Room Temperature Synthesis of Bioactive 1,2,4-Oxadiazoles †
Abstract
:1. Introduction
2. Base-Induced Cyclodehydration of O-acylamidoximes
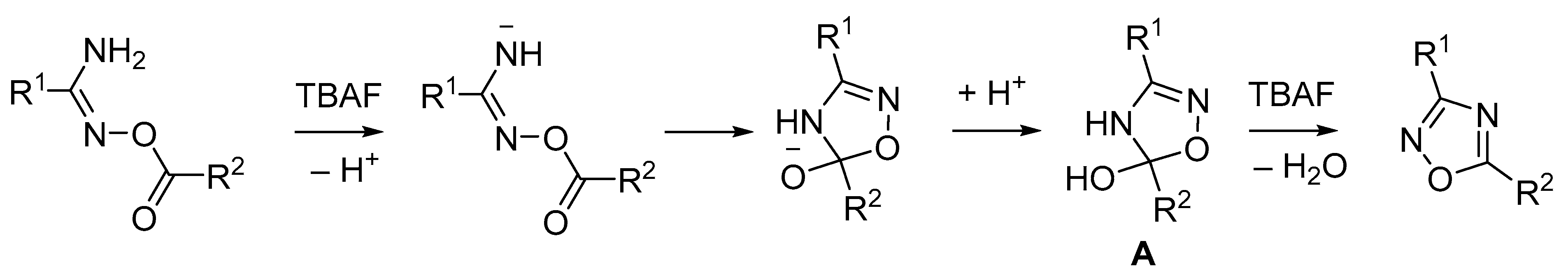
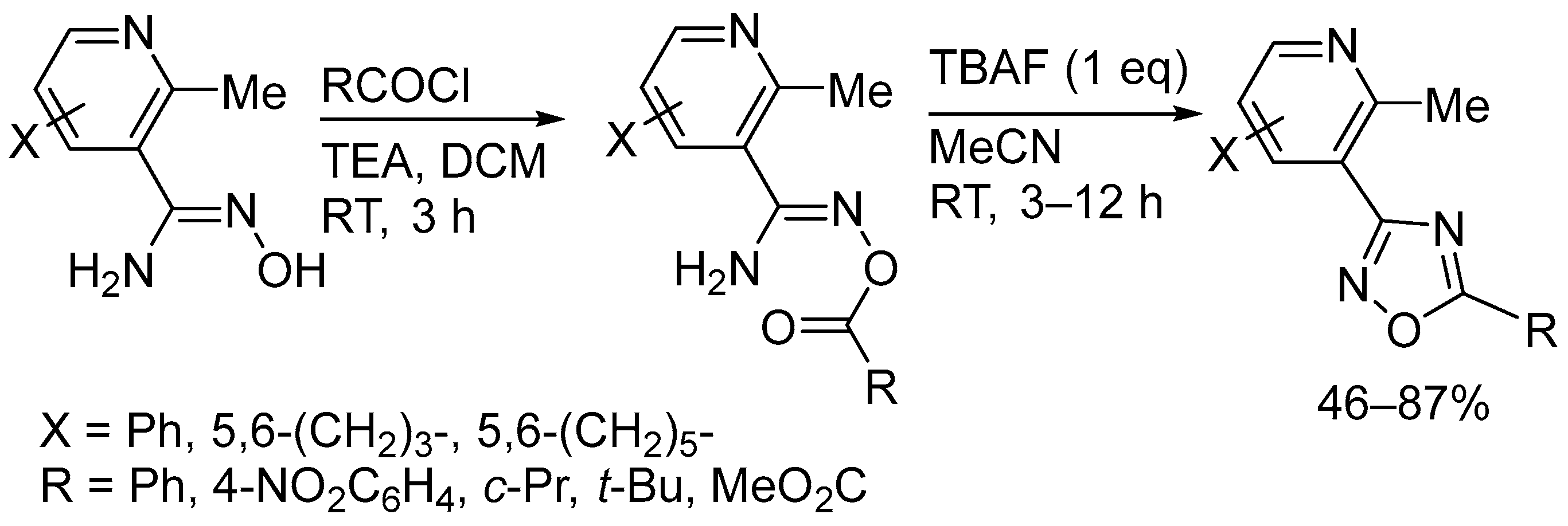
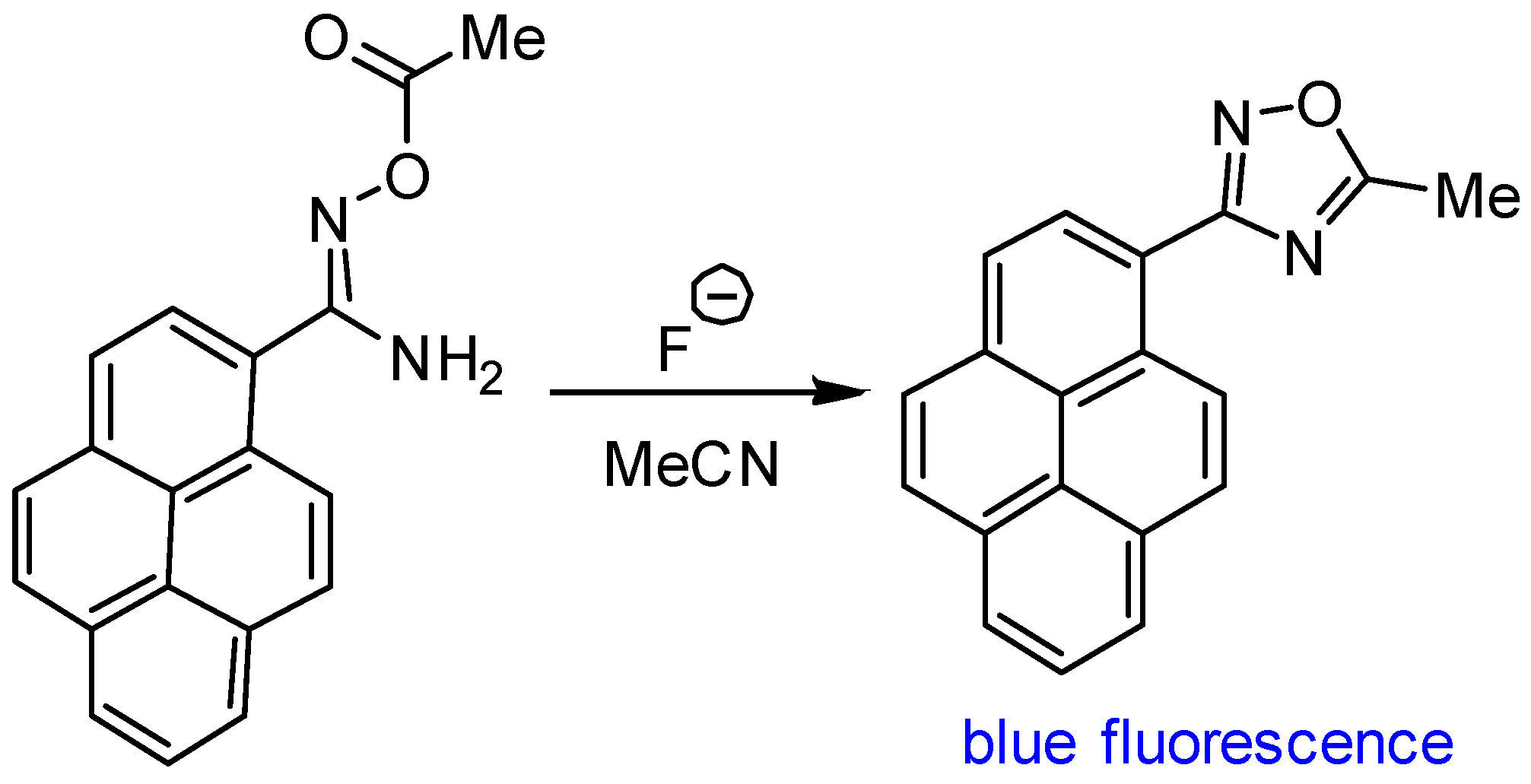
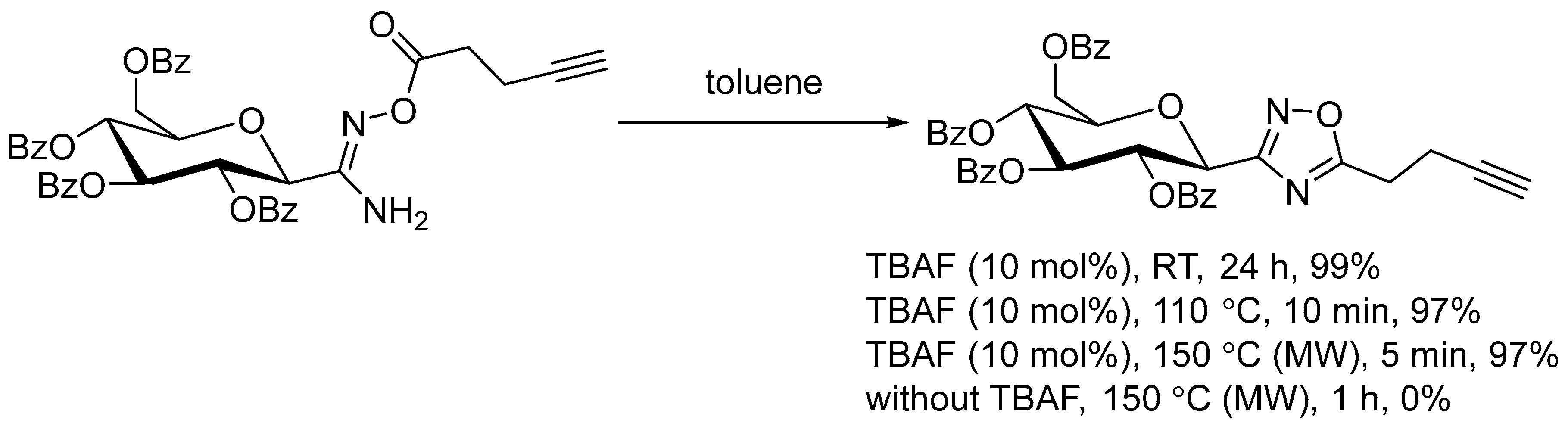
2.1. Tetrabutylammonium Fluoride (TBAF)
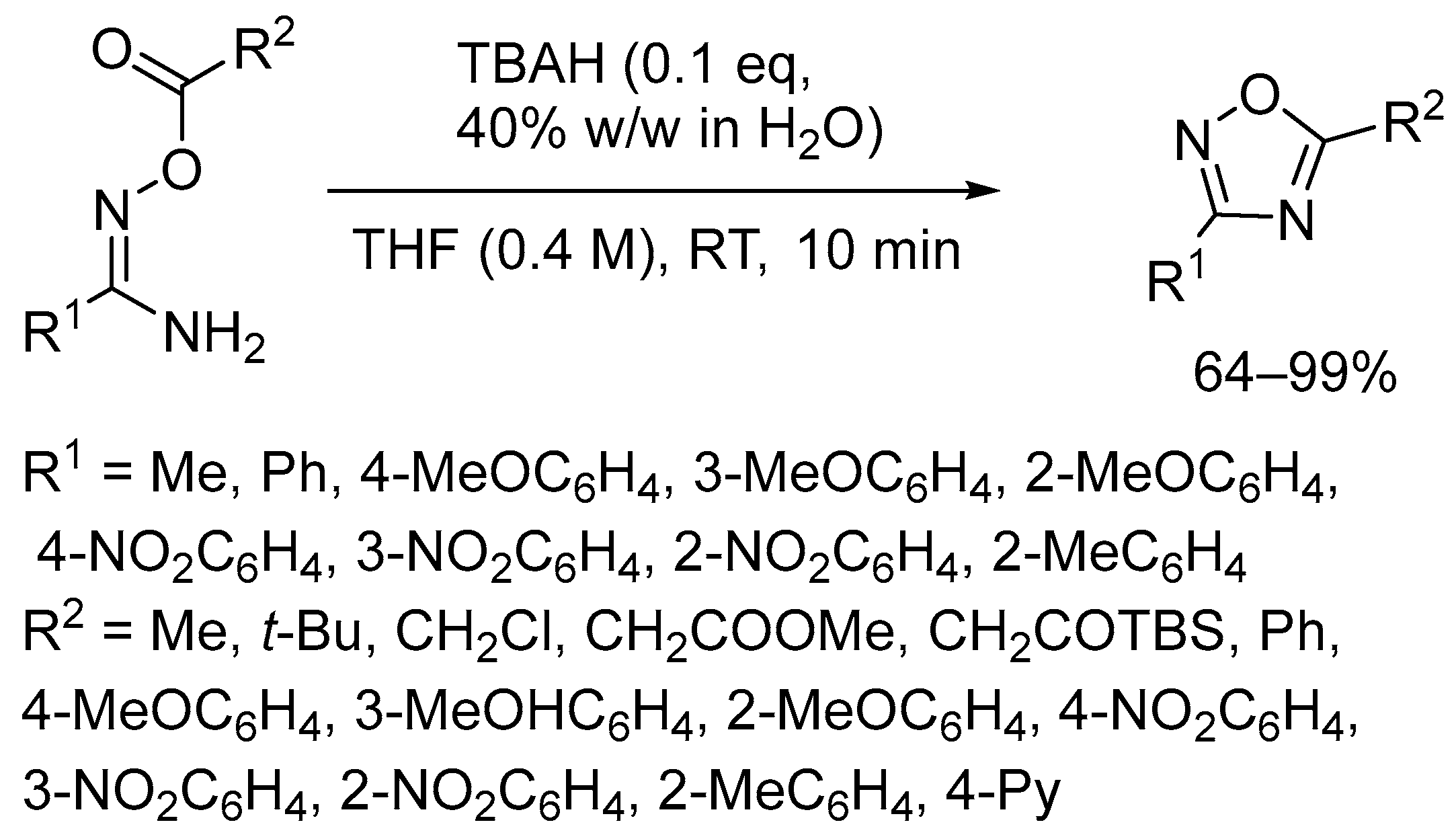
2.2. Tetrabutylammonium Hydroxide (TBAH)
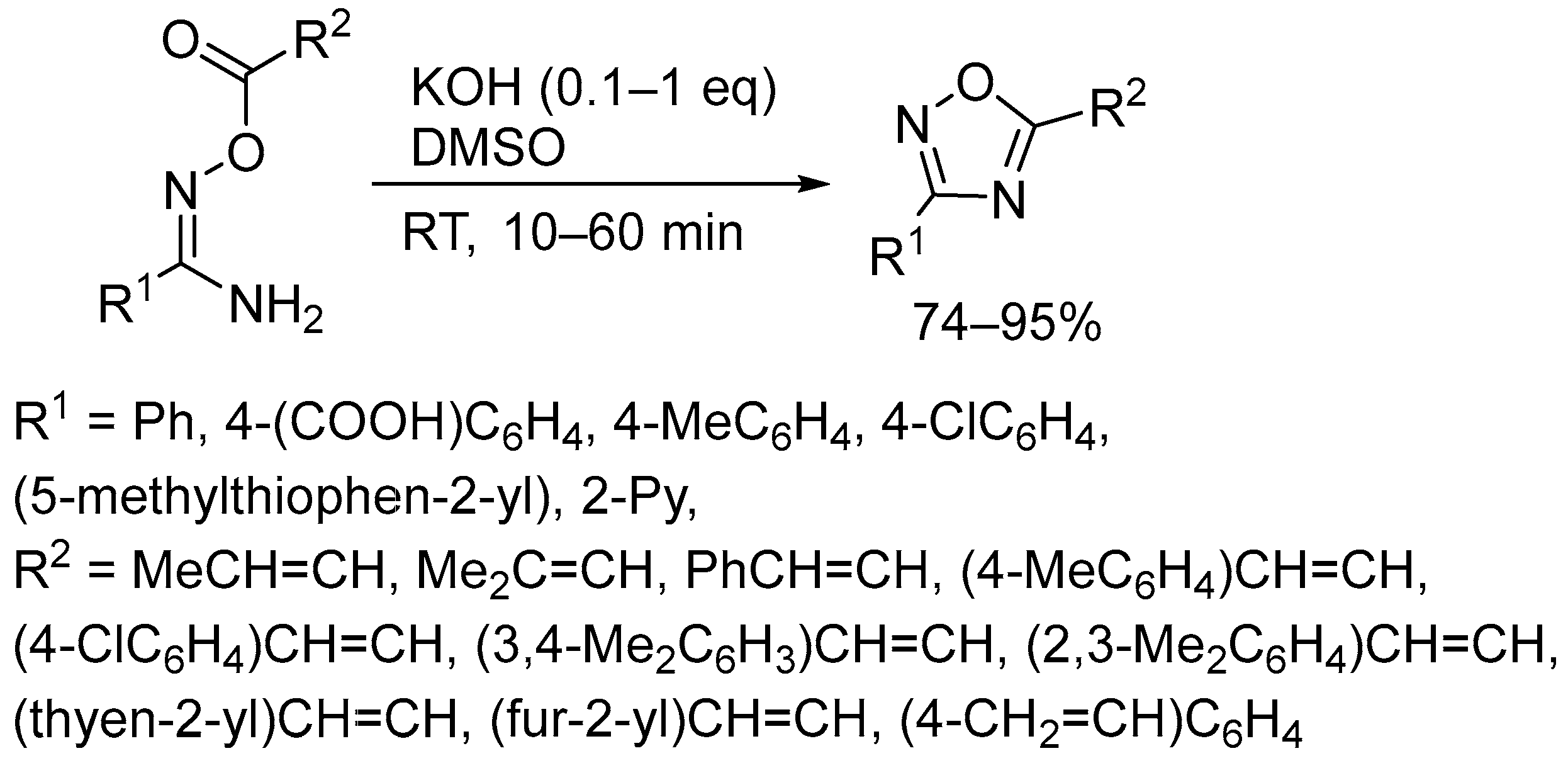
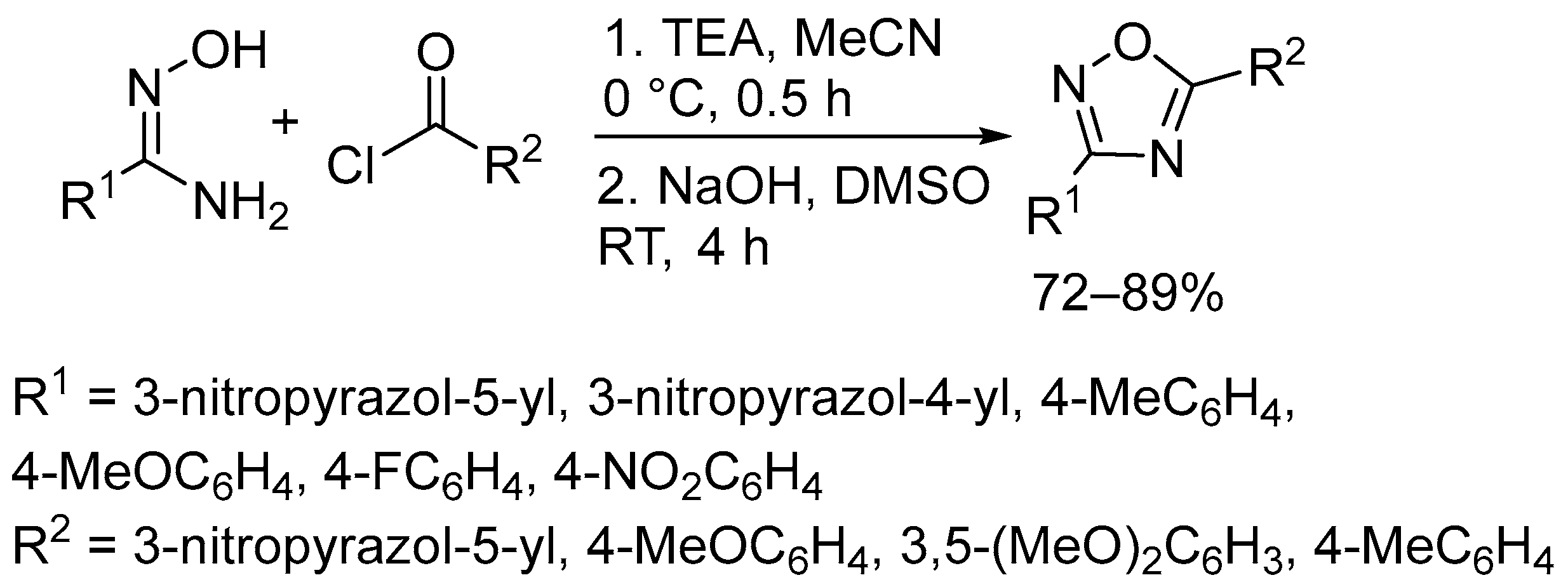
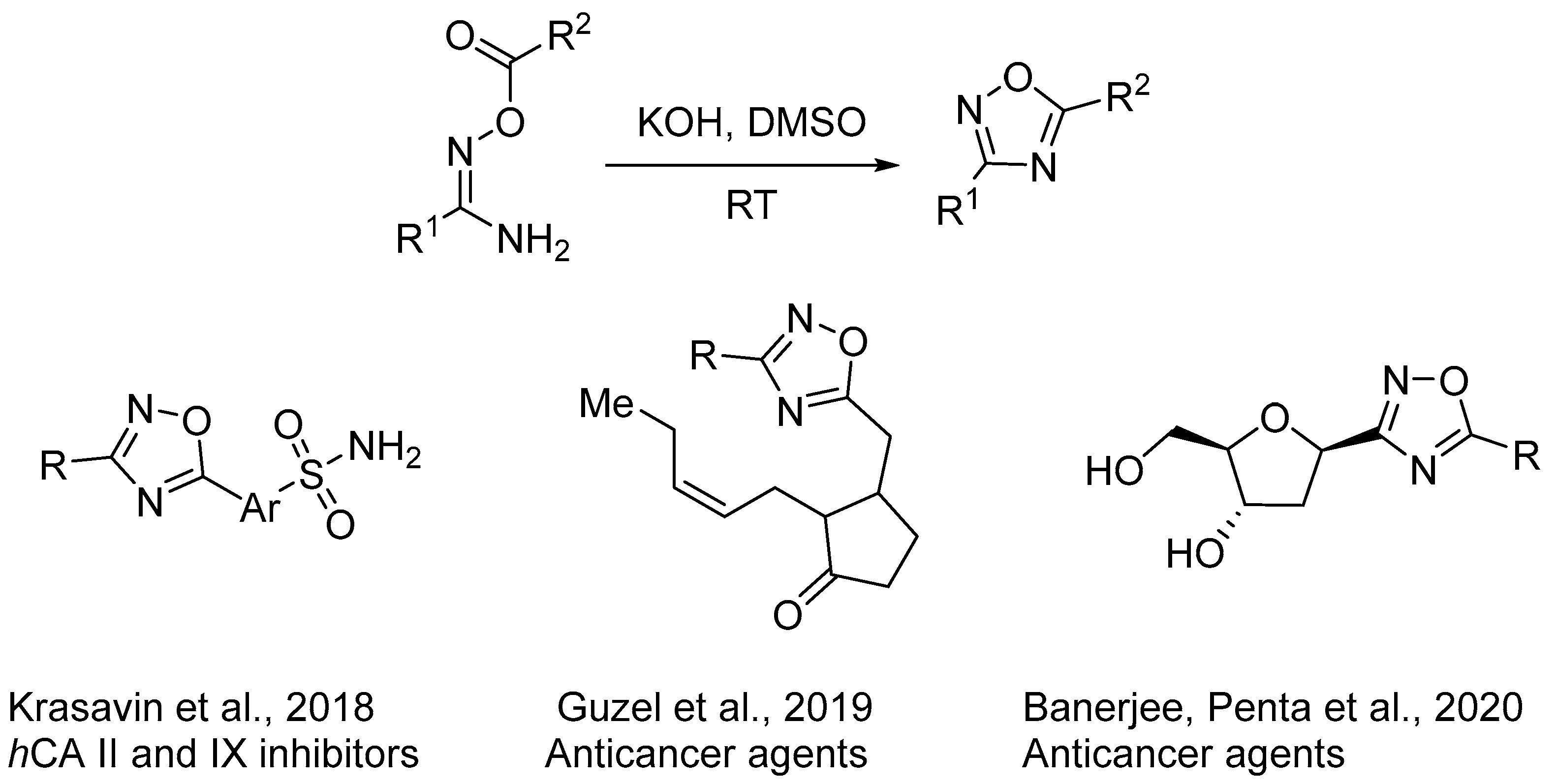
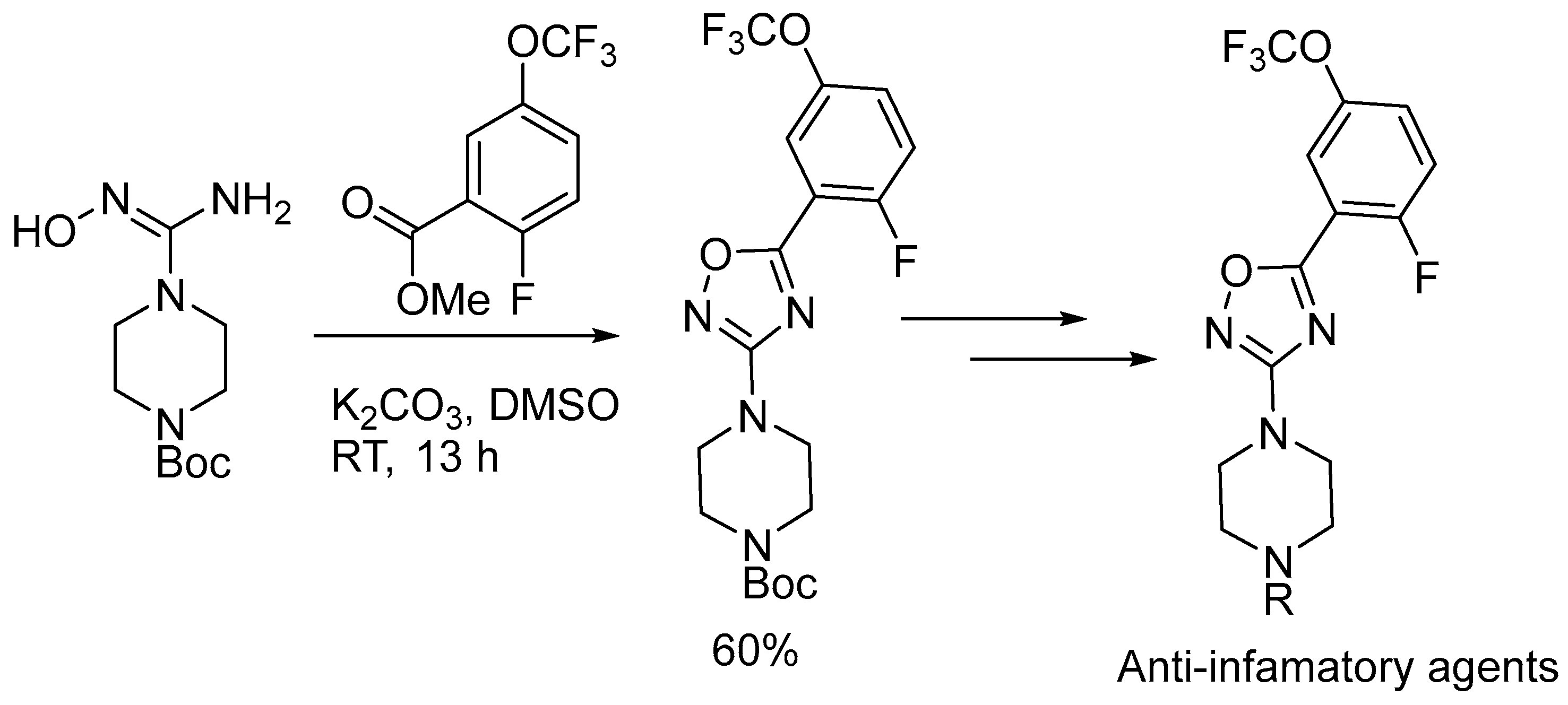
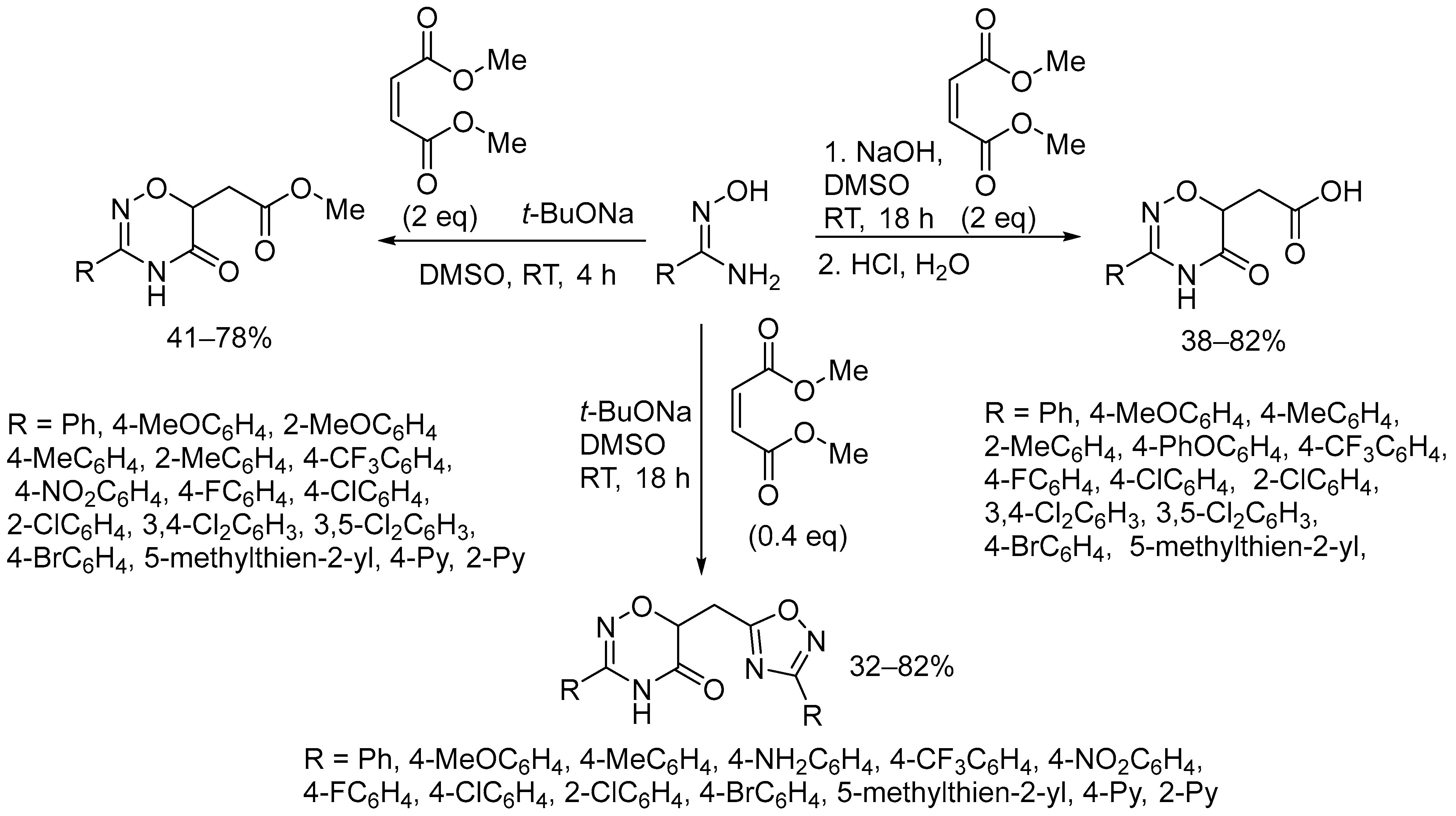
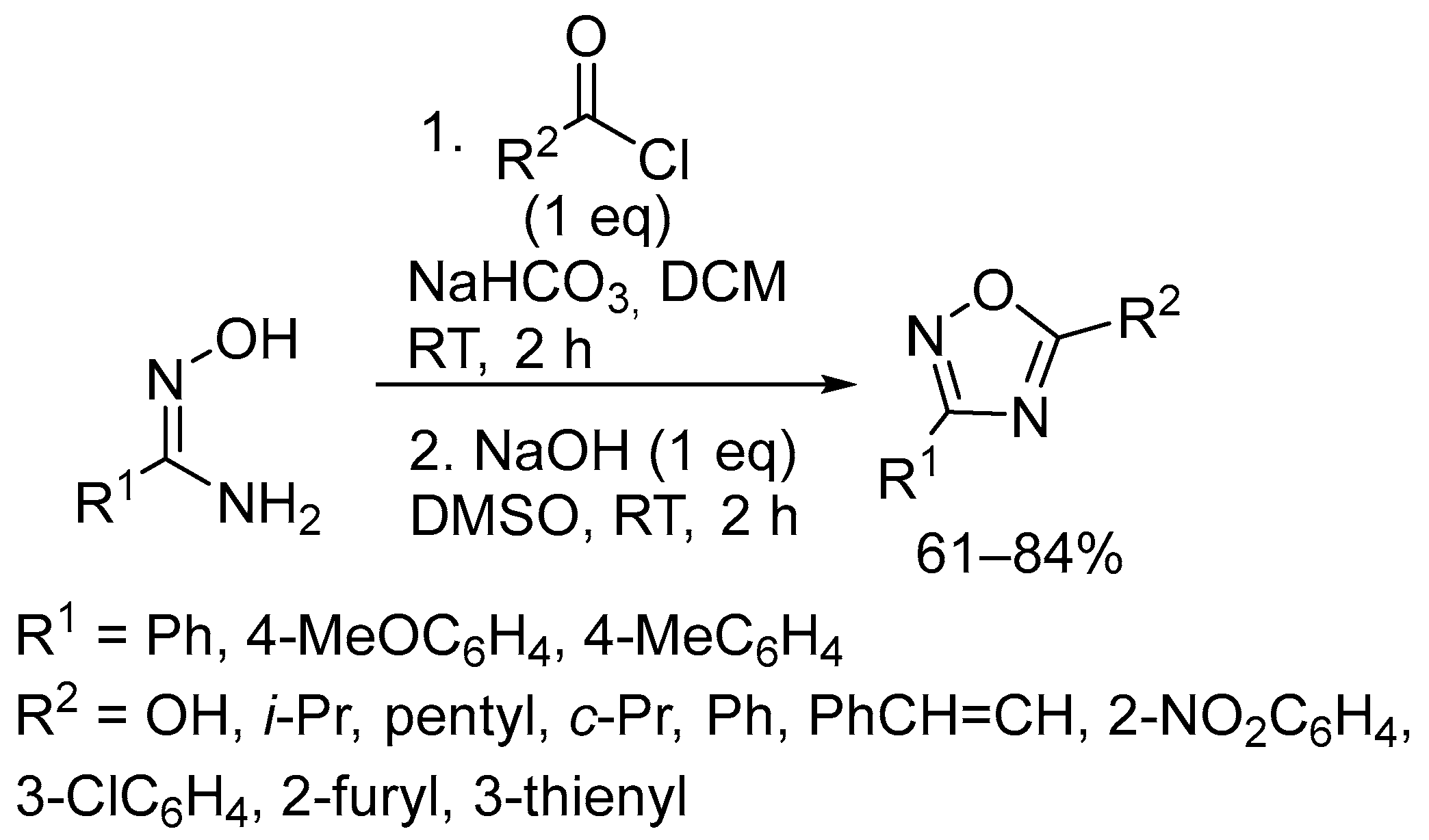
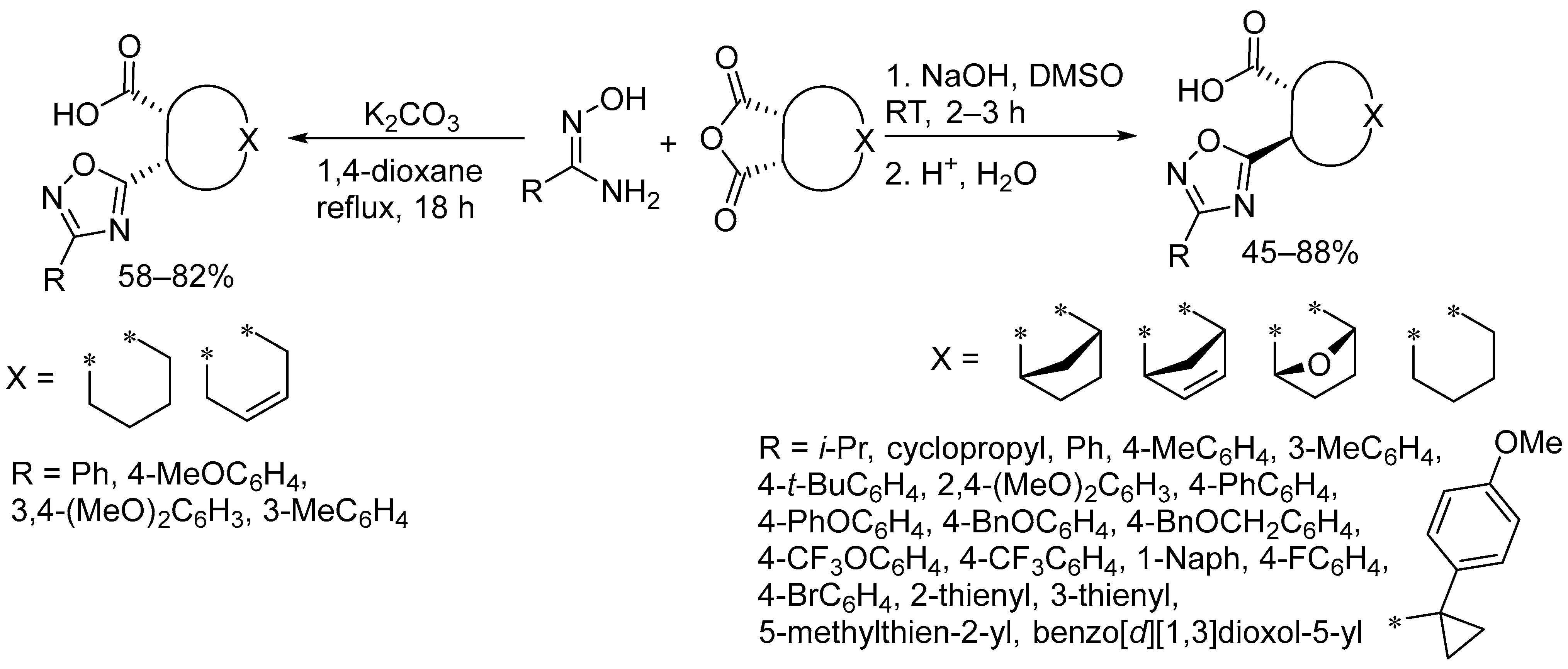
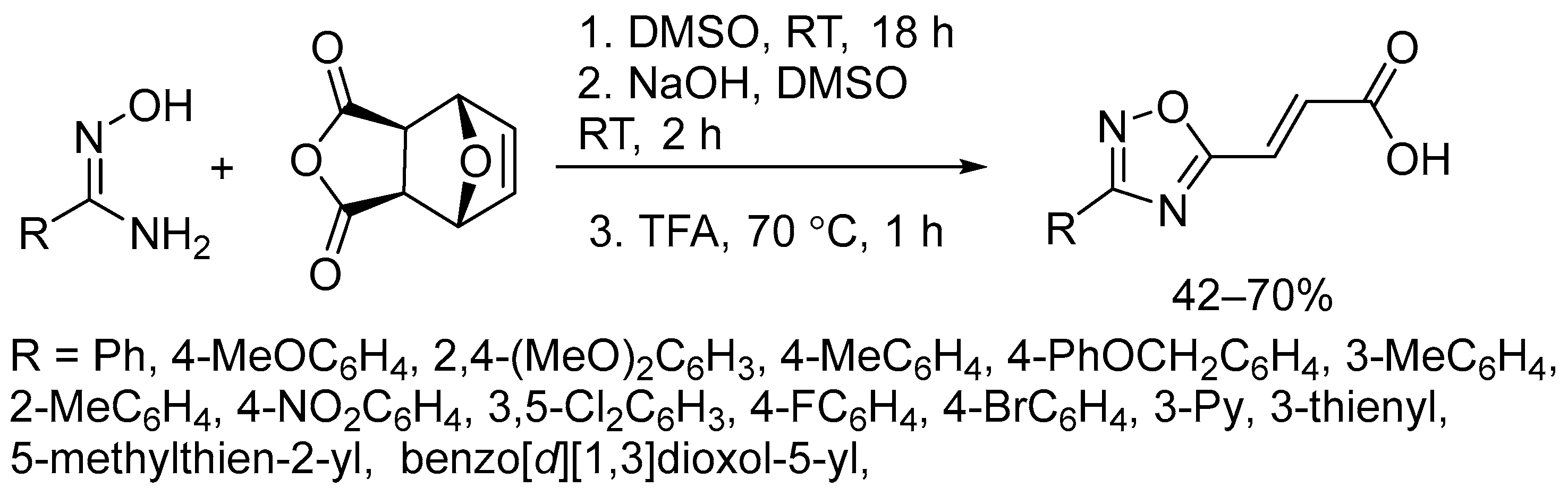
2.3. Alkali Metal Bases
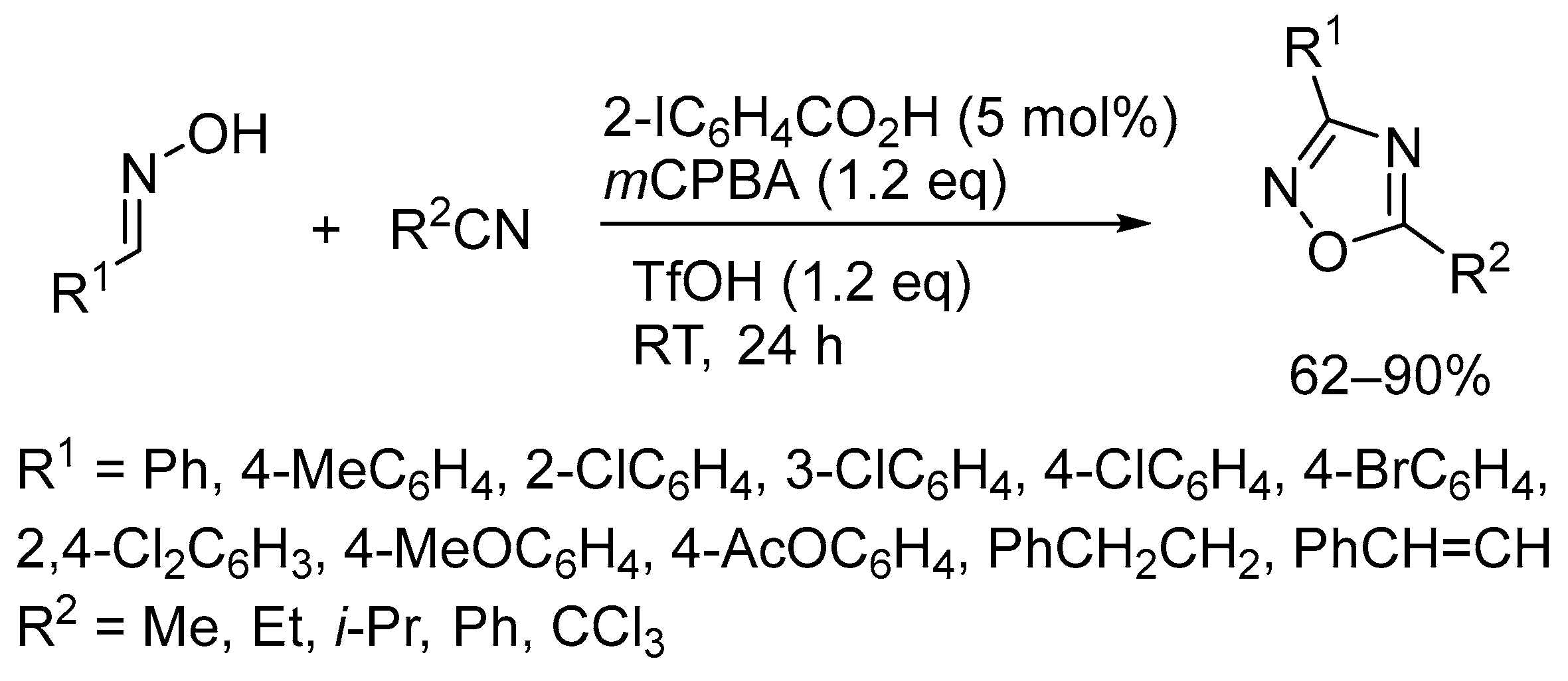
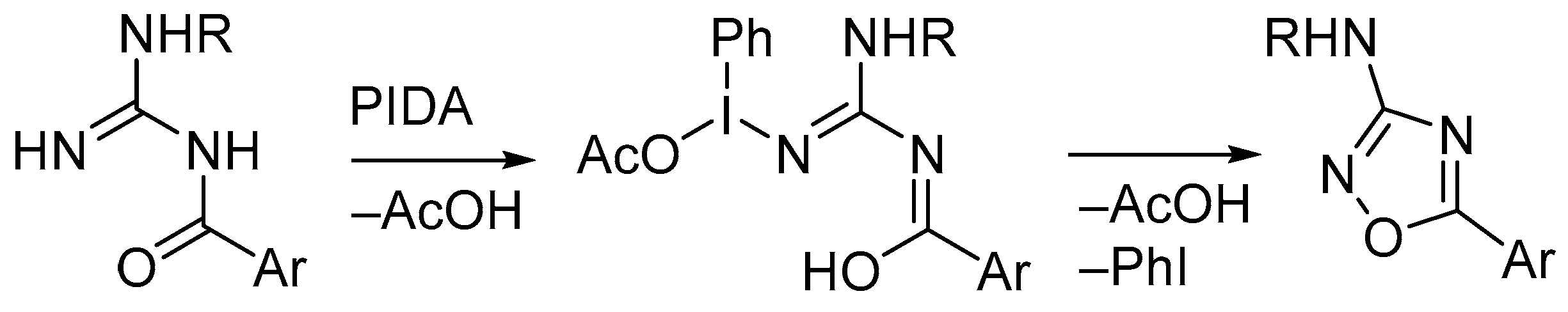
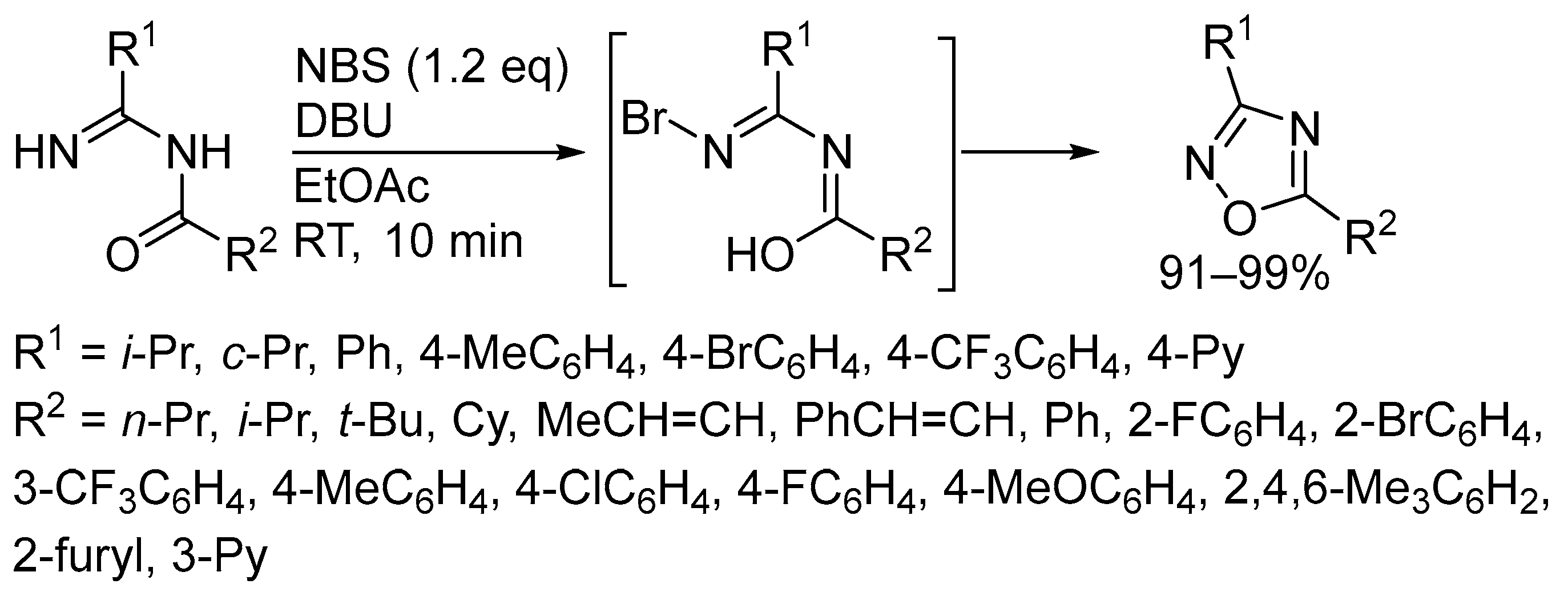
3. Oxidative Cyclization
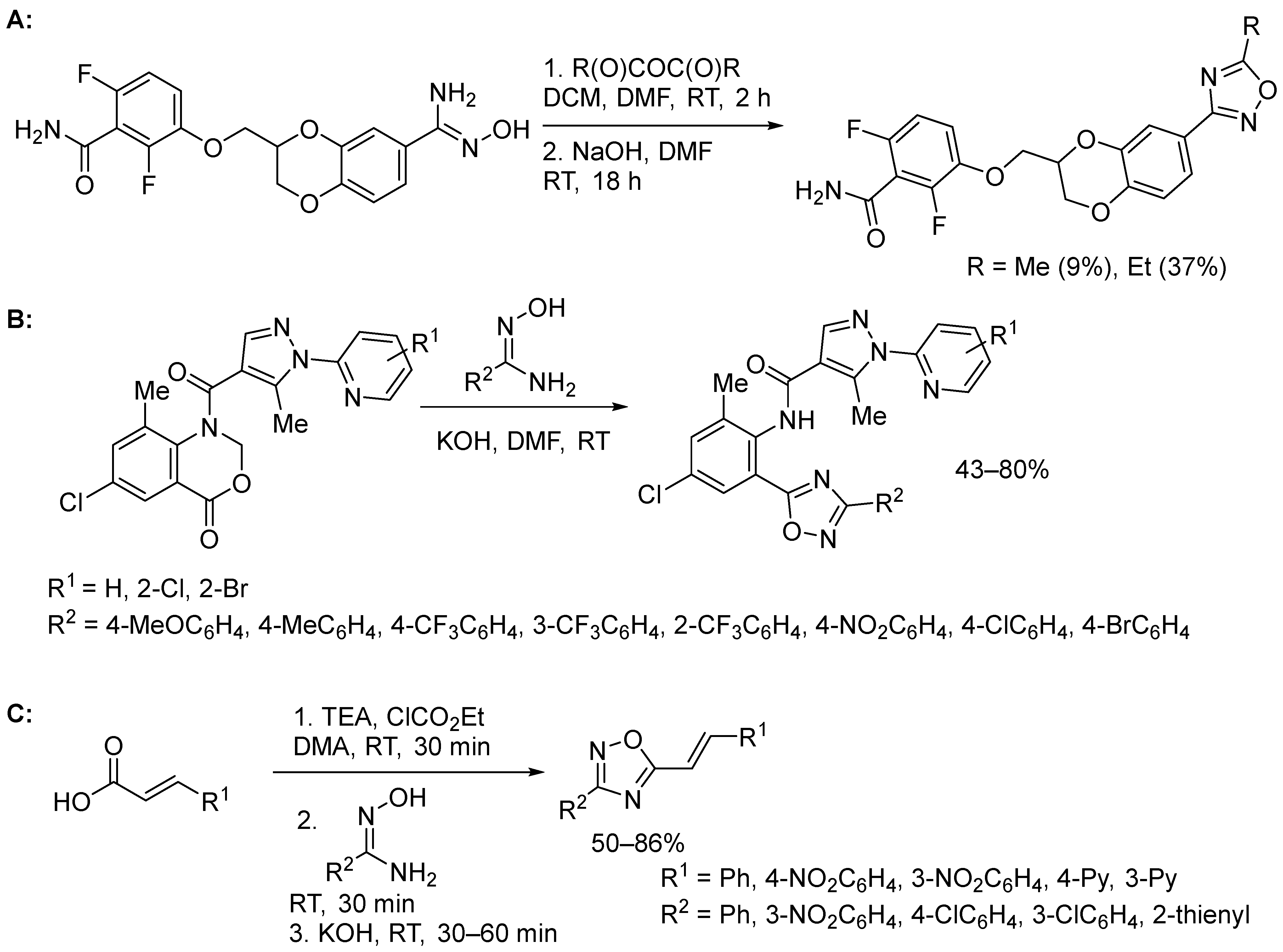
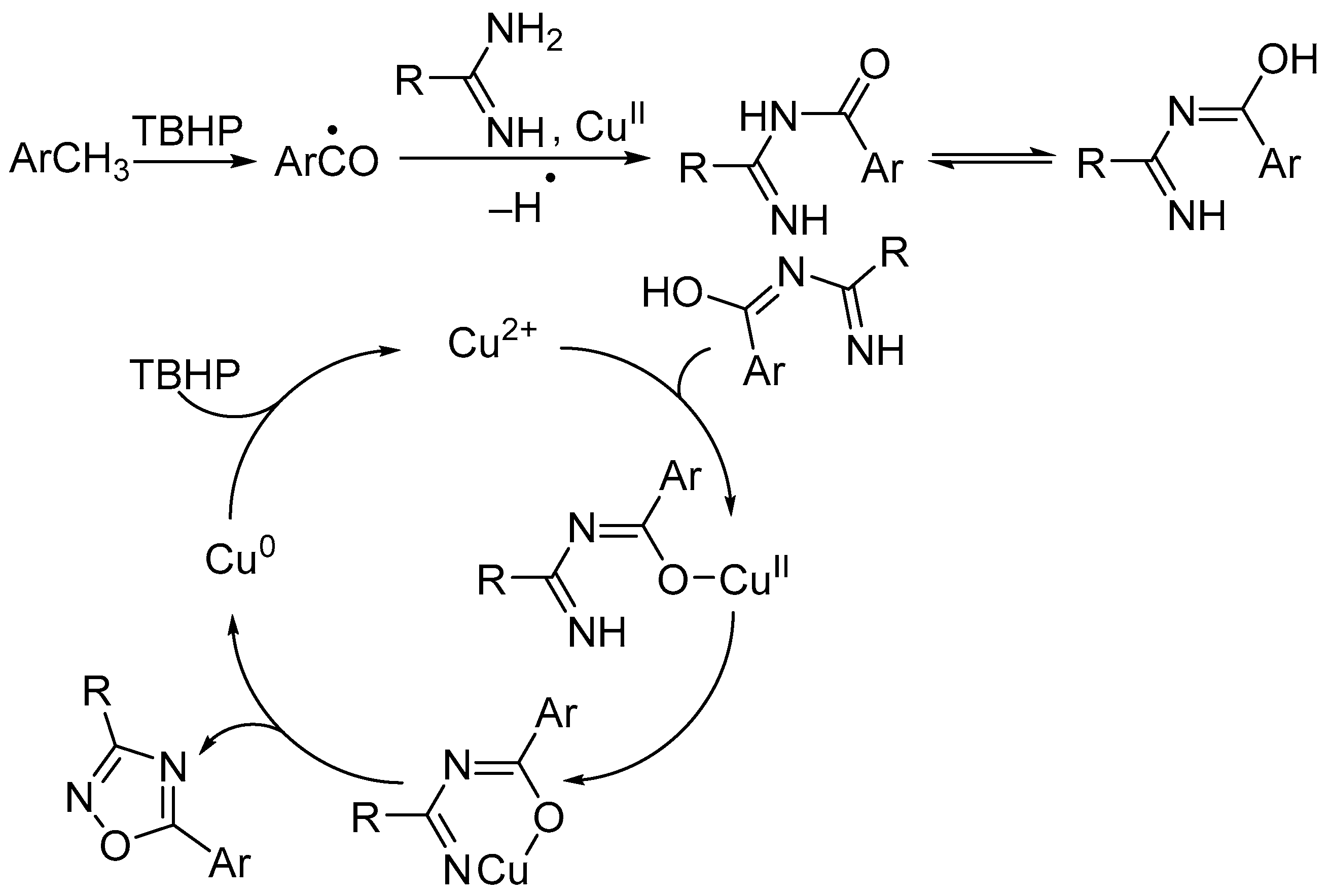
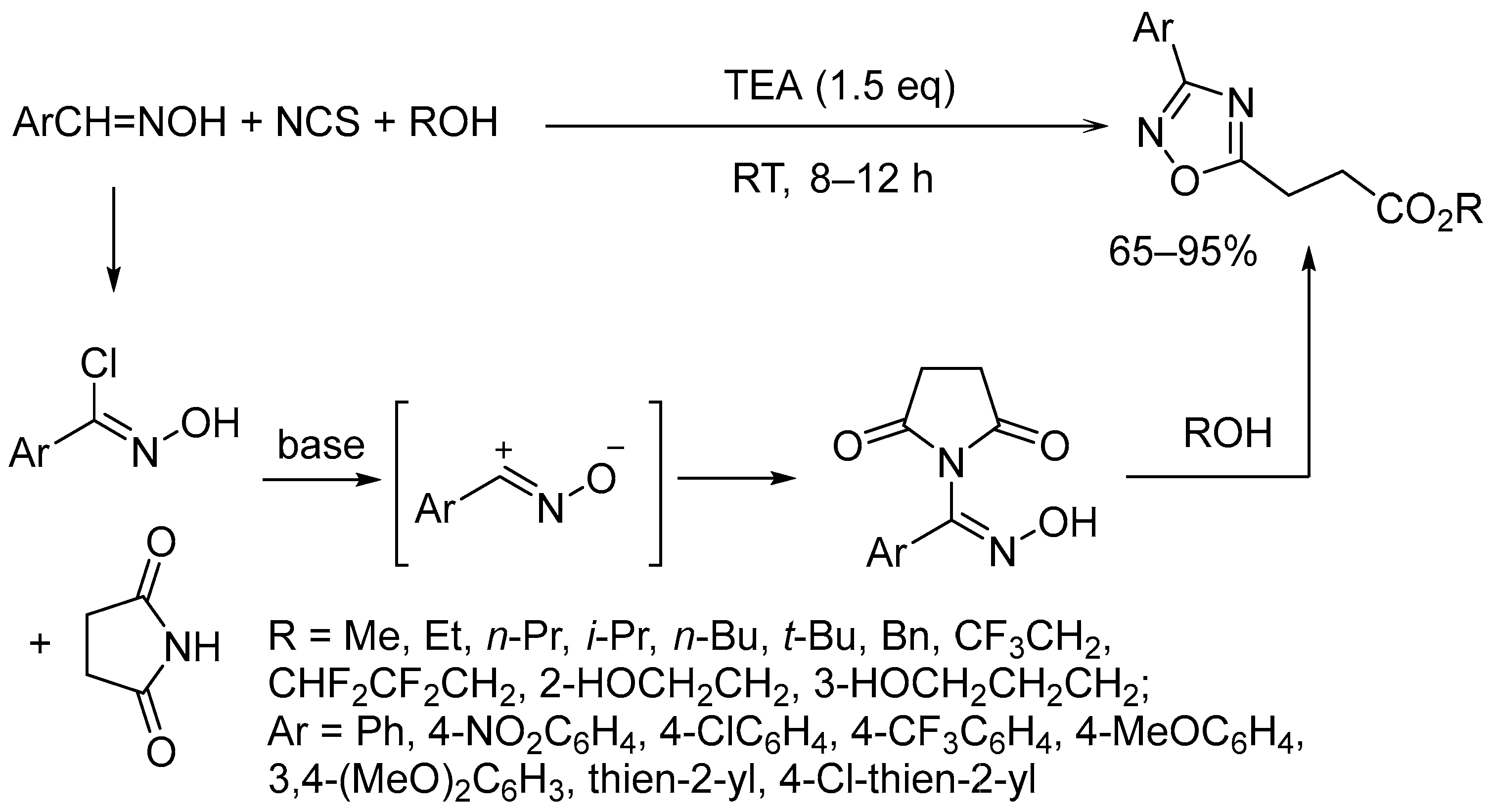
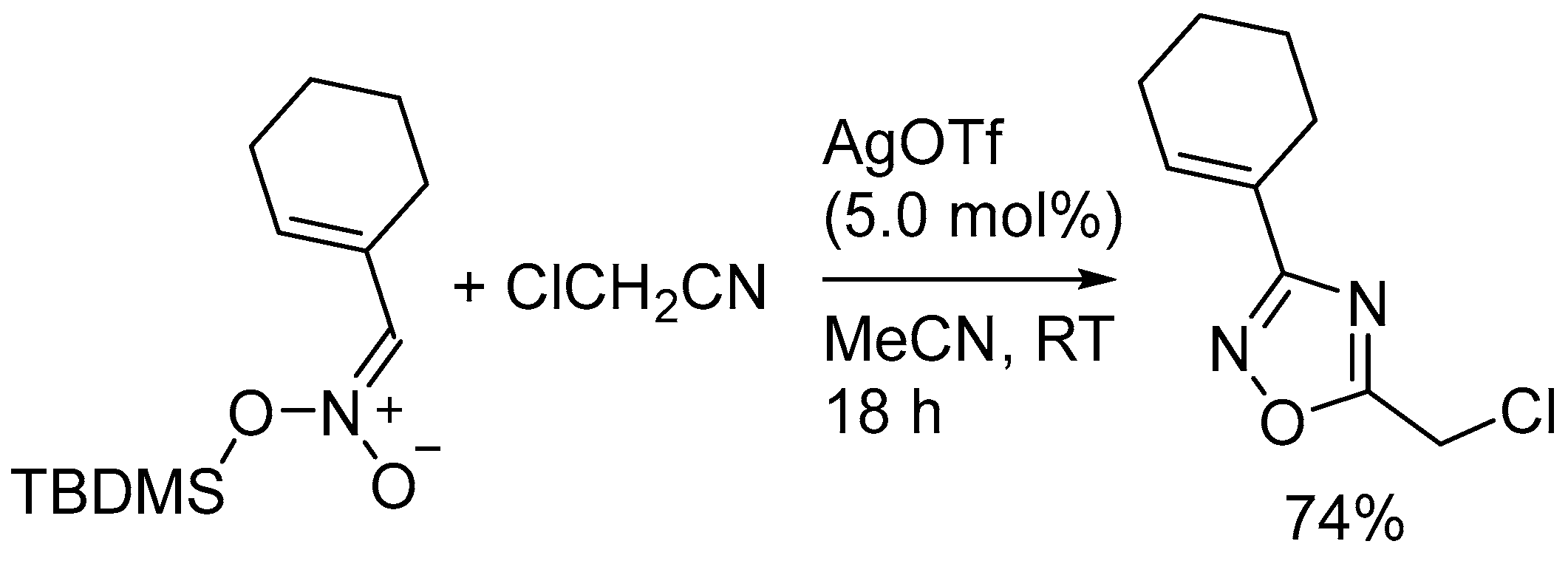
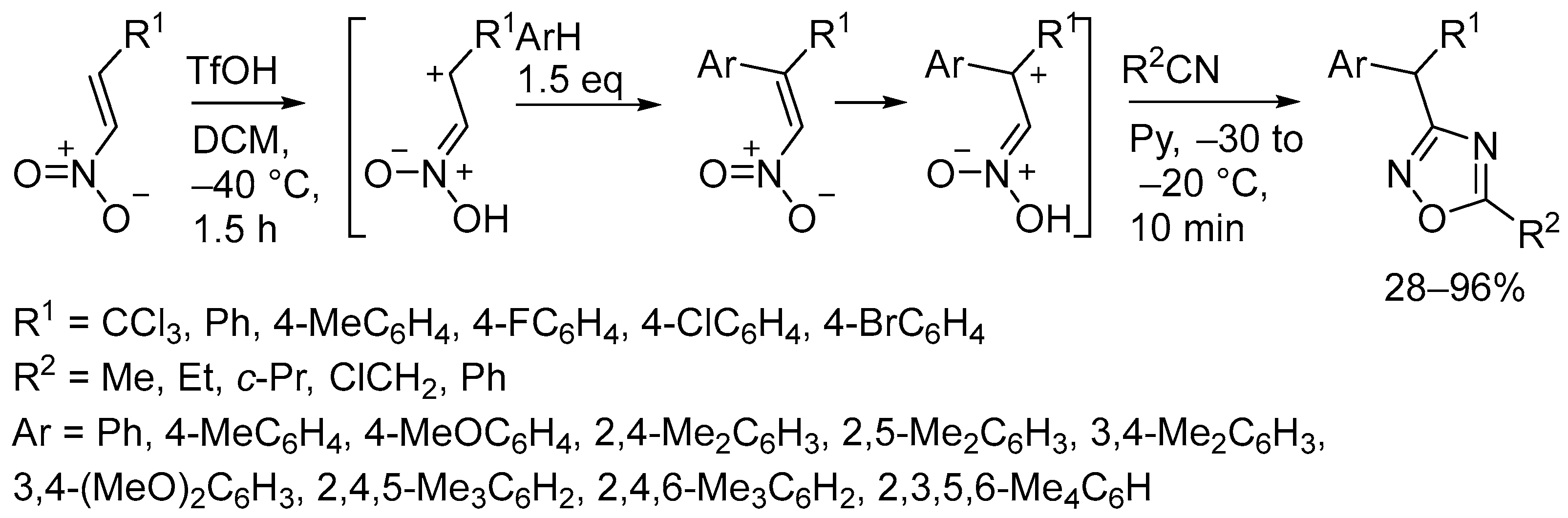
4. Miscellaneous
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Welch, E.M.; Barton, E.R.; Zhuo, J.; Tomizawa, Y.; Friesen, W.J.; Trifillis, P.; Paushkin, S.; Patel, M.; Trotta, C.R.; Hwang, S.; et al. PTC124 Targets Genetic Disorders Caused by Nonsense Mutations. Nature 2007, 447, 87–91. [Google Scholar] [CrossRef] [PubMed]
- Blair, H.A. Naldemedine: A Review in Opioid-Induced Constipation. Drugs 2019, 79, 1241–1247. [Google Scholar] [CrossRef] [PubMed]
- Shoji, N.; Tanese, K.; Sasaki, A.; Horiuchi, T.; Utsuno, Y.; Fukuda, K.; Hoshino, Y.; Noda, S.; Minami, H.; Asakura, W. Pharmaceuticals and Medical Device Agency Approval Summary: Amenamevir for the Treatment of Herpes Zoster. J. Dermatol. 2020, 47, 683–688. [Google Scholar] [CrossRef] [PubMed]
- Cohen, J.A.; Comi, G.; Selmaj, K.W.; Bar-Or, A.; Arnold, D.L.; Steinman, L.; Hartung, H.-P.; Montalban, X.; Kubala Havrdová, E.; Cree, B.A.C.; et al. Safety and Efficacy of Ozanimod versus Interferon Beta-1a in Relapsing Multiple Sclerosis (RADIANCE): A Multicentre, Randomised, 24-Month, Phase 3 Trial. Lancet Neurol. 2019, 18, 1021–1033. [Google Scholar] [CrossRef]
- De Caterina, A.; Harper, A.R.; Cuculi, F. Critical Evaluation of the Efficacy and Tolerability of Azilsartan. Vasc. Health Risk Manag. 2012, 8, 299–305. [Google Scholar] [CrossRef] [Green Version]
- Svetel, M.; Tomić, A.; Kresojević, N.; Kostić, V. Pharmacokinetic Drug Evaluation of Opicapone for the Treatment of Parkinson’s Disease. Expert Opin. Drug Metab. Toxicol. 2018, 14, 353–360. [Google Scholar] [CrossRef]
- Palumbo Piccionello, A.; Pibiri, I.; Pace, A.; Buscemi, S.; Vivona, N. 1,2,4-Oxadiazoles. In Comprehensive Heterocyclic Chemistry IV; Elsevier: Amsterdam, The Netherlands, 2022; pp. 147–189. [Google Scholar]
- Wang, J.-J.; Sun, W.; Jia, W.-D.; Bian, M.; Yu, L.-J. Research Progress on the Synthesis and Pharmacology of 1,3,4-Oxadiazole and 1,2,4-Oxadiazole Derivatives: A Mini Review. J. Enzym. Inhib. Med. Chem. 2022, 37, 2304–2319. [Google Scholar] [CrossRef]
- Biernacki, K.; Daśko, M.; Ciupak, O.; Kubiński, K.; Rachon, J.; Demkowicz, S. Novel 1,2,4-Oxadiazole Derivatives in Drug Discovery. Pharmaceuticals 2020, 13, 111. [Google Scholar] [CrossRef]
- Hendawy, O.M. A Comprehensive Review of Recent Advances in the Biological Activities of 1,2,4-oxadiazoles. Arch. Pharm. 2022, 355, e2200045. [Google Scholar] [CrossRef]
- Hassan, A.; Khan, A.H.; Saleem, F.; Ahmad, H.; Khan, K.M. A Patent Review of Pharmaceutical and Therapeutic Applications of Oxadiazole Derivatives for the Treatment of Chronic Diseases (2013–2021). Expert Opin. Ther. Pat. 2022, 32, 969–1001. [Google Scholar] [CrossRef]
- Baykov, S.; Semenov, A.; Tarasenko, M.; Boyarskiy, V.P. Application of Amidoximes for the Heterocycles Synthesis. Tetrahedron Lett. 2020, 61, 152403. [Google Scholar] [CrossRef]
- Matore, B.W.; Banjare, P.; Guria, T.; Roy, P.P.; Singh, J. Oxadiazole Derivatives: Histone Deacetylase Inhibitors in Anticancer Therapy and Drug Discovery. Eur. J. Med. Chem. Rep. 2022, 5, 100058. [Google Scholar] [CrossRef]
- Krasavin, M.; Shetnev, A.; Sharonova, T.; Baykov, S.; Kalinin, S.; Nocentini, A.; Sharoyko, V.; Poli, G.; Tuccinardi, T.; Presnukhina, S.; et al. Continued Exploration of 1,2,4-Oxadiazole Periphery for Carbonic Anhydrase-Targeting Primary Arene Sulfonamides: Discovery of Subnanomolar Inhibitors of Membrane-Bound HCA IX Isoform That Selectively Kill Cancer Cells in Hypoxic Environment. Eur. J. Med. Chem. 2019, 164, 92–105. [Google Scholar] [CrossRef] [PubMed]
- Xue, D.; Xu, Y.; Kyani, A.; Roy, J.; Dai, L.; Sun, D.; Neamati, N. Discovery and Lead Optimization of Benzene-1,4-Disulfonamides as Oxidative Phosphorylation Inhibitors. J. Med. Chem. 2022, 65, 343–368. [Google Scholar] [CrossRef]
- Caroff, E.; Meyer, E.A.; Äänismaa, P.; Froidevaux, S.; Keller, M.; Piali, L. Design, Synthesis, and Pharmacological Evaluation of Benzimidazolo-Thiazoles as Potent CXCR3 Antagonists with Therapeutic Potential in Autoimmune Diseases: Discovery of ACT-672125. J. Med. Chem. 2022, 65, 11533–11549. [Google Scholar] [CrossRef]
- Macabuag, N.; Esmieu, W.; Breccia, P.; Jarvis, R.; Blackaby, W.; Lazari, O.; Urbonas, L.; Eznarriaga, M.; Williams, R.; Strijbosch, A.; et al. Developing HDAC4-Selective Protein Degraders To Investigate the Role of HDAC4 in Huntington’s Disease Pathology. J. Med. Chem. 2022, 65, 12445–12459. [Google Scholar] [CrossRef]
- Wang, M.; Liu, T.; Chen, S.; Wu, M.; Han, J.; Li, Z. Design and Synthesis of 3-(4-Pyridyl)-5-(4-Sulfamido-Phenyl)-1,2,4-Oxadiazole Derivatives as Novel GSK-3β Inhibitors and Evaluation of Their Potential as Multifunctional Anti-Alzheimer Agents. Eur. J. Med. Chem. 2021, 209, 112874. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Chen, S.; Du, J.; Xing, S.; Li, R.; Li, Z. Design, Synthesis, and Biological Evaluation of Novel (4-(1,2,4-Oxadiazol-5-yl)Phenyl)-2-Aminoacetamide Derivatives as Multifunctional Agents for the Treatment of Alzheimer’s Disease. Eur. J. Med. Chem. 2022, 227, 113973. [Google Scholar] [CrossRef]
