Patterns of Chromosomal Variation, Homoeologous Exchange, and Their Relationship with Genomic Features in Early Generations of a Synthetic Rice Segmental Allotetraploid
Abstract
1. Introduction
2. Results
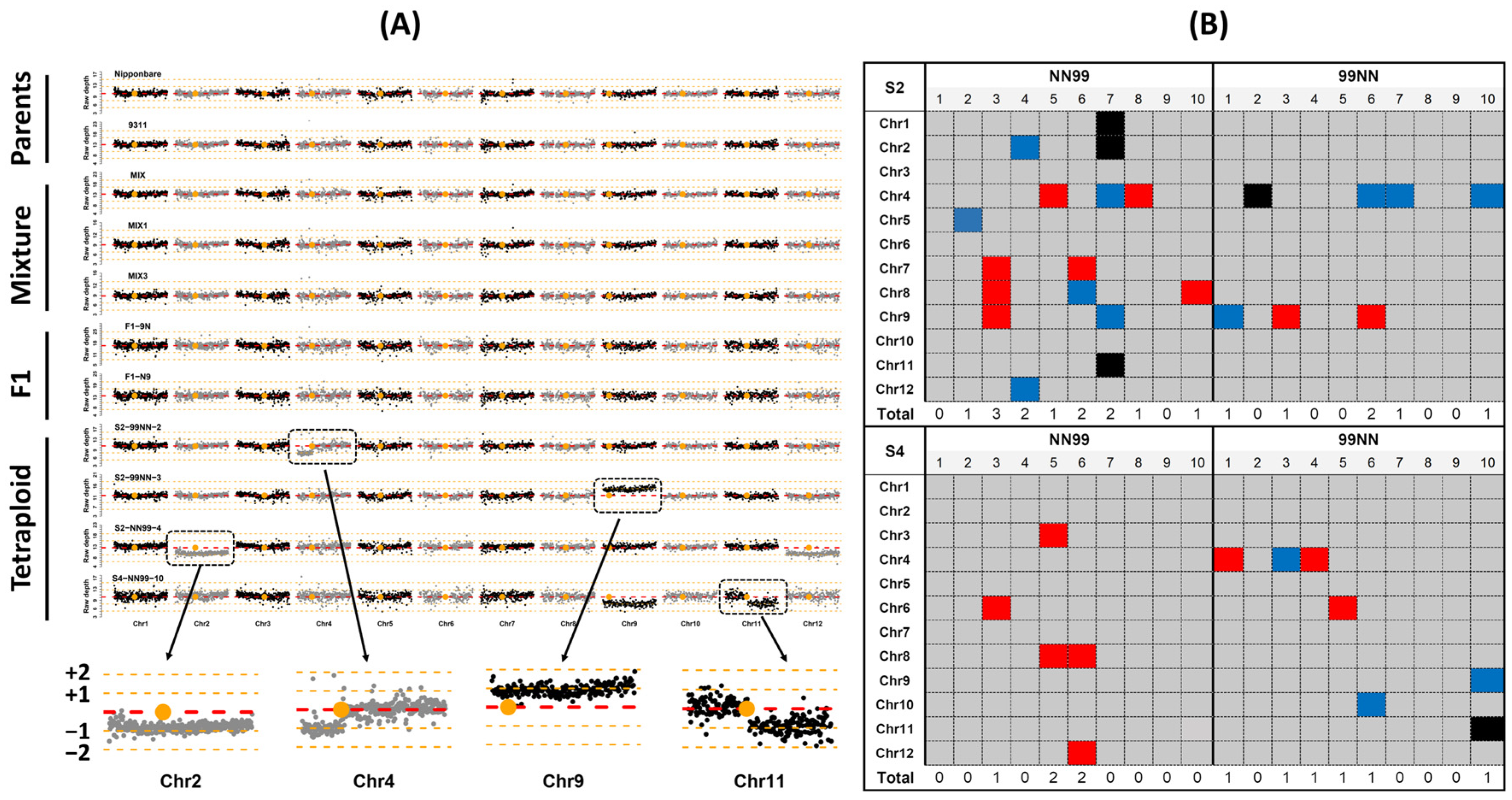
2.1. Patterns of Whole-Chromosome Gain and Loss in Early Generations of the Synthetic Rice Segmental Allotetraploids
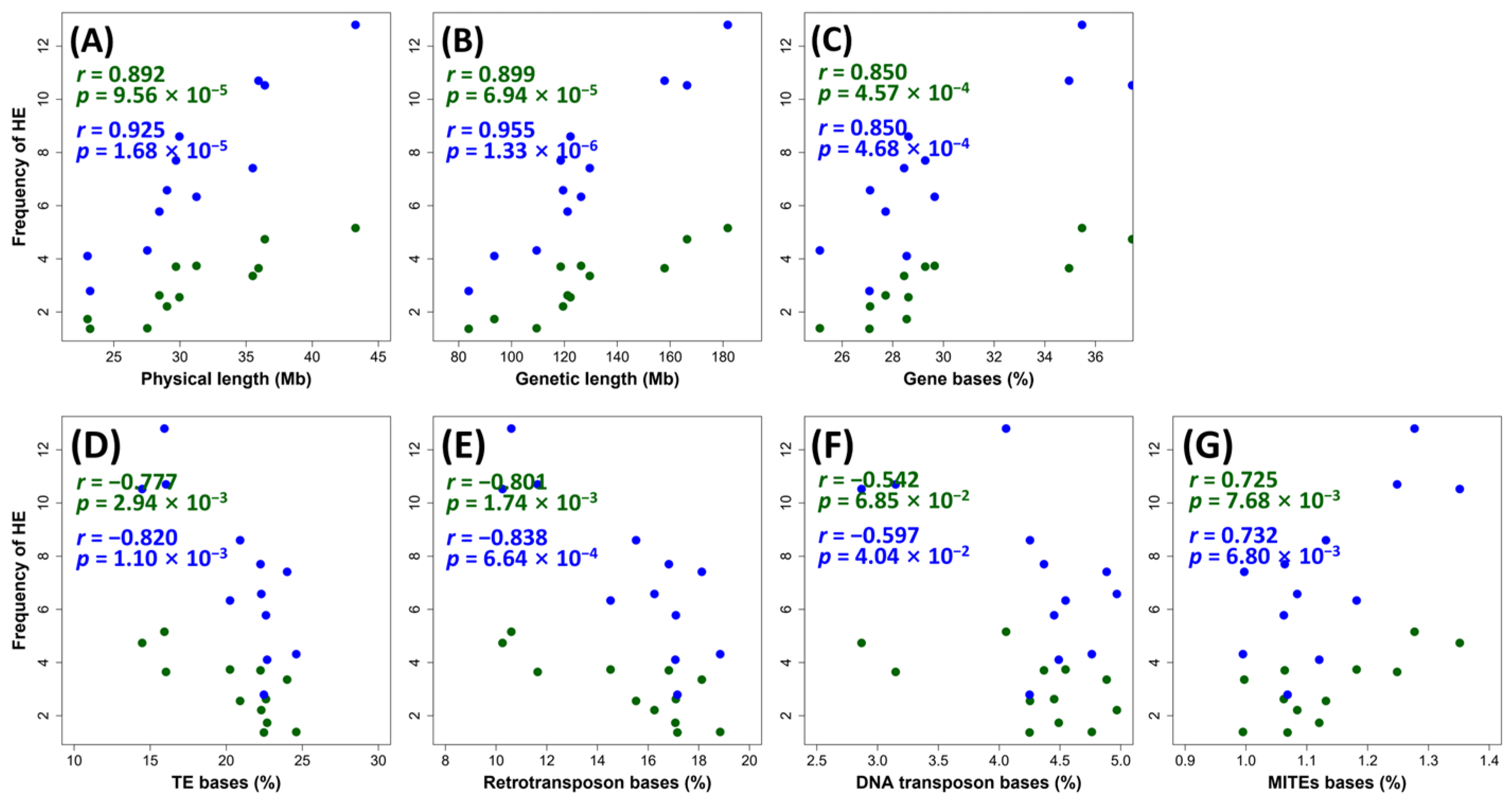
2.2. Relationships between Homoeologous Exchange and Genomic Features
2.3. Relationship between Homoeologous Exchange and Homologous Recombination Rates at Local Scale
2.4. Differential Contributions of Local Sequence Variants, TE Contents, and TE-Insertion Polymorphisms to HE Frequency
3. Discussion
4. Materials and Methods
4.1. Comparative Genomic Analyses of Parental Genomes
4.2. DNA Extraction, Sequencing, and Data Preprocessing
4.3. Identification of SNPs in Syntenic Regions between Nipponbare and 9311
4.4. Allelic/Homeologous Specific Counting
4.5. Chromosome Depth Painting and Identification of Chromosome Number Changes
4.6. Detection of Homoeologous Exchanges (HEs)
4.7. Calculation of Percentages of Gene and TE Bases on Chromosomes
4.8. Estimation of Local Recombination Rate
4.9. Detection of TEs and TIPs between the Two Parents
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Adams, K.L.; Wendel, J.F. Polyploidy and genome evolution in plants. Curr. Opin. Plant Biol. 2005, 8, 135–141. [Google Scholar] [CrossRef] [PubMed]
- Jiao, Y.; Wickett, N.J.; Ayyampalayam, S.; Chanderbali, A.S.; Landherr, L.; Ralph, P.E.; Tomsho, L.P.; Hu, Y.; Liang, H.; Soltis, P.S.; et al. Ancestral polyploidy in seed plants and angiosperms. Nature 2011, 473, 97–100. [Google Scholar] [CrossRef] [PubMed]
- Soltis, P.S.; Marchant, D.B.; Van de Peer, Y.; Soltis, D.E. Polyploidy and genome evolution in plants. Curr. Opin. Genet. Dev. 2015, 35, 119–125. [Google Scholar] [CrossRef] [PubMed]
- Van De Peer, Y.; Mizrachi, E.; Marchal, K. The evolutionary significance of polyploidy. Nat. Rev. Genet. 2017, 18, 411–424. [Google Scholar] [CrossRef]
- Salman-Minkov, A.; Sabath, N.; Mayrose, I. Whole-genome duplication as a key factor in crop domestication. Nat. Plants 2016, 2, 16115. [Google Scholar] [CrossRef]
