In Silico Screening and Molecular Dynamics Simulation Studies in the Identification of Natural Compound Inhibitors Targeting the Human Norovirus RdRp Protein to Fight Gastroenteritis
Abstract
:1. Introduction
2. Results and Discussion
3. Material and Methods
3.1. Target Protein and Compound Library Preparation
3.2. Structure-Based Virtual Screening (SBVS)
3.3. Estimation of Physicochemical, and ADMET Properties
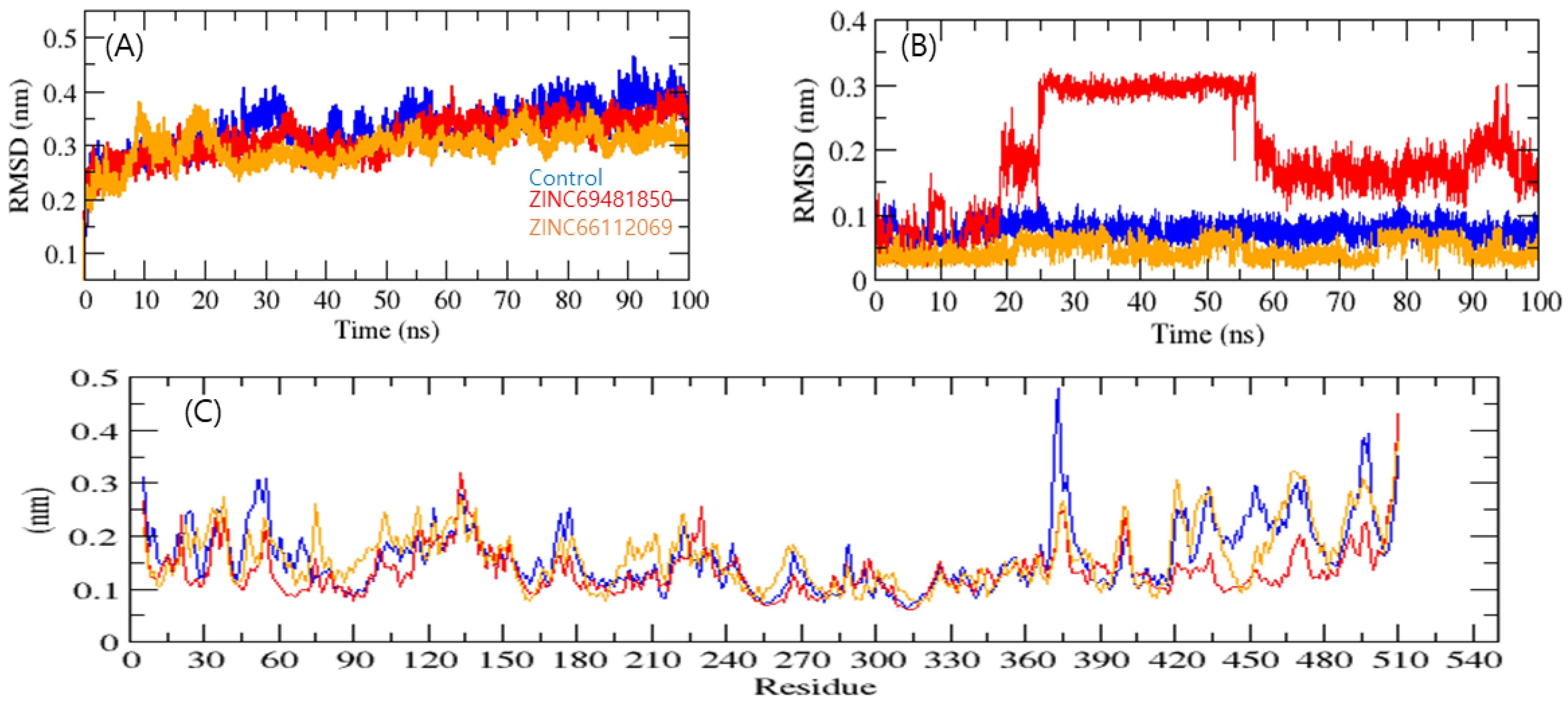
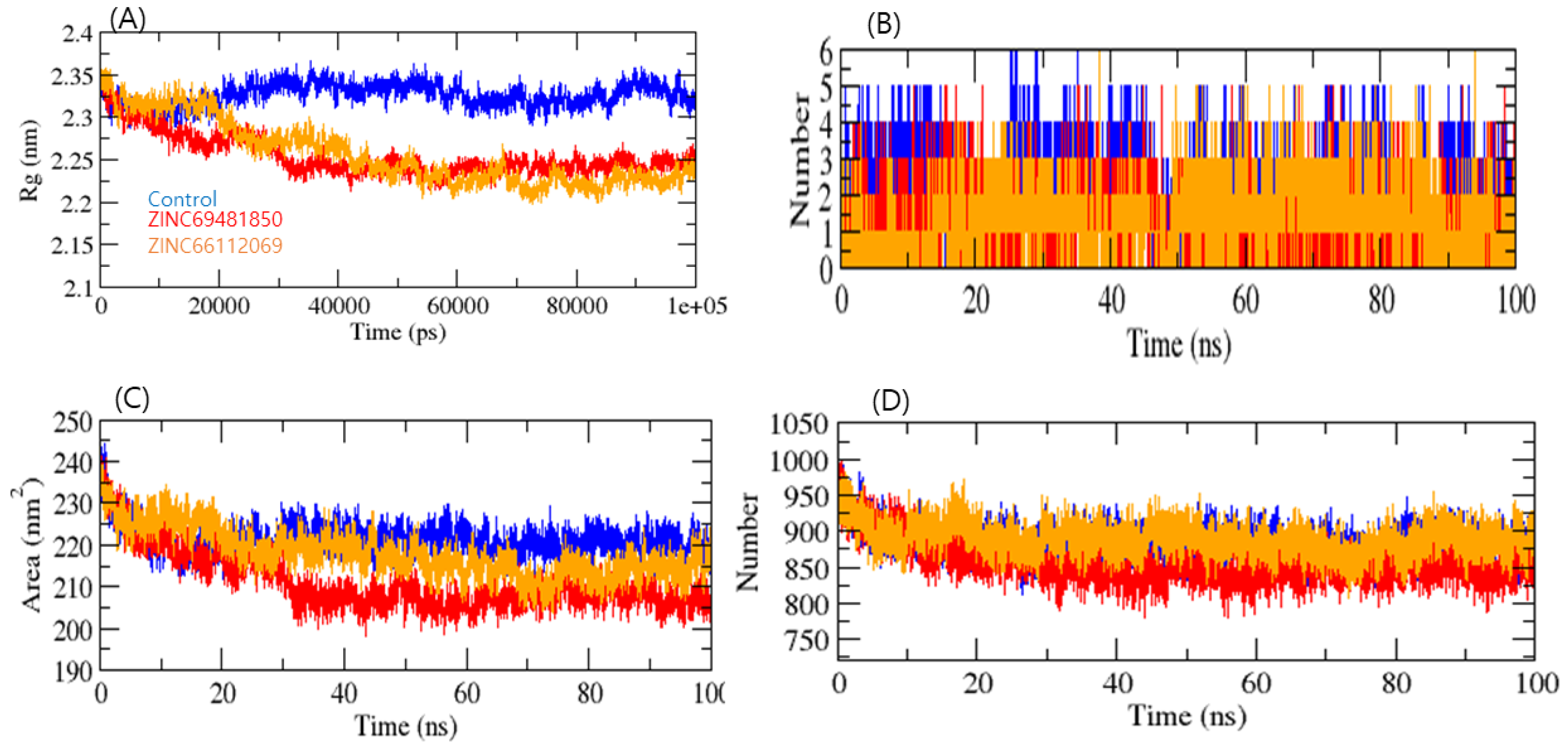
3.4. Molecular Dynamics (MD) Simulation
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Lopman, B.A.; Steele, D.; Kirkwood, C.D.; Parashar, U.D. The Vast and Varied Global Burden of Norovirus: Prospects for Prevention and Control. PLoS Med. 2016, 13, e1001999. [Google Scholar] [CrossRef] [PubMed]
- Petrignani, M.; Verhoef, L.; de Graaf, M.; Richardus, J.H.; Koopmans, M. Chronic sequelae and severe complications of norovirus infection: A systematic review of literature. J. Clin. Virol. 2018, 105, 1–10. [Google Scholar] [CrossRef]
- Shah, M.P.; Hall, A.J. Norovirus Illnesses in Children and Adolescents. Infect. Dis. Clin. N. Am. 2018, 32, 103–118. [Google Scholar] [CrossRef] [PubMed]
- Khayat, A.A.; Telega, G.W. Persistent elevation of aminotransferases in liver transplant in association with chronic norovirus infection. Clin. Mol. Hepatol. 2019, 25, 408–411. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lucero, Y.; Vidal, R.; O’Ryan, G.M. Norovirus vaccines under development. Vaccine 2018, 36, 5435–5441. [Google Scholar] [CrossRef]
- Ebenezer, O.; Jordaan, M.A.; Damoyi, N.; Shapi, M. Discovery of Potential Inhibitors for RNA-Dependent RNA Polymerase of Norovirus: Virtual Screening, and Molecular Dynamics. Int. J. Mol. Sci. 2020, 22, 171. [Google Scholar] [CrossRef]
