itzhaki, H.; Naveh, L.; Lindahl, M.; Cook, M.; Adam, Z. Identification and characterization of DegP, a serine protease associated with the luminal side of the thylakoid membrane. J. Biol. Chem. 1998, 273, 7094–7098. [Google Scholar] [CrossRef]
- Zienkiewicz, M.; Ferenc, A.; Wasilewska, W.; Romanowska, E. High light stimulates Deg1-dependent cleavage of the minor LHCII antenna proteins CP26 and CP29 and the PsbS protein in Arabidopsis thaliana. Planta 2012, 235, 279–288. [Google Scholar] [CrossRef]
- Golldack, D.; Vera, P.; Dietz, K.J. Expression of subtilisin-like serine proteases in Arabidopsis thaliana is cell-specific and responds to jasmonic acid and heavy metals with developmental differences. Physiol. Plantarum 2003, 118, 64–73. [Google Scholar] [CrossRef] [PubMed]
- Zhao, G.R.; Liu, J.Y.; Du, X.M. Molecular cloning and characterization of cotton cDNAs expressed in developing fiber cells. Biosci. Biotechnol. Biochem. 2001, 65, 2789–2793. [Google Scholar] [CrossRef]
- Baron, K.N.; Schroeder, D.F.; Stasolla, C. GEm-Related 5 (GER5), an ABA and stress-responsive GRAM domain protein regulating seed development and inflorescence architecture. Plant Sci. 2014, 223, 153–166. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.D.; Ni, M. Hyposensitive to light, an alpha/beta fold protein, acts downstream of elongated hypocotyl 5 to regulate seedling de-etiolation. Mol. Plant 2011, 4, 116–126. [Google Scholar] [CrossRef] [PubMed]
- Kulich, I.; Cole, R.; Drdova, E.; Cvrckova, F.; Soukup, A.; Fowler, J.; Zarsky, V. Arabidopsis exocyst subunits SEC8 and EXO70A1 and exocyst interactor ROH1 are involved in the localized deposition of seed coat pectin. New Phytol. 2010, 188, 615–625. [Google Scholar] [CrossRef] [PubMed]
- Cheng, C.; An, L.; Li, F.; Ahmad, W.; Aslam, M.; Ul Haq, M.Z.; Yan, Y.; Ahmad, R.M. Wide-range portrayal of AP2/ERF transcription factor family in Maize (Zea mays L.) development and stress responses. Genes 2023, 14, 194. [Google Scholar] [CrossRef]
- Guo, X.; Ullah, A.; Siuta, D.; Kukfisz, B.; Iqbal, S. Role of WRKY transcription factors in regulation of abiotic stress responses in Cotton. Life 2022, 12, 1410. [Google Scholar] [CrossRef]
- Li, W.; Zhong, J.; Zhang, L.; Wang, Y.; Song, P.; Liu, W.; Li, X.; Han, D. Overexpression of a Fragaria vesca MYB transcription factor gene (FvMYB82) increases salt and cold tolerance in Arabidopsis thaliana. Int. J. Mol. Sci. 2022, 23, 10538. [Google Scholar] [CrossRef]
- Mijiti, M.; Wang, Y.; Wang, L.; Habuding, X. Tamarix hispida NAC transcription factor ThNAC4 confers salt and drought stress tolerance to transgenic Tamarix and Arabidopsis. Plants 2022, 11, 2647. [Google Scholar] [CrossRef]
- Kim, J.H.; Nguyen, N.H.; Jeong, C.Y.; Nguyen, N.T.; Hong, S.W.; Lee, H. Loss of the R2R3 MYB, AtMyb73, causes hyper-induction of the SOS1 and SOS3 genes in response to high salinity in Arabidopsis. J Plant Physiol. 2013, 170, 1461–1465. [Google Scholar] [CrossRef]
- Tang, J.; Liu, Q.Q.; Yuan, H.Y.; Zhang, Y.X.; Huang, S.Z. Molecular analysis of a novel alkaline metal salt (NaCl)-responsive WRKY transcription factor gene IlWRKY1 from the halophyte Iris lactea var. chinensis. Int. Biodeter. Biodegr. 2018, 127, 139–145. [Google Scholar] [CrossRef]
- Yang, S.D.; Seo, P.J.; Yoon, H.K.; Park, C.M. The Arabidopsis NAC transcription factor VNI2 integrates abscisic acid signals into leaf senescence via the COR/RD genes. Plant Cell 2011, 23, 2155–2168. [Google Scholar] [CrossRef] [PubMed]
- Du, M.; Zhao, J.; Tzeng, D.T.W.; Liu, Y.; Deng, L.; Yang, T.; Zhai, Q.; Wu, F.; Huang, Z.; Zhou, M.; et al. MYC2 orchestrates a hierarchical transcriptional cascade that regulates jasmonate-mediated plant immunity in tomato. Plant Cell 2017, 29, 1883–1906. [Google Scholar] [CrossRef]
