Characteristics of a Rollable Dielectric Barrier Discharge Plasma and Its Effects on Spinach-Seed Germination
Abstract
:1. Introduction
2. Results and Discussions
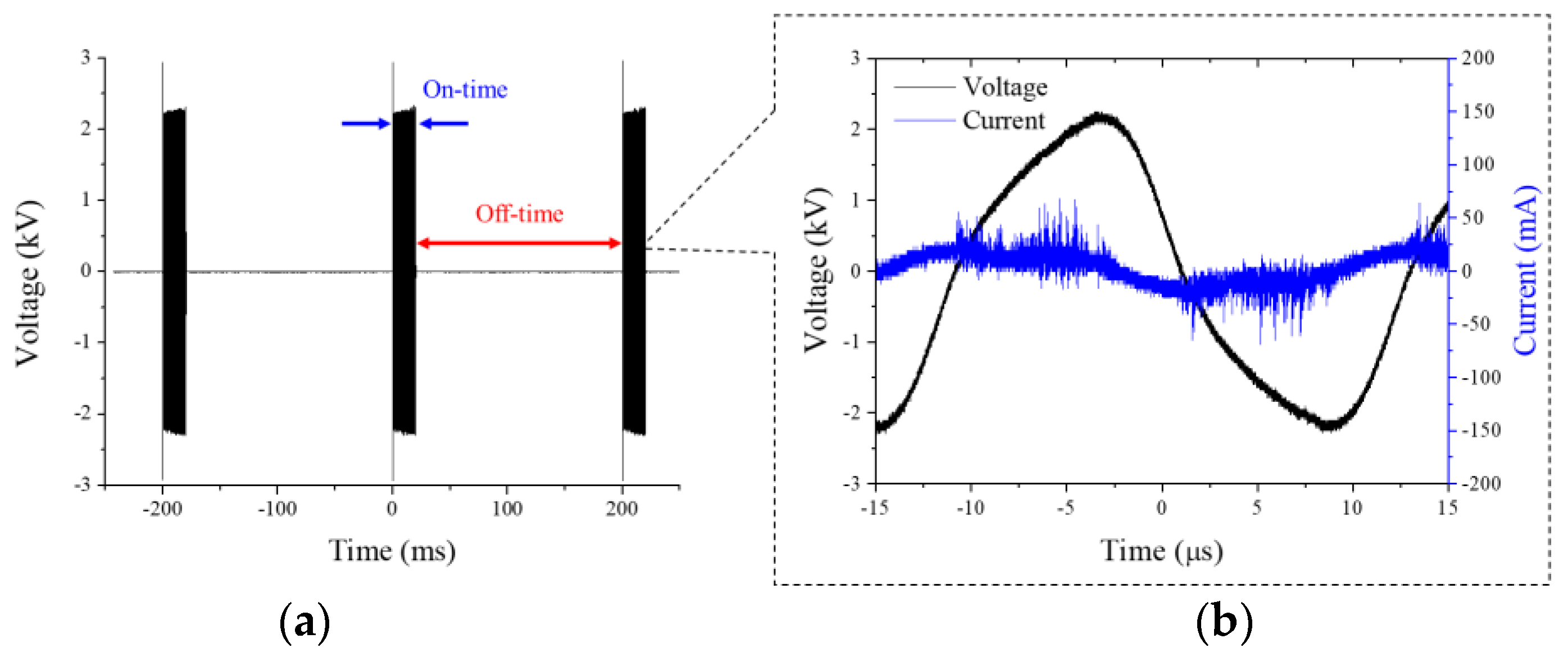
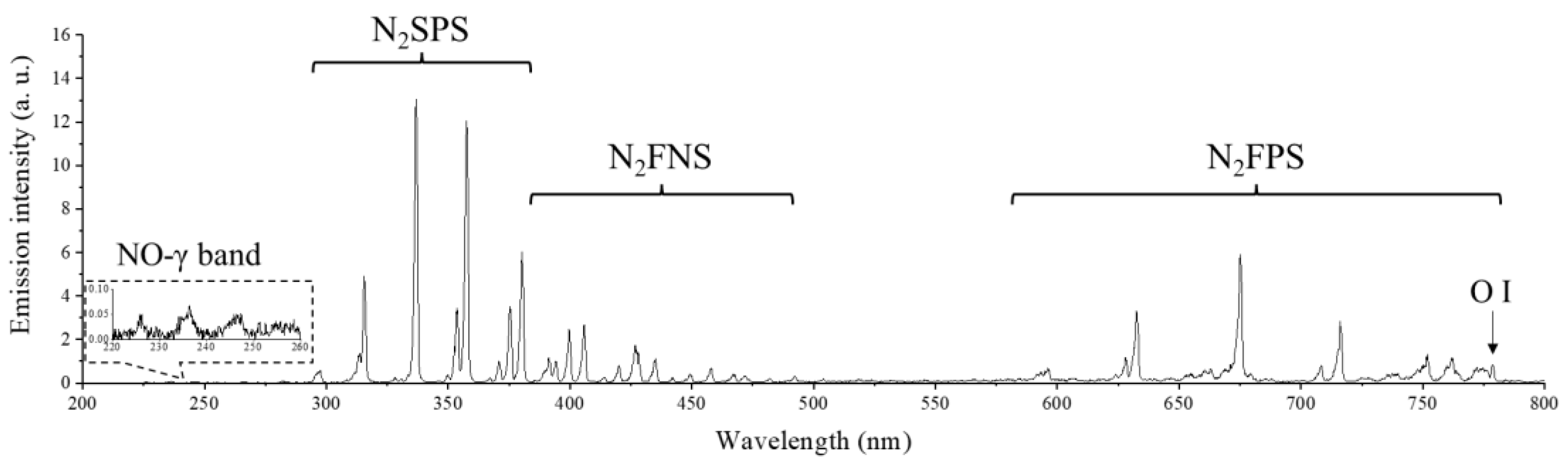
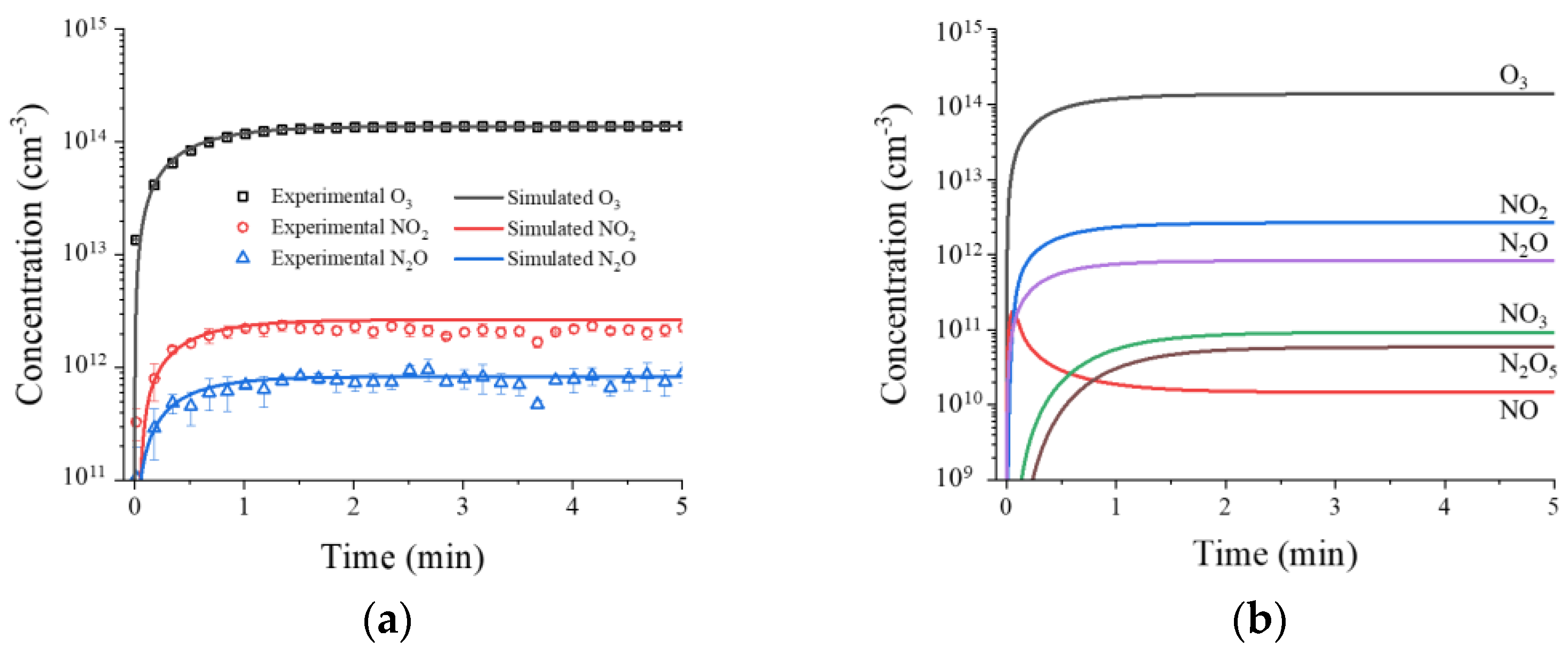
2.1. Physical and Chemical Properties of RDBD
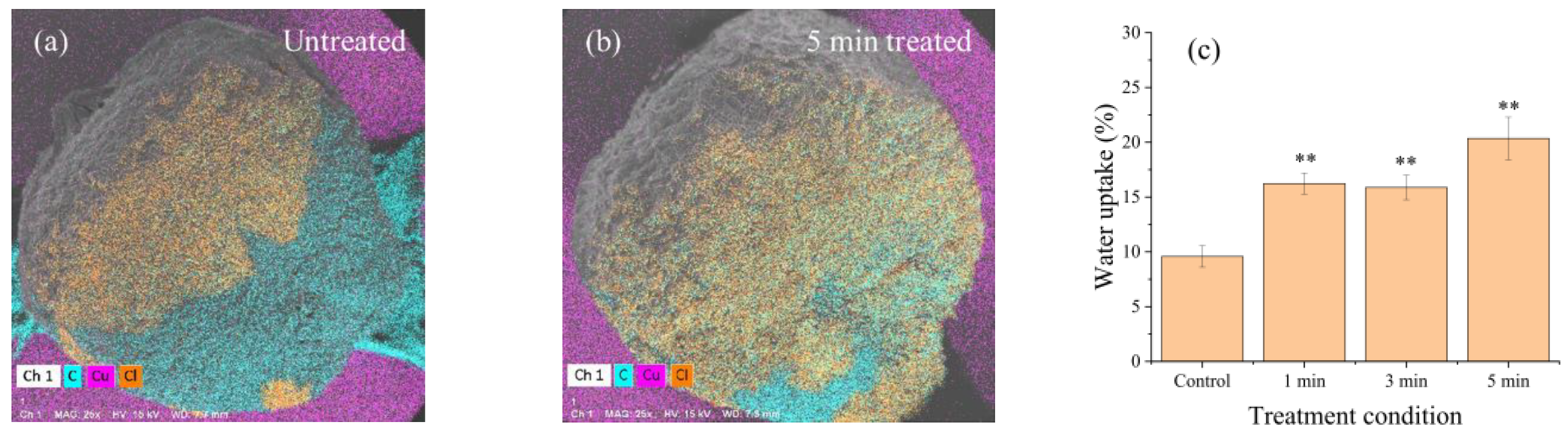
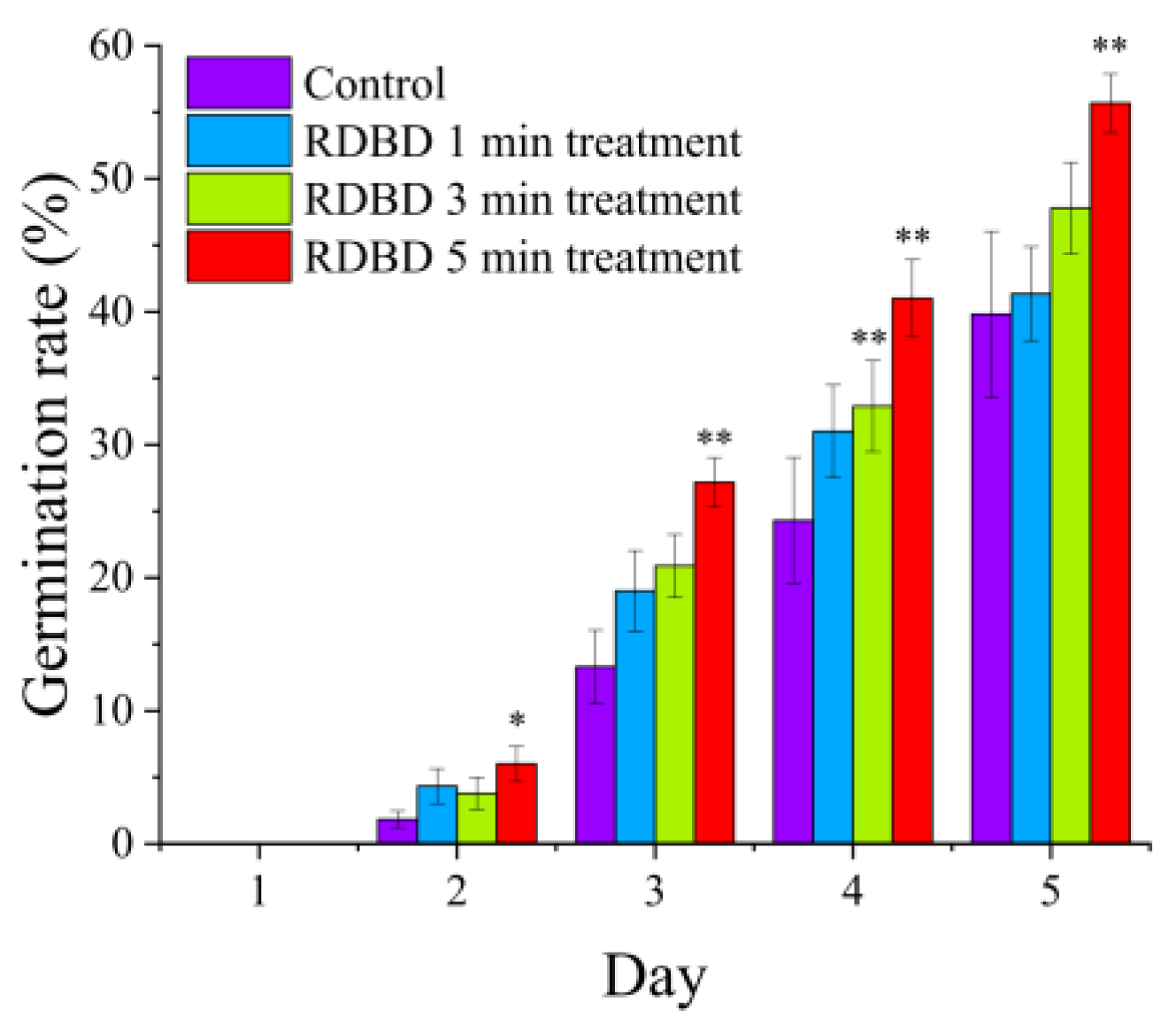
2.2. Improvement of Water Uptake and Germination Rates in Seeds Using RDBD
3. Materials and Methods
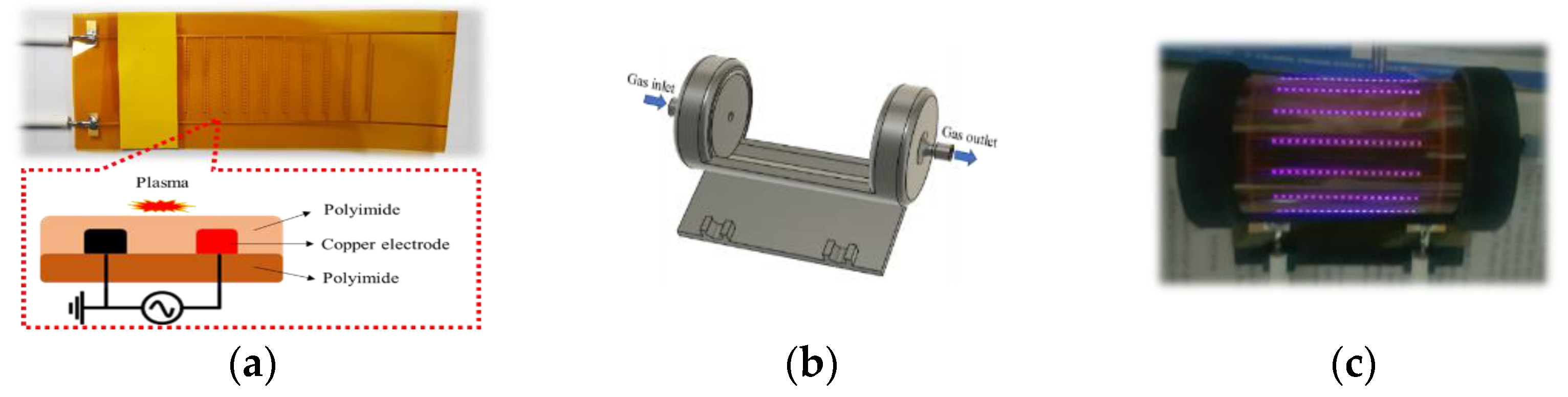
3.1. Rollable DBD Source
3.2. Electrical and Optical Properties of Rollable DBD
3.3. Chemical Species Measurement in FTIR Spectroscopy
3.4. Zero-Dimension (0D) Chemical Species Simulation
3.5. Spinach Seed Treatment and Germination
3.6. Measurement of Water Uptake of Seeds
3.7. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Choi, E.H.; Kaushik, N.K.; Hong, Y.J.; Lim, J.S.; Choi, J.S.; Han, I. Plasma Bioscience for Medicine, Agriculture and Hygiene Applications. J. Korean Phys. Soc. 2022, 80, 817–851. [Google Scholar] [CrossRef]
- Keidar, M. Plasma for Cancer Treatment. Plasma Sources Sci. Technol. 2015, 24, 33001. [Google Scholar] [CrossRef]
- Sarangapani, C.; Patange, A.; Bourke, P.; Keener, K.; Cullen, P.J. Recent Advances in the Application of Cold Plasma Technology in Foods. Annu. Rev. Food Sci. Technol. 2018, 9, 609–629. [Google Scholar] [CrossRef] [PubMed]
- Ganesan, A.R.; Tiwari, U.; Ezhilarasi, P.N.; Rajauria, G. Application of Cold Plasma on Food Matrices: A Review on Current and Future Prospects. J. Food Process. Preserv. 2021, 45, e15070. [Google Scholar] [CrossRef]