- Yates, M.K.; Seley-Radtke, K.L. The evolution of antiviral nucleoside analogues: A review for chemists and non-chemists. Part II: Complex modifications to the nucleoside scaffold. Antiviral. Res. 2019, 162, 5–21. [Google Scholar] [CrossRef] [PubMed]
- Mastrangelo, E.; Pezzullo, M.; Tarantino, D.; Petazzi, R.; Germani, F.; Kramer, D.; Robel, I.; Rohayem, J.; Bolognesi, M.; Milani, M. Structure-based inhibition of Norovirus RNA-dependent RNA polymerases. J. Mol. Biol. 2012, 419, 198–210. [Google Scholar] [CrossRef]
- Kawsar, S.M.A.; Hosen, M.A.; Ahmad, S.; El Bakri, Y.; Laaroussi, H.; Ben Hadda, T.; Almalki, F.A.; Ozeki, Y.; Goumri-Said, S. Potential SARS-CoV-2 RdRp inhibitors of cytidine derivatives: Molecular docking, molecular dynamic simulations, ADMET, and POM analyses for the identification of pharmacophore sites. PLoS ONE 2022, 28, e0273256. [Google Scholar] [CrossRef]
- Bassetto, M.; Van Dycke, J.; Neyts, J.; Brancale, A.; Rocha-Pereira, J. Targeting the Viral Polymerase of Diarrhea-Causing Viruses as a Strategy to Develop a Single Broad-Spectrum Antiviral Therapy. Viruses 2019, 11, 173. [Google Scholar] [CrossRef] [Green Version]
- Alam, A.; Agrawal, G.P.; Khan, S.; Khalilullah, H.; Saifullah, M.K.; Arshad, M.F. Towards the discovery of potential RdRp inhibitors for the treatment of COVID-19: Structure guided virtual screening, computational ADME and molecular dynamics study. Struct. Chem. 2022, 33, 1569–1583. [Google Scholar] [CrossRef]
- Murali, M.; Gowtham, H.G.; Ansari, M.A.; Alomary, M.N.; Alghamdi, S.; Almehmadi, M.; Singh, S.B.; Shilpa, N.; Aiyaz, M.; Kalegowda, N.; et al. Repositioning Therapeutics for SARS-CoV-2: Virtual Screening of Plant-based Anti-HIV Compounds as Possible Inhibitors against COVID-19 Viral RdRp. Curr. Pharm. Des. 2022, 28, 969–980. [Google Scholar] [CrossRef]