- Stankiewicz, A.; Kaczorowska, K.; Bugno, R.; Kozioł, A.; Paluchowska, M.H.; Burnat, G.; Chruścicka, B.; Chorobik, P.; Brański, P.; Wierońska, J.M.; et al. New 1,2,4-Oxadiazole Derivatives with Positive MGlu 4 Receptor Modulation Activity and Antipsychotic-like Properties. J. Enzym. Inhib. Med. Chem. 2022, 37, 211–225. [Google Scholar] [CrossRef]
- Verma, S.K.; Verma, R.; Verma, S.; Vaishnav, Y.; Tiwari, S.P.; Rakesh, K.P. Anti-Tuberculosis Activity and Its Structure-Activity Relationship (SAR) Studies of Oxadiazole Derivatives: A Key Review. Eur. J. Med. Chem. 2021, 209, 112886. [Google Scholar] [CrossRef] [PubMed]
- Dhameliya, T.M.; Chudasma, S.J.; Patel, T.M.; Dave, B.P. A Review on Synthetic Account of 1,2,4-Oxadiazoles as Anti-Infective Agents. Mol. Divers. 2022, 26, 2967–2980. [Google Scholar] [CrossRef]
- De, S.S.; Khambete, M.P.; Degani, M.S. Oxadiazole Scaffolds in Anti-Tuberculosis Drug Discovery. Bioorg. Med. Chem. Lett. 2019, 29, 1999–2007. [Google Scholar] [CrossRef] [PubMed]
- Verma, S.K.; Verma, R.; Kumar, K.S.S.; Banjare, L.; Shaik, A.B.; Bhandare, R.R.; Rakesh, K.P.; Rangappa, K.S. A Key Review on Oxadiazole Analogs as Potential Methicillin-Resistant Staphylococcus Aureus (MRSA) Activity: Structure-Activity Relationship Studies. Eur. J. Med. Chem. 2021, 219, 113442. [Google Scholar] [CrossRef] [PubMed]
- Mohammad, B.D.; Baig, M.S.; Bhandari, N.; Siddiqui, F.A.; Khan, S.L.; Ahmad, Z.; Khan, F.S.; Tagde, P.; Jeandet, P. Heterocyclic Compounds as Dipeptidyl Peptidase-IV Inhibitors with Special Emphasis on Oxadiazoles as Potent Anti-Diabetic Agents. Molecules 2022, 27, 6001. [Google Scholar] [CrossRef] [PubMed]
- Nara, S.J.; Jogi, S.; Cheruku, S.; Kandhasamy, S.; Jaipuri, F.; Kathi, P.K.; Reddy, S.; Sarodaya, S.; Cook, E.M.; Wang, T.; et al. Discovery of BMS-986339, a Pharmacologically Differentiated Farnesoid X Receptor Agonist for the Treatment of Nonalcoholic Steatohepatitis. J. Med. Chem. 2022, 65, 8948–8960. [Google Scholar] [CrossRef] [PubMed]
- Di Leva, F.S.; Festa, C.; Carino, A.; De Marino, S.; Marchianò, S.; Di Marino, D.; Finamore, C.; Monti, M.C.; Zampella, A.; Fiorucci, S.; et al. Discovery of ((1,2,4-Oxadiazol-5-yl)Pyrrolidin-3-yl)Ureidyl Derivatives as Selective Non-Steroidal Agonists of the G-Protein Coupled Bile Acid Receptor-1. Sci. Rep. 2019, 9, 2504. [Google Scholar] [CrossRef] [Green Version]
- Gay, E.A.; Guan, D.; Van Voorhies, K.; Vasukuttan, V.; Mathews, K.M.; Besheer, J.; Jin, C. Discovery and Characterization of the First Nonpeptide Antagonists for the Relaxin-3/RXFP3 System. J. Med. Chem. 2022, 65, 7959–7974. [Google Scholar] [CrossRef]
- Gao, J.; Liu, X.; Zhang, B.; Mao, Q.; Zhang, Z.; Zou, Q.; Dai, X.; Wang, S. Design, Synthesis and Biological Evaluation of 1-Alkyl-5/6-(5-Oxo-4,5-Dihydro-1,2,4-Oxadiazol-3-Yl)-1H-Indole-3-Carbonitriles as Novel Xanthine Oxidase Inhibitors. Eur. J. Med. Chem. 2020, 190, 112077. [Google Scholar] [CrossRef]
- Lee, E.C.Y.; McRiner, A.J.; Georgiadis, K.E.; Liu, J.; Wang, Z.; Ferguson, A.D.; Levin, B.; von Rechenberg, M.; Hupp, C.D.; Monteiro, M.I.; et al. Discovery of Novel, Potent Inhibitors of Hydroxy Acid Oxidase 1 (HAO1) Using DNA-Encoded Chemical Library Screening. J. Med. Chem. 2021, 64, 6730–6744. [Google Scholar] [CrossRef]
- Pitcher, N.P.; Harjani, J.R.; Zhao, Y.; Jin, J.; Knight, D.R.; Li, L.; Putsathit, P.; Riley, T.V.; Carter, G.P.; Baell, J.B. Development of 1,2,4-Oxadiazole Antimicrobial Agents to Treat Enteric Pathogens within the Gastrointestinal Tract. ACS Omega 2022, 7, 6737–6759. [Google Scholar] [CrossRef]
- Chuang, C.; Collibee, S.; Ashcraft, L.; Wang, W.; Vander Wal, M.; Wang, X.; Hwee, D.T.; Wu, Y.; Wang, J.; Chin, E.R.; et al. Discovery of Aficamten (CK-274), a Next-Generation Cardiac Myosin Inhibitor for the Treatment of Hypertrophic Cardiomyopathy. J. Med. Chem. 2021, 64, 14142–14152. [Google Scholar] [CrossRef] [PubMed]
- Taylor, S.J.; Abeywardane, A.; Liang, S.; Xiong, Z.; Proudfoot, J.R.; Farmer, B.S.; Gao, D.A.; Heim-Riether, A.; Smith-Keenan, L.L.; Muegge, I.; et al. Indole Inhibitors of MMP-13 for Arthritic Disorders. ACS Omega 2021, 6, 18635–18650. [Google Scholar] [CrossRef] [PubMed]
- Terrett, J.A.; Chen, H.; Shore, D.G.; Villemure, E.; Larouche-Gauthier, R.; Déry, M.; Beaumier, F.; Constantineau-Forget, L.; Grand-Maître, C.; Lépissier, L.; et al. Tetrahydrofuran-Based Transient Receptor Potential Ankyrin 1 (TRPA1) Antagonists: Ligand-Based Discovery, Activity in a Rodent Asthma Model, and Mechanism-of-Action via Cryogenic Electron Microscopy. J. Med. Chem. 2021, 64, 3843–3869. [Google Scholar] [CrossRef] [PubMed]
- Andersen, K.; Jørgensen, A.; Bræstrup, C. Oxadiazoles as Bioisosteric Transformations of Carboxylic Functionalities. Part I. Eur. J. Med. Chem. 1994, 29, 393–399. [Google Scholar] [CrossRef]
- Andersen, K.E.; Lundt, B.F.; Jørgensen, A.S.; Braestrup, C. Oxadiazoles as Bioisosteric Transformations of Carboxylic Functionalities. II. Eur. J. Med. Chem. 1996, 31, 417–425. [Google Scholar] [CrossRef]
- Sun, S.; Jia, Q.; Zhang, Z. Applications of Amide Isosteres in Medicinal Chemistry. Bioorg. Med. Chem. Lett. 2019, 29, 2535–2550. [Google Scholar] [CrossRef] [PubMed]
- Kumari, S.; Carmona, A.V.; Tiwari, A.K.; Trippier, P.C. Amide Bond Bioisosteres: Strategies, Synthesis, and Successes. J. Med. Chem. 2020, 63, 12290–12358. [Google Scholar] [CrossRef]
- Diana, G.D.; Volkots, D.L.; Nitz, T.J.; Bailey, T.R.; Long, M.A.; Vescio, N.; Aldous, S.; Pevear, D.C.; Dutko, F.J. Oxadiazoles as Ester Bioisosteric Replacements in Compounds Related to Disoxaril. Antirhinovirus Activity. J. Med. Chem. 1994, 37, 2421–2436. [Google Scholar] [CrossRef] [PubMed]