- Laroque, D.A.; Seó, S.T.; Valencia, G.A.; Laurindo, J.B.; Carciofi, B.A.M. Cold Plasma in Food Processing: Design, Mechanisms, and Application. J. Food Eng. 2022, 312, 110748. [Google Scholar] [CrossRef]
- Zhang, B.; Tan, C.; Zou, F.; Sun, Y.; Shang, N.; Wu, W. Impacts of Cold Plasma Technology on Sensory, Nutritional and Safety Quality of Food: A Review. Foods 2022, 11, 2818. [Google Scholar] [CrossRef]
- Vandamme, M.; Robert, E.; Lerondel, S.; Sarron, V.; Ries, D.; Dozias, S.; Sobilo, J.; Gosset, D.; Kieda, C.; Legrain, B.; et al. ROS Implication in a New Antitumor Strategy Based on Non-Thermal Plasma. Int. J. Cancer 2012, 130, 2185–2194. [Google Scholar] [CrossRef]
- Mumtaz, S.; Rana, J.N.; Choi, E.H.; Han, I. Microwave Radiation and the Brain: Mechanisms, Current Status, and Future Prospects. Int. J. Mol. Sci. 2022, 23, 9288. [Google Scholar] [CrossRef]
- Chen, Z.; Chen, G.; Obenchain, R.; Zhang, R.; Bai, F.; Fang, T.; Wang, H.; Lu, Y.; Wirz, R.E.; Gu, Z. Cold Atmospheric Plasma Delivery for Biomedical Applications. Mater. Today 2022, 54, 153–188. [Google Scholar] [CrossRef]
- Laroussi, M. Cold Plasma in Medicine and Healthcare: The New Frontier in Low Temperature Plasma Applications. Front. Phys. 2020, 8, 74. [Google Scholar] [CrossRef]
- Dubey, S.K.; Parab, S.; Alexander, A.; Agrawal, M.; Achalla, V.P.K.; Pal, U.N.; Pandey, M.M.; Kesharwani, P. Cold Atmospheric Plasma Therapy in Wound Healing. Process Biochem. 2022, 112, 112–123. [Google Scholar] [CrossRef]
