Metabiotics Signature through Genome Sequencing and In Vitro Inhibitory Assessment of a Novel Lactococcus lactis Strain UTNCys6-1 Isolated from Amazonian Camu-Camu Fruits
Abstract
:1. Introduction
2. Results and Discussion
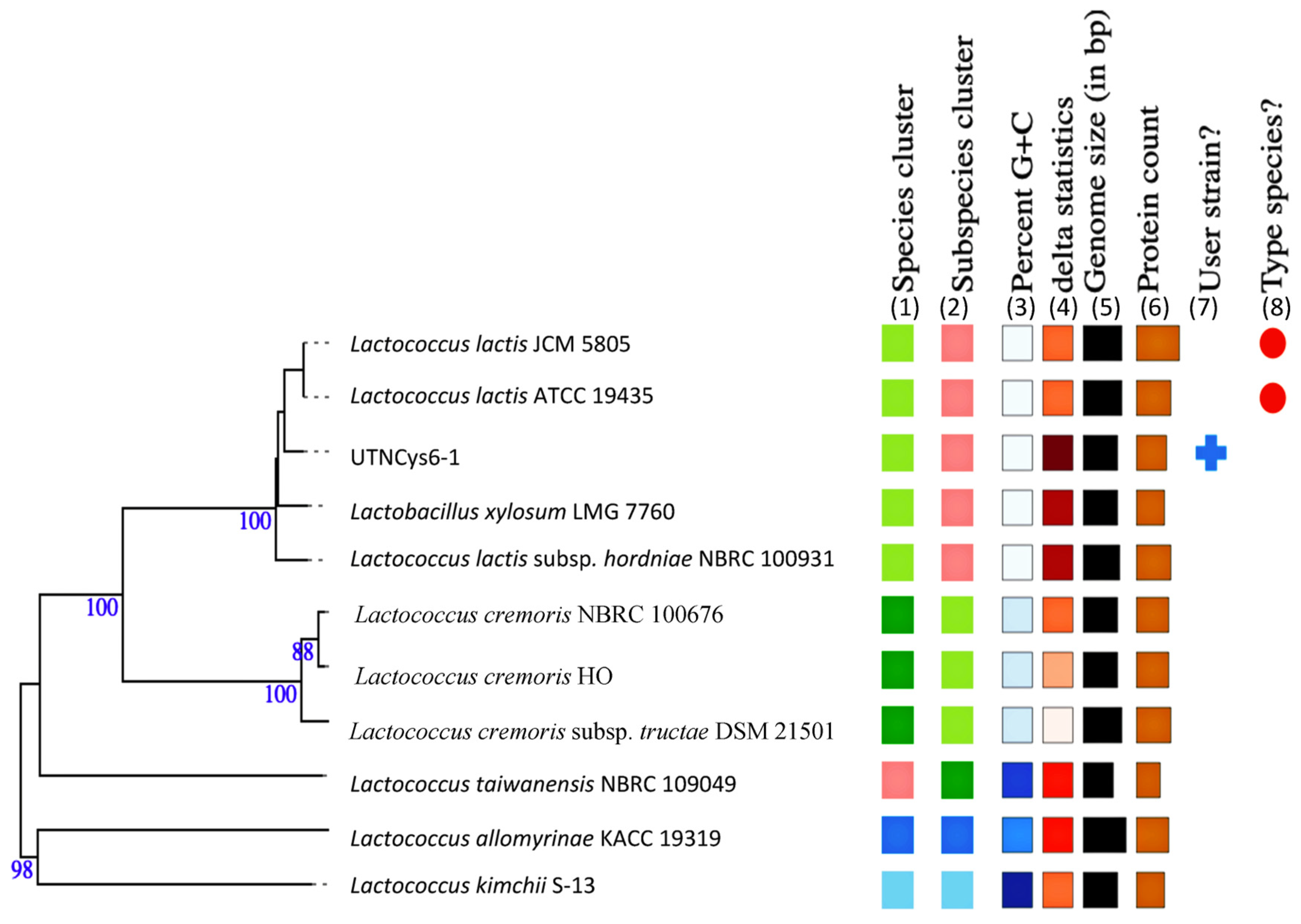
2.1. Species Identification and Phylogenetic Relationship
2.2. Gene Prediction and Functional Annotation of Enzymes Involved in Different Metabolic Pathways
2.3. Prediction of CRISPR Elements, Prophages, ARGs, VFs, GIs, ISs and Pathogenicity
2.4. Pangenome Comparison Analysis
2.5. Metabolite Gene Cluster (MGC) Prediction
2.6. BGC Organization Predicted from Genome Study
2.7. In Vitro Characteristics
2.7.1. Metabolic Profile and Antibiotic Susceptibility
2.7.2. Inhibitory Activity against Foodborne Pathogens
3. Materials and Methods
3.1. Bacterial Isolation and Selection
3.2. De Novo Assembly and Workflow Sequencing
3.3. Typing and Species Relatedness
3.4. General Genome Features, Gene Prediction and Functional Annotation
3.5. In Silico Analysis
3.5.1. Prediction of CRISPR Sequences, Prophages, ARGs, VFs, GIs, ISs and Pathogenicity
3.5.2. Pangenome Analysis
3.5.3. Primary and Secondary Metabolites and Bacteriocin-Encoding Gene Prediction
3.6. In Vitro Analysis
3.6.1. Physiological Characteristics and Antibiotic Susceptibility
3.6.2. Generation of EPSs
3.6.3. PPE Extraction and Size Estimation Using Tricine-SDS-PAGE Analysis
3.6.4. In Vitro Antimicrobial Activity of PPE and EPSs
4. Conclusions
Supplementary Materials
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shenderov, B.A. Metabiotics: Novel idea or natural development of probiotic conception. Microb. Ecol. Health Dis. 2013, 24, 20399. [Google Scholar] [CrossRef] [PubMed]
- Sharma, M.; Shukla, G. Administration of metabiotics extracted from probiotic Lactobacillus rhamnosus MD 14 inhibit experimental colorectal carcinogenesis by targeting wnt/β-catenin pathway. Front. Oncol. 2020, 10, 746. [Google Scholar] [CrossRef] [PubMed]
- Pihurov, M.; Pacularu-Burada, B.; Cotârlet, M.; Vasile, M.A.; Bahrim, G.E. Novel insights for metabiotics production by using artisanal probiotic cultures. Microorganisms 2021, 9, 2184. [Google Scholar] [CrossRef] [PubMed]
- Roberfroid, M.; Gibson, G.R.; Hoyles, L.; McCartney, A.L.; Rastall, R.; Rowland, I.; Wolvers, D.; Watzl, B.; Szajewska, H.; Stahl, B.; et al. Prebiotic effects: Metabolic and health benefits. Br. J. Nutr. 2010, 104 (Suppl. 2), S1–S63. [Google Scholar] [CrossRef] [Green Version]
- Qiao, W.; Liu, F.; Wan, X.; Qiao, Y.; Li, R.; Wu, Z.; Saris, P.; Xu, H.; Qiao, M. Genomic features and construction of streamlined genome chassis of Nisin Z producer Lactococcus lactis N8. Microorganisms 2021, 10, 47. [Google Scholar] [CrossRef]
- Rodríguez, L.G.R.; Mohamed, F.; Bleckwedel, J.; Medina, R.; De Vuyst, L.; Hebert, E.M.; Mozzi, F. Diversity and functional properties of lactic acid bacteria isolated from wild fruits and flowers present in Northern Argentina. Front. Microbiol. 2019, 10, 1091. [Google Scholar] [CrossRef]
