A Mixture of Cervus elaphus sibiricus and Glycine max (L.) Merrill Inhibits Ovariectomy-Induced Bone Loss Via Regulation of Osteogenic Molecules in a Mouse Model
Abstract
1. Introduction
2. Results
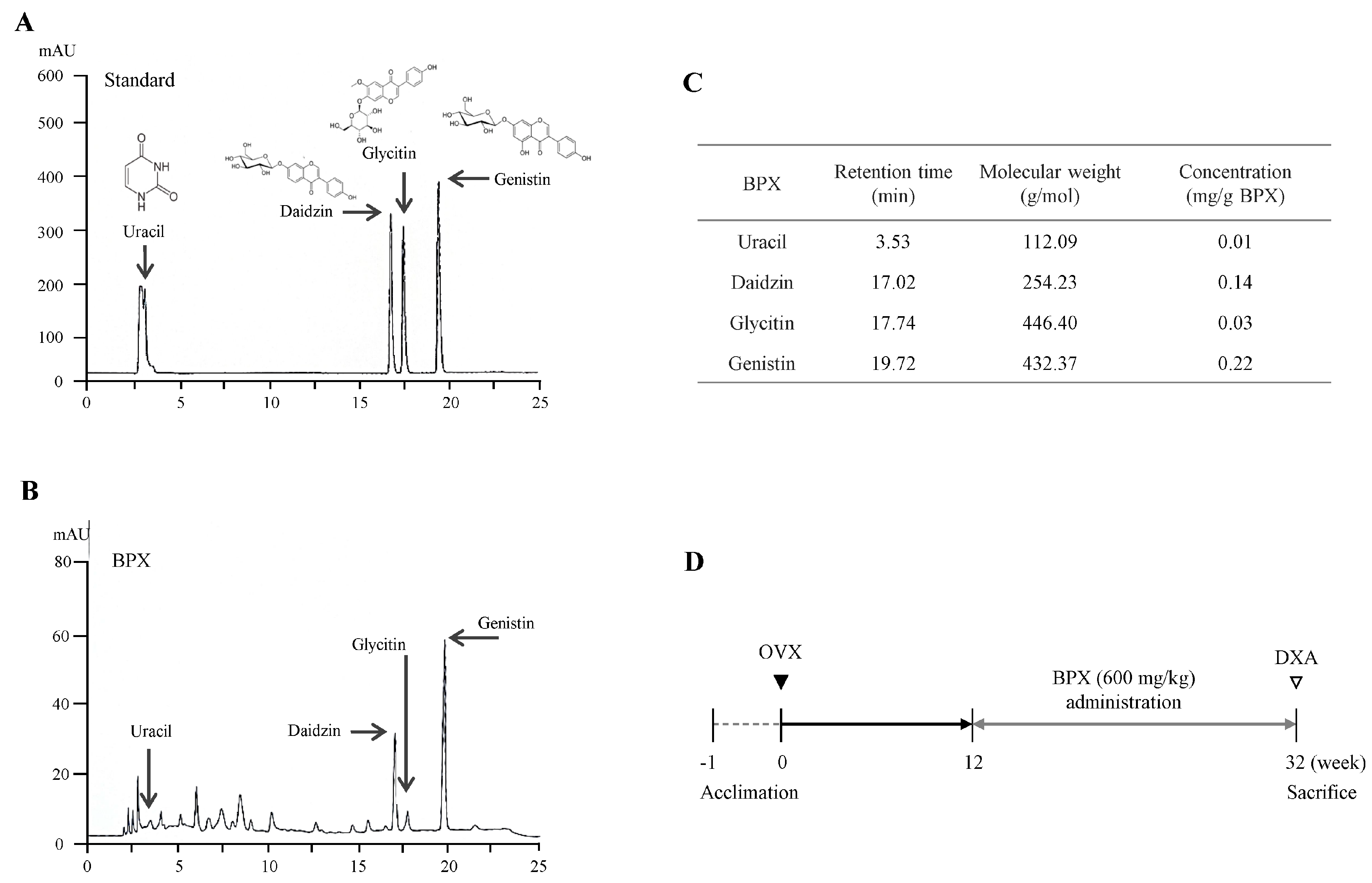
2.1. Fingerprinting of BPX
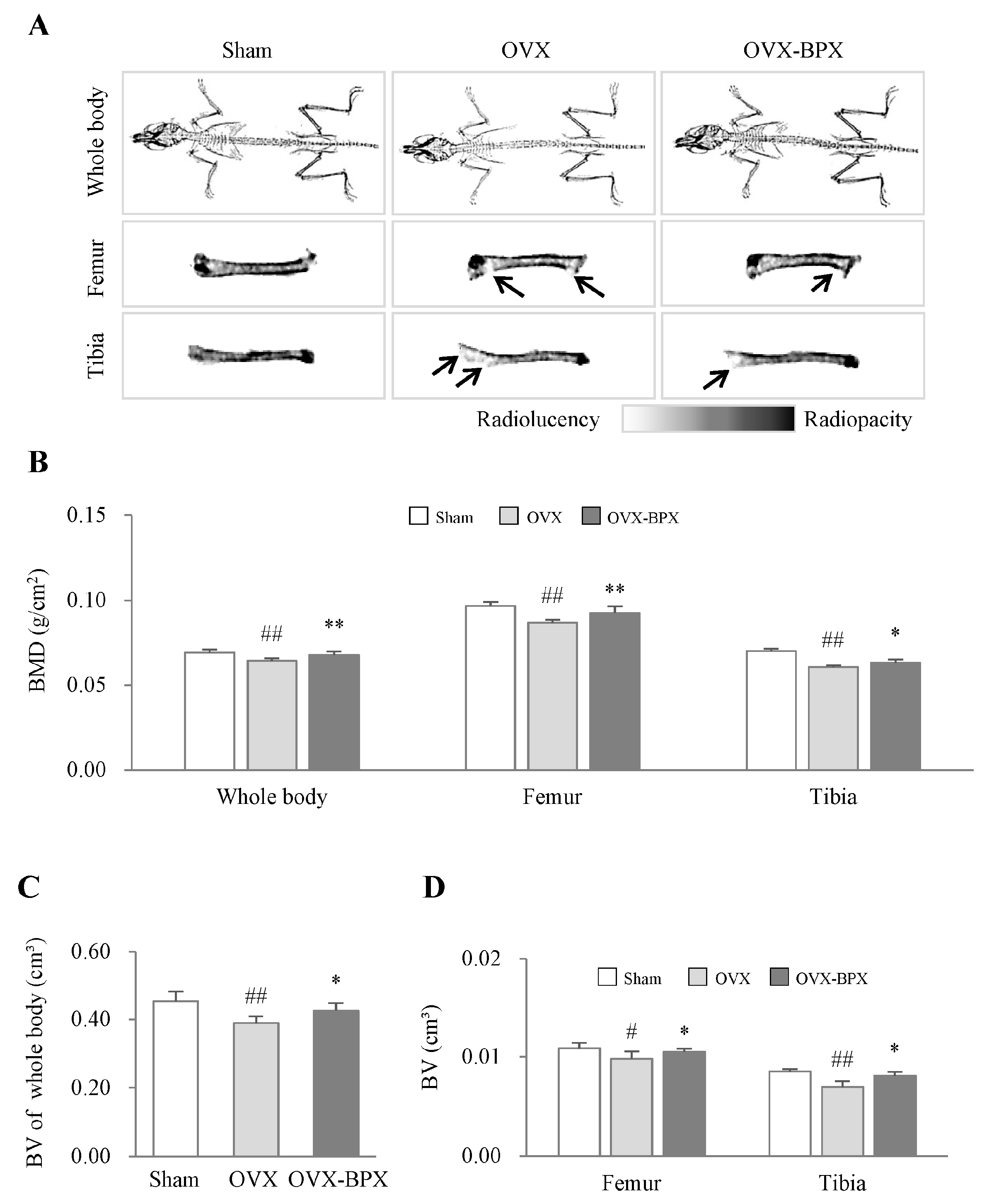
2.2. BPX Attenuated OVX-Induced Bone Loss
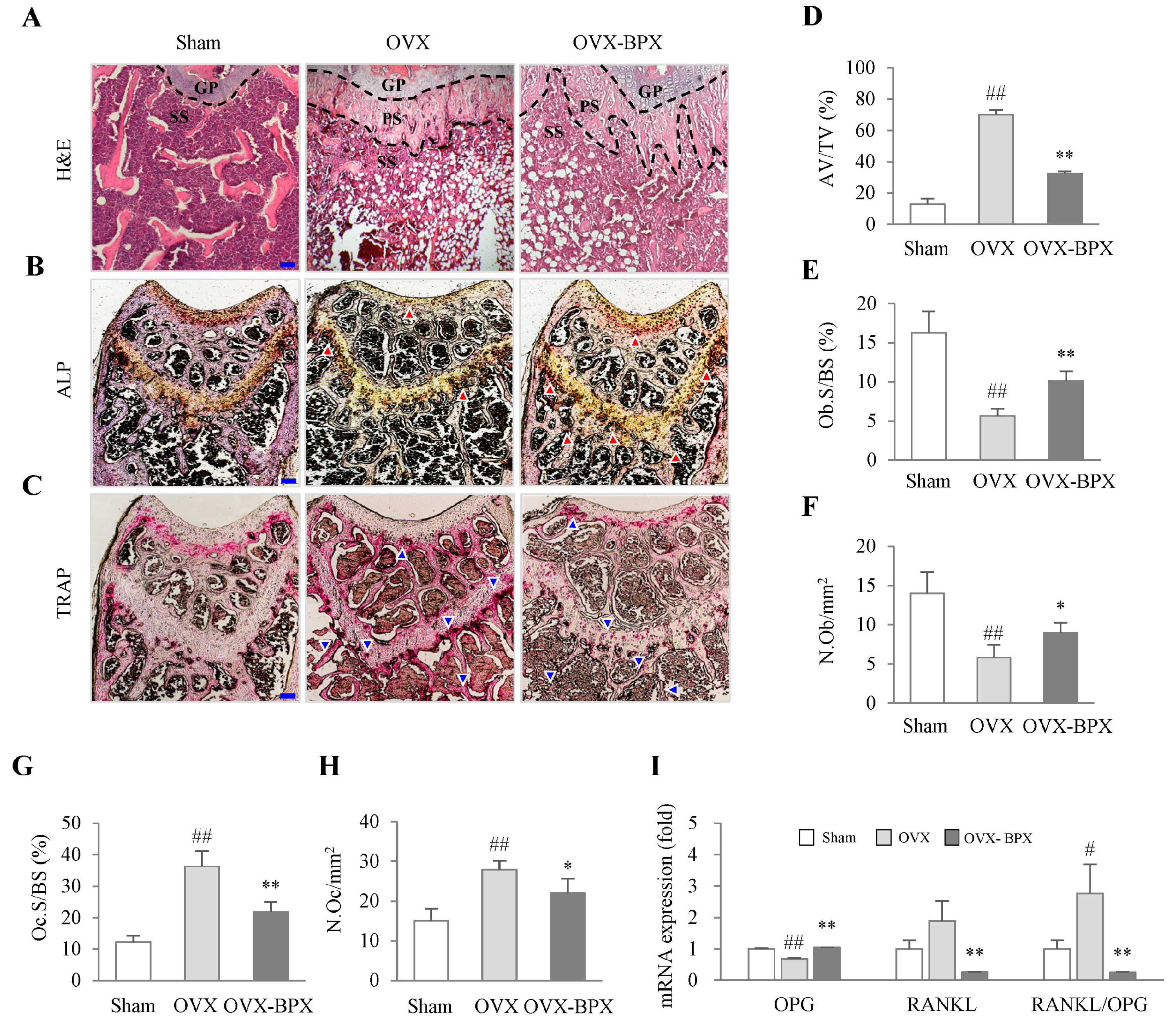
2.3. BPX Attenuated Histological Alterations in the Femur
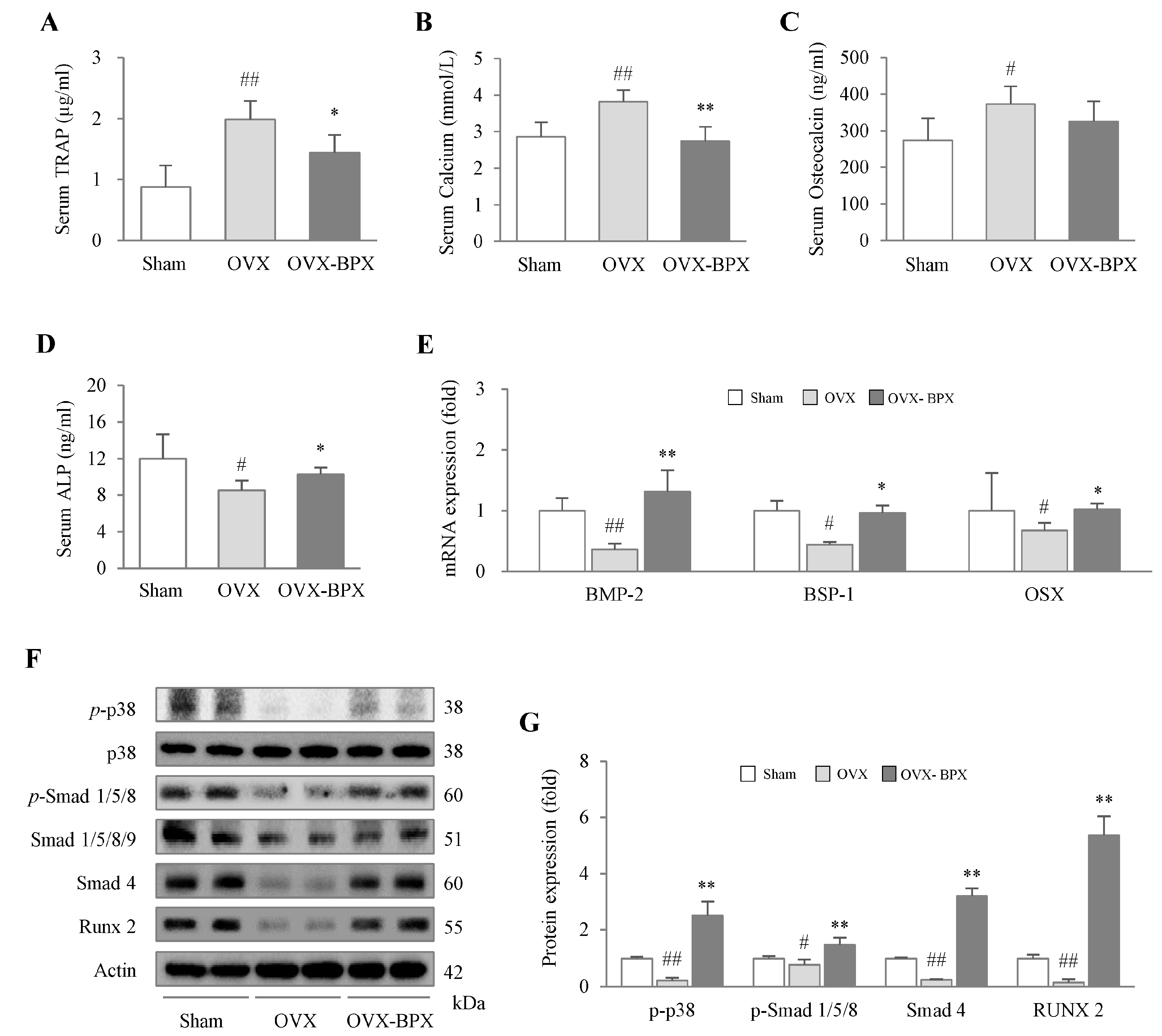
2.4. BPX Attenuated Alterations in Bone Formation and Resorption Markers in Serum
2.5. BPX Modulated the BMP Pathway and Its Related Molecules
3. Discussion
4. Materials and Methods
4.1. Chemicals and Reagents
4.2. BPX Preparation and Fingerprinting
4.3. Animals and Ovariectomy
4.4. Drug Treatment
4.5. Dual-Energy X-ray Absorptiometry (DXA) Analysis
4.6. Histological Analysis
4.7. Enzyme-Linked Immunosorbent Assay (ELISA)
4.8. Western Blot Analysis
4.9. Quantitative Real-Time PCR Analysis
4.10. Cell Culture and Cytotoxicity
4.11. ALP Staining and Activity Assay in MC3T3-E1 Cell
4.12. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Klibanski, A.; Adams-Campbell, L.; Bassford, T.; Blair, S.N.; Boden, S.D.; Dickersin, K.; Gifford, D.R.; Glasse, L.; Goldring, S.R.; Hruska, K. Osteoporosis prevention, diagnosis, and therapy. J. Am. Med. Assoc. 2001, 285, 785–795. [Google Scholar]
- Salari, N.; Ghasemi, H.; Mohammadi, L.; Rabieenia, E.; Shohaimi, S.; Mohammadi, M. The global prevalence of osteoporosis in the world: A comprehensive systematic review and meta-analysis. J. Orthop. Surg. Res. 2021, 16, 609. [Google Scholar] [CrossRef] [PubMed]
- Kemmak, A.R.; Rezapour, A.; Jahangiri, R.; Nikjoo, S.; Farabi, H.; Soleimanpour, S. Economic burden of osteoporosis in the world: A systematic review. Med. J. Islam. Repub. Iran 2020, 34, 154. [Google Scholar]