- Pankaj, S.K.; Keener, K.M. Cold Plasma: Background, Applications and Current Trends. Curr. Opin. Food Sci. 2017, 16, 49–52. [Google Scholar] [CrossRef]
- Lim, J.S.; Hong, Y.J.; Ghimire, B.; Choi, J.; Mumtaz, S.; Choi, E.H. Measurement of Electron Density in Transient Spark Discharge by Simple Interferometry. Results Phys. 2021, 20, 103693. [Google Scholar] [CrossRef]
- Lim, J.S.; Kim, R.H.; Hong, Y.J.; Lamichhane, P.; Adhikari, B.C.; Choi, J.; Choi, E.H. Interactions between Atmospheric Pressure Plasma Jet and Deionized Water Surface. Results Phys. 2020, 19, 103569. [Google Scholar] [CrossRef]
- Pavlovich, M.J.; Chang, H.W.; Sakiyama, Y.; Clark, D.S.; Graves, D.B. Ozone Correlates with Antibacterial Effects from Indirect Air Dielectric Barrier Discharge Treatment of Water. J. Phys. D Appl. Phys. 2013, 46, 145001. [Google Scholar] [CrossRef]
- Kaushik, N.K.; Kaushik, N.; Bhartiya, P.; Nguyen, L.N.; Choi, E.H. Glycolytic Inhibitor Induces Metabolic Crisis in Solid Cancer Cells to Enhance Cold Plasma-Induced Cell Death. Plasma Process. Polym. 2021, 18, 2000187. [Google Scholar] [CrossRef]
- Lin, A.; Truong, B.; Patel, S.; Kaushik, N.; Choi, E.H.; Fridman, G.; Fridman, A.; Miller, V. Nanosecond-Pulsed Dbd Plasma-Generated Reactive Oxygen Species Trigger Immunogenic Cell Death in A549 Lung Carcinoma Cells through Intracellular Oxidative Stress. Int. J. Mol. Sci. 2017, 18, 966. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.; Park, S.; Choe, W. Surface Plasma with an Inkjet-Printed Patterned Electrode for Low-Temperature Applications. Sci. Rep. 2021, 11, 12206. [Google Scholar] [CrossRef]