- Iglesias-Fernandez, R.; Barrero-Sicilia, C.; Carrillo-Barral, N.; Onate-Sanchez, L.; Carbonero, P. Arabidopsis thaliana bZIP44: A transcription factor affecting seed germination and expression of the mannanase-encoding gene AtMAN7. Plant J. 2013, 74, 767–780. [Google Scholar] [CrossRef]
- Tatematsu, K.; Nakabayashi, K.; Kamiya, Y.; Nambara, E. Transcription factor AtTCP14 regulates embryonic growth potential during seed germination in Arabidopsis thaliana. Plant J. 2008, 53, 42–52. [Google Scholar] [CrossRef]
- Oh, J.; Park, E.; Song, K.; Bae, G.; Choi, G. Phytochrome interacting factor8 inhibits phytochrome A-mediated far-red light responses in Arabidopsis. Plant Cell 2020, 32, 186–205. [Google Scholar] [CrossRef]
- Wang, H.; Wei, T.; Wang, X.; Zhang, L.; Yang, M.; Chen, L.; Song, W.; Wang, C.; Chen, C. Transcriptome analyses from mutant Salvia miltiorrhiza reveals important roles for SmGASA4 during plant development. Int. J. Mol. Sci. 2018, 19, 2088. [Google Scholar] [CrossRef] [PubMed]
- Jain, M.; Khurana, J.P. Transcript profiling reveals diverse roles of auxin-responsive genes during reproductive development and abiotic stress in rice. FEBS J. 2009, 276, 3148–3162. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, S.; Du, S.; Wang, G.; Zhang, J.; Jiang, J. Effects of exogenous (K+) potassium application on plant hormones in the roots of Tamarix ramosissima under NaCl stress. Genes 2022, 13, 1803. [Google Scholar] [CrossRef] [PubMed]
- Herzog, M.; Dorne, A.M.; Grellet, F. GASA, a gibberellin-regulated gene family from Arabidopsis thaliana related to the tomato GAST1 gene. Plant Mol. Biol. 1995, 27, 743–752. [Google Scholar] [CrossRef]
- Liscum, E.; Reed, J.W. Genetics of Aux/IAA and ARF action in plant growth and development. Plant Mol. Biol. 2002, 49, 387–400. [Google Scholar] [CrossRef]
- Hao, Y.; Hu, G.; Breitel, D.; Liu, M.; Mila, I.; Frasse, P.; Fu, Y.; Aharoni, A.; Bouzayen, M.; Zouine, M. Auxin response factor SlARF2 is an essential component of the regulatory mechanism controlling fruit ripening in tomato. PLoS Genet. 2015, 11, E1005649. [Google Scholar] [CrossRef]
- Veach, Y.K.; Martin, R.C.; Mok, D.W.S.; Malbeck, J.; Vankova, R.; Mok, M.C. O-glucosylation of cis-zeatin in maize. Characterization of genes, enzymes, and endogenous cytokinins. Plant Physiol. 2003, 131, 1374–1380. [Google Scholar] [CrossRef]
- Riefler, M.; Novak, O.; Strnad, M.; Schmuelling, T. Arabidopsis cytokinin receptor mutants reveal functions in shoot growth, leaf senescence, seed size, germination, root development, and cytokinin metabolism. Plant Cell 2006, 18, 40–54. [Google Scholar] [CrossRef]
- Smirnova, E.; Marquis, V.; Poirier, L.; Aubert, Y.; Zumsteg, J.; Menard, R.; Miesch, L.; Heitz, T. Jasmonic acid oxidase 2 hydroxylates jasmonic acid and represses basal defense and resistance responses against Botrytis cinerea infection. Mol. Plant 2017, 10, 1159–1173. [Google Scholar] [CrossRef] [PubMed]
- Chini, A.; Fonseca, S.; Fernandez, G.; Adie, B.; Chico, J.M.; Lorenzo, O.; Garcia-Casado, G.; Lopez-Vidriero, I.; Lozano, F.M.; Ponce, M.R.; et al. The JAZ family of repressors is the missing link in jasmonate signalling. Nature 2007, 448, 666–671. [Google Scholar] [CrossRef]
- Tian, X.; Wang, Z.; Li, X.; Lv, T.; Liu, H.; Wang, L.; Niu, H.; Bu, Q. Characterization and functional analysis of pyrabactin resistance-like abscisic acid receptor family in rice. Rice 2015, 8, 28. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, T.; Nishimura, N.; Kitahata, N.; Kuromori, T.; Ito, T.; Asami, T.; Shinozaki, K.; Hirayama, T. ABA-hypersensitive germination3 encodes a protein phosphatase 2C (AtPP2CA) that strongly regulates abscisic acid signaling during germination among Arabidopsis protein phosphatase 2Cs. Plant Physiol. 2006, 140, 115–126. [Google Scholar] [CrossRef] [PubMed]
- O’Malley, R.C.; Rodriguez, F.I.; Esch, J.J.; Binder, B.M.; O’Donnell, P.; Klee, H.J.; Bleecker, A.B. Ethylene-binding activity, gene expression levels, and receptor system output for ethylene receptor family members from Arabidopsis and tomato. Plant J. 2005, 41, 651–659. [Google Scholar] [CrossRef]
