Molecular and Cellular Regulations in the Development of the Choroidal Circulation System
Abstract
:1. Introduction
2. The General Development of the Ocular System
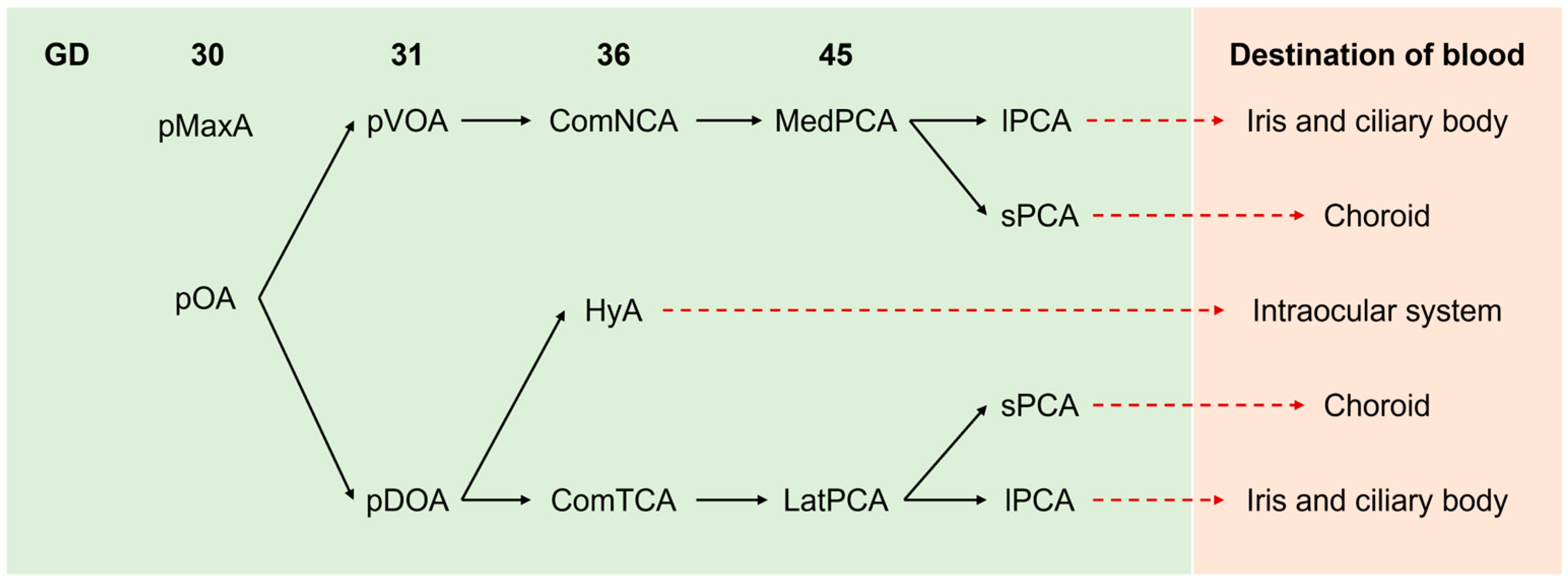
3. The Embryonic Blood Supply to the Eyes
4. Overview of Intraocular Vascularization
5. Anatomy of the Choroid
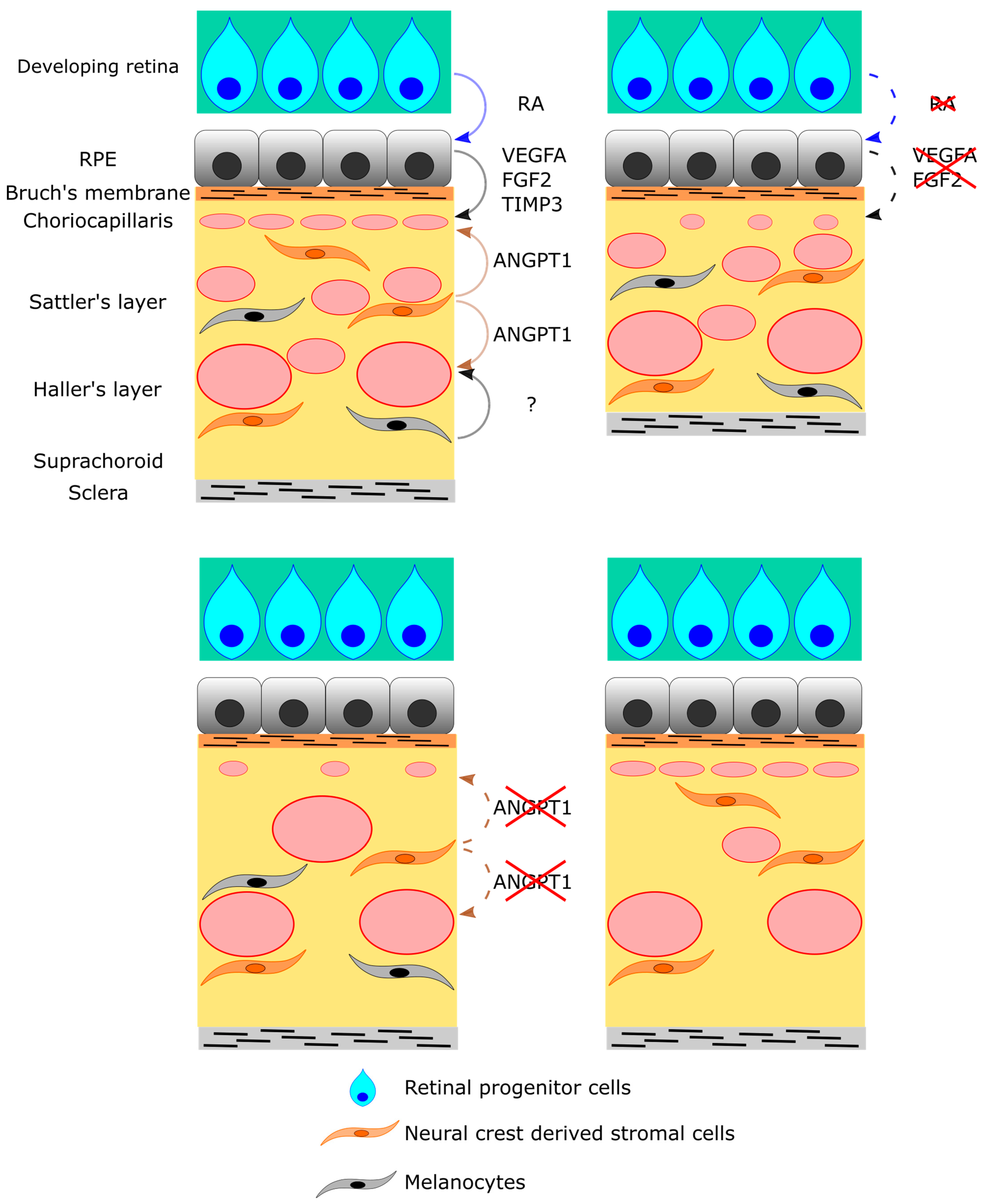
6. Cellular and Molecular Regulation of Choroid Development
7. RPE Cells
| Model Mouse | Genetic Modification | Phenotype | Relevant Disease | Ref |
|---|---|---|---|---|
| Tyrp1-Fgf9 | Overexpression in RPE | Lack of RPE Lack of choroid | [40] | |
| βB1-Tgfb1 | Overexpression in lens | Lack of RPE Choriocapillaris atrophy | [41] | |
| Best1-rtTA;TRE-Cre;Vegfaflox/flox | Knockout in RPE (drug-inducible) | Hypoplasia of choriocapillaris Hypoplasia of large vessels | [42] | |
| Tyrp1-Cretg/0;Vegfaflox/flox | Knockout in RPE | Hypoplasia of choriocapillaris | [43] | |
| Best1-Cre;Vegfaflox/flox | Knockout in RPE | Hypoplasia of choriocapillaris Elongation of axial length Decreased refraction | Myopia | [44] |
| Best1-Cre;Vhlflox/flox | Knockout in RPE | Vasodilatation | [44] | |
| Tyrp1-trFgfr1 | Knockout in RPE | Immature choriocapillaris | [39] | |
| Lrp2267/− | Heterozygote knockout with the missense mutant at amino acid position 2721 of the LRP2 (line 267) | Enlarged and exophthalmic eyes Thinning of retina Hyperplasia of non-pigmented epithelium of the ciliary body Elongation of axial length | Donnai–Barrow syndrome | [53] |
| Best1-Cre;Lrp2flox/flox | Knockout in RPE | Enlarged eyes Thinning of retina Elongation of axial length Hypoplasia of choriocapillaris Thinning of sclera | Donnai–Barrow syndromeSevere myopia | [44] |
| Chx10-Cre;Lrp2flox/flox | Knockout in neural retina | No phenotype | [44] | |
| Timp3 KO | Total knockout | Dilated choroidal vessels | Sorsby fundus dystrophy | [55] |
| Aldh1a KO | Total knockout | Hypoplasia of choriocapillaris (Dorsal specific) | [59] | |
| Dct-Cre;Sox9flox/flox | Knockout in RPE | Hypoplasia of choriocapillaris | [60] | |
| Wnt1-Cre;Angpt1flox/flox | Knockout in neural crest cells | At birth Hypoplasia of choriocapillaris, dilated venules Reduction in vortex vein number 1 year of age Pachyvessels in the choroid RPE dysplasia Subretinal choroidal neovascularization | Polypoidal choroidal vasculopathy Central serous chorioretinopathy | [61] |
| Mitfmi-bw/Mitfmi-bw | Knockout in melanocytes | Normal choriocapillaris Reduced branching of posterior ciliary arteries and collected venules | Waardenburg syndrome type 2 | [62] |
| Ihh KO | Total knockout | Misshapen eyes Hypopigmentation in RPE Immature sclera Choroid thinning | [63] |
8. Retinal Progenitor Cells
9. Neural Crest-Derived Stromal Cells
10. Melanocytes
11. The Choroid as a Regulator of Ocular Development
12. Summary
Author Contributions
Funding
Conflicts of Interest
References