- Xiong, Z.; Gaeta, R.T.; Pires, J.C. Homoeologous shuffling and chromosome compensation maintain genome balance in resynthesized allopolyploid Brassica napus. Proc. Natl. Acad. Sci. USA 2011, 108, 7908–7913. [Google Scholar] [CrossRef]
- Zhang, H.K.; Bian, Y.; Gou, X.W.; Zhu, B.; Xu, C.M.; Qi, B.; Li, N.; Rustgi, S.; Zhou, H.; Han, F.P.; et al. Persistent whole-chromosome aneuploidy is generally associated with nascent allohexaploid wheat. Proc. Natl. Acad. Sci. USA 2013, 110, 3447–3452. [Google Scholar] [CrossRef]
- Chester, M.; Gallagher, J.P.; Symonds, V.V.; Cruz da Silva, A.V.; Mavrodiev, E.V.; Leitch, A.R.; Soltis, P.S.; Soltis, D.E. Extensive chromosomal variation in a recently formed natural allopolyploid species, Tragopogon miscellus (Asteraceae). Proc. Natl. Acad. Sci. USA 2012, 109, 1176–1181. [Google Scholar] [CrossRef]
- Gou, X.W.; Bian, Y.; Zhang, A.; Zhang, H.K.; Wang, B.; Lv, R.L.; Li, J.Z.; Zhu, B.; Gong, L.; Liu, B. Transgenerationally Precipitated Meiotic Chromosome Instability Fuels Rapid Karyotypic Evolution and Phenotypic Diversity in an Artificially Constructed Allotetraploid Wheat (AADD). Mol. Biol. Evol. 2018, 35, 1078–1091. [Google Scholar] [CrossRef]
- Lv, R.L.; Wang, C.Y.; Wang, R.S.; Wang, X.F.; Zhao, J.; Wang, B.; Aslam, T.; Han, F.P.; Liu, B. Chromosomal instability and phenotypic variation in a specific lineage derived from a synthetic allotetraploid wheat. Front. Plant Sci. 2022, 13, 981234. [Google Scholar] [CrossRef]
- Bomblies, K. When everything changes at once: Finding a new normal after genome duplication. Proc. R. Soc. B 2020, 287, 20202154. [Google Scholar] [CrossRef] [PubMed]
- Gaeta, R.T.; Chris Pires, J. Homoeologous recombination in allopolyploids: The polyploid ratchet. New Phytol. 2010, 186, 18–28. [Google Scholar] [CrossRef] [PubMed]
- Gonzalo, A. All Ways Lead to Rome-Meiotic Stabilization Can Take Many Routes in Nascent Polyploid Plants. Genes 2022, 13, 147. [Google Scholar] [CrossRef]
- Lloyd, A.; Blary, A.; Charif, D.; Charpentier, C.; Tran, J.; Balzergue, S.; Delannoy, E.; Rigaill, G.; Jenczewski, E. Homoeologous exchanges cause extensive dosage-dependent gene expression changes in an allopolyploid crop. New Phytol. 2018, 217, 367–377. [Google Scholar] [CrossRef]