- Tenea, G.N.; Hurtado, P.; Ortega, C. Inhibitory effect of substances produced by native Lactococcus lactis strains of tropical fruits towards food pathogens. Prev. Nutr. Food Sci. 2018, 23, 260–268. [Google Scholar] [CrossRef]
- Tenea, G.N.; Suárez, J. Probiotic potential and technological properties of bacteriocinogenic Lactococcus lactis subsp. lactis UTNGt28 from a native Amazonian fruit as a yogurt starter culture. Microorganisms 2020, 8, 733. [Google Scholar] [CrossRef]
- Tenea, G.N. Decoding the gene variants of two native probiotic Lactiplantibacillus plantarum strains through whole-genome resequencing: Insights into bacterial adaptability to stressors and antimicrobial strength. Genes 2022, 13, 443. [Google Scholar] [CrossRef]
- Arellano-Acuña, E.; Rojas-Zavaleta, I.; Paucar-Menacho, L.M. Camu-camu (Myrciaria dubia): Fruta tropical de excelentes propiedades funcionales que ayudan a mejorar la calidad de vida. Sci. Agropecu. 2016, 7, 433–443. [Google Scholar] [CrossRef] [Green Version]
- Altschul, S.F.; Gish, W.; Miller, W.; Myers, E.W.; Lipman, D.J. Basic local alignment search tool. J. Mol. Biol. 1990, 215, 403–410. [Google Scholar] [CrossRef] [PubMed]
- Ondov, B.D.; Treangen, T.J.; Melsted, P.; Mallonee, A.B.; Bergman, N.H.; Koren, S.; Phillippy, A.M. Mash: Fast genome and metagenome distance estimation using MinHash. Genome Biol. 2016, 17, 132. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meier-Kolthoff, J.P.; Göker, M. TYGS is an automated high-throughput platform for state-of-the-art genome-based taxonomy. Nat. Commun. 2019, 10, 2182. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sanders, J.W.; Leenhouts, K.J.; Haandrikman, A.J.; Venema, G.; Kok, J. Stress response in Lactococcus lactis: Cloning, expression analysis, and mutation of the lactococcal superoxide dismutase gene. J. Bacteriol. 1995, 177, 5254–5260. [Google Scholar] [CrossRef] [Green Version]
- Bolotin, A.; Wincker, P.; Mauger, S.; Jaillon, O.; Malarme, K.; Weissenbach, J.; Ehrlich, S.D.; Sorokin, A. The complete genome sequence of the lactic acid bacterium Lactococcus lactis ssp. lactis IL1403. Genome Res. 2001, 11, 731–753. [Google Scholar] [CrossRef] [PubMed]
- Vido, K.; Le Bars, D.; Mistou, M.Y.; Anglade, P.; Gruss, A.; Gaudu, P. Proteome analyses of heme-dependent respiration in Lactococcus lactis: Involvement of the proteolytic system. J. Bacteriol. 2004, 186, 1648–1657. [Google Scholar] [CrossRef] [Green Version]
- Horvath, P.; Coûté-Monvoisin, A.C.; Romero, D.A.; Boyaval, P.; Fremaux, C.; Barrangou, R. Comparative analysis of CRISPR loci in lactic acid bacteria genomes. Int. J. Food Microbiol. 2009, 131, 62–70. [Google Scholar] [CrossRef]
- Millen, A.M.; Samson, J.E.; Tremblay, D.M.; Magadán, A.H.; Rousseau, G.M.; Moineau, S.; Romero, D.A. Lactococcus lactis type III-A CRISPR-Cas system cleaves bacteriophage RNA. RNA Biol. 2019, 16, 461–468. [Google Scholar] [CrossRef] [Green Version]