- Ritchie, T.J.; Macdonald, S.J.F.; Peace, S.; Pickett, S.D.; Luscombe, C.N. The Developability of Heteroaromatic and Heteroaliphatic Rings—Do Some Have a Better Pedigree as Potential Drug Molecules than Others? MedChemComm 2012, 3, 1062. [Google Scholar] [CrossRef]
- Boström, J.; Hogner, A.; Llinàs, A.; Wellner, E.; Plowright, A.T. Oxadiazoles in Medicinal Chemistry. J. Med. Chem. 2012, 55, 1817–1830. [Google Scholar] [CrossRef]
- Tsiulin, P.A.; Sosnina, V.V.; Krasovskaya, G.G.; Danilova, A.S.; Baikov, S.V.; Kofanov, E.R. Formation and Cyclization of N′-(Benzoyloxy)Benzenecarboximidamides. Russ. J. Org. Chem. 2011, 47, 1874–1877. [Google Scholar] [CrossRef]
- Valeur, E.; Bradley, M. Amide Bond Formation: Beyond the Myth of Coupling Reagents. Chem. Soc. Rev. 2009, 38, 606–631. [Google Scholar] [CrossRef] [PubMed]
- Abdildinova, A.; Gong, Y.-D. Current Parallel Solid-Phase Synthesis of Drug-like Oxadiazole and Thiadiazole Derivatives for Combinatorial Chemistry. ACS Comb. Sci. 2018, 20, 309–329. [Google Scholar] [CrossRef]
- Joullie, M.M.; Lassen, K.M. Evolution of Amide Bond Formation. Arkivoc 2010, 2010, 189–250. [Google Scholar] [CrossRef] [Green Version]
- Gangloff, A.R.; Litvak, J.; Shelton, E.J.; Sperandio, D.; Wang, V.R.; Rice, K.D. Synthesis of 3,5-Disubstituted-1,2,4-Oxadiazoles Using Tetrabutylammonium Fluoride as a Mild and Efficient Catalyst. Tetrahedron Lett. 2001, 42, 1441–1443. [Google Scholar] [CrossRef]
- Koufaki, M.; Kiziridi, C.; Nikoloudaki, F.; Alexis, M.N. Design and Synthesis of 1,2-Dithiolane Derivatives and Evaluation of Their Neuroprotective Activity. Bioorg. Med. Chem. Lett. 2007, 17, 4223–4227. [Google Scholar] [CrossRef] [PubMed]
- Clark, J.H. Fluoride Ion as a Base in Organic Synthesis. Chem. Rev. 1980, 80, 429–452. [Google Scholar] [CrossRef]
- Hameed, A.; Alharthy, R.D.; Iqbal, J.; Langer, P. The Role of Naked Fluoride Ion as Base or Catalyst in Organic Synthesis. Tetrahedron 2016, 72, 2763–2812. [Google Scholar] [CrossRef]
- Rice, K.D.; Nuss, J.M. An Improved Synthesis of 1,2,4-Oxadiazoles on Solid Support. Bioorg. Med. Chem. Lett. 2001, 11, 753–755. [Google Scholar] [CrossRef]
- Tamura, M.; Ise, Y.; Okajima, Y.; Nishiwaki, N.; Ariga, M. Facile Synthesis of 3-Carbamoyl-1,2,4-Oxadiazoles. Synthesis 2006, 2006, 3453–3461. [Google Scholar] [CrossRef]
- Lukyanov, S.M.; Bliznets, I.V.; Shorshnev, S.V. Synthesis of Sterically Hindered 3-(Azolyl)Pyridines. Arkivoc 2008, 2009, 21–45. [Google Scholar] [CrossRef] [Green Version]
- Huck, J.; Roumestant, M.L.; Martinez, J. From Beta-N-Tert-Butyloxycarbonyl N-Carboxyanhydrides to Beta-Aminoacid Derivatives. J. Pept. Res. 2003, 62, 233–237. [Google Scholar] [CrossRef] [PubMed]
- Meyer, E.; Joussef, A.C.; de Souza, L.d.B.P. Synthesis of New 1,2,4- and 1,3,4-Oxadiazole Derivatives as Potential Nonpeptide Angiotensin II Receptor Antagonists. Synth. Commun. 2006, 36, 729–741. [Google Scholar] [CrossRef]
- Benmansour, F.; Eydoux, C.; Querat, G.; de Lamballerie, X.; Canard, B.; Alvarez, K.; Guillemot, J.-C.; Barral, K. Novel 2-Phenyl-5-[(E)-2-(Thiophen-2-yl)Ethenyl]-1,3,4-Oxadiazole and 3-Phenyl-5-[(E)-2-(Thiophen-2-yl)Ethenyl]-1,2,4-Oxadiazole Derivatives as Dengue Virus Inhibitors Targeting NS5 Polymerase. Eur. J. Med. Chem. 2016, 109, 146–156. [Google Scholar] [CrossRef]
- Galbiati, A.; Zana, A.; Coser, C.; Tamborini, L.; Basilico, N.; Parapini, S.; Taramelli, D.; Conti, P. Development of Potent 3-Br-Isoxazoline-Based Antimalarial and Antileishmanial Compounds. ACS Med. Chem. Lett. 2021, 12, 1726–1732. [Google Scholar] [CrossRef] [PubMed]
- Kiss, L.E.; Ferreira, H.S.; Torrão, L.; Bonifácio, M.J.; Palma, P.N.; Soares-da-Silva, P.; Learmonth, D.A. Discovery of a Long-Acting, Peripherally Selective Inhibitor of Catechol-O-Methyltransferase. J. Med. Chem. 2010, 53, 3396–3411. [Google Scholar] [CrossRef] [PubMed]
- Mori, M.; Dasso Lang, M.C.; Saladini, F.; Palombi, N.; Kovalenko, L.; De Forni, D.; Poddesu, B.; Friggeri, L.; Giannini, A.; Malancona, S.; et al. Synthesis and Evaluation of Bifunctional Aminothiazoles as Antiretrovirals Targeting the HIV-1 Nucleocapsid Protein. ACS Med. Chem. Lett. 2019, 10, 463–468. [Google Scholar] [CrossRef]
- Schwertz, G.; Witschel, M.C.; Rottmann, M.; Bonnert, R.; Leartsakulpanich, U.; Chitnumsub, P.; Jaruwat, A.; Ittarat, W.; Schäfer, A.; Aponte, R.A.; et al. Antimalarial Inhibitors Targeting Serine Hydroxymethyltransferase (SHMT) with in Vivo Efficacy and Analysis of Their Binding Mode Based on X-Ray Cocrystal Structures. J. Med. Chem. 2017, 60, 4840–4860. [Google Scholar] [CrossRef]
- Käsnänen, H.; Myllymäki, M.J.; Minkkilä, A.; Kataja, A.O.; Saario, S.M.; Nevalainen, T.; Koskinen, A.M.P.; Poso, A. 3-Heterocycle-Phenyl N -Alkylcarbamates as FAAH Inhibitors: Design, Synthesis and 3D-QSAR Studies. ChemMedChem 2010, 5, 213–231. [Google Scholar] [CrossRef]
- Wurm, K.W.; Bartz, F.; Schulig, L.; Bodtke, A.; Bednarski, P.J.; Link, A. Replacing the Oxidation-sensitive Triaminoaryl Chemotype of Problematic KV7 Channel Openers: Exploration of a Nicotinamide Scaffold. Arch. Pharm. 2023, 356, e2200473. [Google Scholar] [CrossRef]
- Kovács, D.; Wölfling, J.; Szabó, N.; Szécsi, M.; Kovács, I.; Zupkó, I.; Frank, É. An Efficient Approach to Novel 17-5′-(1′,2′,4′)-Oxadiazolyl Androstenes via the Cyclodehydration of Cytotoxic O-Steroidacylamidoximes, and an Evaluation of Their Inhibitory Action on 17α-Hydroxylase/C17,20-Lyase. Eur. J. Med. Chem. 2013, 70, 649–660. [Google Scholar] [CrossRef]
- Gonda, T.; Bérdi, P.; Zupkó, I.; Fülöp, F.; Szakonyi, Z. Stereoselective Synthesis, Synthetic and Pharmacological Application of Monoterpene-Based 1,2,4- and 1,3,4-Oxadiazoles. Int. J. Mol. Sci. 2017, 19, 81. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ontoria, J.M.; Bufi, L.L.; Torrisi, C.; Bresciani, A.; Giomini, C.; Rowley, M.; Serafini, S.; Bin, H.; Hao, W.; Steinkühler, C.; et al. Identification of a Series of 4-[3-(Quinolin-2-yl)-1,2,4-Oxadiazol-5-yl]Piperazinyl Ureas as Potent Smoothened Antagonist Hedgehog Pathway Inhibitors. Bioorg. Med. Chem. Lett. 2011, 21, 5274–5282. [Google Scholar] [CrossRef] [PubMed]