- Gaudinier, A.; Rodriguez-Medina, J.; Zhang, L.; Olson, A.; Liseron-Monfils, C.; Bagman, A.M.; Foret, J.; Abbitt, S.; Tang, M.; Li, B.; et al. Transcriptional regulation of nitrogen-associated metabolism and growth. Nature 2018, 563, 259–264. [Google Scholar] [CrossRef]
- Blumwald, E. Sodium transport and salt tolerance in plants. Curr. Opin. Cell Biol. 2000, 12, 431–434. [Google Scholar] [CrossRef]
- Park, H.J.; Kim, W.Y.; Yun, A.D.J. A New insight of salt stress signaling in plant. Mol. Cells 2016, 39, 447–459. [Google Scholar] [CrossRef]
- Ren, W.; Wang, Q.; Chen, L.; Ren, Y. Transcriptome and metabolome analyses of salt stress response in Cotton (Gossypium hirsutum) seed pretreated with NaCl. Agronomy 2022, 12, 1849. [Google Scholar] [CrossRef]
- Siddique, M.H.; Babar, N.I.; Zameer, R.; Muzammil, S.; Nahid, N.; Ijaz, U.; Masroor, A.; Nadeem, M.; Rashid, M.A.R.; Hashem, A.; et al. Genome-wide identification, genomic organization, and characterization of potassium transport-related genes in Cajanus cajan and their role in abiotic stress. Plants 2021, 10, 2238. [Google Scholar] [CrossRef]
- Chen, J.; Ran, Q.; Yang, Z.; Zhou, Y.; Yuan, Z.; Lai, H.; Wang, J.; Nie, G.; Zhu, Y. Genome-wide identification and expression profile of the HD-Zip transcription factor family associated with seed germination and abiotic stress response in Miscanthus sinensis. Genes 2022, 13, 2256. [Google Scholar] [CrossRef]
- Hou, C.; Tian, W.; Kleist, T.; He, K.; Garcia, V.; Bai, F.; Hao, Y.; Luan, S.; Li, L. DUF221 proteins are a family of osmosensitive calcium-permeable cation channels conserved across eukaryotes. Cell Res. 2014, 24, 632–635. [Google Scholar] [CrossRef] [PubMed]
- Jojoa-Cruz, S.; Saotome, K.; Murthy, S.E.; Tsui, C.C.A.; Sansom, M.S.; Patapoutian, A.; Ward, A.B. Cryo-EM structure of the mechanically activated ion channel OSCA1.2. Elife 2018, 7, e41845. [Google Scholar] [CrossRef] [PubMed]
- Kushwaha, H.R.; Singh, A.K.; Sopory, S.K.; Singla-Pareek, S.L.; Pareek, A. Genome wide expression analysis of CBS domain containing proteins in Arabidopsis thaliana (L.) Heynh and Oryza sativa L. reveals their developmental and stress regulation. BMC Genom. 2009, 10, 200. [Google Scholar] [CrossRef]
- Fujimoto, S.Y.; Ohta, M.; Usui, A.; Shinshi, H.; Ohme-Takagi, M. Arabidopsis ethylene responsive element binding factors act as transcriptional activators or repressors of GCC box mediated gene expression. Plant Cell 2000, 12, 393–404. [Google Scholar] [PubMed]
- Aranda-Sicilia, M.N.; Cagnac, O.; Chanroj, S.; Sze, H.; Rodriguez-Rosales, M.P.; Venema, K. Arabidopsis KEA2, a homolog of bacterial KefC, encodes a K(+)/H(+) antiporter with a chloroplast transit peptide. Biochim. Biophys. Acta 2012, 1818, 2362–2371. [Google Scholar] [CrossRef]
- Ni, D.Q.; Zook, J.; Klewer, D.A.; Nieman, R.A.; Soll, J.; Fromme, P. Isolation, folding and structural investigations of the amino acid transporter OEP16. Protein Expr. Purif. 2011, 80, 157–168. [Google Scholar] [CrossRef] [PubMed]
- Viotti, C.; Luoni, L.; Morandini, P.; De Michelis, M.I. Characterization of the interaction between the plasma membrane H-ATPase of Arabidopsis thaliana and a novel interactor (PPI1). FEBS J. 2005, 272, 5864–5871. [Google Scholar] [CrossRef]
- Yokoi, S.; Quintero, F.J.; Cubero, B.; Ruiz, M.T.; Bressan, R.A.; Hasegawa, P.M.; Pardo, J.M. Differential expression and function of Arabidopsis thaliana NHX Na(+)/H(+) antiporters in the salt stress response. Plant J. 2002, 30, 529–539. [Google Scholar] [CrossRef]
- Cheng, N.H.; Pittman, J.K.; Barkla, B.J.; Shigaki, T.; Hirschi, K.D. The Arabidopsis cax1 mutant exhibits impaired ion homeostasis, development, and hormonal responses and reveals interplay among vacuolar transporters. Plant Cell 2003, 15, 347–364. [Google Scholar] [CrossRef]