- Lu, S.; Wang, J.; Chitsaz, F.; Derbyshire, M.K.; Geer, R.C.; Gonzales, N.R.; Gwadz, M.; Hurwitz, D.I.; Marchler, G.H.; Song, J.S.; et al. CDD/SPARCLE: The conserved domain database in 2020. Nucleic Acids Res. 2020, 48, D265–D268. [Google Scholar] [CrossRef] [Green Version]
- Mahony, J.; Bottacini, F.; van Sinderen, D.; Fitzgerald, G.F. Progress in lactic acid bacterial phage research. Microb. Cell Fact. 2014, 13 (Suppl. 1), 1–12. [Google Scholar] [CrossRef] [Green Version]
- Blattner, F.R.; Plunkett, G., III; Bloch, C.A.; Perna, N.T.; Burland, V.; Riley, M.; Collado-Vides, J.; Glasner, J.D.; Rode, C.K.; Mayhew, G.F.; et al. The complete genome sequence of Escherichia coli K-12. Science 1997, 277, 1453–1462. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Flórez, A.B.; Danielsen, M.; Korhonen, J.; Zycka, J.; von Wright, A.; Bardowski, J.; Mayo, B. Antibiotic survey of Lactococcus lactis strains to six antibiotics by Etest, and establishment of new susceptibility-resistance cut-off values. J. Dairy Res. 2007, 74, 262–268. [Google Scholar] [CrossRef] [PubMed]
- EFSA. Guidance on the characterisation of microorganisms used as feed additives or as production organisms. EFSA J. 2018, 16, e05206. [Google Scholar]
- Champoux, J.J. DNA topoisomerases: Structure, function, and mechanism. Annu. Rev. Biochem. 2001, 70, 369–413. [Google Scholar] [CrossRef] [Green Version]
- Mills, D.A.; McKay, L.L.; Dunny, G.M. Splicing of a group II intron involved in the conjugative transfer of pRS01 in lactococci. J. Bacteriol. 1996, 178, 3531–3538. [Google Scholar] [CrossRef] [Green Version]
- Gosalbes, M.J.; Monedero, V.; Alpert, C.A.; Pérez-Martínez, G. Establishing a model to study the regulation of the lactose operon in Lactobacillus casei. FEMS Microbiol. Lett. 1997, 148, 83–89. [Google Scholar] [CrossRef]
- Sadaie, Y.; Yata, K.; Fujita, M.; Sagai, H.; Itaya, M.; Kasahara, Y.; Ogasawara, N. Nucleotide sequence and analysis of the phoB-rrnE-groESL region of the Bacillus subtilis chromosome. Microbiology 1997, 143, 1861–1866. [Google Scholar] [CrossRef] [Green Version]
- Yoshida, K.; Shindo, K.; Sano, H.; Seki, S.; Fujimura, M.; Yanai, N.; Miwa, Y.; Fujita, Y. Sequencing of a 65 kb region of the Bacillus subtilis genome containing the lic and cel loci, and creation of a 177 kb contig covering the gnt-sacXY region. Microbiology 1996, 142, 3113–3123. [Google Scholar] [CrossRef] [Green Version]
- Wiltshire, M.D.; Foster, S.J. Identification and analysis of Staphylococcus aureus components expressed by a model system of growth in serum. Infect. Immun. 2001, 69, 5198–5202. [Google Scholar] [CrossRef] [Green Version]
- Jia, B.; Hao, L.; Xuan, Y.H.; Jeon, C.O. New insight into the diversity of SemiSWEET sugar transporters and the homologs in prokaryotes. Front. Genet. 2018, 9, 180. [Google Scholar] [CrossRef] [Green Version]