- Tutone, M.; Almerico, A.M. Computational Approaches: Drug Discovery and Design in Medicinal Chemistry and Bioinformatics. Molecules 2021, 26, 7500. [Google Scholar] [CrossRef] [PubMed]
- Atiya, A.; Shahidi, H.; Mohammad, T.; Sharaf, S.E.; Abdulmonem, W.A.; Ashraf, G.M.; Elasbali, A.M.; Alharethi, S.H.; Alhumaydhi, F.A.; Baeesa, S.S.; et al. A virtual screening investigation to identify bioactive natural compounds as potential inhibitors of cyclin-dependent kinase 9. J. Biomol. Struct. Dyn. 2022, 23, 1–12. [Google Scholar] [CrossRef]
- Asseri, A.H.; Alam, J.; Alzahrani, F.; Khames, A.; Pathan, M.T.; Abourehab, M.A.S.; Hosawi, S.; Ahmed, R.; Sultana, S.A.; Alam, N.F.; et al. Toward the Identification of Natural Antiviral Drug Candidates against Merkel Cell Polyomavirus: Computational Drug Design Approaches. Pharmaceuticals 2022, 15, 501. [Google Scholar] [CrossRef]
- Abduljaleel, Z.; Shahzad, N.; Aziz, S.A.; Malik, S.M. Monoclonal antibody designed for SARS-nCoV-2 spike protein of receptor binding domain on antigenic targeted epitopes for inhibition to prevent viral entry. Mol. Divers. 2022, 26, 1–14. [Google Scholar] [CrossRef]
- Maia, E.H.B.; Assis, L.C.; de Oliveira, T.A.; da Silva, A.M.; Taranto, A.G. Structure-Based Virtual Screening: From Classical to Artificial Intelligence. Front. Chem. 2020, 8, 343. [Google Scholar] [CrossRef] [PubMed]
- Shamshad, H.; Saeed, M.; Ul-Haq, Z.; Halim, S.A.; Gul, S.; Mirza, A.Z. Relative assessment of different statistical instruments and measures for the prediction of promising outcomes using docking, virtual screening and ADMET analysis against HIV-RT. J. Biomol. Struct. Dyn. 2022, 40, 7680–7692. [Google Scholar] [CrossRef]
- Shawky, A.M.; Ibrahim, N.A.; Abourehab, M.A.S.; Abdalla, A.N.; Gouda, A.M. Pharmacophore-based virtual screening, synthesis, biological evaluation, and molecular docking study of novel pyrrolizines bearing urea/thiourea moieties with potential cytotoxicity and CDK inhibitory activities. J. Enzyme. Inhib. Med. Chem. 2021, 36, 15–33. [Google Scholar] [CrossRef] [PubMed]