- Mason, A.S.; Wendel, J.F. Homoeologous Exchanges, Segmental Allopolyploidy, and Polyploid Genome Evolution. Front. Genet. 2020, 11, 1014. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Lv, R.; Zhang, Z.; Yang, C.; Xun, H.; Liu, B.; Gong, L. Homoeologous exchange enables rapid evolution of tolerance to salinity and hyper-osmotic stresses in a synthetic allotetraploid wheat. J. Exp. Bot. 2022, 73, 7488–7502. [Google Scholar] [CrossRef]
- Wu, Y.; Lin, F.; Zhou, Y.; Wang, J.; Sun, S.; Wang, B.; Zhang, Z.B.; Li, G.; Lin, X.Y.; Wang, X.T.; et al. Genomic mosaicism due to homoeologous exchange generates extensive phenotypic diversity in nascent allopolyploids. Natl. Sci. Rev. 2021, 8, nwaa277. [Google Scholar] [CrossRef]
- Chalhoub, B.; Denoeud, F.; Liu, S.Y.; Parkin, I.A.P.; Tang, H.B.; Wang, X.Y.; Chiquet, J.; Belcram, H.; Tong, C.B.; Samans, B.; et al. Early allopolyploid evolution in the post-Neolithic Brassica napus oilseed genome. Science 2014, 345, 950–953. [Google Scholar] [CrossRef]
- Osborn, T.C.; Pires, J.C.; Birchler, J.A.; Auger, D.L.; Chen, Z.J.; Lee, H.S.; Comai, L.; Madlung, A.; Doerge, R.W.; Colot, V.; et al. Understanding mechanisms of novel gene expression in polyploids. Trends Genet. 2003, 19, 141–147. [Google Scholar] [CrossRef]
- Pires, J.C.; Zhao, J.W.; Schranz, M.E.; Leon, E.J.; Quijada, P.A.; Lukens, L.N.; Osborn, T.C. Flowering time divergence and genomic rearrangements in resynthesized Brassica polyploids (Brassicaceae). Biol. J. Linn. Soc. 2004, 82, 675–688. [Google Scholar] [CrossRef]
- Stein, A.; Coriton, O.; Rousseau-Gueutin, M.; Samans, B.; Schiessl, S.V.; Obermeier, C.; Parkin, I.A.P.; Chevre, A.M.; Snowdon, R.J. Mapping of homoeologous chromosome exchanges influencing quantitative trait variation in Brassica napus. Plant Biotechnol. J. 2017, 15, 1478–1489. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Miao, H.X.; Liu, J.H.; Xu, B.Y.; Yao, X.M.; Xu, C.Y.; Zhao, S.C.; Fang, X.D.; Jia, C.H.; Wang, J.Y.; et al. Musa balbisiana genome reveals subgenome evolution and functional divergence. Nat. Plants 2019, 5, 810–821. [Google Scholar] [CrossRef] [PubMed]