- Sliz, P.; Engelmann, R.; Hengstenberg, W.; Pai, E.F. The structure of enzyme IIA lactose from Lactococcus lactis reveals a new fold and points to possible interactions of a multicomponent system. Structure 1997, 5, 775–788. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jamal, Z.; Miot-Sertier, C.; Thibau, F.; Dutilh, L.; Lonvaud-Funel, A.; Ballestra, P.; Le Marrec, C.; Dols-Lafargue, M. Distribution and functions of phosphotransferase system genes in the genome of the lactic acid bacterium Oenococcus oeni. Appl. Environ. Microbiol. 2013, 79, 3371–3379. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sperisen, P.; Schmid, C.D.; Bucher, P.; Zilian, O. Stealth proteins: In silico identification of a novel protein family rendering bacterial pathogens invisible to host immune defense. PLoS Comput. Biol. 2005, 1, e63. [Google Scholar] [CrossRef] [PubMed]
- Brandsma, J.B.; van de Kraats, I.; Abee, T.; Zwietering, M.H.; Meijer, W.C. Arginine metabolism in sugar deprived Lactococcus lactis enhances survival and cellular activity, while supporting flavour production. Food Microbiol. 2012, 29, 27–32. [Google Scholar] [CrossRef]
- Benjdia, A.; Balty, C.; Berteau, O. Radical SAM enzymes in the biosynthesis of ribosomally synthesized and post-translationally modified peptides (RiPPs). Front. Chem. 2017, 5, 87. [Google Scholar] [CrossRef] [Green Version]
- Nilsen, T.; Nes, I.F.; Holo, H. Enterolysin A, a cell wall-degrading bacteriocin from Enterococcus faecalis LMG 2333. Appl. Environ. Microbiol. 2003, 69, 2975–2984. [Google Scholar] [CrossRef] [Green Version]
- Angeles, D.M.; Scheffers, D.J. The Cell Wall of Bacillus subtilis. Curr. Issues Mol. Biol. 2021, 41, 539–596. [Google Scholar] [CrossRef]
- Abdulkarim, I.H.; Mohammed, S.S.D.; Orukotan, A.A. Gene identification for bacteriocin production by lactic acid bacteria isolated from fermented foods. Asian J. Biochem. Genet. 2020, 3, 1–12. [Google Scholar] [CrossRef]
- Poorinmohammad, N.; Hamedi, J.; Masoudi-Nejad, A. Genome-scale exploration of transcriptional regulation in the nisin Z producer Lactococcus lactis subsp. Lactis IO-1. Sci. Rep. 2020, 10, 3787. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Looijesteijn, P.J.; Boels, I.C.; Kleerebezem, M.; Hugenholtz, J. Regulation of exopolysaccharide production by Lactococcus lactis subsp. cremoris by the sugar source. Appl. Environ. Microbiol. 1999, 65, 5003–5008. [Google Scholar] [CrossRef] [Green Version]
- Xiao, H.; Wang, Q.; Bang-Berthelsen, C.H.; Jensen, P.R.; Solem, C. Harnessing adaptive evolution to achieve superior mannitol production by Lactococcus lactis using its native metabolism. J. Agric. Food Chem. 2020, 68, 4912–4921. [Google Scholar] [CrossRef]
- Gao, Y.; Liu, Y.; Sun, M.; Zhang, H.; Mu, G.; Tuo, Y. Physiological function analysis of Lactobacillus plantarum Y44 based on genotypic and phenotypic characteristics. J. Dairy Sci. 2020, 103, 5916–5930. [Google Scholar] [CrossRef] [PubMed]
- Jodłowski, G.; Strzelec, E. Use of glycerol waste in lactic acid bacteria metabolism for the production of lactic acid: State of the art in Poland. Open Chem. 2021, 19, 998–1008. [Google Scholar] [CrossRef]