- Saint-Geniez, M.; D’Amore, P.A. Development and Pathology of the Hyaloid, Choroidal and Retinal Vasculature. Int. J. Dev. Biol. 2004, 48, 1045–1058. [Google Scholar] [CrossRef] [Green Version]
- Gilmour, D.F. Familial Exudative Vitreoretinopathy and Related Retinopathies. Eye Lond. Engl. 2015, 29, 1–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sarkar, H.; Moosajee, M. Choroideremia: Molecular Mechanisms and Therapies. Trends Mol. Med. 2022, 28, 378–387. [Google Scholar] [CrossRef] [PubMed]
- Kazmierczak de Camargo, J.P.; de Barros Prezia, G.N.; Shiokawa, N.; Sato, M.T.; Rosati, R.; Boldt, A.B.W. A New Insights on the Regulatory Gene Network Disturbed in Central Areolar Choroidal Dystrophy-Beyond Classical Gene Candidates. Front. Genet. 2022, 13, 886461. [Google Scholar] [CrossRef] [PubMed]
- Balfoort, B.M.; Buijs, M.J.N.; Ten Asbroek, A.L.M.A.; Bergen, A.A.B.; Boon, C.J.F.; Ferreira, E.A.; Houtkooper, R.H.; Wagenmakers, M.A.E.M.; Wanders, R.J.A.; Waterham, H.R.; et al. A Review of Treatment Modalities in Gyrate Atrophy of the Choroid and Retina (GACR). Mol. Genet. Metab. 2021, 134, 96–116. [Google Scholar] [CrossRef] [PubMed]
- Fitzpatrick, D.R.; van Heyningen, V. Developmental Eye Disorders. Curr. Opin. Genet. Dev. 2005, 15, 348–353. [Google Scholar] [CrossRef] [PubMed]
- Yun, S.; Saijoh, Y.; Hirokawa, K.E.; Kopinke, D.; Murtaugh, L.C.; Monuki, E.S.; Levine, E.M. Lhx2 Links the Intrinsic and Extrinsic Factors That Control Optic Cup Formation. Dev. Camb. Engl. 2009, 136, 3895–3906. [Google Scholar] [CrossRef] [Green Version]
- Jasrapuria-Agrawal, S.; Lwigale, P.Y. Neural Crest Cells in Ocular Development. In Neural Crest Cells; Elsevier: Amsterdam, The Netherlands, 2014; pp. 189–203. ISBN 978-0-12-401730-6. [Google Scholar]
- Swamynathan, S.K. Ocular Surface Development and Gene Expression. J. Ophthalmol. 2013, 2013, 103947. [Google Scholar] [CrossRef] [Green Version]
- Johnston, M.C.; Noden, D.M.; Hazelton, R.D.; Coulombre, J.L.; Coulombre, A.J. Origins of Avian Ocular and Periocular Tissues. Exp. Eye Res. 1979, 29, 27–43. [Google Scholar] [CrossRef]
- Le Lièvre, C.S.; Le Douarin, N.M. Mesenchymal Derivatives of the Neural Crest: Analysis of Chimaeric Quail and Chick Embryos. J. Embryol. Exp. Morphol. 1975, 34, 125–154. [Google Scholar] [CrossRef]
- Gage, P.J.; Rhoades, W.; Prucka, S.K.; Hjalt, T. Fate Maps of Neural Crest and Mesoderm in the Mammalian Eye. Investig. Opthalmol. Vis. Sci. 2005, 46, 4200. [Google Scholar] [CrossRef]
- Lwigale, P.Y. Corneal Development. In Progress in Molecular Biology and Translational Science; Elsevier: Amsterdam, The Netherlands, 2015; Volume 134, pp. 43–59. ISBN 978-0-12-801059-4. [Google Scholar]
- Hall, H.N.; Williamson, K.A.; FitzPatrick, D.R. The Genetic Architecture of Aniridia and Gillespie Syndrome. Hum. Genet. 2019, 138, 881–898. [Google Scholar] [CrossRef] [Green Version]