- Dobbs, M.B.; Buckwalter, J.; Saltzman, C. Osteoporosis: The increasing role of the orthopaedist. Iowa Orthop. J. 1999, 19, 43. [Google Scholar]
- El-Gazzar, A.; Högler, W. Mechanisms of bone fragility: From osteogenesis imperfecta to secondary osteoporosis. Int. J. Mol. Sci. 2021, 22, 625. [Google Scholar] [CrossRef] [PubMed]
- Noirrit-Esclassan, E.; Valera, M.-C.; Tremollieres, F.; Arnal, J.-F.; Lenfant, F.; Fontaine, C.; Vinel, A. Critical role of estrogens on bone homeostasis in both male and female: From physiology to medical implications. Int. J. Mol. Sci. 2021, 22, 1568. [Google Scholar] [CrossRef]
- Streicher, C.; Heyny, A.; Andrukhova, O.; Haigl, B.; Slavic, S.; Schüler, C.; Kollmann, K.; Kantner, I.; Sexl, V.; Kleiter, M. Estrogen regulates bone turnover by targeting RANKL expression in bone lining cells. Sci. Rep. 2017, 7, 6460. [Google Scholar] [CrossRef]
- Chen, X.; Wang, Z.; Duan, N.; Zhu, G.; Schwarz, E.M.; Xie, C. Osteoblast–osteoclast interactions. Connect. Tissue Res. 2018, 59, 99–107. [Google Scholar] [CrossRef]
- Siris, E.; Adler, R.; Bilezikian, J.; Bolognese, M.; Dawson-Hughes, B.; Favus, M.; Harris, S.; Jan de Beur, S.; Khosla, S.; Lane, N.E. The clinical diagnosis of osteoporosis: A position statement from the National Bone Health Alliance Working Group. Osteoporos. Int. 2014, 25, 1439–1443. [Google Scholar] [CrossRef]
- Sözen, T.; Özışık, L.; Başaran, N.Ç. An overview and management of osteoporosis. Eur. J. Rheumatol. 2017, 4, 46. [Google Scholar] [CrossRef]
- Wu, C.-H.; Hung, W.-C.; Chang, L.; Tsai, T.-T.; Chang, Y.-F.; McCloskey, E.V.; Watts, N.B.; McClung, M.R.; Huang, C.-F.; Chen, C.-H. Pharmacologic intervention for prevention of fractures in osteopenic and osteoporotic postmenopausal women: Systemic review and meta-analysis. Bone Rep. 2020, 13, 100729. [Google Scholar] [CrossRef] [PubMed]