- Guo, Y.; Fang, M.; Zhang, L.; Sun, J.; Wang, X.; Tie, J.; Zhou, Q.; Zhang, L.; Luo, H. Study on Flexible Surface Dielectric Barrier Discharge Plasma Film for in Situ Inactivation of Bacteria and Viruses. Appl. Phys. Lett. 2022, 121, 074101. [Google Scholar] [CrossRef]
- Gershman, S.; Harreguy, M.B.; Yatom, S.; Raitses, Y.; Efthimion, P.; Haspel, G. A Low Power Flexible Dielectric Barrier Discharge Disinfects Surfaces and Improves the Action of Hydrogen Peroxide. Sci. Rep. 2021, 11, 4626. [Google Scholar] [CrossRef]
- Sehrawat, R.; Thakur, A.K.; Vikram, A.; Vaid, A.; Rane, R. Effect of Cold Plasma Treatment on Physiological Quality of Okra Seed. J. Hill Agric. 2017, 8, 66–71. [Google Scholar] [CrossRef]
- Bazavan, M.; Iova, I. Temperature Determination of a Cold N2 Discharge Plasma by the Fit of the Experimental Spectra with the Simulated Emission Spectra. Rom. Rep. Phys. 2008, 60, 671–678. [Google Scholar]
- Herzberg, G.; Mrozowski, S. Molecular Spectra and Molecular Structure. I. Spectra of Diatomic Molecules; D. Van Nostrand Company. Inc.: New York, NY, USA, 1951; Volume 19. [Google Scholar]
- Shimizu, T.; Sakiyama, Y.; Graves, D.B.; Zimmermann, J.L.; Morfill, G.E. The Dynamics of Ozone Generation and Mode Transition in Air Surface Micro-Discharge Plasma at Atmospheric Pressure. New J. Phys. 2012, 14, 103028. [Google Scholar] [CrossRef] [Green Version]
- Herron, J.T.; Green, D.S. Chemical Kinetics Database and Predictive Schemes for Nonthermal Humid Air Plasma Chemistry. Part II. Neutral Species Reactions. Plasma Chem. Plasma Process. 2001, 21, 459–481. [Google Scholar] [CrossRef]