- Davis-Silberman, N.; Ashery-Padan, R. Iris Development in Vertebrates; Genetic and Molecular Considerations. Brain Res. 2008, 1192, 17–28. [Google Scholar] [CrossRef]
- Cvekl, A.; Tamm, E.R. Anterior Eye Development and Ocular Mesenchyme: New Insights from Mouse Models and Human Diseases. BioEssays 2004, 26, 374–386. [Google Scholar] [CrossRef] [Green Version]
- Pilon, N. Treatment and Prevention of Neurocristopathies. Trends Mol. Med. 2021, 27, 451–468. [Google Scholar] [CrossRef]
- McMenamin, P.G.; Shields, G.T.; Seyed-Razavi, Y.; Kalirai, H.; Insall, R.H.; Machesky, L.M.; Coupland, S.E. Melanoblasts Populate the Mouse Choroid Earlier in Development Than Previously Described. Investig. Opthalmol. Vis. Sci. 2020, 61, 33. [Google Scholar] [CrossRef]
- Tawfik, H.A.; Abdulhafez, M.H.; Fouad, Y.A.; Dutton, J.J. Embryologic and Fetal Development of the Human Eyelid. Ophthal. Plast. Reconstr. Surg. 2016, 32, 407–414. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tao, H.; Shimizu, M.; Kusumoto, R.; Ono, K.; Noji, S.; Ohuchi, H. A Dual Role of FGF10 in Proliferation and Coordinated Migration of Epithelial Leading Edge Cells during Mouse Eyelid Development. Development 2005, 132, 3217–3230. [Google Scholar] [CrossRef] [Green Version]
- Findlater, G.S.; McDougall, R.D.; Kaufman, M.H. Eyelid Development, Fusion and Subsequent Reopening in the Mouse. J. Anat. 1993, 183 Pt 1, 121–129. [Google Scholar] [PubMed]
- de la Cuadra-Blanco, C.; Peces-Peña, M.D.; Mérida-Velasco, J.R. Morphogenesis of the Human Lacrimal Gland. J. Anat. 2003, 203, 531–536. [Google Scholar] [CrossRef] [PubMed]
- Garg, A.; Zhang, X. Lacrimal Gland Development: From Signaling Interactions to Regenerative Medicine: Lacrimal Gland Development and Regeneration. Dev. Dyn. 2017, 246, 970–980. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bertulli, L.; Robert, T. Embryological Development of the Human Cranio-Facial Arterial System: A Pictorial Review. Surg. Radiol. Anat. 2021, 43, 961–973. [Google Scholar] [CrossRef] [PubMed]
- Toma, N. Anatomy of the Ophthalmic Artery: Embryological Consideration. Neurol. Med. Chir. (Tokyo) 2016, 56, 585–591. [Google Scholar] [CrossRef] [Green Version]
- Lang, R.A.; Bishop, J.M. Macrophages Are Required for Cell Death and Tissue Remodeling in the Developing Mouse Eye. Cell 1993, 74, 453–462. [Google Scholar] [CrossRef] [PubMed]
- Silbert, M.; Gurwood, A.S. Persistent Hyperplastic Primary Vitreous. Clin. Eye Vis. Care 2000, 12, 131–137. [Google Scholar] [CrossRef]
- Dorrell, M.I.; Aguilar, E.; Friedlander, M. Retinal Vascular Development Is Mediated by Endothelial Filopodia, a Preexisting Astrocytic Template and Specific R-Cadherin Adhesion. Investig. Ophthalmol. Vis. Sci. 2002, 43, 3500–3510. [Google Scholar]
- Gariano, R.F.; Gardner, T.W. Retinal Angiogenesis in Development and Disease. Nature 2005, 438, 960–966. [Google Scholar] [CrossRef]