- Clarke, B.L. Effects of Estrogens and SERMs on Bone Metabolism: Clinical Aspects. In Osteoporosis; Springer: Berlin/Heidelberg, Germany, 2020; pp. 239–257. [Google Scholar]
- Chan, C.C. Selective estrogen receptor modulators. In Gynecology Drug Therapy; Springer: Berlin/Heidelberg, Germany, 2016; pp. 105–110. [Google Scholar]
- Graves, L.L.; Bukata, S.V.; Aghazadehsanai, N.; Chang, T.I.; Garrett, N.R.; Friedlander, A.H. Patients receiving parenteral bisphosphonates for malignant disease and having developed an atypical femoral fracture are at risk of concomitant osteonecrosis of the jaw: An evidence-based review. J. Oral Maxillofac. Surg. 2016, 74, 2403–2408. [Google Scholar] [CrossRef] [PubMed]
- Salari, P.; Abdollahi, M. Long term bisphosphonate use in osteoporotic patients; a step forward, two steps back. J. Pharm. Pharm. Sci. 2012, 15, 305–317. [Google Scholar] [CrossRef]
- Prentice, R.L. Postmenopausal Hormone Therapy and the Risks of Coronary Heart Disease, Breast Cancer, and Stroke, Seminars in reproductive medicine; Thieme Medical Publishers: New York, NY, USA, 2014; pp. 419–425. [Google Scholar]
- Sølling, A.S.K.; Harsløf, T.; Langdahl, B. Current status of bone-forming therapies for the management of osteoporosis. Drugs Aging 2019, 36, 625–638. [Google Scholar] [CrossRef]
- Vukicevic, S.; Oppermann, H.; Verbanac, D.; Jankolija, M.; Popek, I.; Curak, J.; Brkljacic, J.; Pauk, M.; Erjavec, I.; Francetic, I. The clinical use of bone morphogenetic proteins revisited: A novel biocompatible carrier device OSTEOGROW for bone healing. Int. Orthop. 2014, 38, 635–647. [Google Scholar] [CrossRef] [PubMed]