- Kapetanovic, I.M. Computer-aided drug discovery and development (CADDD): In silico-chemico-biological approach. Chem. Biol. Interact. 2008, 171, 165–176. [Google Scholar] [CrossRef] [Green Version]
- Venkataraman, S.; Prasad, B.; Selvarajan, R. RNA Dependent RNA Polymerases: Insights from Structure, Function and Evolution. Viruses 2018, 10, 76. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deval, J.; Jin, Z.; Chuang, Y.C.; Kao, C.C. Structure(s), function(s), and inhibition of the RNA-dependent RNA polymerase of noroviruses. Virus Res. 2017, 234, 21–33. [Google Scholar] [CrossRef]
- Ebenezer, O.; Damoyi, N.; Jordaan, M.A.; Shapi, M. Unveiling of Pyrimidindinones as Potential Anti-Norovirus Agents—A Pharmacoinformatic-Based Approach. Molecules 2022, 27, 380. [Google Scholar] [CrossRef]
- Giancotti, G.; Rigo, I.; Pasqualetto, G.; Young, M.T.; Neyts, J.; Rocha-Pereira, J.; Brancale, A.; Ferla, S.; Bassetto, M. A new antiviral scaffold for human norovirus identified with computer-aided approaches on the viral polymerase. Sci. Rep. 2019, 9, 18413. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meng, X.Y.; Zhang, H.X.; Mezei, M.; Cui, M. Molecular docking: A powerful approach for structure-based drug discovery. Curr. Comput. Aided Drug. Des. 2011, 7, 146–157. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.F.; Tan, M.; Chhabra, M.; Dai, Y.C.; Meller, J.; Jiang, X. Inhibition of histo-blood group antigen binding as a novel strategy to block norovirus infections. PLoS ONE 2013, 8, e69379. [Google Scholar] [CrossRef] [Green Version]