- Krebs, M.; Beyhl, D.; Goerlich, E.; Al-Rasheid, K.A.S.; Marten, I.; Stierhof, Y.D.; Hedrich, R.; Schumacher, K. Arabidopsis V-ATPase activity at the tonoplast is required for efficient nutrient storage but not for sodium accumulation. Proc. Natl. Acad. Sci. USA 2010, 107, 3251–3256. [Google Scholar] [CrossRef]
- Grotz, N.; Fox, T.; Connolly, E.; Park, W.; Guerinot, M.L.; Eide, D. Identification of a family of zinc transporter genes from Arabidopsis that respond to zinc deficiency. Proc. Natl. Acad. Sci. USA 1998, 95, 7220–7224. [Google Scholar] [CrossRef] [PubMed]
- Stewart, R.R.; Bewley, J.D. Lipid peroxidation associated with accelerated aging of soybean axes. Plant Physiol. 1980, 65, 245–248. [Google Scholar] [CrossRef]
- Hammerschmidt, R.; Nuckles, E.M.; Kuc, J. Association of enhanced peroxidase activity with induced systemic resistance of cucumber to Colletotrichum lagenarium. Physiol. Plant Pathol. 1982, 20, 73–82. [Google Scholar] [CrossRef]
- Chen, X.M. Comparison of three different methods of determining catalase activities in cut flowers. Chin. J. Trop. Agr. 2002, 22, 13–16. [Google Scholar]
- Shen, W.B.; Xu, L.L.; Ye, M.B.; Zhang, R.X. Study on determination of ASP activity. Plant Physiol. Comm. 1996, 32, 203–205. [Google Scholar]
- Dubois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F. Colorimetric method for determination of sugars and related substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Li, H.S. Principles and Techniques of Plant Physiological Biochemical Experimental; High Education Press: Beijing, China, 2000; pp. 260–261. [Google Scholar]
- Shi, H.T. Experimental Guidance of Plant Stress Physiology; Science Press: Beijing, China, 2016; pp. 14–15. [Google Scholar]
- Li, M.F.; Sun, P.; Kang, T.L.; Xing, H.; Yang, D.L.; Zhang, J.L.; Paré, P.W. Mapping podophyllotoxin biosynthesis and growth related transcripts with high elevation in Sinopodophyllum hexandrum. Ind. Crops Prod. 2018, 124, 510–518. [Google Scholar] [CrossRef]
- Chen, S.; Zhou, Y.; Chen, Y.; Gu, J. fastp: An ultra-fast all-in-one FASTQ preprocessor. Bioinformatics 2018, 34, i884–i890. [Google Scholar] [CrossRef] [PubMed]
- Grabherr, M.G.; Haas, B.J.; Yassour, M.; Levin, J.Z.; Thompson, D.A.; Amit, I.; Adiconis, X.; Fan, L.; Raychowdhury, R.; Zeng, Q.D.; et al. Full-length transcriptome assembly from RNA-Seq data without a reference genome. Nat. Biotechnol. 2011, 29, 644–652. [Google Scholar] [CrossRef] [PubMed]
- Love, M.I.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for RNAseq data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef]
- Conesa, A.; Gotz, S.; Garcia-Gomez, J.M.; Terol, J.; Talon, M.; Robles, M. Blast2GO: A universal tool for annotation, visualization and analysis in functional genomics research. Bioinformatics 2005, 21, 3674–3676. [Google Scholar] [CrossRef] [PubMed]
- Willems, E.; Leyns, L.; Vandesompele, J. Standardization of real-time PCR gene expression data from independent biological replicates. Anal. Biochem. 2008, 379, 127–129. [Google Scholar] [CrossRef]


| CK | 300 mmol/L NaCl | |
|---|---|---|
| Filtered data | ||
| Data of reads number (million) | 51.43 ± 12.23 | 65.36 ± 26.19 |
| Data of reads number×read length (million) | 7714 ± 1835 | 9803 ± 3928 |
| Q20 (%) | 97.18 ± 0.28 | 96.99 ± 0.04 |
| Q30 (%) | 92.33 ± 0.56 | 91.93 ± 0.08 |
| Mapped data | ||
| Data of unique mapped reads (million) | 42.12 ± 10.02 | 53.26 ± 21.33 |
| Data of multiple mapped reads (million) | 0.81± 0.11 | 0.80 ± 0.33 |
| Mapping ratio (%) | 83.47 ± 3.08 | 82.71 ± 4.12 |
| Gene Name | SwissProt ID | Protein Name | log2FC (NaCl vs. CK) |
|---|---|---|---|
| ALDH7B4 | Q9SYG7 | Aldehyde dehydrogenase family 7 member B4 | 4.13 |
| ALDH10A8 | Q9S795 | Aminoaldehyde dehydrogenase ALDH10A8 | 3.91 |
| ANN2 | Q9XEE2 | Annexin D2 | 4.33 |
| ANN5 | Q9C9X3 | Annexin D5 | 4.51 |