- Mukwaya, E.; Xu, F.; Wong, M.-S.; Zhang, Y. Chinese herbal medicine for bone health. Pharm. Biol. 2014, 52, 1223–1228. [Google Scholar] [CrossRef]
- Peng, Z.; Xu, R.; You, Q. Role of Traditional Chinese Medicine in Bone Regeneration and Osteoporosis. Front. Bioeng. Biotechnol. 2022, 10, 911326. [Google Scholar] [CrossRef]
- Park, H.I.; Lee, K.H. Comparison of the effects of deer antler, old antler, and antler glue on osteoporosis in ovariectomized rats. J. Acupunct. Res. 2018, 35, 21–27. [Google Scholar] [CrossRef]
- Lee, J.M.; Kim, N.H.; Lee, E.-J. The Effects of Oral Administration of Deer Antler Extracts on an Osteoporosis-induced Animal Model: A Systematic Review and Meta-analysis. J. Korean Med. Rehabil. 2022, 32, 65–81. [Google Scholar] [CrossRef]
- Salam, M.A.; Raslan, H.M.; Mohamed, D.A.; Elgendy, A.; Hussein, R.A.; Moguib, O.; Abdelhadi, M.; El, R.A.E.-S.S.; Fouda, K.; El Sherity, S.Y. Effect of Soybean on Bone Health and Some Metabolic Parameters in Postmenopausal Egyptian Women. Pharmacogn. J. 2021, 13, 3. [Google Scholar] [CrossRef]
- Lips, P.; van Schoor, N.M. Quality of life in patients with osteoporosis. Osteoporos. Int. 2005, 16, 447–455. [Google Scholar] [CrossRef] [PubMed]
- Green, J.R. Antitumor effects of bisphosphonates. Cancer Interdiscip. Int. J. Am. Cancer Soc. 2003, 97 (Suppl. 3), 840–847. [Google Scholar] [CrossRef] [PubMed]
- Fleisch, H.; Reszka, A.; Rodan, G.; Rogers, M. Bisphosphonates: Mechanisms of action. Princ. Bone Biol. 2002, 1361, XLIII. [Google Scholar]
- Reyes, C.; Hitz, M.; Prieto-Alhambra, D.; Abrahamsen, B. Risks and benefits of bisphosphonate therapies. J. Cell. Biochem. 2016, 117, 20–28. [Google Scholar] [CrossRef]
- Food and Drug Administration. Guidelines for Preclinical and Clinical Evaluation of Agents Used in the Prevention or Treatment of Postmenopausal Osteoporosis; Division of Metabolism and Endocrine Drug Products: Rockville, MD, USA, 1994. [Google Scholar]