- Edger, P.P.; Poorten, T.; VanBuren, R.; Hardigan, M.A.; Colle, M.; McKain, M.R.; Smith, R.D.; Teresi, S.; Nelson, A.D.L.; Wai, C.M.; et al. Origin and evolution of the octoploid strawberry genome. Nat. Genet. 2019, 51, 541–547. [Google Scholar] [CrossRef]
- Lashermes, P.; Combes, M.C.; Hueber, Y.; Severac, D.; Dereeper, A. Genome rearrangements derived from homoeologous recombination following allopolyploidy speciation in coffee. Plant J. 2014, 78, 674–685. [Google Scholar] [CrossRef] [PubMed]
- Bertioli, D.J.; Jenkins, J.; Clevenger, J.; Dudchenko, O.; Gao, D.Y.; Seijo, G.; Leal-Bertioli, S.C.M.; Ren, L.H.; Farmer, A.D.; Pandey, M.K.; et al. The genome sequence of segmental allotetraploid peanut Arachis hypogaea. Nat. Genet. 2019, 51, 877–884. [Google Scholar] [CrossRef]
- He, Z.S.; Wang, L.H.; Harper, A.L.; Havlickova, L.; Pradhan, A.K.; Parkin, I.A.P.; Bancroft, I. Extensive homoeologous genome exchanges in allopolyploid crops revealed by mRNAseq-based visualization. Plant Biotechnol. J. 2017, 15, 594–604. [Google Scholar] [CrossRef]
- Liu, H.X.; Maclean, C.J.; Zhang, J.Z. Evolution of the Yeast Recombination Landscape. Mol. Biol. Evol. 2019, 36, 412–422. [Google Scholar] [CrossRef]
- Piegu, B.; Guyot, R.; Picault, N.; Roulin, A.; Sanyal, A.; Kim, H.; Collura, K.; Brar, D.S.; Jackson, S.; Wing, R.A.; et al. Doubling genome size without polyploidization: Dynamics of retrotransposition-driven genomic expansions in Oryza australiensis, a wild relative of rice. Genome Res. 2006, 16, 1262–1269. [Google Scholar] [CrossRef]
- Kejnovsky, E.; Hawkins, J.S.; Feschotte, C.D. Plant Transposable Elements: Biology and Evolution. In Plant Genome Diversity; Wendel, J., Greilhuber, J., Dolezel, J., Leitch, I., Eds.; Springer: Vienna, Austria, 2012; Volume 1, pp. 17–34. [Google Scholar]
- Huang, X.; Lu, G.; Zhao, Q.; Liu, X.; Han, B. Genome-wide analysis of transposon insertion polymorphisms reveals intraspecific variation in cultivated rice. Plant Physiol. 2008, 148, 25–40. [Google Scholar] [CrossRef]
- Liu, Z.; Zhao, H.; Yan, Y.; Wei, M.X.; Zheng, Y.C.; Yue, E.K.; Alam, M.S.; Smartt, K.O.; Duan, M.H.; Xu, J.H. Extensively Current Activity of Transposable Elements in Natural Rice Accessions Revealed by Singleton Insertions. Front. Plant Sci. 2021, 12, 745526. [Google Scholar] [CrossRef]