| B2 | P37707 | B2 protein | 4.38 |
| RD22 | Q08298 | BURP domain protein RD22 | 4.60 |
| CAMBP25 | O80683 | Calmodulin-binding protein 25 | 4.19 |
| CTL1 | Q9MA41 | Chitinase-like protein 1 | 3.03 |
| RD19A | P43296 | Cysteine protease RD19A | 3.95 |
| PCC13 | P22242 | Desiccation-related protein PCC13-62 | −2.53 |
| PCC27 | P22241 | Desiccation-related protein PCC27-45 | 3.94 |
| FLZ13 | Q8GRN0 | FCS-Like Zinc finger 13 | −2.97 |
| GRP1 | Q03878 | Glycine-rich RNA-binding protein | 5.23 |
| RBG7 | Q03250 | Glycine-rich RNA-binding protein 7 | 3.30 |
| HMGB2 | O49596 | High mobility group B protein 2 | 3.61 |
| IQM4 | O64851 | IQ domain-containing protein IQM4 | 8.89 |
| NRP1 | Q9ZQ80 | Nodulin-related protein 1 | 3.65 |
| ATP1 | Q9LU63 | Probable pterin-4-alpha-carbinolamine dehydratase | 4.80 |
| ARP1 | Q9M1S3 | Probable RNA-binding protein ARP1 | −1.09 |
| ERD7 | O48832 | Protein EARLY-RESPONSIVE TO DEHYDRATION 7 | 8.98 |
| NFD4 | F4I9E1 | Protein NUCLEAR FUSION DEFECTIVE 4 | 4.68 |
| AVP1 | P31414 | Pyrophosphate-energized vacuolar membrane proton pump 1 | 3.98 |
| REM4.1 | Q93YN8 | Remorin 4.1 | 1.16 |
| ALDH5F1 | Q9SAK4 | Succinate-semialdehyde dehydrogenase | 4.24 |
| TIL | Q9FGT8 | Temperature-induced lipocalin-1 | 4.08 |
| WSD1 | Q93ZR6 | Wax ester synthase/diacylglycerol acyltransferase 1 | −1.11 |
| At2g40140 | Q9XEE6 | Zinc finger CCCH domain-containing protein 29 | 1.30 |
| Os07g0682400 | Q0D3J9 | Zinc finger CCCH domain-containing protein 53 | 3.70 |
| ZFNL | Q9SWF9 | Zinc finger CCCH domain-containing protein ZFN-like | 5.48 |
| Gene Name | SwissProt ID | Protein Name | log2FC (NaCl vs. CK) |
|---|---|---|---|
| SOD (3) | |||
| SODCP | P07505 | Superoxide dismutase (Cu-Zn), chloroplastic | 3.98 |
| FSD2 | Q9LU64 | Superoxide dismutase (Fe) 2, chloroplastic | 3.62 |
| SODA | P11796 | Superoxide dismutase (Mn), mitochondrial | 8.98 |
| POD (17) | |||
| At5g06290 | Q9C5R8 | 2-Cys peroxiredoxin BAS1-like, chloroplastic | 3.39 |
| APX3 | Q42564 | L-ascorbate peroxidase 3 | 3.31 |
| APX1 | P48534 | L-ascorbate peroxidase, cytosolic | 1.08 |
| PER12 | Q96520 | Peroxidase 12 | 5.22 |
| PER23 | O80912 | Peroxidase 23 | −3.11 |
| PER31 | Q9LHA7 | Peroxidase 31 | 5.22 |
| PER42 | Q9SB81 | Peroxidase 42 | 3.83 |
| PER52 | Q9FLC0 | Peroxidase 52 | 1.52 |
| poxN1 | Q9XIV8 | Peroxidase N1 | −3.93 |
| PRDX1 | Q06830 | Peroxiredoxin-1 | 9.68 |
| PRDX2 | A9PCL4 | Peroxiredoxin-2 | 3.52 |
| PRXIIE | Q949U7 | Peroxiredoxin-2E, chloroplastic | 6.02 |
| PEX5 | Q9FMA3 | Peroxisome biogenesis protein 5 | 3.81 |
| PEX11C | Q9LQ73 | Peroxisomal membrane protein 11C | 4.38 |
| PEX13 | Q9SRR0 | Peroxisomal membrane protein 13 | 8.99 |
| PEX14 | Q9FXT6 | Peroxisomal membrane protein PEX14 | 4.29 |
| GPX1 | P52032 | Phospholipid hydroperoxide glutathione peroxidase 1 | 3.70 |
| CAT (3) | |||
| CATA | Q9AXH0 | Catalase | 3.80 |
| CAT1 | P17598 | Catalase isozyme 1 | 3.78 |
| PNC1 | P22195 | Cationic peroxidase 1 | 3.52 |
| Gene Name | SwissProt ID | Protein Name | log2FC (NaCl vs. CK) |
|---|---|---|---|
| Glucose (11) | |||
| G6pc2 | Q9Z186 | Glucose-6-phosphatase 2 | 9.28 |
| G6PDC | Q43839 | Glucose-6-phosphate 1-dehydrogenase, chloroplastic | 3.29 |
| GAPB | P12859 | Glyceraldehyde-3-phosphate dehydrogenase B | 3.73 |
| GAPA1 | P25856 | Glyceraldehyde-3-phosphate dehydrogenase GAPA1 | 3.85 |
| GAPC2 | Q9FX54 | Glyceraldehyde-3-phosphate dehydrogenase GAPC2 | 3.65 |
| PGMP | Q9M4G5 | Phosphoglucomutase, chloroplastic | 3.47 |
| PGM1 | Q9ZSQ4 | Phosphoglucomutase, cytoplasmic | 3.20 |
| Gcg | P55095 | Pro-glucagon | 12.87 |
| PSL5 | Q9FN05 | Probable glucan 1,3-alpha-glucosidase | 3.31 |
| UGD3 | Q9AUV6 | UDP-glucose 6-dehydrogenase 3 | 3.50 |
| UGPA | P19595 | UTP--glucose-1-phosphate uridylyltransferase | 4.88 |
| Sucrose (3) | |||
| INVE | Q9FK88 | Alkaline/neutral invertase E, chloroplastic | 8.90 |