- Jee, W.; Yao, W. Overview: Animal models of osteopenia and osteoporosis. J. Musculoskelet. Neuronal. Interact. 2001, 1, 193–207. [Google Scholar]
- Kimmel, D.B. Animal models for in vivo experimentation in osteoporosis research. In Osteoporosis; Elsevier: Amsterdam, The Netherlands, 2001; pp. 29–47. [Google Scholar]
- Leslie, W.D.; Majumdar, S.R.; Morin, S.N.; Lix, L.M. Change in bone mineral density is an indicator of treatment-related antifracture effect in routine clinical practice: A registry-based cohort study. Ann. Intern. Med. 2016, 165, 465–472. [Google Scholar] [CrossRef] [PubMed]
- Banefelt, J.; Timoshanko, J.; Söreskog, E.; Ortsäter, G.; Moayyeri, A.; Åkesson, K.E.; Spångéus, A.; Libanati, C. Total Hip Bone Mineral Density as an Indicator of Fracture Risk in Bisphosphonate-Treated Patients in a Real-World Setting. J. Bone Miner. Res. 2022, 37, 52–58. [Google Scholar] [CrossRef]
- Langdahl, B.; Ferrari, S.; Dempster, D.W. Bone modeling and remodeling: Potential as therapeutic targets for the treatment of osteoporosis. Ther. Adv. Musculoskelet. Dis. 2016, 8, 225–235. [Google Scholar] [CrossRef]
- Manolagas, S.C.; O’brien, C.A.; Almeida, M. The role of estrogen and androgen receptors in bone health and disease. Nat. Rev. Endocrinol. 2013, 9, 699. [Google Scholar] [CrossRef]
- Olsson, A.; Oturai, A.B.; Søndergaard, H.B.; Sellebjerg, F.; Oturai, P.S. Bone microarchitecture and bone mineral density in multiple sclerosis. Acta Neurol. Scand. 2018, 137, 363–369. [Google Scholar] [CrossRef]
- Ambrosi, T.H.; Scialdone, A.; Graja, A.; Gohlke, S.; Jank, A.-M.; Bocian, C.; Woelk, L.; Fan, H.; Logan, D.W.; Schürmann, A. Adipocyte accumulation in the bone marrow during obesity and aging impairs stem cell-based hematopoietic and bone regeneration. Cell Stem Cell 2017, 20, 771–784. [Google Scholar] [CrossRef]