- Pitulescu, M.E.; Schmidt, I.; Benedito, R.; Adams, R.H. Inducible Gene Targeting in the Neonatal Vasculature and Analysis of Retinal Angiogenesis in Mice. Nat. Protoc. 2010, 5, 1518–1534. [Google Scholar] [CrossRef] [PubMed]
- Okabe, K.; Kobayashi, S.; Yamada, T.; Kurihara, T.; Tai-Nagara, I.; Miyamoto, T.; Mukouyama, Y.; Sato, T.N.; Suda, T.; Ema, M.; et al. Neurons Limit Angiogenesis by Titrating VEGF in Retina. Cell 2014, 159, 584–596. [Google Scholar] [CrossRef] [Green Version]
- Rao, S.; Chun, C.; Fan, J.; Kofron, J.M.; Yang, M.B.; Hegde, R.S.; Ferrara, N.; Copenhagen, D.R.; Lang, R.A. A Direct and Melanopsin-Dependent Fetal Light Response Regulates Mouse Eye Development. Nature 2013, 494, 243–246. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, M.-T.T.; Vemaraju, S.; Nayak, G.; Odaka, Y.; Buhr, E.D.; Alonzo, N.; Tran, U.; Batie, M.; Upton, B.A.; Darvas, M.; et al. An Opsin 5-Dopamine Pathway Mediates Light-Dependent Vascular Development in the Eye. Nat. Cell Biol. 2019, 21, 420–429. [Google Scholar] [CrossRef] [PubMed]
- Milde, F.; Lauw, S.; Koumoutsakos, P.; Iruela-Arispe, M.L. The Mouse Retina in 3D: Quantification of Vascular Growth and Remodeling. Integr. Biol. Quant. Biosci. Nano Macro. 2013, 5, 1426–1438. [Google Scholar] [CrossRef] [Green Version]
- Gerhardt, H.; Golding, M.; Fruttiger, M.; Ruhrberg, C.; Lundkvist, A.; Abramsson, A.; Jeltsch, M.; Mitchell, C.; Alitalo, K.; Shima, D.; et al. VEGF Guides Angiogenic Sprouting Utilizing Endothelial Tip Cell Filopodia. J. Cell Biol. 2003, 161, 1163–1177. [Google Scholar] [CrossRef]
- Nickla, D.L.; Wallman, J. The Multifunctional Choroid. Prog. Retin. Eye Res. 2010, 29, 144–168. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hasegawa, T.; McLeod, D.S.; Bhutto, I.A.; Prow, T.; Merges, C.A.; Grebe, R.; Lutty, G.A. The Embryonic Human Choriocapillaris Develops by Hemo-Vasculogenesis. Dev. Dyn. Off. Publ. Am. Assoc. Anat. 2007, 236, 2089–2100. [Google Scholar] [CrossRef] [Green Version]
- Lutty, G.A.; McLeod, D.S. Development of the Hyaloid, Choroidal and Retinal Vasculatures in the Fetal Human Eye. Prog. Retin. Eye Res. 2018, 62, 58–76. [Google Scholar] [CrossRef] [PubMed]
- Rousseau, B.; Larrieu-Lahargue, F.; Bikfalvi, A.; Javerzat, S. Involvement of Fibroblast Growth Factors in Choroidal Angiogenesis and Retinal Vascularization. Exp. Eye Res. 2003, 77, 147–156. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Overbeek, P.A. Regulation of Choroid Development by the Retinal Pigment Epithelium. Mol. Vis. 2001, 7, 277–282. [Google Scholar] [PubMed]
- Ohlmann, A.; Scholz, M.; Koch, M.; Tamm, E.R. Epithelial-Mesenchymal Transition of the Retinal Pigment Epithelium Causes Choriocapillaris Atrophy. Histochem. Cell Biol. 2016, 146, 769–780. [Google Scholar] [CrossRef]