- European Food Safety Authority. Guidance on the assessment of bacterial susceptibility to antimicrobials of human and veterinary importance. EFSA J. 2012, 10, 2740–2749. [Google Scholar] [CrossRef]
- Gueimonde, M.; Sánchez, B.; de los Reyes-Gavilán, C.G.; Margolles, A. Antibiotic resistance in probiotic bacteria. Front. Microbiol. 2013, 4, 202. [Google Scholar] [CrossRef] [Green Version]
- Poelarends, G.J.; Mazurkiewicz, P.; Konings, W.N. Multidrug transporters and antibiotic resistance in Lactococcus lactis. Biochim. Biophys. Acta 2002, 10, 1555. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khelissa, S.; Chihib, N.E.; Gharsallaoui, A. Conditions of nisin production by Lactococcus lactis subsp. lactis and its main uses as a food preservative. Arch. Microbiol. 2021, 203, 465–480. [Google Scholar] [CrossRef]
- Koral, G.; Tuncer, Y. Nisin Z-producing Lactococcus lactis subsp. lactis GYI32 isolated from Boza. J. Food Process. Preserv. 2014, 38, 1044–1053. [Google Scholar] [CrossRef]
- Choi, H.J.; Cheigh, C.I.; Kim, S.B.; Pyun, Y.R. Production of a nisin-like bacteriocin by Lactococcus lactis subsp. lactis A164 isolated from Kimchi. J. Appl. Microbiol. 2000, 88, 563–571. [Google Scholar] [CrossRef]
- Juturu, V.; Wu, J.C. Microbial production of bacteriocins: Latest research development and applications. Biotechnol. Adv. 2018, 36, 2187–2200. [Google Scholar] [CrossRef]
- Abdalla, A.K.; Ayyash, M.M.; Olaimat, A.N.; Osaili, T.M.; Al-Nabulsi, A.A.; Shah, N.P.; Holley, R. Exopolysaccharides as antimicrobial agents: Mechanism and spectrum of activity. Front. Microbiol. 2021, 12, 664395. [Google Scholar] [CrossRef] [PubMed]
- Nehal, F.; Sahnoun, M.; Smaoui, S.; Jaouadi, B.; Bejar, S.; Mohammed, S. Characterization, high production and antimicrobial activity of exopolysaccharides from Lactococcus lactis F-mou. Microb. Pathogen. 2019, 132, 10–19. [Google Scholar] [CrossRef] [PubMed]
- Domingos-Lopes, M.; Lamosa, P.; Stanton, C.; Ross, R.P.; Silva, C. Isolation and characterization of an exopolysaccharide-producing Leuconostoc citreum strain from artisanal cheese. Lett. Appl. Microbiol. 2018, 67, 570–578. [Google Scholar] [CrossRef] [PubMed]
- Vurture, G.W.; Sedlazeck, F.J.; Nattestad, M.; Underwood, C.J.; Fang, H.; Gurtowski, J.; Schatz, M.C. GenomeScope: Fast reference-free genome profiling from short reads. Bioinformatics 2017, 33, 2202–2204. [Google Scholar] [CrossRef] [Green Version]
- Simao, F.A.; Waterhouse, R.M.; Ioannidis, P.; Kriventseva, E.V.; Zdobnov, E.M. BUSCO: Assessing genome assembly and annotation completeness with single-copy orthologs. Bioinformatics 2015, 31, 3210–3212. [Google Scholar] [CrossRef] [Green Version]
- Goris, J.; Konstantinidis, K.T.; Klappenbach, J.A.; Coenye, T.; Vandamme, P.; Tiedje, J.M. DNA-DNA hybridization values and their relationship to whole-genome sequence similarities. Int. J. Syst. Evol. Microbiol. 2007, 57, 81–91. [Google Scholar] [CrossRef] [Green Version]