- Rice, K.D.; Kim, M.H.; Bussenius, J.; Anand, N.K.; Blazey, C.M.; Bowles, O.J.; Canne-Bannen, L.; Chan, D.S.-M.; Chen, B.; Co, E.W.; et al. Pyrazolopyrimidines as Dual Akt/P70S6K Inhibitors. Bioorg. Med. Chem. Lett. 2012, 22, 2693–2697. [Google Scholar] [CrossRef]
- Chobanian, H.R.; Guo, Y.; Liu, P.; Chioda, M.; Lanza, T.J.; Chang, L.; Kelly, T.M.; Kan, Y.; Palyha, O.; Guan, X.-M.; et al. Discovery of MK-7725, A Potent, Selective Bombesin Receptor Subtype-3 Agonist for the Treatment of Obesity. ACS Med. Chem. Lett. 2012, 3, 252–256. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- He, S.; Ye, Z.; Dobbelaar, P.H.; Bakshi, R.K.; Hong, Q.; Dellureficio, J.P.; Sebhat, I.K.; Guo, L.; Liu, J.; Jian, T.; et al. Discovery of Highly Potent and Efficacious MC4R Agonists with Spiroindane N-Me-1,2,4-Triazole Privileged Structures for the Treatment of Obesity. Bioorg. Med. Chem. Lett. 2010, 20, 6524–6532. [Google Scholar] [CrossRef] [PubMed]
- Houck, J.D.; Dawson, T.K.; Kennedy, A.J.; Kharel, Y.; Naimon, N.D.; Field, S.D.; Lynch, K.R.; Macdonald, T.L. Structural Requirements and Docking Analysis of Amidine-Based Sphingosine Kinase 1 Inhibitors Containing Oxadiazoles. ACS Med. Chem. Lett. 2016, 7, 487–492. [Google Scholar] [CrossRef] [Green Version]
- Congdon, M.D.; Childress, E.S.; Patwardhan, N.N.; Gumkowski, J.; Morris, E.A.; Kharel, Y.; Lynch, K.R.; Santos, W.L. Structure–Activity Relationship Studies of the Lipophilic Tail Region of Sphingosine Kinase 2 Inhibitors. Bioorg. Med. Chem. Lett. 2015, 25, 4956–4960. [Google Scholar] [CrossRef] [Green Version]
- Gilmore, J.L.; Sheppeck, J.E.; Watterson, S.H.; Haque, L.; Mukhopadhyay, P.; Tebben, A.J.; Galella, M.A.; Shen, D.R.; Yarde, M.; Cvijic, M.E.; et al. Discovery and Structure–Activity Relationship (SAR) of a Series of Ethanolamine-Based Direct-Acting Agonists of Sphingosine-1-Phosphate (S1P1). J. Med. Chem. 2016, 59, 6248–6264. [Google Scholar] [CrossRef]
- Meng, Q.; Zhao, B.; Xu, Q.; Xu, X.; Deng, G.; Li, C.; Luan, L.; Ren, F.; Wang, H.; Xu, H.; et al. Indole-Propionic Acid Derivatives as Potent, S1P3-Sparing and EAE Efficacious Sphingosine-1-Phosphate 1 (S1P1) Receptor Agonists. Bioorg. Med. Chem. Lett. 2012, 22, 2794–2797. [Google Scholar] [CrossRef]
- Nakamura, T.; Asano, M.; Sekiguchi, Y.; Mizuno, Y.; Tamaki, K.; Kimura, T.; Nara, F.; Kawase, Y.; Shimozato, T.; Doi, H.; et al. Discovery of CS-2100, a Potent, Orally Active and S1P3-Sparing S1P1 Agonist. Bioorg. Med. Chem. Lett. 2012, 22, 1788–1792. [Google Scholar] [CrossRef] [PubMed]
- O’Daniel, P.I.; Peng, Z.; Pi, H.; Testero, S.A.; Ding, D.; Spink, E.; Leemans, E.; Boudreau, M.A.; Yamaguchi, T.; Schroeder, V.A.; et al. Discovery of a New Class of Non-β-Lactam Inhibitors of Penicillin-Binding Proteins with Gram-Positive Antibacterial Activity. J. Am. Chem. Soc. 2014, 136, 3664–3672. [Google Scholar] [CrossRef]
- Koufaki, M.; Kiziridi, C.; Alexi, X.; Alexis, M.N. Design and Synthesis of Novel Neuroprotective 1,2-Dithiolane/Chroman Hybrids. Bioorg. Med. Chem. 2009, 17, 6432–6441. [Google Scholar] [CrossRef] [PubMed]
- Koufaki, M.; Theodorou, E.; Alexi, X.; Alexis, M.N. Synthesis of a Second Generation Chroman/Catechol Hybrids and Evaluation of Their Activity in Protecting Neuronal Cells from Oxidative Stress-Induced Cell Death. Bioorg. Med. Chem. 2010, 18, 3898–3909. [Google Scholar] [CrossRef]
- Zhang, H.; Ye, N.; Zhou, S.; Guo, L.; Zheng, L.; Liu, Z.; Gao, B.; Zhen, X.; Zhang, A. Identification of N -Propylnoraporphin-11-yl 5-(1,2-Dithiolan-3-yl)Pentanoate as a New Anti-Parkinson’s Agent Possessing a Dopamine D 2 and Serotonin 5-HT 1A Dual-Agonist Profile. J. Med. Chem. 2011, 54, 4324–4338. [Google Scholar] [CrossRef] [PubMed]
- Fox, B.M.; Iio, K.; Li, K.; Choi, R.; Inaba, T.; Jackson, S.; Sagawa, S.; Shan, B.; Tanaka, M.; Yoshida, A.; et al. Discovery of Pyrrolopyridazines as Novel DGAT1 Inhibitors. Bioorg. Med. Chem. Lett. 2010, 20, 6030–6033. [Google Scholar] [CrossRef]
- Hencken, C.P.; Jones-Brando, L.; Bordón, C.; Stohler, R.; Mott, B.T.; Yolken, R.; Posner, G.H.; Woodard, L.E. Thiazole, Oxadiazole, and Carboxamide Derivatives of Artemisinin Are Highly Selective and Potent Inhibitors of Toxoplasma Gondii. J. Med. Chem. 2010, 53, 3594–3601. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wágner, G.; Wéber, C.; Nyéki, O.; Nógrádi, K.; Bielik, A.; Molnár, L.; Bobok, A.; Horváth, A.; Kiss, B.; Kolok, S.; et al. Hit-to-Lead Optimization of Disubstituted Oxadiazoles and Tetrazoles as MGluR5 NAMs. Bioorg. Med. Chem. Lett. 2010, 20, 3737–3741. [Google Scholar] [CrossRef]
- Malkondu, S.; Altinkaya, N.; Erdemir, S.; Kocak, A. A Reaction-Based Approach for Fluorescence Sensing of Fluoride through Cyclization of an O-Acyl Pyrene Amidoxime Derivative. Sens. Actuators B Chem. 2018, 276, 296–303. [Google Scholar] [CrossRef]
- Cecioni, S.; Argintaru, O.-A.; Docsa, T.; Gergely, P.; Praly, J.-P.; Vidal, S. Probing Multivalency for the Inhibition of an Enzyme: Glycogen Phosphorylase as a Case Study. New J. Chem. 2009, 33, 148–156. [Google Scholar] [CrossRef]
- Otaka, H.; Ikeda, J.; Tanaka, D.; Tobe, M. Construction of 3,5-Substituted 1,2,4-Oxadiazole Rings Triggered by Tetrabutylammonium Hydroxide: A Highly Efficient and Fluoride-Free Ring Closure Reaction of O-Acylamidoximes. Tetrahedron Lett. 2014, 55, 979–981. [Google Scholar] [CrossRef]
- Jones, D.H.; Kay, S.T.; McLellan, J.A.; Kennedy, A.R.; Tomkinson, N.C.O. Regioselective Three-Component Reaction of Pyridine N -Oxides, Acyl Chlorides, and Cyclic Ethers. Org. Lett. 2017, 19, 3512–3515. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fershtat, L.L.; Ananyev, I.V.; Makhova, N.N. Efficient Assembly of Mono- and Bis(1,2,4-Oxadiazol-3-yl)Furoxan Scaffolds via Tandem Reactions of Furoxanylamidoximes. RSC Adv. 2015, 5, 47248–47260. [Google Scholar] [CrossRef]