- Capitelli, M.; Ferreira, C.M.; Gordiets, B.F.; Osipov, A.I. Plasma Kinetics in Atmospheric Gases; Springer Science\& Business Media: Berlin/Heidelberg, Germany, 2013; Volume 31. [Google Scholar]
- Atkinson, R.; Baulch, D.L.; Cox, R.A.; Hampson, R.F.; Kerr, J.A.; Rossi, M.J.; Troe, J. Evaluated Kinetic and Photochemical Data for Atmospheric Chemistry: Supplement VI: IUPAC Subcommittee on Gas Kinetic Data Evaluation for Atmospheric Chemistry. J. Phys. Chem. Ref. Data 1997, 26, 1329–1499. [Google Scholar] [CrossRef] [Green Version]
- Atkinson, R.; Baulch, D.L.; Cox, R.A.; Crowley, J.N.; Hampson, R.F.; Hynes, R.G.; Jenkin, M.E.; Rossi, M.J.; Troe, J. Evaluated Kinetic and Photochemical Data for Atmospheric Chemistry: Volume I-Gas Phase Reactions of Ox, HOx, NOx and SOx Species. Atmos. Chem. Phys. 2004, 4, 1461–1738. [Google Scholar] [CrossRef] [Green Version]
- Murakami, T.; Niemi, K.; Gans, T.; O’Connell, D.; Graham, W.G. Chemical Kinetics and Reactive Species in Atmospheric Pressure Helium--Oxygen Plasmas with Humid-Air Impurities. Plasma Sources Sci. Technol. 2012, 22, 15003. [Google Scholar] [CrossRef]
- Atkinson, R.; Baulch, D.L.; Cox, R.A.; Hampson, R.F., Jr.; Kerr, J.A.; Troe, J. Evaluated Kinetic and Photochemical Data for Atmospheric Chemistry: Supplement IV. IUPAC Subcommittee on Gas Kinetic Data Evaluation for Atmospheric Chemistry. J. Phys. Chem. Ref. Data 1992, 21, 1125–1568. [Google Scholar] [CrossRef]