- Stothard, P.; Grant, J.R.; Van Domselaar, G. Visualizing and comparing circular genomes using the CGView family of tools. Brief Bioinform. 2019, 20, 1576–1582. [Google Scholar] [CrossRef] [Green Version]
- Meier-Kolthoff, J.P.; Auch, A.F.; Klenk, H.-P.; Göker, M. Genome sequence-based species delimitation with confidence intervals and improved distance functions. BMC Bioinform. 2013, 14, 60. [Google Scholar] [CrossRef] [Green Version]
- Seemann, T. Prokka: Rapid prokaryotic genome annotation. Bioinformatics 2014, 30, 2068–2069. [Google Scholar] [CrossRef] [Green Version]
- Jones, P.; Binns, D.; Chang, H.Y.; Fraser, M.; Li, W.; McAnulla, C.; McWilliam, H.; Maslen, J.; Mitchell, A.; Nuka, G.; et al. InterProScan 5: Genome-scale protein function classification. Bioinformatics 2014, 30, 1236–1240. [Google Scholar] [CrossRef] [Green Version]
- Selengut, J.D.; Haft, D.H.; Davidsen, T.; Ganapathy, A.; Gwinn-Giglio, M.; Nelson, W.C.; Richter, A.R.; White, O. TIGRFAMs and Genome Properties: Tools for the assignment of molecular function and biological process in prokaryotic genomes. Nucleic Acids Res. 2007, 35, D260–D264. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huerta-Cepas, J.; Szklarczyk, D.; Heller, D.; Hernández-Plaza, A.; Forslund, S.K.; Cook, H.; Mende, D.R.; Letunic, I.; Rattei, T.; Jensen, L.J.; et al. EggNOG 5.0: A hierarchical, functionally and phylogenetically annotated orthology resource based on 5090 organisms and 2502 viruses. Nucleic Acids Res. 2019, 47, D309–D314. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grissa, I.; Vergnaud, G.; Pourcel, C. CRISPRFinder: A web tool to identify clustered regularly interspaced short palindromic repeats. Nucleic Acids Res. 2007, 35, W52–W57. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arndt, D.; Grant, J.R.; Marcu, A.; Sajed, T.; Pon, A.; Liang, Y.; Wishart, D.S. PHASTER: A better, faster version of the PHAST phage search tool. Nucleic Acids Res. 2016, 44, W16–W21. [Google Scholar] [CrossRef] [Green Version]
- Jia, B.; Raphenya, A.R.; Alcock, B.; Waglechner, N.; Guo, P.; Tsang, K.K.; Lago, B.A.; Dave, B.M.; Pereira, S.; Sharma, A.N.; et al. CARD 2017: Expansion and model-centric curation of the comprehensive antibiotic resistance database. Nucleic Acids Res. 2017, 45, D566–D573. [Google Scholar] [CrossRef] [PubMed]
- Bortolaia, V.; Kaas, R.S.; Ruppe, E.; Roberts, M.C.; Schwarz, S.; Cattoir, V.; Philippon, A.; Allesoe, R.L.; Rebelo, A.R.; Florensa, A.F.; et al. ResFinder 4.0 for predictions of phenotypes from genotypes. J. Antimicrob. Chemother. 2020, 75, 3491–3500. [Google Scholar] [CrossRef] [PubMed]
- Carattoli, A.; Zankari, E.; García-Fernández, A.; Voldby Larsen, M.; Lund, O.; Villa, L.; Møller Aarestrup, F.; Hasman, H. In silico detection and typing of plasmids using PlasmidFinder and plasmid multilocus sequence typing. Antimicrob. Agents Chemother. 2014, 58, 3895–3903. [Google Scholar] [CrossRef] [Green Version]
- Liu, B.; Zheng, D.; Jin, Q.; Chen, L.; Yang, J. VFDB 2019: A comparative pathogenomic platform with an interactive web interface. Nucleic Acids Res. 2019, 47, D687–D692. [Google Scholar] [CrossRef]