- Kidwell, M.G.; Lisch, D.R. Perspective: Transposable elements, parasitic DNA, and genome evolution. Evolution 2001, 55, 1–24. [Google Scholar] [PubMed]
- Lippman, Z.; Gendrel, A.V.; Black, M.; Vaughn, M.W.; Dedhia, N.; McCombie, W.R.; Lavine, K.; Mittal, V.; May, B.; Kasschau, K.D.; et al. Role of transposable elements in heterochromatin and epigenetic control. Nature 2004, 430, 471–476. [Google Scholar] [CrossRef] [PubMed]
- Bureau, T.E.; Wessler, S.R. Tourist: A large family of small inverted repeat elements frequently associated with maize genes. Plant Cell 1992, 4, 1283–1294. [Google Scholar]
- International Rice Genome Sequencing, P. The map-based sequence of the rice genome. Nature 2005, 436, 793–800. [Google Scholar]
- Wright, S.I.; Agrawal, N.; Bureau, T.E. Effects of recombination rate and gene density on transposable element distributions in Arabidopsis thaliana. Genome Res. 2003, 13, 1897–1903. [Google Scholar] [CrossRef] [PubMed]
- Rizzon, C.; Marais, G.; Gouy, M.; Biemont, C. Recombination rate and the distribution of transposable elements in the Drosophila melanogaster genome. Genome Res. 2002, 12, 400–407. [Google Scholar] [CrossRef]
- Jensen-Seaman, M.I.; Furey, T.S.; Payseur, B.A.; Lu, Y.; Roskin, K.M.; Chen, C.F.; Thomas, M.A.; Haussler, D.; Jacob, H.J. Comparative recombination rates in the rat, mouse, and human genomes. Genome Res. 2004, 14, 528–538. [Google Scholar] [CrossRef]
- Tian, Z.; Rizzon, C.; Du, J.; Zhu, L.; Bennetzen, J.L.; Jackson, S.A.; Gaut, B.S.; Ma, J. Do genetic recombination and gene density shape the pattern of DNA elimination in rice long terminal repeat retrotransposons? Genome Res. 2009, 19, 2221–2230. [Google Scholar] [CrossRef]
- Kent, T.V.; Uzunovic, J.; Wright, S.I. Coevolution between transposable elements and recombination. Philos. Trans. R. Soc. B Biol. Sci. 2017, 372, 20160458. [Google Scholar] [CrossRef]
- Underwood, C.J.; Choi, K. Heterogeneous transposable elements as silencers, enhancers and targets of meiotic recombination. Chromosoma 2019, 128, 279–296. [Google Scholar] [CrossRef]