- Kulikov, A.S.; Larin, A.A.; Fershtat, L.L.; Anikina, L.V.; Pukhov, S.A.; Klochkov, S.G.; Struchkova, M.I.; Romanova, A.A.; Ananyev, I.V.; Makhova, N.N. Synthesis, Structural Characterization and Cytotoxic Activity of Heterocyclic Compounds Containing the Furoxan Ring. Arkivoc 2017, 2017, 250–268. [Google Scholar] [CrossRef]
- Baykov, S.; Sharonova, T.; Osipyan, A.; Rozhkov, S.; Shetnev, A.; Smirnov, A. A Convenient and Mild Method for 1,2,4-Oxadiazole Preparation: Cyclodehydration of O -Acylamidoximes in the Superbase System MOH/DMSO. Tetrahedron Lett. 2016, 57, 2898–2900. [Google Scholar] [CrossRef]
- Tarasenko, M.; Sidneva, V.; Belova, A.; Romanycheva, A.; Sharonova, T.; Baykov, S.; Shetnev, A.; Kofanov, E.; Kuznetsov, M. An Efficient Synthesis and Antimicrobial Evaluation of 5-Alkenyl- and 5-Styryl-1,2,4-Oxadiazoles. Arkivoc 2018, 2018, 458–470. [Google Scholar] [CrossRef]
- Zalivatskaya, A.S.; Ryabukhin, D.S.; Tarasenko, M.V.; Ivanov, A.Y.; Boyarskaya, I.A.; Grinenko, E.V.; Osetrova, L.V.; Kofanov, E.R.; Vasilyev, A.V. Metal-Free Hydroarylation of the Side Chain Carbon–Carbon Double Bond of 5-(2-Arylethenyl)-3-Aryl-1,2,4-Oxadiazoles in Triflic Acid. Beilstein J. Org. Chem. 2017, 13, 883–894. [Google Scholar] [CrossRef] [Green Version]
- Shkineva, T.K.; Vatsadze, I.A.; Khoranyan, T.E.; Lipilin, D.L.; Pivkina, A.N.; Dalinger, I.L. Synthesis of 3(5)-Aryl-5(3)-Pyrazolyl-1,2,4-Oxadiazole Nitro Derivatives. Chem. Heterocycl. Compd. 2021, 57, 828–836. [Google Scholar] [CrossRef]
- Tarasenko, M.V.; Kofanov, E.R.; Baikov, S.V.; Krasovskaya, G.G.; Danilova, A.S. Selective Reduction of 5-Alkenyl-3-(Nitrophenyl)-1,2,4-Oxadiazoles to 5-Alkenyl-3-(Aminophenyl)-1,2,4-Oxadiazoles. Russ. J. Org. Chem. 2017, 53, 1085–1089. [Google Scholar] [CrossRef]
- Krasavin, M.; Shetnev, A.; Sharonova, T.; Baykov, S.; Tuccinardi, T.; Kalinin, S.; Angeli, A.; Supuran, C.T. Heterocyclic Periphery in the Design of Carbonic Anhydrase Inhibitors: 1,2,4-Oxadiazol-5-yl Benzenesulfonamides as Potent and Selective Inhibitors of Cytosolic h CA II and Membrane-Bound h CA IX Isoforms. Bioorg. Chem. 2018, 76, 88–97. [Google Scholar] [CrossRef]
- Sucu, B.O.; Ipek, O.S.; Kurtulus, S.O.; Yazici, B.E.; Karakas, N.; Guzel, M. Synthesis of Novel Methyl Jasmonate Derivatives and Evaluation of Their Biological Activity in Various Cancer Cell Lines. Bioorg. Chem. 2019, 91, 103146. [Google Scholar] [CrossRef]
- Penjarla, S.; Kumar Sabui, S.; Sudhakar Reddy, D.; Banerjee, S.; Yella Reddy, P.; Penta, S.; Sanghvi, Y.S. An Efficient Synthesis Tetrazole and Oxadiazole Analogues of Novel 2′-Deoxy-C-Nucleosides and Their Antitumor Activity. Bioorg. Med. Chem. Lett. 2020, 30, 127612. [Google Scholar] [CrossRef]
- Qiu, L.; Jiang, H.; Yu, Y.; Gu, J.; Wang, J.; Zhao, H.; Huang, T.; Gropler, R.J.; Klein, R.S.; Perlmutter, J.S.; et al. Radiosynthesis and Evaluation of a Fluorine-18 Radiotracer [18 F]FS1P1 for Imaging Sphingosine-1-Phosphate Receptor 1. Org. Biomol. Chem. 2022, 20, 1041–1052. [Google Scholar] [CrossRef]
- Baykov, S.; Sharonova, T.; Shetnev, A.; Rozhkov, S.; Kalinin, S.; Smirnov, A.V. The First One-Pot Ambient-Temperature Synthesis of 1,2,4-Oxadiazoles from Amidoximes and Carboxylic Acid Esters. Tetrahedron 2017, 73, 945–951. [Google Scholar] [CrossRef]
- Pankrat’eva, V.E.; Sharonova, T.V.; Tarasenko, M.V.; Baikov, S.V.; Kofanov, E.R. One-Pot Synthesis of 3,5-Disubstituted 1,2,4-Oxadiazoles Using Catalytic System NaOH–DMSO. Russ. J. Org. Chem. 2018, 54, 1250–1255. [Google Scholar] [CrossRef]
- Sharonova, T.; Pankrat’eva, V.; Savko, P.; Baykov, S.; Shetnev, A. Facile Room-Temperature Assembly of the 1,2,4-Oxadiazole Core from Readily Available Amidoximes and Carboxylic Acids. Tetrahedron Lett. 2018, 59, 2824–2827. [Google Scholar] [CrossRef]
- Tarasenko, M.; Duderin, N.; Sharonova, T.; Baykov, S.; Shetnev, A.; Smirnov, A.V. Room-Temperature Synthesis of Pharmaceutically Important Carboxylic Acids Bearing the 1,2,4-Oxadiazole Moiety. Tetrahedron Lett. 2017, 58, 3672–3677. [Google Scholar] [CrossRef]
- Baykov, S.; Tarasenko, M.; Zelenkov, L.E.; Kasatkina, S.; Savko, P.; Shetnev, A. Diastereoselective Opening of Bridged Anhydrides by Amidoximes Providing Access to 1,2,4-Oxadiazole/Norborna(e)Ne Hybrids. Eur. J. Org. Chem. 2019, 2019, 5685–5693. [Google Scholar] [CrossRef]
- Baykov, S.V.; Tarasenko, M.V.; Semenov, A.V.; Katlenok, E.A.; Shetnev, A.A.; Boyarskiy, V.P. Dualism of 1,2,4-Oxadiazole Ring in Noncovalent Interactions with Carboxylic Group. J. Mol. Struct. 2022, 1262, 132974. [Google Scholar] [CrossRef]
- Presnukhina, S.; Tarasenko, M.; Baykov, S.; Smirnov, S.N.; Boyarskiy, V.P.; Shetnev, A.; Korsakov, M.K. Entry into (E)-3-(1,2,4-Oxadiazol-5-yl)Acrylic Acids via a One-Pot Ring-Opening/Ring-Closing/Retro-Diels-Alder Reaction Sequence. Tetrahedron Lett. 2020, 61, 151543. [Google Scholar] [CrossRef]
- Tarasenko, M.V.; Presnukhina, S.I.; Baikov, S.V.; Shetnev, A.A. Synthesis and Evaluation of Antibacterial Activity of 1,2,4-Oxadiazole-Containing Biphenylcarboxylic Acids. Russ. J. Gen. Chem. 2020, 90, 1611–1619. [Google Scholar] [CrossRef]
- Shetnev, A.A.; Pankratieva, V.E.; Kunichkina, A.S.; Vlasov, A.S.; Proskurina, I.K.; Kotov, A.D.; Korsakov, M.K. Synthesis of 3,5-Disubstituted 1,2,4-Oxadiazoles from Amidoximes and Aldehydes in the Superbasic System NaOH/DMSO. Russ. J. Org. Chem. 2020, 56, 1181–1186. [Google Scholar] [CrossRef]
- Nam, S.; Na, H.G.; Oh, E.H.; Jung, E.; Lee, Y.H.; Jeong, E.J.; Ou, Y.-D.; Zhou, B.; Ahn, S.; Shin, J.S.; et al. Discovery and Synthesis of 1,2,4-Oxadiazole Derivatives as Novel Inhibitors of Zika, Dengue, Japanese Encephalitis, and Classical Swine Fever Virus Infections. Arch. Pharm. Res. 2022, 45, 280–293. [Google Scholar] [CrossRef] [PubMed]