- Bertelli, C.; Laird, M.R.; Williams, K.P.; Lau, B.Y.; Hoad, G.; Winsor, G.L.; Brinkman, F.; Simon Fraser University Research Computing Group. IslandViewer 4: Expanded prediction of genomic islands for larger-scale datasets. Nucleic Acids Res. 2017, 45, W30–W35. [Google Scholar] [CrossRef]
- Siguier, P.; Perochon, J.; Lestrade, L.; Mahillon, J.; Chandler, M. ISfinder: The reference centre for bacterial insertion sequences. Nucleic Acids Res. 2006, 34, D32–D36. [Google Scholar] [CrossRef] [Green Version]
- Cosentino, S.; Voldby Larsen, M.; Møller Aarestrup, F.; Lund, O. PathogenFinder--distinguishing friend from foe using bacterial whole genome sequence data. PLoS ONE 2013, 8, e77302. [Google Scholar] [CrossRef]
- Page, A.J.; Cummins, C.A.; Hunt, M.; Wong, V.K.; Reuter, S.; Holden, M.T.; Fookes, M.; Falush, D.; Keane, J.A.; Parkhill, J. Roary: Rapid large-scale prokaryote pangenome analysis. Bioinformatics 2015, 31, 3691–3693. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Katoh, K.; Standley, D.M. MAFFT multiple sequence alignment software version 7: Improvements in performance and usability. Mol. Biol. Evol. 2013, 30, 772–780. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Andreu, V.P.; Roel-Touris, J.; Dodd, D.; Fischbach, M.A.; Medema, M.H. The gutSMASH web server: Automated identification of primary metabolic gene clusters from the gut microbiota. Nucleic Acids Res. 2021, 49, W263–W270. [Google Scholar] [CrossRef]
- Medema, M.H.; Blin, K.; Cimermancic, P.; de Jager, V.; Zakrzewski, P.; Fischbach, M.A.; Weber, T.; Takano, E.; Breitling, R. AntiSMASH: Rapid identification, annotation and analysis of secondary metabolite biosynthesis gene clusters in bacterial and fungal genome sequences. Nucleic Acids Res. 2011, 39, W339–W346. [Google Scholar] [CrossRef]
- De Jong, A.; van Hijum, S.A.; Bijlsma, J.J.; Kok, J.; Kuipers, O.P. BAGEL: A web-based bacteriocin genome mining tool. Nucleic Acids Res. 2006, 34, W273–W279. [Google Scholar] [CrossRef]
- Darbandi, A.; Asadi, A.; Ari, M.M.; Ohadi, E.; Talebi, M.; Zadeh, M.H.; Emamie, A.D.; Ghanavati, R.; Kakanj, M. Bacteriocins: Properties and potential use as antimicrobials. J. Clin. Lab. Anal. 2022, 36, e24093. [Google Scholar] [CrossRef]
- Llamas-Arriba, M.G.; Hernández-Alcántara, A.M.; Mohedano, M.L.; Chiva, R.; Celador-Lera, L.; Velázquez, E.; Prieto, A.; Dueñas, M.T.; Tamame, M.; López, P. Lactic acid bacteria isolated from fermented doughs in Spain produce dextrans and riboflavin. Foods 2021, 10, 2004. [Google Scholar] [CrossRef]
- Jiang, G.; Li, R.; He, J.; Yang, L.; Chen, J.; Xu, Z.; Zheng, B.; Yang, Y.; Xia, Z.; Tian, Y. Extraction, Structural Analysis, and Biofunctional Properties of Exopolysaccharide from Lactiplantibacillus pentosus B8 Isolated from Sichuan Pickle. Foods 2022, 11, 2327. [Google Scholar] [CrossRef]





| Primary Metabolites | |||
|---|---|---|---|
| Contig. Region | Type | Location (Length) | Most Similar Gene Cluster ClusterBlast */KnownCluster Blast Gene Similarity (%) |