- Cheng, C.-H.; Chen, L.-R.; Chen, K.-H. Osteoporosis due to hormone imbalance: An overview of the effects of estrogen deficiency and glucocorticoid overuse on bone turnover. Int. J. Mol. Sci. 2022, 23, 1376. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Kumar, D.; Lal, A.K. Serum osteocalcin as a diagnostic biomarker for primary osteoporosis in women. J. Clin. Diagn. Res. JCDR 2015, 9, RC04. [Google Scholar] [CrossRef] [PubMed]
- Mederle, O.A.; Balas, M.; Ioanoviciu, S.D.; Gurban, C.-V.; Tudor, A.; Borza, C. Correlations between bone turnover markers, serum magnesium and bone mass density in postmenopausal osteoporosis. Clin. Interv. Aging 2018, 13, 1383. [Google Scholar] [CrossRef] [PubMed]
- Alcantara, E.H.; Shin, M.-Y.; Sohn, H.-Y.; Park, Y.-M.; Kim, T.; Lim, J.-H.; Jeong, H.-J.; Kwon, S.-T.; Kwun, I.-S. Diosgenin stimulates osteogenic activity by increasing bone matrix protein synthesis and bone-specific transcription factor Runx2 in osteoblastic MC3T3-E1 cells. J. Nutr. Biochem. 2011, 22, 1055–1063. [Google Scholar] [CrossRef] [PubMed]
- Stuss, M.; Rieske, P.; Cegłowska, A.; Stêpień-Kłos, W.; Liberski, P.P.; Brzeziańska, E.; Sewerynek, E. Assessment of OPG/RANK/RANKL gene expression levels in peripheral blood mononuclear cells (PBMC) after treatment with strontium ranelate and ibandronate in patients with postmenopausal osteoporosis. J. Clin. Endocrinol. Metab. 2013, 98, E1007–E1011. [Google Scholar] [CrossRef]
- Tobeiha, M.; Moghadasian, M.H.; Amin, N.; Jafarnejad, S. RANKL/RANK/OPG pathway: A mechanism involved in exercise-induced bone remodeling. BioMed Res. Int. 2020, 2020, 6910312. [Google Scholar] [CrossRef]