- Le, Y.-Z.; Bai, Y.; Zhu, M.; Zheng, L. Temporal Requirement of RPE-Derived VEGF in the Development of Choroidal Vasculature. J. Neurochem. 2010, 112, 1584–1592. [Google Scholar] [CrossRef] [Green Version]
- Marneros, A.G.; Fan, J.; Yokoyama, Y.; Gerber, H.P.; Ferrara, N.; Crouch, R.K.; Olsen, B.R. Vascular Endothelial Growth Factor Expression in the Retinal Pigment Epithelium Is Essential for Choriocapillaris Development and Visual Function. Am. J. Pathol. 2005, 167, 1451–1459. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Jeong, H.; Mori, K.; Ikeda, S.-I.; Shoda, C.; Miwa, Y.; Nakai, A.; Chen, J.; Ma, Z.; Jiang, X.; et al. Vascular Endothelial Growth Factor from Retinal Pigment Epithelium Is Essential in Choriocapillaris and Axial Length Maintenance. PNAS Nexus 2022, 1, pgac166. [Google Scholar] [CrossRef] [PubMed]
- Kurihara, T.; Westenskow, P.D.; Bravo, S.; Aguilar, E.; Friedlander, M. Targeted Deletion of Vegfa in Adult Mice Induces Vision Loss. J. Clin. Investig. 2012, 122, 4213–4217. [Google Scholar] [CrossRef] [Green Version]
- Kurihara, T.; Westenskow, P.D.; Gantner, M.L.; Usui, Y.; Schultz, A.; Bravo, S.; Aguilar, E.; Wittgrove, C.; Friedlander, M.S.; Paris, L.P.; et al. Hypoxia-Induced Metabolic Stress in Retinal Pigment Epithelial Cells Is Sufficient to Induce Photoreceptor Degeneration. eLife 2016, 5, e14319. [Google Scholar] [CrossRef]
- Kim, S.A.; Kim, S.J.; Choi, Y.A.; Yoon, H.-J.; Kim, A.; Lee, J. Retinal VEGFA Maintains the Ultrastructure and Function of Choriocapillaris by Preserving the Endothelial PLVAP. Biochem. Biophys. Res. Commun. 2020, 522, 240–246. [Google Scholar] [CrossRef] [PubMed]
- Ford, K.M.; Saint-Geniez, M.; Walshe, T.; Zahr, A.; D’Amore, P.A. Expression and Role of VEGF in the Adult Retinal Pigment Epithelium. Investig. Ophthalmol. Vis. Sci. 2011, 52, 9478–9487. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saint-Geniez, M.; Maldonado, A.E.; D’Amore, P.A. VEGF Expression and Receptor Activation in the Choroid during Development and in the Adult. Investig. Opthalmol. Vis. Sci. 2006, 47, 3135. [Google Scholar] [CrossRef] [Green Version]
- Blaauwgeers, H.G.; Holtkamp, G.M.; Rutten, H.; Witmer, A.N.; Koolwijk, P.; Partanen, T.A.; Alitalo, K.; Kroon, M.E.; Kijlstra, A.; van Hinsbergh, V.W.; et al. Polarized Vascular Endothelial Growth Factor Secretion by Human Retinal Pigment Epithelium and Localization of Vascular Endothelial Growth Factor Receptors on the Inner Choriocapillaris. Evidence for a Trophic Paracrine Relation. Am. J. Pathol. 1999, 155, 421–428. [Google Scholar] [CrossRef] [PubMed]
- Longoni, M.; Kantarci, S.; Donnai, D.; Pober, B.R. Donnai-Barrow Syndrome. In GeneReviews®; Adam, M.P., Everman, D.B., Mirzaa, G.M., Pagon, R.A., Wallace, S.E., Bean, L.J., Gripp, K.W., Amemiya, A., Eds.; University of Washington: Seattle, WA, USA, 1993; Available online: https://www.ncbi.nlm.nih.gov/books/NBK1878/ (accessed on 12 December 2022).