- Xu, C.; Bai, Y.; Lin, X.; Zhao, N.; Hu, L.; Gong, Z.; Wendel, J.F.; Liu, B. Genome-wide disruption of gene expression in allopolyploids but not hybrids of rice subspecies. Mol. Biol. Evol. 2014, 31, 1066–1076. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Sun, Y.; Sun, S.; Li, G.; Wang, J.; Wang, B.; Lin, X.Y.; Huang, M.; Gong, Z.Y.; Sanguinet, K.A.; et al. Aneuploidization under segmental allotetraploidy in rice and its phenotypic manifestation. Theor. Appl. Genet. 2018, 131, 1273–1285. [Google Scholar] [CrossRef] [PubMed]
- Harushima, Y.; Yano, M.; Shomura, A.; Sato, M.; Shimano, T.; Kuboki, Y.; Yamamoto, T.; Lin, S.Y.; Antonio, B.A.; Parco, A.; et al. A high-density rice genetic linkage map with 2275 markers using a single F2 population. Genetics 1998, 148, 479–494. [Google Scholar] [CrossRef]
- Rezvoy, C.; Charif, D.; Gueguen, L.; Marais, G.A.B. MareyMap: An R-based tool with graphical interface for estimating recombination rates. Bioinformatics 2007, 23, 2188–2189. [Google Scholar] [CrossRef]
- Siberchicot, A.; Bessy, A.; Gueguen, L.; Marais, G.A.B. MareyMap Online: A User-Friendly Web Application and Database Service for Estimating Recombination Rates Using Physical and Genetic Maps. Genome Biol. Evol. 2017, 9, 2506–2509. [Google Scholar] [CrossRef] [PubMed]
- Pavelka, N.; Rancati, G.; Zhu, J.; Bradford, W.D.; Saraf, A.; Florens, L.; Sanderson, B.W.; Hattem, G.L.; Li, R. Aneuploidy confers quantitative proteome changes and phenotypic variation in budding yeast. Nature 2010, 468, 321–325. [Google Scholar] [CrossRef] [PubMed]
- Rutledge, S.D.; Cimini, D. Consequences of aneuploidy in sickness and in health. Curr. Opin. Cell Biol. 2016, 40, 41–46. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.B.; Gou, X.W.; Xun, H.W.; Bian, Y.; Ma, X.T.; Li, J.Z.; Li, N.; Gong, L.; Feldman, M.; Liu, B.; et al. Homoeologous exchanges occur through intragenic recombination generating novel transcripts and proteins in wheat and other polyploids. Proc. Natl. Acad. Sci. USA 2020, 117, 14561–14571. [Google Scholar] [CrossRef] [PubMed]
- Gaeta, R.T.; Pires, J.C.; Iniguez-Luy, F.; Leon, E.; Osborn, T.C. Genomic changes in resynthesized Brassica napus and their effect on gene expression and phenotype. Plant Cell 2007, 19, 3403–3417. [Google Scholar] [CrossRef]