- Koromyslova, A.; Tripathi, S.; Morozov, V.; Schroten, H.; Hansman, G.S. Human norovirus inhibition by a human milk oligosaccharide. Virology 2017, 508, 81–89. [Google Scholar] [CrossRef]
- Netzler, N.E.; Enosi Tuipulotu, D.; White, P.A. Norovirus antivirals: Where are we now? Med. Res. Rev. 2019, 39, 860–886. [Google Scholar] [CrossRef] [PubMed]
- Harmalkar, D.S.; Lee, S.J.; Lu, Q.; Kim, M.I.; Park, J.; Lee, H.; Park, M.; Lee, A.; Lee, C.; Lee, K. Identification of novel non-nucleoside vinyl-stilbene analogs as potent norovirus replication inhibitors with a potential host-targeting mechanism. Eur. J. Med. Chem. 2019, 184, 111733. [Google Scholar] [CrossRef]
- Rossignol, J.F.; El-Gohary, Y.M. Nitazoxanide in the treatment of viral gastroenteritis: A randomized double-blind placebo-controlled clinical trial. Aliment Pharmacol. Ther. 2006, 24, 1423–1430. [Google Scholar] [CrossRef]
- Croci, R.; Pezzullo, M.; Tarantino, D.; Milani, M.; Tsay, S.C.; Sureshbabu, R.; Tsai, Y.J.; Mastrangelo, E.; Rohayem, J.; Bolognesi, M.; et al. Structural bases of norovirus RNA dependent RNA polymerase inhibition by novel suramin-related compounds. PLoS ONE 2014, 9, e91765. [Google Scholar] [CrossRef]
- Atanasov, A.G.; Zotchev, S.B.; Dirsch, V.M.; International Natural Product Sciences, T.; Supuran, C.T. Natural products in drug discovery: Advances and opportunities. Nat. Rev. Drug. Discov. 2021, 20, 200–216. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.T.; Hsu, W.C.; Lin, C.C. Antiviral natural products and herbal medicines. J. Tradit. Complement. Med. 2014, 4, 24–35. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mazola, Y.; Guirola, O.; Palomares, S.; Chinea, G.; Menendez, C.; Hernandez, L.; Musacchio, A. A comparative molecular dynamics study of thermophilic and mesophilic beta-fructosidase enzymes. J. Mol. Model. 2015, 21, 228. [Google Scholar] [CrossRef] [PubMed]