| SUS2 | O24301 | Sucrose synthase 2 | 3.82 |
| SUS3 | Q9M111 | Sucrose synthase 3 | −3.29 |
| Fructose (9) | |||
| FKFBP | Q9MB58 | 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase | 3.50 |
| RHVI2 | H2DF88 | Acid beta-fructofuranosidase 2, vacuolar | −1.32 |
| FRK2 | Q42896 | Fructokinase-2 | 5.37 |
| FBP | P46275 | Fructose-1,6-bisphosphatase, chloroplastic | 3.26 |
| F16P2 | P46276 | Fructose-1,6-bisphosphatase, cytosolic | 4.07 |
| FBA2 | Q944G9 | Fructose-bisphosphate aldolase 2, chloroplastic | 3.49 |
| FBA3 | Q9ZU52 | Fructose-bisphosphate aldolase 3, chloroplastic | 1.93 |
| FBA6 | Q9SJQ9 | Fructose-bisphosphate aldolase 6, cytosolic | 1.31 |
| FBA1 | P46256 | Fructose-bisphosphate aldolase, cytoplasmic isozyme 1 | 4.00 |
| Galactose (2) | |||
| GOLS1 | O22893 | Galactinol synthase 1 | 8.16 |
| GOLS2 | C7G304 | Galactinol synthase 2 | 8.35 |
| Trehalose (2) | |||
| TPS6 | Q94AH8 | Alpha,alpha-trehalose-phosphate synthase | 4.20 |
| TPS7 | Q9LMI0 | Probable alpha,alpha-trehalose-phosphate synthase | 3.11 |
| Fucose (2) | |||
| OFUT19 | Q9SH89 | O-fucosyltransferase 19 | 1.08 |
| OFUT39 | Q0WUZ5 | O-fucosyltransferase 39 | 3.78 |
| Starch (12) | |||
| SBEI | Q41058 | 1,4-alpha-glucan-branching enzyme 1 | 3.43 |
| DPE2 | Q8RXD9 | 4-alpha-glucanotransferase DPE2 | 4.63 |
| AMY3 | Q94A41 | Alpha-amylase 3, chloroplastic | 3.49 |
| R1 | Q8LPT9 | Alpha-glucan water dikinase, chloroplastic | 3.37 |
| BAM1 | Q9LIR6 | Beta-amylase 1, chloroplastic | 4.43 |
| BAM3 | O23553 | Beta-amylase 3, chloroplastic | 4.61 |
| ADG2 | P55229 | Glucose-1-phosphate adenylyltransferase large subunit 1 | 3.43 |
| AGPS1 | Q9M462 | Glucose-1-phosphate adenylyltransferase small subunit | 2.91 |
| ISA1 | D0TZF0 | Isoamylase 1, chloroplastic | 5.35 |
| DSP4 | G4LTX4 | Phosphoglucan phosphatase DSP4, amyloplastic | 3.88 |
| GWD3 | Q6ZY51 | Phosphoglucan, water dikinase, chloroplastic | 4.48 |
| SS4 | Q0WVX5 | Probable starch synthase 4, chloroplastic/amyloplastic | 5.33 |
| Protein (38) | |||
| PSMD2 | Q5R9I6 | 26S proteasome non-ATPase regulatory subunit 2 | 4.39 |
| RPN1A | Q9SIV2 | 26S proteasome non-ATPase regulatory subunit 2 homolog A | 3.51 |
| RPN9B | Q8GYA6 | 26S proteasome non-ATPase regulatory subunit 13 homolog B | 8.52 |
| RPN10 | P55034 | 26S proteasome non-ATPase regulatory subunit 4 homolog | 4.94 |
| RPT5A | Q9SEI2 | 26S proteasome regulatory subunit 6A homolog A | 3.39 |
| RPT1A | P0DKJ9 | 26S proteasome regulatory subunit 7A | 5.17 |
| RPT6A | Q9C5U3 | 26S proteasome regulatory subunit 8 homolog A | 4.29 |
| RPT4A | Q9SEI3 | 26S proteasome regulatory subunit 10B homolog A | 8.74 |
| PCS1 | Q9LZL3 | Aspartic proteinase PCS1 | 3.89 |
| APF2 | Q9LNJ3 | Aspartyl protease family protein 2 | 8.63 |
| At5g10770 | Q8S9J6 | Aspartyl protease family protein At5g10770 | 4.31 |
| Cys | Q86GF7 | Crustapain | −10.16 |
| SMAC_06893 | D1ZSU8 | Extracellular metalloprotease SMAC_06893 | −6.78 |
| GGP5 | O82225 | Gamma-glutamyl peptidase 5 | 4.19 |
| LAP2 | Q944P7 | Leucine aminopeptidase 2, chloroplastic | 3.76 |
| Pcsk2 | P21661 | Neuroendocrine convertase 2 | 5.62 |
| maoI | Q07121 | Primary amine oxidase | 3.53 |
| RD19C | Q9SUL1 | Probable cysteine protease RD19C | 5.48 |
| RD21B | Q9FMH8 | Probable cysteine protease RD21B | 2.33 |
| MPPbeta | Q42290 | Probable mitochondrial-processing peptidase subunit beta | 6.03 |
| Prep | Q9QUR6 | Prolyl endopeptidase | −2.29 |
| DEGP1 | O22609 | Protease Do-like 1, chloroplastic | 5.36 |
| DEGP2 | O82261 | Protease Do-like 2, chloroplastic | 3.67 |
| PBG1 | Q7DLR9 | Proteasome subunit beta type-4 | 8.91 |
| PBE2 | Q9LIP2 | Proteasome subunit beta type-5-B | 4.84 |
| PBA1 | Q8LD27 | Proteasome subunit beta type-6 | 4.68 |
| MPA1 | Q8H0S9 | Puromycin-sensitive aminopeptidase | 3.52 |
| Rbp3 | P49194 | Retinol-binding protein 3 | −7.96 |
| RBL6 | Q8VZ48 | RHOMBOID-like protein 6, mitochondrial | 5.28 |