- Baikov, S.V.; Trukhanova, Y.A.; Tarasenko, M.V.; Kinzhalov, M.A. Synthesis and Study of the Structure of Palladium(II) Acyclic Diaminocarbene Complexes Containing a 1,2,4-Oxadiazole Moiety. Russ. J. Gen. Chem. 2020, 90, 1892–1900. [Google Scholar] [CrossRef]
- Ayoup, M.S.; Abu-Serie, M.M.; Abdel-Hamid, H.; Teleb, M. Beyond Direct Nrf2 Activation; Reinvestigating 1,2,4-Oxadiazole Scaffold as a Master Key Unlocking the Antioxidant Cellular Machinery for Cancer Therapy. Eur. J. Med. Chem. 2021, 220, 113475. [Google Scholar] [CrossRef]
- Strelnikova, J.O.; Rostovskii, N.V.; Starova, G.L.; Khlebnikov, A.F.; Novikov, M.S. Rh(II)-Catalyzed Transannulation of 1,2,4-Oxadiazole Derivatives with 1-Sulfonyl-1,2,3-Triazoles: Regioselective Synthesis of 5-Sulfonamidoimidazoles. J. Org. Chem. 2018, 83, 11232–11244. [Google Scholar] [CrossRef] [PubMed]
- Strelnikova, J.O.; Rostovskii, N.V.; Khoroshilova, O.V.; Khlebnikov, A.F.; Novikov, M.S. An Efficient Synthesis of Functionalized 2H-1,3,5-Oxadiazines via Metal-Carbenoid-Induced 1,2,4-Oxadiazole Ring Cleavage. Synthesis 2021, 53, 348–358. [Google Scholar]
- Shetnev, A.; Baykov, S.; Kalinin, S.; Belova, A.; Sharoyko, V.; Rozhkov, A.; Zelenkov, L.; Tarasenko, M.; Sadykov, E.; Korsakov, M.; et al. 1,2,4-Oxadiazole/2-Imidazoline Hybrids: Multi-Target-Directed Compounds for the Treatment of Infectious Diseases and Cancer. Int. J. Mol. Sci. 2019, 20, 1699. [Google Scholar] [CrossRef] [Green Version]
- Shetnev, A.; Osipyan, A.; Baykov, S.; Sapegin, A.; Chirkova, Z.; Korsakov, M.; Petzer, A.; Engelbrecht, I.; Petzer, J.P. Novel Monoamine Oxidase Inhibitors Based on the Privileged 2-Imidazoline Molecular Framework. Bioorg. Med. Chem. Lett. 2019, 29, 40–46. [Google Scholar] [CrossRef]
- Kulkarni, B.; Manjunatha, K.; Joy, M.N.; Sajith, A.M.; Prashantha, C.N.; Pakkath, R.; Alshammari, M.B. Design, Synthesis and Molecular Docking Studies of Some 1-(5-(2-Fluoro-5-(Trifluoromethoxy)Phenyl)-1,2,4-Oxadiazol-3-yl)Piperazine Derivatives as Potential Anti-Inflammatory Agents. Mol. Divers. 2022, 26, 2893–2905. [Google Scholar] [CrossRef]
- Presnukhina, S.I.; Tarasenko, M.V.; Geyl, K.K.; Baykova, S.O.; Baykov, S.V.; Shetnev, A.A.; Boyarskiy, V.P. Unusual Formation of 1,2,4-Oxadiazine Core in Reaction of Amidoximes with Maleic or Fumaric Esters. Molecules 2022, 27, 7508. [Google Scholar] [CrossRef] [PubMed]
- Thacker, P.S.; Angeli, A.; Argulwar, O.S.; Tiwari, P.L.; Arifuddin, M.; Supuran, C.T. Design, Synthesis and Biological Evaluation of Coumarin Linked 1,2,4-Oxadiazoles as Selective Carbonic Anhydrase IX and XII Inhibitors. Bioorg. Chem. 2020, 98, 103739. [Google Scholar] [CrossRef] [PubMed]
- Krasavin, M.; Sharonova, T.; Sharoyko, V.; Zhukovsky, D.; Kalinin, S.; Žalubovskis, R.; Tennikova, T.; Supuran, C.T. Combining Carbonic Anhydrase and Thioredoxin Reductase Inhibitory Motifs within a Single Molecule Dramatically Increases Its Cytotoxicity. J. Enzym. Inhib. Med. Chem. 2020, 35, 665–671. [Google Scholar] [CrossRef] [Green Version]
- Geyl, K.; Baykov, S.; Tarasenko, M.; Zelenkov, L.E.; Matveevskaya, V.; Boyarskiy, V.P. Convenient Entry to N-Pyridinylureas with Pharmaceutically Privileged Oxadiazole Substituents via the Acid-Catalyzed C H Activation of N-Oxides. Tetrahedron Lett. 2019, 60, 151108. [Google Scholar] [CrossRef]
- Baykov, S.; Mikherdov, A.; Novikov, A.; Geyl, K.; Tarasenko, M.; Gureev, M.; Boyarskiy, V. π–π Noncovalent Interaction Involving 1,2,4- and 1,3,4-Oxadiazole Systems: The Combined Experimental, Theoretical, and Database Study. Molecules 2021, 26, 5672. [Google Scholar] [CrossRef]
- Kasatkina, S.O.; Geyl, K.K.; Baykov, S.V.; Novikov, M.S.; Boyarskiy, V.P. “Urea to Urea” Approach: Access to Unsymmetrical Ureas Bearing Pyridyl Substituents. Adv. Synth. Catal. 2022, 364, 1295–1304. [Google Scholar] [CrossRef]
- Kasatkina, S.O.; Geyl, K.K.; Baykov, S.V.; Boyarskaya, I.A.; Boyarskiy, V.P. Catalyst-Free Synthesis of Substituted Pyridin-2-yl, Quinolin-2-yl, and Isoquinolin-1-yl Carbamates from the Corresponding Hetaryl Ureas and Alcohols. Org. Biomol. Chem. 2021, 19, 6059–6065. [Google Scholar] [CrossRef] [PubMed]
- Geyl, K.K.; Baykova, S.O.; Andoskin, P.A.; Sharoyko, V.V.; Eliseeva, A.A.; Baykov, S.V.; Semenov, K.N.; Boyarskiy, V.P. Palladium(II) and Platinum(II) Deprotonated Diaminocarbene Complexes Based on N-(2-Pyridyl)Ureas with Oxadiazole Periphery. Inorganics 2022, 10, 247. [Google Scholar] [CrossRef]
- Geyl, K.K.; Baykov, S.V.; Kalinin, S.A.; Bunev, A.S.; Troshina, M.A.; Sharonova, T.V.; Skripkin, M.Y.; Kasatkina, S.O.; Presnukhina, S.I.; Shetnev, A.A.; et al. Synthesis, Structure, and Antiproliferative Action of 2-Pyridyl Urea-Based Cu(II) Complexes. Biomedicines 2022, 10, 461. [Google Scholar] [CrossRef]
- Soldatova, N.S.; Semenov, A.V.; Geyl, K.K.; Baykov, S.V.; Shetnev, A.A.; Konstantinova, A.S.; Korsakov, M.M.; Yusubov, M.S.; Postnikov, P.S. Copper-Catalyzed Selective N-Arylation of Oxadiazolones by Diaryliodonium Salts. Adv. Synth. Catal. 2021, 363, 3566–3576. [Google Scholar] [CrossRef]
- Tarasenko, M.V.; Kotlyarova, V.D.; Baykov, S.V.; Shetnev, A.A. 2-(1,2,4-Oxadiazol-5-yl)Anilines Based on Amidoximes and Isatoic Anhydrides: Synthesis and Structure Features. Russ. J. Gen. Chem. 2021, 91, 768–778. [Google Scholar] [CrossRef]
- Baykov, S.; Tarasenko, M.; Kotlyarova, V.; Shetnev, A.; Zelenkov, L.E.; Boyarskaya, I.A.; Boyarskiy, V.P. 2-(1,2,4-Oxadiazol-5-yl)Aniline as a New Scaffold for Blue Luminescent Materials. ChemistrySelect 2022, 7, 768–778. [Google Scholar] [CrossRef]
- Knouse, K.W.; Ator, L.E.; Beausoleil, L.E.; Hauseman, Z.J.; Casaubon, R.L.; Ott, G.R. Improved and Expanded One-Pot, Two-Component Boulton-Katritzky Syntheses of N–N Bond Containing Bicyclic Heterocycles. Tetrahedron Lett. 2017, 58, 202–205. [Google Scholar] [CrossRef]