- Maia, E.H.; Campos, V.A.; Dos Reis Santos, B.; Costa, M.S.; Lima, I.G.; Greco, S.J.; Ribeiro, R.I.; Munayer, F.M.; da Silva, A.M.; Taranto, A.G. Octopus: A platform for the virtual high-throughput screening of a pool of compounds against a set of molecular targets. J. Mol. Model. 2017, 23, 26. [Google Scholar] [CrossRef]
- Dallakyan, S.; Olson, A.J. Small-molecule library screening by docking with PyRx. Methods Mol. Biol. 2015, 1263, 243–250. [Google Scholar] [CrossRef]
- López-López, E.; Naveja, J.J.; Medina-Franco, J.L. DataWarrior: An evaluation of the open-source drug discovery tool. Expert Opin. Drug Discov. 2019, 14, 335–341. [Google Scholar] [CrossRef]
- Pol-Fachin, L.; Fernandes, C.L.; Verli, H. GROMOS96 43a1 performance on the characterization of glycoprotein conformational ensembles through molecular dynamics simulations. Carbohydr. Res. 2009, 344, 491–500. [Google Scholar] [CrossRef]
- Malik, M.S.; Alsantali, R.I.; Jamal, Q.M.S.; Seddigi, Z.S.; Morad, M.; Alsharif, M.A.; Hussein, E.M.; Jassas, R.S.; Al-Rooqi, M.M.; Abduljaleel, Z.; et al. New Imidazole-Based N-Phenylbenzamide Derivatives as Potential Anticancer Agents: Key Computational Insights. Front. Chem. 2022, 19, 808556. [Google Scholar] [CrossRef] [PubMed]
- Schuttelkopf, A.W.; van Aalten, D.M. PRODRG: A tool for high-throughput crystallography of protein-ligand complexes. Acta Crystallogr. D Biol. Crystallogr. 2004, 60, 1355–1363. [Google Scholar] [CrossRef] [PubMed] [Green Version]


| Ligand | Binding Energy (Kcal/mol) |
|---|---|
| ZINC69482526 | −9.9 |
| ZINC69482529 | −9.9 |
| ZINC66112069 | −9.7 |
| ZINC69481856 | −9.6 |
| ZINC69482059 | −9.5 |
| ZINC69481850 | −9.4 |
| ZINC69482023 | −9.3 |
| ZINC69481956 | −9.3 |
| ZINC69482024 | −9.3 |
| ZINC69482510 | −9.2 |
| ZINC04097720 | −9.1 |
| ZINC69482364 | −9.1 |
| PPNDS | −9 |