- Jeong, J.Y.; Park, J.; Henins, I.; Babayan, S.E.; Tu, V.J.; Selwyn, G.S.; Ding, G.; Hicks, R.F. Reaction Chemistry in the Afterglow of an Oxygen- Helium, Atmospheric-Pressure Plasma. J. Phys. Chem. A 2000, 104, 8027–8032. [Google Scholar] [CrossRef] [Green Version]
- Baulch, D.L.; Cobos, C.J.; Cox, R.A.; Frank, P.; Hayman, G.; Just, T.; Kerr, J.A.; Murrells, T.; Pilling, M.J.; Troe, J.; et al. Evaluated Kinetic Data for Combustion Modeling. Supplement I. J. Phys. Chem. Ref. Data 1994, 23, 847–848. [Google Scholar] [CrossRef] [Green Version]
- Sander, S.P.; Kircher, C.C. Temperature Dependence of the Reaction NO+ NO3→ 2NO2. Chem. Phys. Lett. 1986, 126, 149–152. [Google Scholar] [CrossRef]
- Eichwald, O.; Yousfi, M.; Hennad, A.; Benabdessadok, M.D. Coupling of Chemical Kinetics, Gas Dynamics, and Charged Particle Kinetics Models for the Analysis of NO Reduction from Flue Gases. J. Appl. Phys. 1997, 82, 4781–4794. [Google Scholar] [CrossRef]
- Johnston, H.S.; Cantrell, C.A.; Calvert, J.G. Unimolecular Decomposition of NO3 to Form NO and O2 and a Review of N2O5/NO3 Kinetics. J. Geophys. Res. Atmos. 1986, 91, 5159–5172. [Google Scholar] [CrossRef]
- Matzing, H. Chemical Kinetics of Flue Gas Cleaning by Irradiation with Electrons. Adv. Chem. Phys. 1991, 80, 315–402. [Google Scholar]
- Gordon, I.E.; Rothman, L.S.; Hargreaves, R.J.; Hashemi, R.; Karlovets, E.V.; Skinner, F.M.; Conway, E.K.; Hill, C.; Kochanov, R.V.; Tan, Y.; et al. The HITRAN2020 Molecular Spectroscopic Database. J. Quant. Spectrosc. Radiat. Transf. 2022, 277, 107949. [Google Scholar] [CrossRef]