- Straniero, V.; Sebastián-Pérez, V.; Hrast, M.; Zanotto, C.; Casiraghi, A.; Suigo, L.; Zdovc, I.; Radaelli, A.; De Giuli Morghen, C.; Valoti, E. Benzodioxane-Benzamides as Antibacterial Agents: Computational and SAR Studies to Evaluate the Influence of the 7-Substitution in FtsZ Interaction. ChemMedChem 2020, 15, 195–209. [Google Scholar] [CrossRef]
- Khallaf, A.; Wang, P.; Liu, H.; Zhuo, S.; Zhu, H. 1,2,4-Oxadiazole Ring–Containing Pyridylpyrazole-4-carboxamides: Synthesis and Evaluation as Novel Insecticides of the Anthranilic Diamide Family. J. Heterocycl. Chem. 2020, 57, 1981–1992. [Google Scholar] [CrossRef]
- Sidneva, V.V.; Tarasenko, M.V.; Kofanov, E.R. One-Pot Synthesis of 3,5-Disubstituted 1,2,4-Oxadiazoles Containing an Alkenyl Moiety. Chem. Heterocycl. Compd. 2022, 58, 349–353. [Google Scholar] [CrossRef]
- Efimova, J.A.; Shetnev, A.A.; Gasilina, O.A.; Tarasenko, M.V.; Korsakov, M.K. Synthesis of 3,5-Disubstituted-1,2,4-Oxadiazole Sulfonamides in the Superbasic t-BuONa/DMAA System. Russ. J. Gen. Chem. 2022, 92, 2982–2988. [Google Scholar] [CrossRef]
- Guo, W.; Huang, K.; Ji, F.; Wu, W.; Jiang, H. A Facile Approach to Synthesize 3,5-Disubstituted-1,2,4-Oxadiazoles via Copper-Catalyzed-Cascade Annulation of Amidines and Methylarenes. Chem. Commun. 2015, 51, 8857–8860. [Google Scholar] [CrossRef]
- Yoshimura, A.; Nguyen, K.C.; Klasen, S.C.; Saito, A.; Nemykin, V.N.; Zhdankin, V.V. Preparation, Structure, and Versatile Reactivity of Pseudocyclic Benziodoxole Triflate, New Hypervalent Iodine Reagent. Chem. Commun. 2015, 51, 7835–7838. [Google Scholar] [CrossRef]
- Yoshimura, A.; Nguyen, K.C.; Klasen, S.C.; Postnikov, P.S.; Yusubov, M.S.; Saito, A.; Nemykin, V.N.; Zhdankin, V.V. Hypervalent Iodine-Catalyzed Synthesis of 1,2,4-Oxadiazoles from Aldoximes and Nitriles. Asian J. Org. Chem. 2016, 5, 1128–1133. [Google Scholar] [CrossRef]
- Qurban, J.; Elsherbini, M.; Alharbi, H.; Wirth, T. Synthesis, Characterisation, and Reactivity of Novel Pseudocyclic Hypervalent Iodine Reagents with Heteroaryl Carbonyl Substituents. Chem. Commun. 2019, 55, 7998–8000. [Google Scholar] [CrossRef]
- Anan, K.; Masui, M.; Hara, S.; Ohara, M.; Kume, M.; Yamamoto, S.; Shinohara, S.; Tsuji, H.; Shimada, S.; Yagi, S.; et al. Discovery of Orally Bioavailable Cyclohexanol-Based NR2B-Selective NMDA Receptor Antagonists with Analgesic Activity Utilizing a Scaffold Hopping Approach. Bioorg. Med. Chem. Lett. 2017, 27, 4194–4198. [Google Scholar] [CrossRef]
- Chilaka, S.K.; Chellu, R.K.; Soda, A.K.; Kurva, S.; Nanubolu, J.B.; Madabhushi, S. Base-Catalyzed Domino Reaction Between Aldoxime and N-Chlorosuccinimide in Alcohol: One-Pot Synthesis of Alkyl 3-(3-Aryl-1,2,4-oxadiazol-5-yl)Propanoates. Adv. Synth. Catal. 2022, 364, 2944–2950. [Google Scholar] [CrossRef]
- Lu, K.; Duan, L.; Xu, B.; Yin, W.; Wu, D.; Zhao, Y.; Gong, P. Facile Synthesis of 3-Amino-5-Aryl-1,2,4-Oxadiazoles via PIDA-Mediated Intramolecular Oxidative Cyclization. RSC Adv. 2016, 6, 54277–54280. [Google Scholar] [CrossRef]
- Li, E.; Wang, M.; Wang, Z.; Yu, W.; Chang, J. NBS-Mediated Practical Cyclization of N-Acyl Amidines to 1,2,4-Oxadiazoles via Oxidative N–O Bond Formation. Tetrahedron 2018, 74, 4613–4618. [Google Scholar] [CrossRef]
- Lade, J.J.; Patil, B.N.; Vadagaonkar, K.S.; Chaskar, A.C. Oxidative Cyclization of Amidoximes and Thiohydroximic Acids: A Facile and Efficient Strategy for Accessing 3,5-Disubstituted 1,2,4-Oxadiazoles and 1,4,2-Oxathiazoles. Tetrahedron Lett. 2017, 58, 2103–2108. [Google Scholar] [CrossRef]
- Jiang, C.; Li, M.; Xu, L.; Yi, Y.; Ye, J.; Hu, A. Electrochemical Synthesis of 1,2,4-Oxadiazoles from Amidoximes through Dehydrogenative Cyclization. Org. Biomol. Chem. 2021, 19, 10611–10616. [Google Scholar] [CrossRef] [PubMed]
- Jakubiec, D.; Walczak, K.Z. A Convenient Synthesis of 5-(1,2,4-Oxadiazol-5-yl)Pyrimidine-2,4(1H,3H)-Diones. Tetrahedron Lett. 2011, 52, 6890–6891. [Google Scholar] [CrossRef]
- Chalyk, B.A.; Sosedko, A.S.; Volochnyuk, D.M.; Tolmachev, A.A.; Gavrilenko, K.S.; Liashuk, O.S.; Grygorenko, O.O. Regioselective Synthesis of Isoxazole and 1,2,4-Oxadiazole-Derived Phosphonates via [3 + 2] Cycloaddition. Org. Biomol. Chem. 2018, 16, 9152–9164. [Google Scholar] [CrossRef] [PubMed]
- Kivrak, A.; Zora, M. A Novel Synthesis of 1,2,4-Oxadiazoles and Isoxazoles. Tetrahedron 2014, 70, 817–831. [Google Scholar] [CrossRef]
- Nikodemiak, P.; Koert, U. Metal-Catalyzed Synthesis of Functionalized 1,2,4-Oxadiazoles from Silyl Nitronates and Nitriles. Adv. Synth. Catal. 2017, 359, 1708–1716. [Google Scholar] [CrossRef]
- Golushko, A.A.; Khoroshilova, O.V.; Vasilyev, A.V. Synthesis of 1,2,4-Oxadiazoles by Tandem Reaction of Nitroalkenes with Arenes and Nitriles in the Superacid TfOH. J. Org. Chem. 2019, 84, 7495–7500. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Bi, F.; Yang, Z.; Xue, Q.; Wang, B. 5-Nitrotetrazol and 1,2,4-Oxadiazole Methylene-Bridged Energetic Compounds: Synthesis, Crystal Structures and Performances. Molecules 2021, 26, 7072. [Google Scholar] [CrossRef] [PubMed]
- Likhite, N.; Lakshminarasimhan, T.; Rao, M.H.V.R.; Shekarappa, V.; Sidar, S.; Subramanian, V.; Fraunhoffer, K.J.; Leung, S.; Vaidyanathan, R. A Scalable Synthesis of 2-(1,2,4-Oxadiazol-3-yl)Propan-2-Amine Hydrobromide Using a Process Safety-Driven Protecting Group Strategy. Org. Process Res. Dev. 2016, 20, 1328–1335. [Google Scholar] [CrossRef]
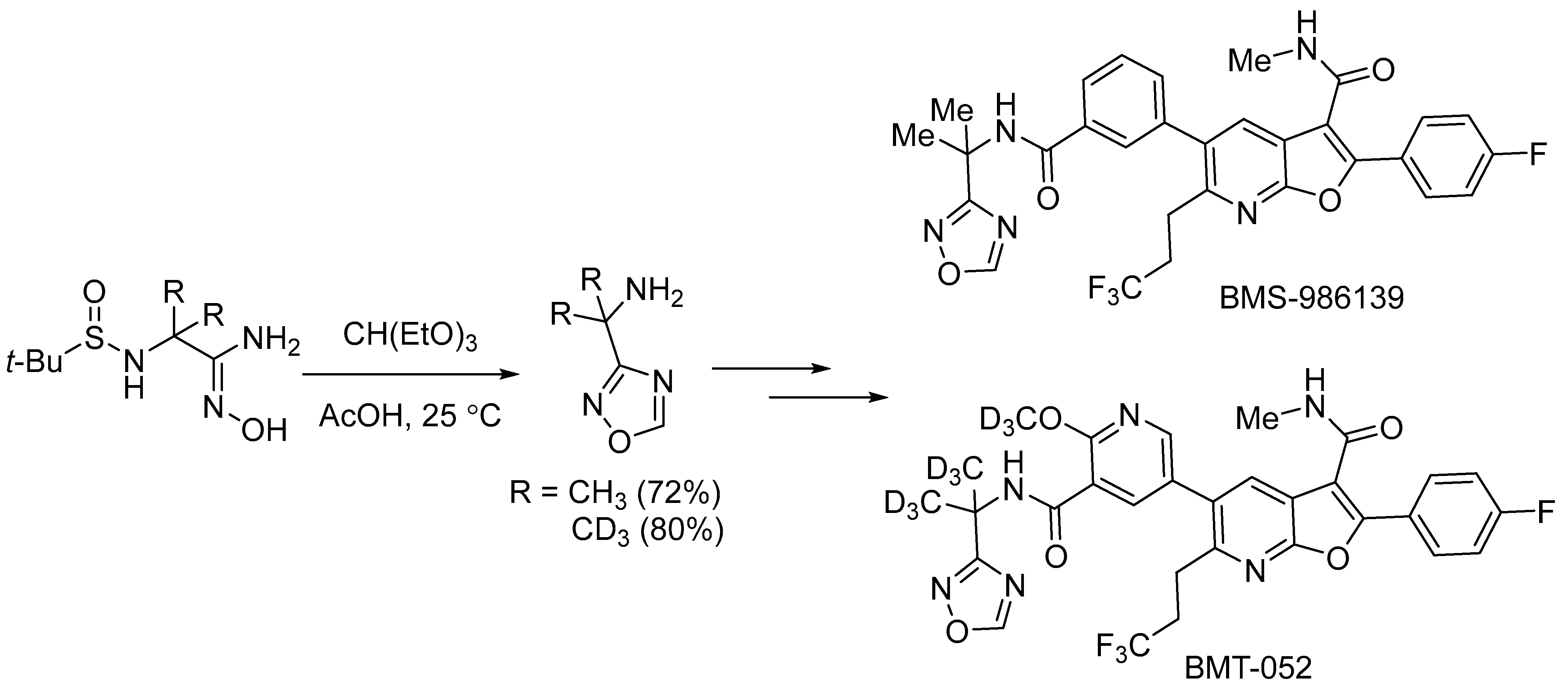
- Parcella, K.; Eastman, K.; Yeung, K.-S.; Grant-Young, K.A.; Zhu, J.; Wang, T.; Zhang, Z.; Yin, Z.; Parker, D.; Mosure, K.; et al. Improving Metabolic Stability with Deuterium: The Discovery of BMT-052, a Pan-Genotypic HCV NS5B Polymerase Inhibitor. ACS Med. Chem. Lett. 2017, 8, 771–774. [Google Scholar] [CrossRef]
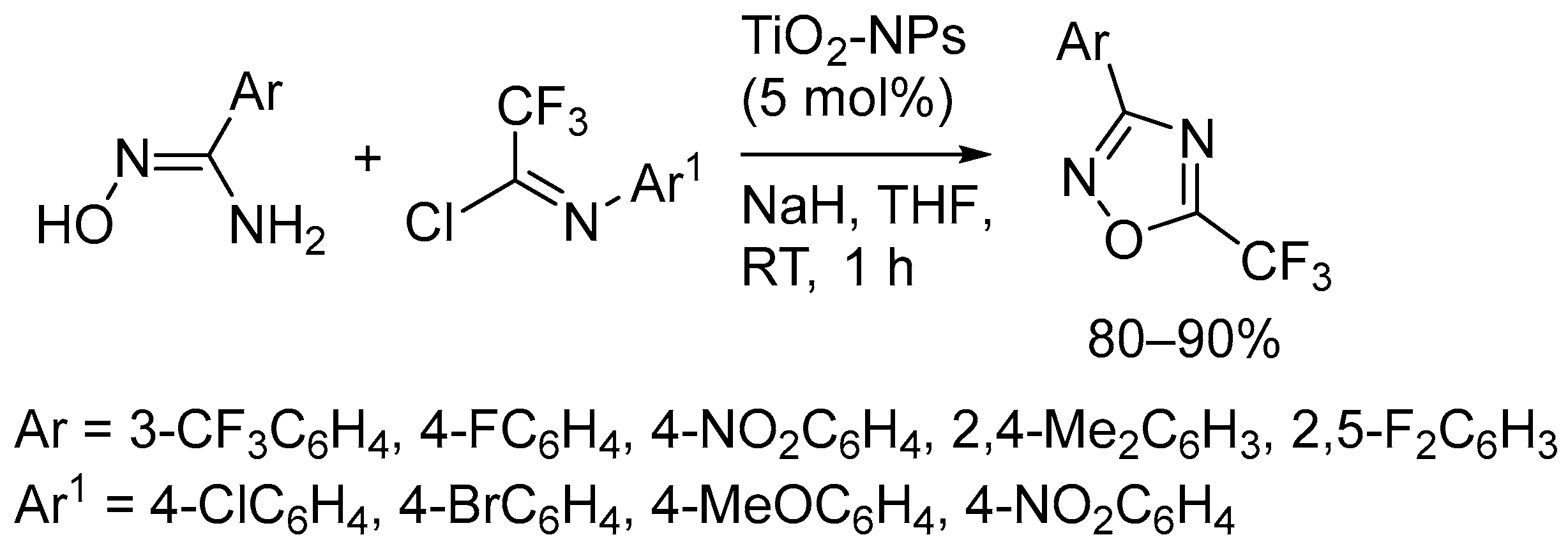