| 1.1 | GR_AA_metabolism | 89,383–125,845 nt | Not match |
| (total: 36,463 nt) | |||
| 1.2 | TPP_fatty_acids | 253,778–280,874 nt | Not match |
| (total: 27,097 nt) | |||
| 1.3 | TPP_AA_metabolism | 719,434–745,727 nt | Threonine to propionate (20%) |
| (total: 26,294 nt) | |||
| 3.1 | Arginine2_Hcarbonate | 85,098–111,226 nt | Arginine to hydrogen carbonate of P. aeruginosa (100%) |
| (total: 26,129 nt) | |||
| 4.1 | Others_HGD_unassigned | 36,177–60,487 nt | Not match |
| (total: 24,311 nt) | |||
| Secondary metabolites | |||
| 1.1 | Beta lactone | 93,313–125,845 nt | 100/nisin A |
| (total: 32,533 nt) | |||
| 1.2 | Lanthipeptide-class I | 127,000–153,031 nt | |
| (total: 26,032 nt) | |||
| 1.3 | T3PKS | 1,112,828–1,153,982 nt | |
| (total: 41,155 nt) | |||
| 2.1 | RaS-RiPP | 31,471–53,861 nt | Not match |
| (total: 22,391 nt) | |||
| 5.1 | RaS-RiPP | 12,025–34,680 nt | Not match |
| (total: 22,656 nt) | |||
| Indicator Strain | Average Diameter of the Inhibition Zone (mm) | |||||
|---|---|---|---|---|---|---|
| PPE | EPSQ | EPSS | ||||
| UTNCys6-1 | L. LAC | UTNCys6-1 | L. LAC | UTNCys6-1 | L. LAC | |
| S. aureus ATCC1026 | 9.33 ± 0.58 Db | 10.33 ± 0.58 Aa | 9.33 ± 0.58 Bb | 9.33 ± 0.58 Bb | 9.17 ± 0.29 Cb | 9.17 ± 0.29 Cb |
| S. aureus ATCC43300 | 9.33 ± 0.58 Dd | 10.33 ± 0.58 Ac | 9.33 ± 0.58 Bd | 9.33 ± 0.58 Bd | 12.33 ± 0.58 Bb | 13.17 ± 0.29 Ba |
| L. monocytogenes ATCC19115 | 18.03 ± 0.05 Aa | 9.33 ± 0.58 Be | 11.33 ± 0.58 Ac | 10.17 ± 0.29 Ad | 12.67 ± 0.58 Bb | 9.33 ± 0.58 Ce |
| S. enterica subsp. enterica ATCC51741 | 12.33 ± 0.94 Ca | 9.33 ± 0.58 Bb | 8.33 ± 0.58 Bc | 8.33 ± 0.58 Cc | 12.33 ± 0.94 Ba | 12.33 ± 0.94 Ba |
| E. coli ATCC25922 | 9.33 ± 0.58 Db | 10.33 ± 0.58 Aa | 8.33 ± 0.58 Bc | 8.33 ± 0.58 Cc | 9.33 ± 0.58 Cb | 9.33 ± 0.58 Cb |
| E. hormechei UTNB3Sh1 | 14.67 ± 0.94 Ba | 9.33 ± 0.58 Bd | 8.33 ± 0.58 Be | 8.33 ± 0.58 Ce | 14.33 ± 0.58 Ab | 13.67 ± 0.58 Ac |
| MRS broth (negative control) | 6.01 ± 0.20 *Ea | 6.01 ± 0.20 *Ca | 6.01 ± 0.20 *Ca | 6.01 ± 0.20 *Da | 6.01 ± 0.20 *Da | 6.01 ± 0.20 *Da |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tenea, G.N. Metabiotics Signature through Genome Sequencing and In Vitro Inhibitory Assessment of a Novel Lactococcus lactis Strain UTNCys6-1 Isolated from Amazonian Camu-Camu Fruits. Int. J. Mol. Sci. 2023, 24, 6127. https://doi.org/10.3390/ijms24076127
Tenea GN. Metabiotics Signature through Genome Sequencing and In Vitro Inhibitory Assessment of a Novel Lactococcus lactis Strain UTNCys6-1 Isolated from Amazonian Camu-Camu Fruits. International Journal of Molecular Sciences. 2023; 24(7):6127. https://doi.org/10.3390/ijms24076127
Chicago/Turabian StyleTenea, Gabriela N. 2023. "Metabiotics Signature through Genome Sequencing and In Vitro Inhibitory Assessment of a Novel Lactococcus lactis Strain UTNCys6-1 Isolated from Amazonian Camu-Camu Fruits" International Journal of Molecular Sciences 24, no. 7: 6127. https://doi.org/10.3390/ijms24076127