| SCPL49 | P32826 | Serine carboxypeptidase-like 49 | 4.84 |
| SBT1.2 | O64495 | Subtilisin-like protease SBT1.2 | −1.73 |
| SBT1.4 | Q9LVJ1 | Subtilisin-like protease SBT1.4 | 8.46 |
| SBT1.6 | O49607 | Subtilisin-like protease SBT1.6 | 3.21 |
| SBT1.7 | O65351 | Subtilisin-like protease SBT1.7 | 0.77 |
| SBT2.5 | O64481 | Subtilisin-like protease SBT2.5 | 4.43 |
| SPDS1 | Q96556 | Spermidine synthase 1 | 5.04 |
| ALEU | Q8H166 | Thiol protease aleurain | 3.66 |
| TPP2 | F4JVN6 | Tripeptidyl-peptidase 2 | 4.06 |
| Gene Name | SwissProt ID | Protein Name | log2FC (NaCl vs. CK) |
|---|---|---|---|
| F16 | Q8W4Z5 | CASP-like protein F16 | 9.63 |
| FIP1 | Q9SE96 | GEM-like protein 1 | 5.00 |
| At5g13200 | Q9LYV6 | GEM-like protein 5 | 7.09 |
| At5g23350 | Q9FMW6 | GEM-like protein 6 | −1.28 |
| KAI2 | Q9SZU7 | Probable esterase KAI2 | 9.61 |
| ROH1 | Q9CAK4 | Protein ROH1 | 3.61 |
| Gene Name | SwissProt ID | Protein Name | log2FC (NaCl vs. CK) |
|---|---|---|---|
| MYB (2) | |||
| MYB73 | O23160 | Transcription factor MYB73 | 5.75 |
| MYB1R1 | Q2V9B0 | Transcription factor MYB1R1 | 3.32 |
| WRKY (7) | |||
| WRKY4 | Q9XI90 | Probable WRKY transcription factor 4 | 4.19 |
| WRKY23 | O22900 | WRKY transcription factor 23 | 1.15 |
| WRKY24 | Q6IEQ7 | WRKY transcription factor WRKY24 | 5.42 |
| WRKY33 | Q8S8P5 | Probable WRKY transcription factor 33 | 4.71 |
| WRKY40 | Q9SAH7 | Probable WRKY transcription factor 40 | −1.20 |
| WRKY49 | Q9FHR7 | Probable WRKY transcription factor 49 | 1.48 |
| WRKY71 | Q93WV4 | WRKY transcription factor 71 | 2.01 |
| NAC (4) | |||
| NAC083 | Q9FY93 | NAC domain-containing protein 83 | 3.76 |
| NAC091 | Q9LKG8 | NAC domain-containing protein 91 | 5.15 |
| NAC100 | Q9FLJ2 | NAC domain-containing protein 100 | 1.34 |
| JA2L | A0A3Q7HH64 | NAC domain-containing protein JA2L | 1.64 |
| bZIP (1) | |||
| BZIP44 | C0Z2L5 | bZIP transcription factor 44 | 5.39 |
| TCP (1) | |||
| TCP14 | Q93Z00 | Transcription factor TCP14 | 3.78 |
| UNE (1) | |||
| UNE10 | Q8GZ38 | Transcription factor UNE10 | 4.16 |
| Gene Name | SwissProt ID | Protein Name | log2FC (NaCl vs. CK) |
|---|---|---|---|
| GA (1) | |||
| GASA2 | P46688 | Gibberellin-regulated protein 2 | 4.35 |
| IAA (14) | |||
| ABP19A | Q9ZRA4 | Auxin-binding protein ABP19a | 3.50 |
| AUX22D | O24542 | Auxin-induced protein 22D | 3.32 |
| AUX12KD | Q05349 | Auxin-repressed 12.5 kDa protein | 3.57 |
| ARF2A | Q2LAJ3 | Auxin response factor 2A | −2.28 |
| ARF2B | K4DF01 | Auxin response factor 2B | 3.98 |
| ARF6 | Q9ZTX8 | Auxin response factor 6 | 4.48 |
| IAA4 | P33077 | Auxin-responsive protein IAA4 | 3.62 |
| IAA8 | Q38826 | Auxin-responsive protein IAA8 | 4.07 |
| IAA9 | Q38827 | Auxin-responsive protein IAA9 | 4.24 |
| IAA14 | Q38832 | Auxin-responsive protein IAA14 | 3.85 |
| SAUR71 | Q9SGU2 | Auxin-responsive protein SAUR71 | −1.28 |
| LAX2 | Q9FEL7 | Auxin transporter-like protein 2 | 2.89 |
| TET4 | Q9LSS4 | Tetraspanin-4 | 4.97 |
| TET8 | Q8S8Q6 | Tetraspanin-8 | 3.93 |
| CTK (3) | |||
| CISZOG2 | Q8RXA5 | Cis-zeatin O-glucosyltransferase 2 | −1.09 |
| AHK3 | Q9C5U1 | Histidine kinase 3 | 4.69 |
| ARR4 | O82798 | Two-component response regulator ARR4 | 1.09 |
| JA (2) | |||
| JOX2 | Q9FFF6 | Jasmonate-induced oxygenase 2 | 2.21 |
| TIFY6B | Q9LVI4 | Protein TIFY 6B | 3.61 |
| ABA (6) | |||
| PYL3 | Q6EN42 | Abscisic acid receptor PYL3 | 3.73 |
| PYL4 | O80920 | Abscisic acid receptor PYL4 | −1.14 |
| GRDP1 | Q9ZQ47 | Glycine-rich domain-containing protein 1 | 3.49 |
| PP2CA | P49598 | Protein phosphatase 2C 37 | 5.90 |
| PP2C51 | Q65XK7 | Protein phosphatase 2C 51 | 1.43 |
| SAL1 | Q42546 | SAL1 phosphatase | 3.31 |
| ETH (10) | |||
| RAV1 | Q9ZWM9 | AP2/ERF and B3 domain-containing transcription factor | 3.47 |
| ETR2 | Q0WPQ2 | Ethylene receptor 2 | −2.03 |
| ERF4 | Q9LW49 | Ethylene-responsive transcription factor 4 | 9.00 |