- Darehkordi, A.; Ramezani, M.; Rahmani, F. TiO2-Nanoparticles Catalyzed Synthesis of New Trifluoromethyl-4,5-Dihydro-1,2,4-Oxadiazoles and Trifluoromethyl-1,2,4-Oxadiazoles. J. Heterocycl. Chem. 2018, 55, 1702–1708. [Google Scholar] [CrossRef]
- Zarei, M. A Mild and Efficient One-Pot Preparation of 1,2,4-Oxadiazoles from Nitriles and Carboxylic Acids Using Vilsmeier Reagent. ChemistrySelect 2018, 3, 11273–11276. [Google Scholar] [CrossRef]
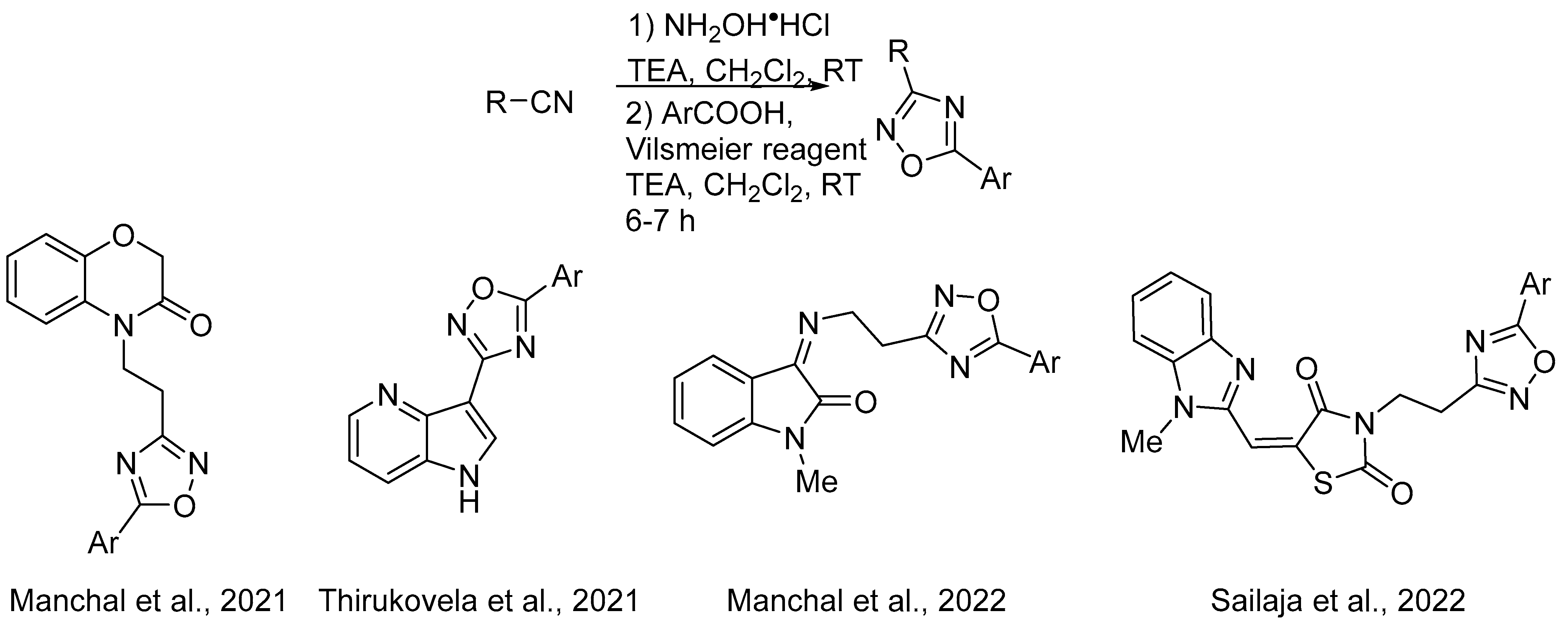
- Nagaraju, A.; Kumar Nukala, S.; Narasimha Swamy Thirukovela, T.; Manchal, R. In Vitro Anticancer and In Silico Studies of Some 1,4-Benzoxazine-1,2,4-oxadiazole Hybrids. ChemistrySelect 2021, 6, 3318–3321. [Google Scholar] [CrossRef]
- Sagam, R.R.; Nukala, S.K.; Nagavath, R.; Sirassu, N.; Gundepaka, P.; Manchal, R.; Thirukovela, N.S. In-vitro Anticancer and Molecular Docking Studies of 4-Azaindole-1,2,4-Oxadiazole Hybrids. ChemistrySelect 2021, 6, 7670–7673. [Google Scholar] [CrossRef]
- Boda, S.; Nukala, S.K.; Manchal, R. One-Pot Synthesis of Some New Isatin-1,2,4-oxadiazole Hybrids as VEGFR-2 Aiming Anticancer Agents. ChemistrySelect 2022, 7, e202200972. [Google Scholar] [CrossRef]
- Kudapa, V.; Saritha, B.; Sailaja, B.B.V. New Molecular Hybrids Containing Benzimidazole, Thiazolidine-2,4-Dione and 1,2,4-Oxadiazole as EGFR Directing Cytotoxic Agents. Tetrahedron 2022, 124, 132991. [Google Scholar] [CrossRef]
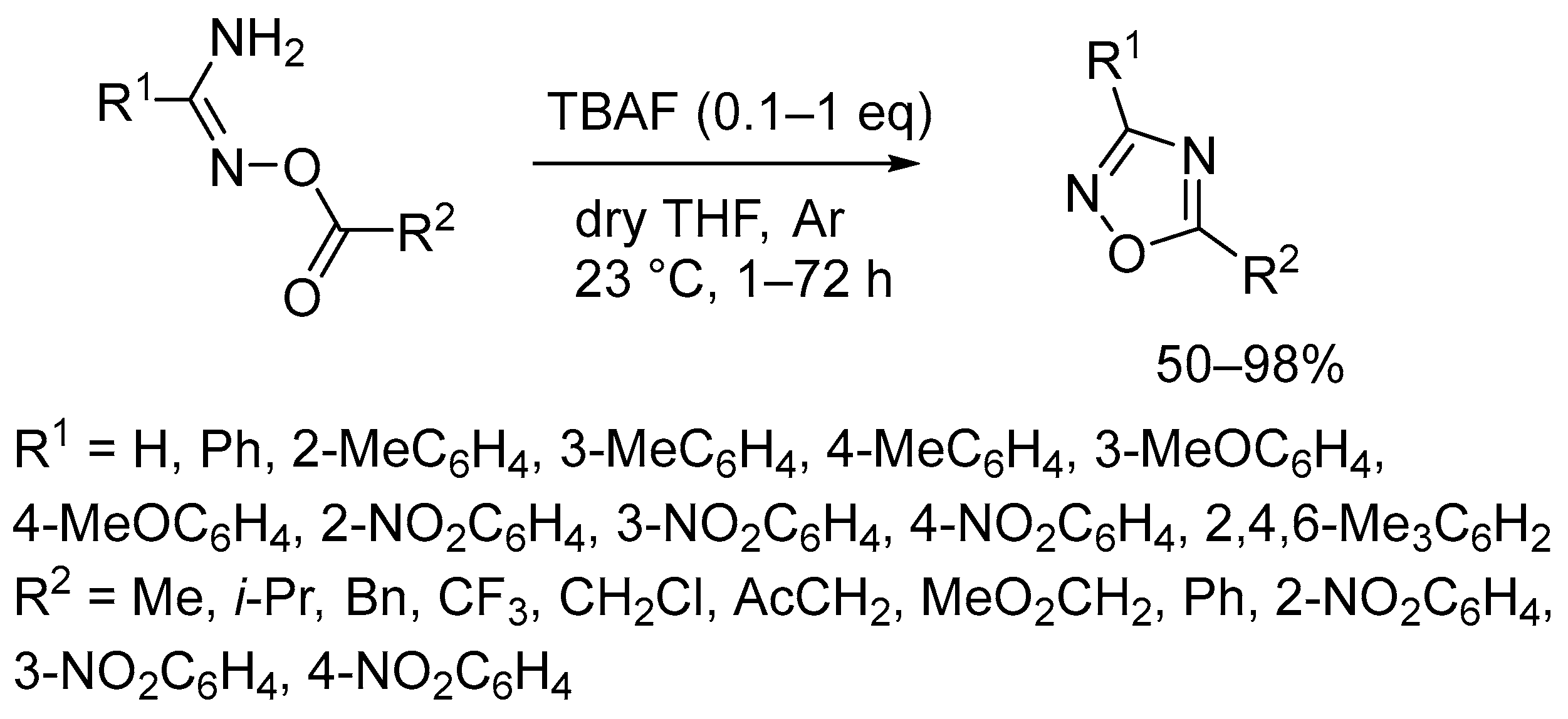





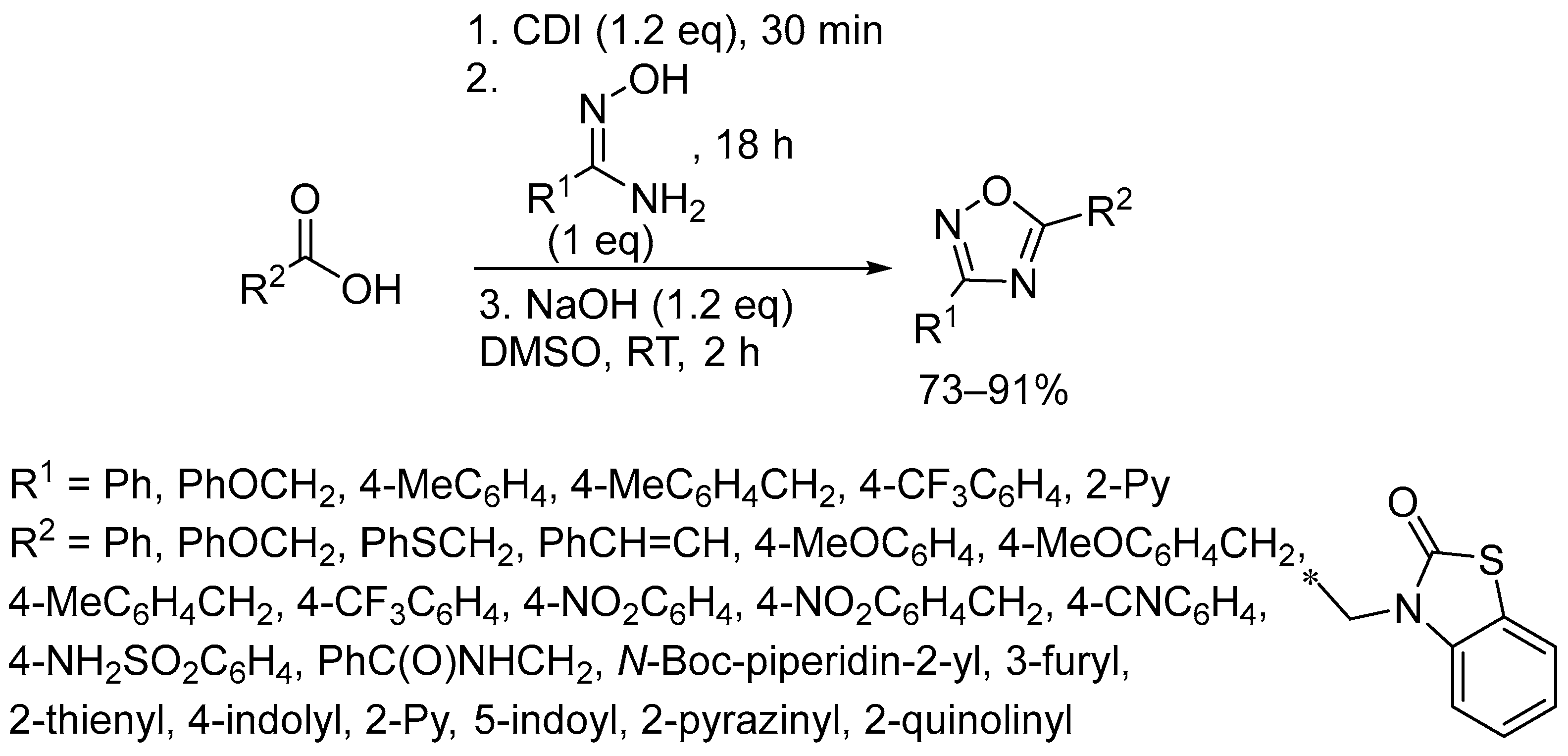
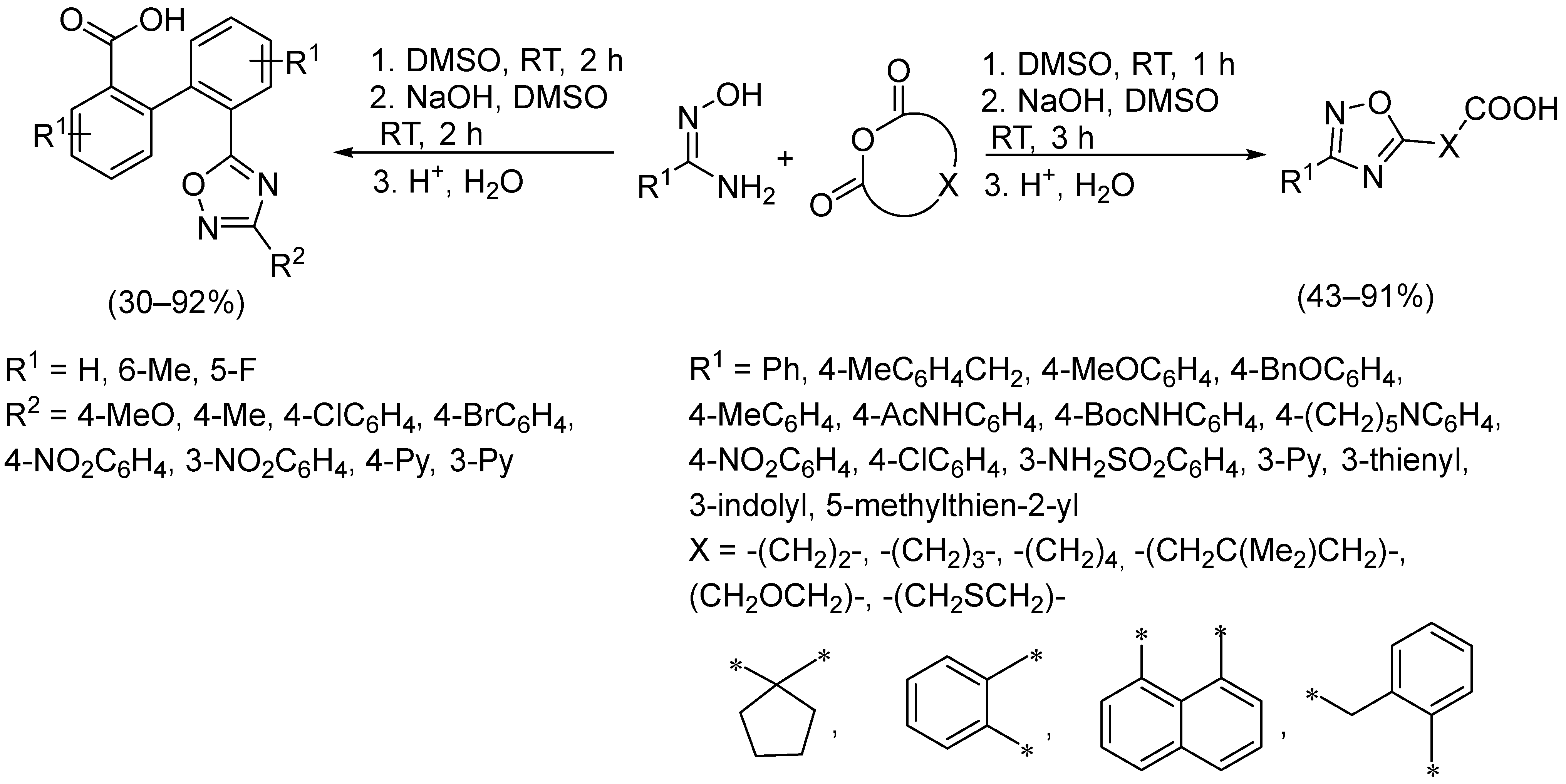












| Activity | Scaffold | Ref. |
|---|---|---|
| Potential angiotensin II receptor antagonist (biological evaluation was not performed) |  | [54] |
| Inhibitors of Dengue virus nonstructural protein 5 (NS5) polymerase | 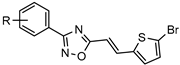 | [55] |
| Antimalarial and antileishmanial compounds |  | [56] |
| Catechol–O–methyltransferase (COMT) inhibitor marketed as anti-Parkinson drug (Opicapone) | 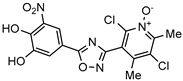 | [57] |
| Antiretroviral agent targeting the HIV-1 nucleocapsid protein | 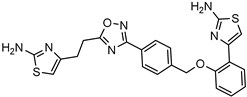 | [58] |
| Inhibitor of serine hydroxymethyltransferase for the treatment of malarial | 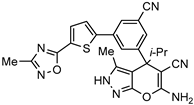 | [59] |
| Fatty acid amide hydrolase (FAAH) inhibitors for the treatment of pain and CNS disorders |  | [60] |
| Kv7 channel opener for the treatment of epilepsy and pain |  | [61] |
| Antiproliferative action against malignant human adherent cell lines | 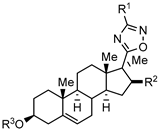 | [62] |
| Antiproliferative action against malignant human adherent cell lines |  | [63] |
| Hedgehog pathway inhibitors for the anticancer therapy | 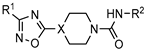 | [64] |
| Dual akt and p70S6 kinases inhibitor for the anticancer therapy (solid tumors) | 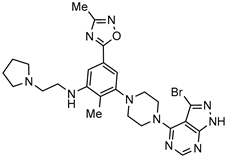 | [65] |
| Bombesin receptor subtype-3 agonist for the obesity treatment | 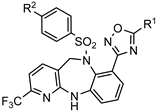 | [66] |
| Melanocortin-4 receptor (MC4R) agonist for the obesity treatment | 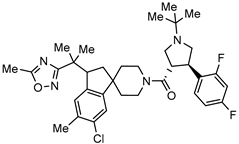 | [67] |
| Sphingosine kinase 1 inhibitors for modulating the production of sphingosine-1-phosphate (S1P)—a potential treatment of cancer, sickle cell disease, atherosclerosis, asthma, diabetes, and fibrosis |  | [68] |
| Sphingosine kinase 2 inhibitors for modulating the production of sphingosine-1-phosphate (S1P)—a potential treatment of cancer, sickle cell disease, atherosclerosis, asthma, diabetes, and fibrosis | 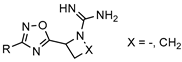 | [69] |
| Sphingosine-1-phosphate receptor-1 (S1PR1) agonists for the therapy of autoimmune diseases (preliminary, relapsing, remitting multiple sclerosis) and the prevention of transplant rejections |  | [70] |
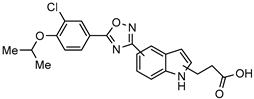 | [71] | |
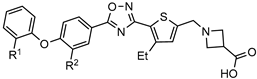 | [72] | |
| Inhibitor of penicillin binding proteins with antibacterial activity against Gram-positive strains | 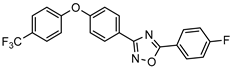 | [73] |
| Neuronal cells protectors preventing the oxidative stress-induced cell death of glutamate-challenged HT22 hippocampal neurons |  | [74] |
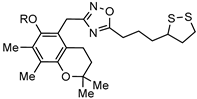 | ||
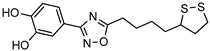 | [47] | |
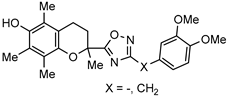 | [75] | |
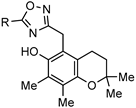 | ||
| Serotonin 5-HT1A and dopamine D2 dual-receptor agonist for the treatment of Parkinson’s diseases |  | [76] |
| Acyl CoA:diacylglycerol acyltransferase (DGAT1) inhibitors for the therapy of obesity and obesity-induced diabetes | 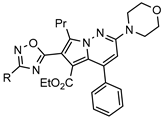 | [77] |
| Agents for the treatment Toxoplasma gondii infections | 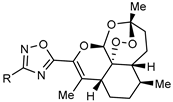 | [78] |
| Negative allosteric modulators (NAMs) of metabotropic glutamate receptor 5 (mGluR5) for neurodegenerative diseases treatment | 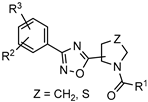 | [79] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baykov, S.V.; Shetnev, A.A.; Semenov, A.V.; Baykova, S.O.; Boyarskiy, V.P. Room Temperature Synthesis of Bioactive 1,2,4-Oxadiazoles. Int. J. Mol. Sci. 2023, 24, 5406. https://doi.org/10.3390/ijms24065406
Baykov SV, Shetnev AA, Semenov AV, Baykova SO, Boyarskiy VP. Room Temperature Synthesis of Bioactive 1,2,4-Oxadiazoles. International Journal of Molecular Sciences. 2023; 24(6):5406. https://doi.org/10.3390/ijms24065406
Chicago/Turabian StyleBaykov, Sergey V., Anton A. Shetnev, Artem V. Semenov, Svetlana O. Baykova, and Vadim P. Boyarskiy. 2023. "Room Temperature Synthesis of Bioactive 1,2,4-Oxadiazoles" International Journal of Molecular Sciences 24, no. 6: 5406. https://doi.org/10.3390/ijms24065406
APA StyleBaykov, S. V., Shetnev, A. A., Semenov, A. V., Baykova, S. O., & Boyarskiy, V. P. (2023). Room Temperature Synthesis of Bioactive 1,2,4-Oxadiazoles. International Journal of Molecular Sciences, 24(6), 5406. https://doi.org/10.3390/ijms24065406