- Park, S.; Choe, W.; Jo, C. Interplay among Ozone and Nitrogen Oxides in Air Plasmas: Rapid Change in Plasma Chemistry. Chem. Eng. J. 2018, 352, 1014–1021. [Google Scholar] [CrossRef]
- Bruggeman, P.J.; Sadeghi, N.; Schram, D.C.; Linss, V. Gas Temperature Determination from Rotational Lines in Non-Equilibrium Plasmas: A Review. Plasma Sources Sci. Technol. 2020, 23, 023001. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Q.Y.; Shi, D.Q.; Xu, W.; Miao, C.Y.; Ma, C.Y.; Ren, C.S.; Zhang, C.; Yi, Z. Determination of Vibrational and Rotational Temperatures in Highly Constricted Nitrogen Plasmas by Fitting the Second Positive System of N2 Molecules. AIP Adv. 2020, 5, 57158. [Google Scholar] [CrossRef] [Green Version]
- Fan, L.; Liu, X.; Ma, Y.; Xiang, Q. Effects of Plasma-Activated Water Treatment on Seed Germination and Growth of Mung Bean Sprouts. J. Taibah Univ. Sci. 2020, 14, 823–830. [Google Scholar] [CrossRef]
| No. | Reaction | Rate Coefficient (cm3/s or cm6/s) | Ref |
|---|---|---|---|
| R1 | [25] | ||
| R2 | [25] | ||
| R3 | [25] | ||
| R4 | [26] | ||
| R5 | [27] | ||
| R6 | [27] | ||
| R7 | [25] | ||
| R8 | [26] | ||
| R9 | [28] | ||
| R10 | [28] | ||
| R11 | [28] | ||
| R12 | [25] | ||
| R13 | [26] | ||
| R14 | [26] | ||
| R15 | [26] | ||
| R16 | [29] | ||
| R17 | [25] | ||
| R18 | [30] | ||
| R19 | [31] | ||
| R20 | [25] | ||
| R21 | [25] | ||
| R22 | [32] | ||
| R23 | [33] | ||
| R24 | [26] | ||
| R25 | [25] | ||
| R26 | [25] | ||
| R27 | [34] | ||
| R28 | [34] | ||
| R29 | [34] | ||
| R30 | [26] | ||
| R31 | [26] | ||
| R32 | [28] | ||
| R33 | [33] | ||
| R34 | [26] | ||
| R35 | [26] | ||
| R36 | [28] | ||