- Moser, S.C.; van der Eerden, B.C. Osteocalcin—A versatile bone-derived hormone. Front. Endocrinol. 2019, 9, 794. [Google Scholar] [CrossRef]
- Halloran, D.; Durbano, H.W.; Nohe, A. Bone morphogenetic protein-2 in development and bone homeostasis. J. Dev. Biol. 2020, 8, 19. [Google Scholar] [CrossRef]
- Donoso, O.; Pino, A.M.; Seitz, G.; Osses, N.; Rodríguez, J.P. Osteoporosis-associated alteration in the signalling status of BMP-2 in human MSCs under adipogenic conditions. J. Cell. Biochem. 2015, 116, 1267–1277. [Google Scholar] [CrossRef]
- Turgeman, G.; Zilberman, Y.; Zhou, S.; Kelly, P.; Moutsatsos, I.K.; Kharode, Y.P.; Borella, L.E.; Bex, F.J.; Komm, B.S.; Bodine, P.V. Systemically administered rhBMP-2 promotes MSC activity and reverses bone and cartilage loss in osteopenic mice. J. Cell. Biochem. 2002, 86, 461–474. [Google Scholar] [CrossRef] [PubMed]
- Govender, S.; Csimma, C.; Genant, H.K.; Valentin-Opran, A.; Amit, Y.; Arbel, R.; Aro, H.; Atar, D.; Bishay, M.; Börner, M.G. Recombinant human bone morphogenetic protein-2 for treatment of open tibial fractures: A prospective, controlled, randomized study of four hundred and fifty patients. JBJS 2002, 84, 2123–2134. [Google Scholar] [CrossRef]
- Hwang, J.-S.; Seo, B.-I.; Park, J.-H.; Roh, S.-S.; Kim, M.-R.; Kim, S.-M.; Koo, J.-S. Effect of cervi cornu on treatment of osteoporosis in ovariectomized rats. Korea J. Herbol. 2010, 25, 1–10. [Google Scholar]
- Meng, H.; Qu, X.; Li, N.; Yuan, S.; Lin, Z. Effects of pilose antler and antler glue on osteoporosis of ovariectomized rats. J. Chin. Med. Mater. 2009, 32, 179–182. [Google Scholar]
- Ma, D.; Qin, L.; Wang, P.; Katoh, R. Soy isoflavone intake inhibits bone resorption and stimulates bone formation in menopausal women: Meta-analysis of randomized controlled trials. Eur. J. Clin. Nutr. 2008, 62, 155–161. [Google Scholar] [CrossRef] [PubMed]