- Pober, B.R.; Longoni, M.; Noonan, K.M. A Review of Donnai-Barrow and Facio-Oculo-Acoustico-Renal (DB/FOAR) Syndrome: Clinical Features and Differential Diagnosis. Birt. Defects Res. Part A Clin. Mol. Teratol. 2009, 85, 76–81. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Christ, A.; Christa, A.; Klippert, J.; Eule, J.C.; Bachmann, S.; Wallace, V.A.; Hammes, A.; Willnow, T.E. LRP2 Acts as SHH Clearance Receptor to Protect the Retinal Margin from Mitogenic Stimuli. Dev. Cell 2015, 35, 36–48. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, Z.; Mao, C.; Jia, Y.; Fu, Y.; Kong, W. Extracellular Matrix Dynamics in Vascular Remodeling. Am. J. Physiol. Cell Physiol. 2020, 319, C481–C499. [Google Scholar] [CrossRef] [PubMed]
- Janssen, A.; Hoellenriegel, J.; Fogarasi, M.; Schrewe, H.; Seeliger, M.; Tamm, E.; Ohlmann, A.; May, C.A.; Weber, B.H.F.; Stöhr, H. Abnormal Vessel Formation in the Choroid of Mice Lacking Tissue Inhibitor of Metalloprotease-3. Investig. Ophthalmol. Vis. Sci. 2008, 49, 2812–2822. [Google Scholar] [CrossRef] [PubMed]
- Anand-Apte, B.; Chao, J.R.; Singh, R.; Stöhr, H. Sorsby Fundus Dystrophy: Insights from the Past and Looking to the Future. J. Neurosci. Res. 2019, 97, 88–97. [Google Scholar] [CrossRef] [Green Version]
- Weber, B.H.; Vogt, G.; Pruett, R.C.; Stöhr, H.; Felbor, U. Mutations in the Tissue Inhibitor of Metalloproteinases-3 (TIMP3) in Patients with Sorsby’s Fundus Dystrophy. Nat. Genet. 1994, 8, 352–356. [Google Scholar] [CrossRef]
- Hess, K.; Raming, K.; Gliem, M.; Charbel Issa, P.; Herrmann, P.; Holz, F.G.; Pfau, M. Choriocapillaris Flow Signal Impairment in Sorsby Fundus Dystrophy. Ophthalmol. J. Int. Ophtalmol. Int. J. Ophthalmol. Z. Augenheilkd. 2022, 245, 265–274. [Google Scholar] [CrossRef] [PubMed]
- Raming, K.; Gliem, M.; Charbel Issa, P.; Birtel, J.; Herrmann, P.; Holz, F.G.; Pfau, M.; Hess, K. Visual Dysfunction and Structural Correlates in Sorsby Fundus Dystrophy. Am. J. Ophthalmol. 2022, 234, 274–284. [Google Scholar] [CrossRef] [PubMed]
- Cohen-Tayar, Y.; Cohen, H.; Mitiagin, Y.; Abravanel, Z.; Levy, C.; Idelson, M.; Reubinoff, B.; Itzkovitz, S.; Raviv, S.; Kaestner, K.H.; et al. Pax6 Regulation of Sox9 in the Mouse Retinal Pigmented Epithelium Controls Its Timely Differentiation and Choroid Vasculature Development. Dev. Camb. Engl. 2018, 145, dev163691. [Google Scholar] [CrossRef] [Green Version]
- Liu, P.; Lavine, J.A.; Fawzi, A.; Quaggin, S.E.; Thomson, B.R. Angiopoietin-1 Is Required for Vortex Vein and Choriocapillaris Development in Mice. Arterioscler. Thromb. Vasc. Biol. 2022, 42, 1413–1427. [Google Scholar] [CrossRef]
- Shibuya, H.; Watanabe, R.; Maeno, A.; Ichimura, K.; Tamura, M.; Wakana, S.; Shiroishi, T.; Ohba, K.; Takeda, K.; Tomita, H.; et al. Melanocytes Contribute to the Vasculature of the Choroid. Genes Genet. Syst. 2018, 93, 51–58. [Google Scholar] [CrossRef] [Green Version]