| R37 | [28] | ||
| R38 | [26] | ||
| R39 | [26] | ||
| R40 | [26] | ||
| R41 | [26] | ||
| R42 | [25] | ||
| R43 | [26] | ||
| R44 | [26] | ||
| R45 | [26] | ||
| R46 | [35] | ||
| R47 | [28] | ||
| R48 | [28] | ||
| R49 | [28] | ||
| R50 | [36] | ||
| R51 | [36] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lim, J.S.; Kim, D.; Ki, S.; Mumtaz, S.; Shaik, A.M.; Han, I.; Hong, Y.J.; Park, G.; Choi, E.H. Characteristics of a Rollable Dielectric Barrier Discharge Plasma and Its Effects on Spinach-Seed Germination. Int. J. Mol. Sci. 2023, 24, 4638. https://doi.org/10.3390/ijms24054638
Lim JS, Kim D, Ki S, Mumtaz S, Shaik AM, Han I, Hong YJ, Park G, Choi EH. Characteristics of a Rollable Dielectric Barrier Discharge Plasma and Its Effects on Spinach-Seed Germination. International Journal of Molecular Sciences. 2023; 24(5):4638. https://doi.org/10.3390/ijms24054638
Chicago/Turabian StyleLim, Jun Sup, Daeun Kim, Sehoon Ki, Sohail Mumtaz, Abdul Munnaf Shaik, Ihn Han, Young June Hong, Gyungsoon Park, and Eun Ha Choi. 2023. "Characteristics of a Rollable Dielectric Barrier Discharge Plasma and Its Effects on Spinach-Seed Germination" International Journal of Molecular Sciences 24, no. 5: 4638. https://doi.org/10.3390/ijms24054638
APA StyleLim, J. S., Kim, D., Ki, S., Mumtaz, S., Shaik, A. M., Han, I., Hong, Y. J., Park, G., & Choi, E. H. (2023). Characteristics of a Rollable Dielectric Barrier Discharge Plasma and Its Effects on Spinach-Seed Germination. International Journal of Molecular Sciences, 24(5), 4638. https://doi.org/10.3390/ijms24054638