- Yelina, N.; Diaz, P.; Lambing, C.; Henderson, I.R. Epigenetic control of meiotic recombination in plants. Sci. China Life Sci. 2015, 58, 223–231. [Google Scholar] [CrossRef]
- Marcais, G.; Delcher, A.L.; Phillippy, A.M.; Coston, R.; Salzberg, S.L.; Zimin, A. MUMmer4: A fast and versatile genome alignment system. PLoS Comput. Biol. 2018, 14, e1005944. [Google Scholar] [CrossRef] [PubMed]
- Goel, M.; Sun, H.Q.; Jiao, W.B.; Schneeberger, K. SyRI: Finding genomic rearrangements and local sequence differences from whole-genome assemblies. Genome Biol. 2019, 20, 277. [Google Scholar] [CrossRef] [PubMed]
- Bolger, A.M.; Lohse, M.; Usadel, B. Trimmomatic: A flexible trimmer for Illumina sequence data. Bioinformatics 2014, 30, 2114–2120. [Google Scholar] [CrossRef]
- Li, H.; Durbin, R. Fast and accurate short read alignment with Burrows-Wheeler transform. Bioinformatics 2009, 25, 1754–1760. [Google Scholar] [CrossRef]
- McKenna, A.; Hanna, M.; Banks, E.; Sivachenko, A.; Cibulskis, K.; Kernytsky, A.; Garimella, K.; Altshuler, D.; Gabriel, S.; Daly, M.; et al. The Genome Analysis Toolkit: A MapReduce framework for analyzing next-generation DNA sequencing data. Genome Res. 2010, 20, 1297–1303. [Google Scholar] [CrossRef] [PubMed]
- Castel, S.E.; Levy-Moonshine, A.; Mohammadii, P.; Banks, E.; Lappalainenii, T. Tools and best practices for data processing in allelic expression analysis. Genome Biol. 2015, 16, 195. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, S.; Buell, C.R. The TIGR Plant Repeat Databases: A collective resource for the identification of repetitive sequences in plants. Nucleic Acids Res. 2004, 32, D360–D363. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Sun, Z.F.; Wang, J.; Huang, H.J.; Kocher, J.P.; Wang, L.G. CrossMap: A versatile tool for coordinate conversion between genome assemblies. Bioinformatics 2014, 30, 1006–1007. [Google Scholar] [CrossRef]
- Kofler, R.; Gomez-Sanchez, D.; Schlotterer, C. PoPoolationTE2: Comparative Population Genomics of Transposable Elements Using Pool-Seq. Mol. Biol. Evol. 2016, 33, 2759–2764. [Google Scholar] [CrossRef]

| Pearson Correlation | ||||
|---|---|---|---|---|