| Molecule Name | Mol Weight | cLogP | cLogS | H-Bond | Drug Likeness Score | Mutagenic | Tumorigenic | Reproductive Effective | Irritant | Drug Score | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Acceptor | Donor | ||||||||||
| ZINC69482510 | 392.493 | 4.5121 | −4.402 | 4 | 1 | 1.0176 | N | N | N | N | 0.51537 |
| ZINC66112069 | 436.59 | 4.4475 | −4.742 | 4 | 2 | 0.4587 | N | N | N | N | 0.433376 |
| ZINC69482023 | 414.651 | 4.4768 | −5.443 | 3 | 2 | 0.37333 | N | N | N | N | 0.312927 |
| ZINC69482526 | 544.639 | 1.2624 | −4.575 | 9 | 2 | −2.3645 | N | N | N | N | 0.294769 |
| ZINC69482059 | 497.737 | 4.4729 | −6.538 | 4 | 0 | −0.333 | N | N | N | N | 0.255883 |
| ZINC69482024 | 430.65 | 3.5501 | −4.936 | 4 | 3 | −2.6117 | N | N | N | N | 0.248718 |
| ZINC69481856 | 532.631 | 3.1858 | −5.326 | 7 | 0 | −3.4332 | N | N | N | N | 0.238752 |
| ZINC69482529 | 613.769 | 2.2649 | −5.844 | 9 | 2 | −1.6313 | N | N | N | N | 0.220738 |
| ZINC69481956 | 472.707 | 5.4045 | −5.643 | 4 | 2 | 0.36826 | N | N | N | N | 0.177611 |
| ZINC69481850 | 520.704 | 3.2548 | −4.713 | 7 | 4 | −0.02161 | N | N | N | N | 0.14069 |
| ZINC04097720 | 426.726 | 7.5888 | −6.968 | 1 | 0 | −3.3053 | N | N | N | N | 0.132645 |
| ZINC69482364 | 424.754 | 8.5403 | −7.379 | 0 | 0 | −7.4295 | N | N | N | N | 0.119384 |
| Compound | Structure | H-Bonded Residues | Van der Waals Interactions |
|---|---|---|---|
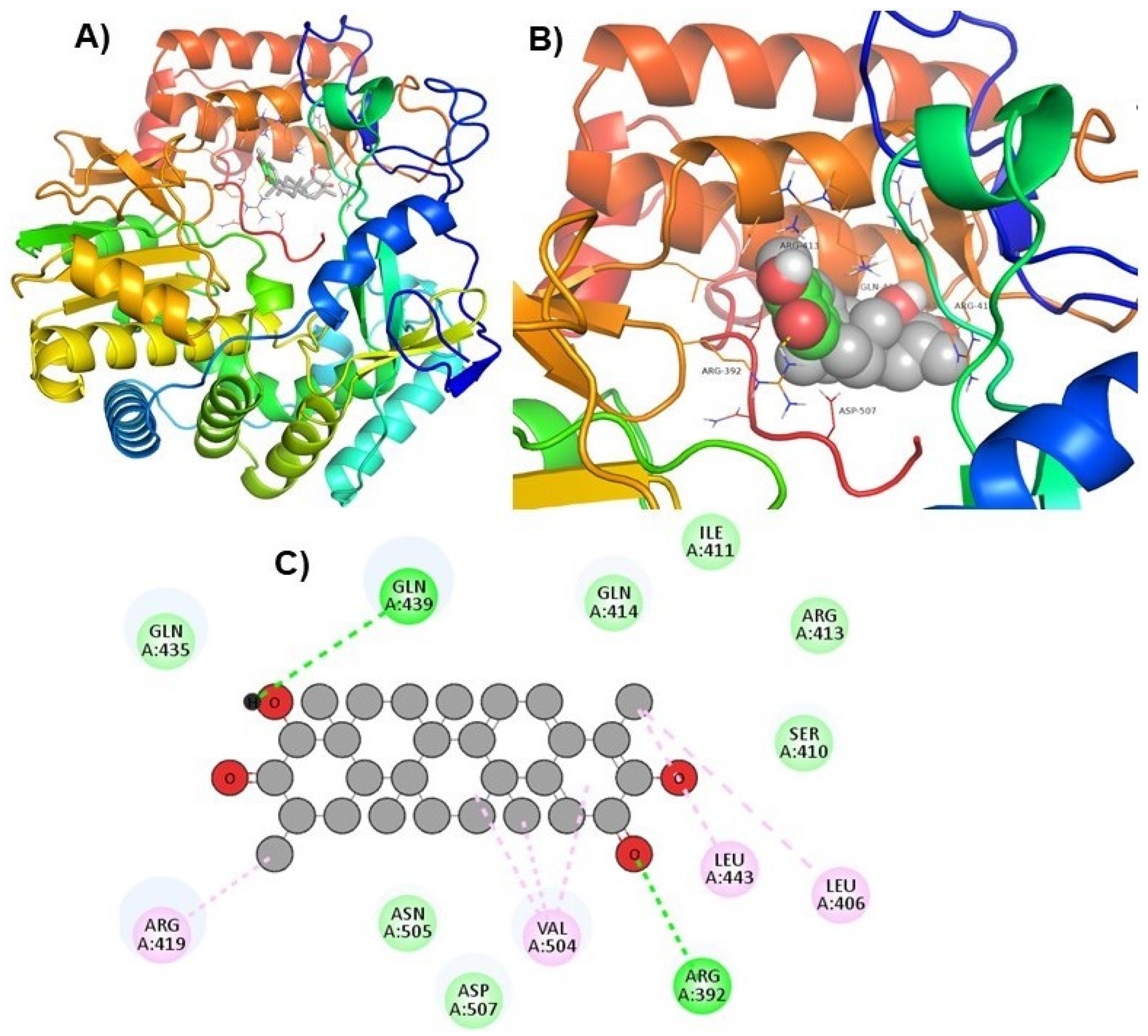
| ZINC66112069 | 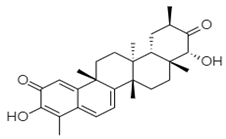 | Arg392 and Gln439 | Ser410, Ile411, Arg413, Gln414, Gln435, Asp507, and Asn505 |
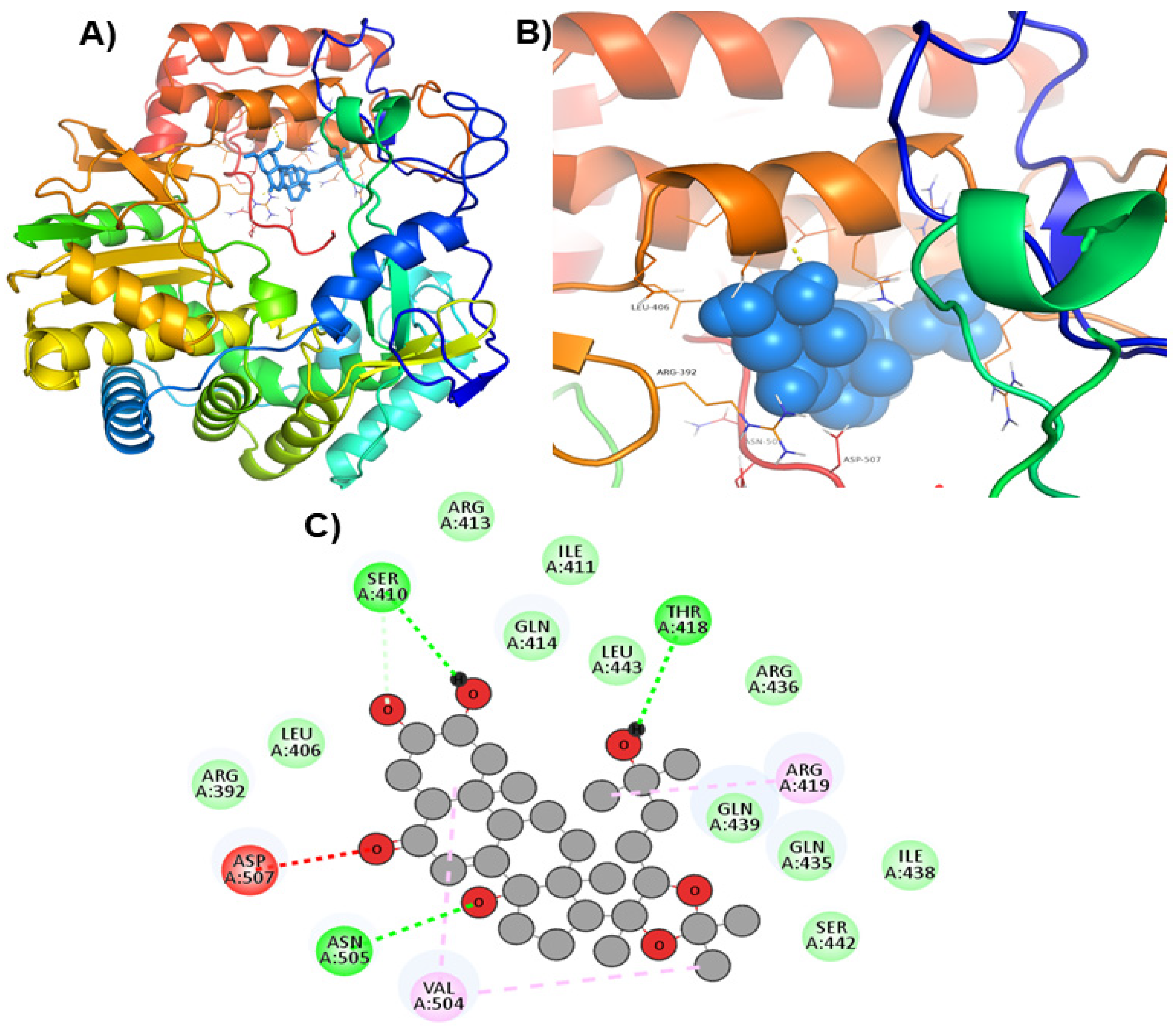
| ZINC69481850 | 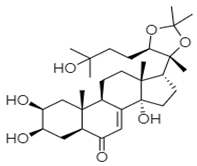 | Ser410, Thr418, and Asn505 | Arg392, Leu406, Ile411, Arg413, Gln414, Gln435, Arg436, Ile438, Gln439, Ser442, and Leu443 |