| ERF5 | Q40478 | Ethylene-responsive transcription factor 5 | 0.83 |
| ERF013 | Q9CAP4 | Ethylene-responsive transcription factor ERF013 | −2.74 |
| ERF016 | Q9C591 | Ethylene-responsive transcription factor ERF016 | 2.11 |
| ERF113 | Q9LYU3 | Ethylene-responsive transcription factor ERF113 | 1.95 |
| RAP2-2 | Q9LUM4 | Ethylene-responsive transcription factor RAP2-2 | 4.50 |
| RAP2-4 | Q8H1E4 | Ethylene-responsive transcription factor RAP2-4 | 3.65 |
| EIN3 | O24606 | Protein ETHYLENE INSENSITIVE 3 | 4.05 |
| Gene Name | SwissProt ID | Protein Name | log2FC (NaCl vs. CK) |
|---|---|---|---|
| AHA10 | Q43128 | ATPase 10, plasma membrane-type | 3.98 |
| CSC1 | Q5XEZ5 | Calcium permeable stress-gated cation channel 1 | 4.65 |
| CLC-B | P92942 | Chloride channel protein CLC-b | 4.68 |
| CLC-C | Q96282 | Chloride channel protein CLC-c | 3.91 |
| ATX1 | Q94BT9 | Copper transport protein ATX1 | 6.95 |
| PAA2 | B9DFX7 | Copper-transporting ATPase PAA2, chloroplastic | 8.69 |
| RAN1 | Q9S7J8 | Copper-transporting ATPase RAN1 | 4.28 |
| ERD4 | A9LIW2 | CSC1-like protein ERD4 | 3.81 |
| KEA2 | O65272 | K(+) efflux antiporter 2, chloroplastic | 5.20 |
| MOT1 | Q9SL95 | Molybdate transporter 1 | −2.18 |
| NEW1 | Q08972 | [NU+] prion formation protein 1 | −9.03 |
| OEP16 | Q41050 | Outer envelope pore protein 16, chloroplastic | 3.58 |
| OEP162 | Q0WMZ5 | Outer envelope pore protein 16-2, chloroplastic | 1.59 |
| PHO1-H1 | Q93ZF5 | Phosphate transporter PHO1 homolog 1 | −1.21 |
| PPI1 | O23144 | Proton pump-interactor 1 | 3.54 |
| Atp2a3 | Q64518 | Sarcoplasmic/endoplasmic reticulum calcium ATPase 3 | 9.37 |
| NHX2 | Q56XP4 | Sodium/hydrogen exchanger 2 | 3.07 |
| ATP1A3 | P13637 | Sodium/potassium-transporting ATPase subunit alpha-3 | −9.32 |
| CAX1 | Q39253 | Vacuolar cation/proton exchanger 1 | 2.30 |
| CAX3 | Q93Z81 | Vacuolar cation/proton exchanger 3 | 3.94 |
| VPS2.1 | Q9SKI2 | Vacuolar protein sorting-associated protein 2 homolog 1 | 4.27 |
| VPE | P49043 | Vacuolar-processing enzyme | 1.64 |
| VSR1 | P93026 | Vacuolar-sorting receptor 1 | 4.04 |
| VATL | Q96473 | V-type proton ATPase 16 kDa proteolipid subunit | 4.97 |
| VHA-a2 | Q9SJT7 | V-type proton ATPase subunit a2 | 4.06 |
| VATB1 | Q43432 | V-type proton ATPase subunit B 1 | 3.65 |
| VHA-C | Q9SDS7 | V-type proton ATPase subunit C | 5.36 |
| VHA-D | Q9XGM1 | V-type proton ATPase subunit D | 4.70 |
| VHA-d2 | Q9LHA4 | V-type proton ATPase subunit d2 | 4.43 |
| VATE | Q9SWE7 | V-type proton ATPase subunit E | 4.41 |
| VHA-H | Q9LX65 | V-type proton ATPase subunit H | 4.04 |
| ZIP1 | O81123 | Zinc transporter 1 | −1.58 |
| ZIP4 | O04089 | Zinc transporter 4, chloroplastic | −1.06 |
| ZIP5 | Q6L8G0 | Zinc transporter 5 | −2.14 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, X.; Li, J.; Su, H.; Sun, P.; Zhang, Z.; Li, M.; Xing, H. Physiological and Transcriptional Responses of Apocynum venetum to Salt Stress at the Seed Germination Stage. Int. J. Mol. Sci. 2023, 24, 3623. https://doi.org/10.3390/ijms24043623
Li X, Li J, Su H, Sun P, Zhang Z, Li M, Xing H. Physiological and Transcriptional Responses of Apocynum venetum to Salt Stress at the Seed Germination Stage. International Journal of Molecular Sciences. 2023; 24(4):3623. https://doi.org/10.3390/ijms24043623
Chicago/Turabian StyleLi, Xin, Jinjuan Li, Hongyan Su, Ping Sun, Zhen Zhang, Mengfei Li, and Hua Xing. 2023. "Physiological and Transcriptional Responses of Apocynum venetum to Salt Stress at the Seed Germination Stage" International Journal of Molecular Sciences 24, no. 4: 3623. https://doi.org/10.3390/ijms24043623
APA StyleLi, X., Li, J., Su, H., Sun, P., Zhang, Z., Li, M., & Xing, H. (2023). Physiological and Transcriptional Responses of Apocynum venetum to Salt Stress at the Seed Germination Stage. International Journal of Molecular Sciences, 24(4), 3623. https://doi.org/10.3390/ijms24043623