| S2 | S4 | |||
| r | p-Value | r | p-Value | |
| SNPs | −0.200 | 9.65 × 10−5 ** | −0.188 | 2.39 × 10−4 ** |
| Small indels (1–99 bp) | −0.042 | 0.408 | 0.011 | 0.828 |
| Large indels (>100 bp) | −0.057 | 0.269 | −0.045 | 0.378 |
| TIPs | −0.167 | 1.15 × 10−3 ** | −0.170 | 9.94 × 10−4 ** |
| Retrotransposon TIPs | −0.261 | 3.04 × 10−7 ** | −0.238 | 3.27 × 10−6 ** |
| DNA transposon TIPs | −0.073 | 0.158 | −0.104 | 0.045 * |
| MITE TIPs | 0.158 | 2.24 × 10−3 ** | 0.135 | 9.03 × 10−3 ** |
| Shared TEs | −0.300 | 3.13 × 10−9 ** | −0.269 | 1.24 × 10−7 ** |
| Shared retrotransposons | −0.334 | 3.13 × 10−11 ** | −0.313 | 5.82 × 10−10 ** |
| Shared DNA transposons | −0.031 | 0.550 | 0.050 | 0.335 |
| Shared MITEs | 0.117 | 0.024 * | 0.098 | 0.059 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, G.; Wu, Y.; Bai, Y.; Zhao, N.; Jiang, Y.; Li, N.; Lin, X.; Liu, B.; Xu, C. Patterns of Chromosomal Variation, Homoeologous Exchange, and Their Relationship with Genomic Features in Early Generations of a Synthetic Rice Segmental Allotetraploid. Int. J. Mol. Sci. 2023, 24, 6065. https://doi.org/10.3390/ijms24076065
Li G, Wu Y, Bai Y, Zhao N, Jiang Y, Li N, Lin X, Liu B, Xu C. Patterns of Chromosomal Variation, Homoeologous Exchange, and Their Relationship with Genomic Features in Early Generations of a Synthetic Rice Segmental Allotetraploid. International Journal of Molecular Sciences. 2023; 24(7):6065. https://doi.org/10.3390/ijms24076065
Chicago/Turabian StyleLi, Guo, Ying Wu, Yan Bai, Na Zhao, Yuhui Jiang, Ning Li, Xiuyun Lin, Bao Liu, and Chunming Xu. 2023. "Patterns of Chromosomal Variation, Homoeologous Exchange, and Their Relationship with Genomic Features in Early Generations of a Synthetic Rice Segmental Allotetraploid" International Journal of Molecular Sciences 24, no. 7: 6065. https://doi.org/10.3390/ijms24076065
APA StyleLi, G., Wu, Y., Bai, Y., Zhao, N., Jiang, Y., Li, N., Lin, X., Liu, B., & Xu, C. (2023). Patterns of Chromosomal Variation, Homoeologous Exchange, and Their Relationship with Genomic Features in Early Generations of a Synthetic Rice Segmental Allotetraploid. International Journal of Molecular Sciences, 24(7), 6065. https://doi.org/10.3390/ijms24076065