- Dakubo, G.D.; Mazerolle, C.; Furimsky, M.; Yu, C.; St-Jacques, B.; McMahon, A.P.; Wallace, V.A. Indian Hedgehog Signaling from Endothelial Cells Is Required for Sclera and Retinal Pigment Epithelium Development in the Mouse Eye. Dev. Biol. 2008, 320, 242–255. [Google Scholar] [CrossRef] [Green Version]
- Akwii, R.G.; Sajib, M.S.; Zahra, F.T.; Mikelis, C.M. Role of Angiopoietin-2 in Vascular Physiology and Pathophysiology. Cells 2019, 8, 471. [Google Scholar] [CrossRef] [Green Version]
- Heier, J.S.; Singh, R.P.; Wykoff, C.C.; Csaky, K.G.; Lai, T.Y.Y.; Loewenstein, A.; Schlottmann, P.G.; Paris, L.P.; Westenskow, P.D.; Quezada-Ruiz, C. The Angiopoietin/Tie pathway in retinal vascular diseases: A Review. Retina Phila. Pa 2021, 41, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Brelen, M.E.; Tsujikawa, M.; Chen, H.; Chu, W.K.; Lai, T.Y.Y.; Ng, D.S.C.; Sayanagi, K.; Hara, C.; Hashida, N.; et al. Identification of ANGPT2 as a New Gene for Neovascular Age-Related Macular Degeneration and Polypoidal Choroidal Vasculopathy in the Chinese and Japanese Populations. Investig. Ophthalmol. Vis. Sci. 2017, 58, 1076–1083. [Google Scholar] [CrossRef] [Green Version]
- Chen, Z.J.; Ma, L.; Brelen, M.E.; Chen, H.; Tsujikawa, M.; Lai, T.Y.; Ho, M.; Sayanagi, K.; Hara, C.; Hashida, N.; et al. Identification of TIE2 as a Susceptibility Gene for Neovascular Age-Related Macular Degeneration and Polypoidal Choroidal Vasculopathy. Br. J. Ophthalmol. 2021, 105, 1035–1040. [Google Scholar] [CrossRef]
- Schellevis, R.L.; Breukink, M.B.; Gilissen, C.; Boon, C.J.F.; Hoyng, C.B.; de Jong, E.K.; den Hollander, A.I. Exome Sequencing in Patients with Chronic Central Serous Chorioretinopathy. Sci. Rep. 2019, 9, 6598. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fachinger, G.; Deutsch, U.; Risau, W. Functional Interaction of Vascular Endothelial-Protein-Tyrosine Phosphatase with the Angiopoietin Receptor Tie-2. Oncogene 1999, 18, 5948–5953. [Google Scholar] [CrossRef] [Green Version]
- Motohashi, H.; Hozawa, K.; Oshima, T.; Takeuchi, T.; Takasaka, T. Dysgenesis of Melanocytes and Cochlear Dysfunction in Mutant Microphthalmia (Mi) Mice. Hear. Res. 1994, 80, 10–20. [Google Scholar] [CrossRef]
- Yajima, I.; Sato, S.; Kimura, T.; Yasumoto, K.; Shibahara, S.; Goding, C.R.; Yamamoto, H. An L1 Element Intronic Insertion in the Black-Eyed White (Mitf[Mi-Bw]) Gene: The Loss of a Single Mitf Isoform Responsible for the Pigmentary Defect and Inner Ear Deafness. Hum. Mol. Genet. 1999, 8, 1431–1441. [Google Scholar] [CrossRef] [Green Version]
- Rishi, P.; Multani, P.; Prasan, V.V.; Rishi, E.; Attiku, Y. Choroidal Thickness in Waardenburg Syndrome. GMS Ophthalmol. Cases 2019, 9, Doc22. [Google Scholar] [CrossRef] [PubMed]
- Shields, C.L.; Nickerson, S.J.; Al-Dahmash, S.; Shields, J.A. Waardenburg Syndrome: Iris and Choroidal Hypopigmentation: Findings on Anterior and Posterior Segment Imaging. JAMA Ophthalmol. 2013, 131, 1167–1173. [Google Scholar] [CrossRef] [Green Version]