| PPNDS * |  | Arg413, Asp507, and Glu510 | Glu168, Arg392, Ser410, Ile411, Gln414, Arg419, and Val509, |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Obaid, R.J.; Shafie, A.; Malik, M.S.; Al-Rooqi, M.M.; Moussa, Z.; Abdulaziz, O.; Aljuaid, A.; Allahyani, M.; Almehmadi, M.; Anjum, F.; et al. In Silico Screening and Molecular Dynamics Simulation Studies in the Identification of Natural Compound Inhibitors Targeting the Human Norovirus RdRp Protein to Fight Gastroenteritis. Int. J. Mol. Sci. 2023, 24, 5003. https://doi.org/10.3390/ijms24055003
Obaid RJ, Shafie A, Malik MS, Al-Rooqi MM, Moussa Z, Abdulaziz O, Aljuaid A, Allahyani M, Almehmadi M, Anjum F, et al. In Silico Screening and Molecular Dynamics Simulation Studies in the Identification of Natural Compound Inhibitors Targeting the Human Norovirus RdRp Protein to Fight Gastroenteritis. International Journal of Molecular Sciences. 2023; 24(5):5003. https://doi.org/10.3390/ijms24055003
Chicago/Turabian StyleObaid, Rami J., Alaa Shafie, M. Shaheer Malik, Munirah M. Al-Rooqi, Ziad Moussa, Osama Abdulaziz, Abdulelah Aljuaid, Mamdouh Allahyani, Mazen Almehmadi, Farah Anjum, and et al. 2023. "In Silico Screening and Molecular Dynamics Simulation Studies in the Identification of Natural Compound Inhibitors Targeting the Human Norovirus RdRp Protein to Fight Gastroenteritis" International Journal of Molecular Sciences 24, no. 5: 5003. https://doi.org/10.3390/ijms24055003
APA StyleObaid, R. J., Shafie, A., Malik, M. S., Al-Rooqi, M. M., Moussa, Z., Abdulaziz, O., Aljuaid, A., Allahyani, M., Almehmadi, M., Anjum, F., & Ahmed, S. A. (2023). In Silico Screening and Molecular Dynamics Simulation Studies in the Identification of Natural Compound Inhibitors Targeting the Human Norovirus RdRp Protein to Fight Gastroenteritis. International Journal of Molecular Sciences, 24(5), 5003. https://doi.org/10.3390/ijms24055003