- Gallo, D.; Zannoni, G.F.; Apollonio, P.; Martinelli, E.; Ferlini, C.; Passetti, G.; Riva, A.; Morazzoni, P.; Bombardelli, E.; Scambia, G. Characterization of the pharmacologic profile of a standardized soy extract in the ovariectomized rat model of menopause: Effects on bone, uterus, and lipid profile. Menopause 2005, 12, 589–600. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baek, D.-C.; Hwang, S.-J.; Lee, J.-S.; Wang, J.-H.; Son, C.-G.; Lee, E.-J. A Mixture of Cervus elaphus sibiricus and Glycine max (L.) Merrill Inhibits Ovariectomy-Induced Bone Loss Via Regulation of Osteogenic Molecules in a Mouse Model. Int. J. Mol. Sci. 2023, 24, 4876. https://doi.org/10.3390/ijms24054876
Baek D-C, Hwang S-J, Lee J-S, Wang J-H, Son C-G, Lee E-J. A Mixture of Cervus elaphus sibiricus and Glycine max (L.) Merrill Inhibits Ovariectomy-Induced Bone Loss Via Regulation of Osteogenic Molecules in a Mouse Model. International Journal of Molecular Sciences. 2023; 24(5):4876. https://doi.org/10.3390/ijms24054876
Chicago/Turabian StyleBaek, Dong-Cheol, Seung-Ju Hwang, Jin-Seok Lee, Jing-Hua Wang, Chang-Gue Son, and Eun-Jung Lee. 2023. "A Mixture of Cervus elaphus sibiricus and Glycine max (L.) Merrill Inhibits Ovariectomy-Induced Bone Loss Via Regulation of Osteogenic Molecules in a Mouse Model" International Journal of Molecular Sciences 24, no. 5: 4876. https://doi.org/10.3390/ijms24054876
APA StyleBaek, D.-C., Hwang, S.-J., Lee, J.-S., Wang, J.-H., Son, C.-G., & Lee, E.-J. (2023). A Mixture of Cervus elaphus sibiricus and Glycine max (L.) Merrill Inhibits Ovariectomy-Induced Bone Loss Via Regulation of Osteogenic Molecules in a Mouse Model. International Journal of Molecular Sciences, 24(5), 4876. https://doi.org/10.3390/ijms24054876