- Lehmann, G.L.; Hanke-Gogokhia, C.; Hu, Y.; Bareja, R.; Salfati, Z.; Ginsberg, M.; Nolan, D.J.; Mendez-Huergo, S.P.; Dalotto-Moreno, T.; Wojcinski, A.; et al. Single-Cell Profiling Reveals an Endothelium-Mediated Immunomodulatory Pathway in the Eye Choroid. J. Exp. Med. 2020, 217, e20190730. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Voigt, A.P.; Mulfaul, K.; Mullin, N.K.; Flamme-Wiese, M.J.; Giacalone, J.C.; Stone, E.M.; Tucker, B.A.; Scheetz, T.E.; Mullins, R.F. Single-Cell Transcriptomics of the Human Retinal Pigment Epithelium and Choroid in Health and Macular Degeneration. Proc. Natl. Acad. Sci. USA 2019, 116, 24100–24107. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carmeliet, P. Mechanisms of Angiogenesis and Arteriogenesis. Nat. Med. 2000, 6, 389–395. [Google Scholar] [CrossRef] [PubMed]

| Developmental Stages | Events in Each Tissue | |||||
|---|---|---|---|---|---|---|
| Human | Mouse | Retina | Lens | Cornea | Iris and Ciliary Body | Periocular Organs |
| ~3 WG | ~E 8.25 | Optic sulcus | ||||
| ~3.5 WG | ~E 8.5 | Optic vesicle | Lens placode | |||
| ~4 GW | ~E 9.5 | |||||
| ~5 GW | ~E 10 | Optic cup | Lens pit | |||
| ~6 GW | ~E 11.5 | Eyelid formation | ||||
| ~6.5 GW | ~E 13.5 | Lacrimal grand formation | ||||
| ~10 GW | ~E 14.5 | Neural retina and RPE | Lens vesicle | Endothelium formation | ||
| ~15 GW | Lacrimal grand maturation (human) | |||||
| ~16 GW | ~E 15.5 | Epithelium formation | ||||
| ~18 GW | ~E 17 | Formation of both tissues | ||||
| ~36 GW | Eyelid separation (human) | |||||
| Birth | Lacrimal grand maturation (mouse) | |||||
| ~Day 12 | Eyelid separation (mouse) | |||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Imanishi, S.; Tomita, Y.; Negishi, K.; Tsubota, K.; Kurihara, T. Molecular and Cellular Regulations in the Development of the Choroidal Circulation System. Int. J. Mol. Sci. 2023, 24, 5371. https://doi.org/10.3390/ijms24065371
Imanishi S, Tomita Y, Negishi K, Tsubota K, Kurihara T. Molecular and Cellular Regulations in the Development of the Choroidal Circulation System. International Journal of Molecular Sciences. 2023; 24(6):5371. https://doi.org/10.3390/ijms24065371
Chicago/Turabian StyleImanishi, Satoshi, Yohei Tomita, Kazuno Negishi, Kazuo Tsubota, and Toshihide Kurihara. 2023. "Molecular and Cellular Regulations in the Development of the Choroidal Circulation System" International Journal of Molecular Sciences 24, no. 6: 5371. https://doi.org/10.3390/ijms24065371
APA StyleImanishi, S., Tomita, Y., Negishi, K., Tsubota, K., & Kurihara, T. (2023). Molecular and Cellular Regulations in the Development of the Choroidal Circulation System. International Journal of Molecular Sciences, 24(6), 5371. https://doi.org/10.3390/ijms24065371









