First In Vivo Insights on the Effects of Tempol-Methoxycinnamate, a New UV Filter, as Alternative to Octyl Methoxycinnamate, on Zebrafish Early Development
Abstract
:1. Introduction
2. Results
2.1. Effect on Development, Hatching, and Growth Parameters
2.1.1. Effect on Embryo Morphology
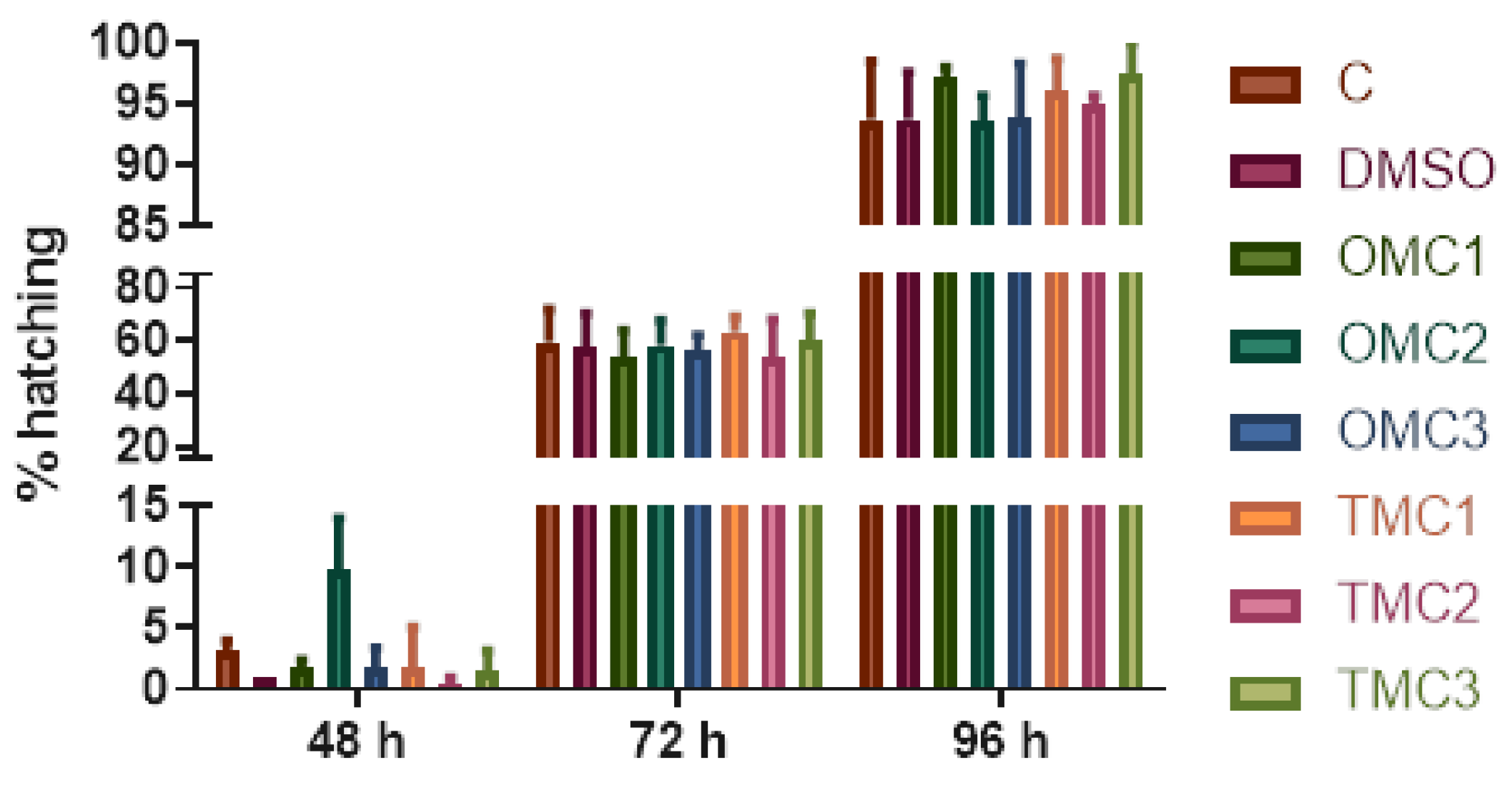
2.1.2. Effects of Treatments on Hatching Rate
2.1.3. Alcian Blue–Alizarin Red Double Staining
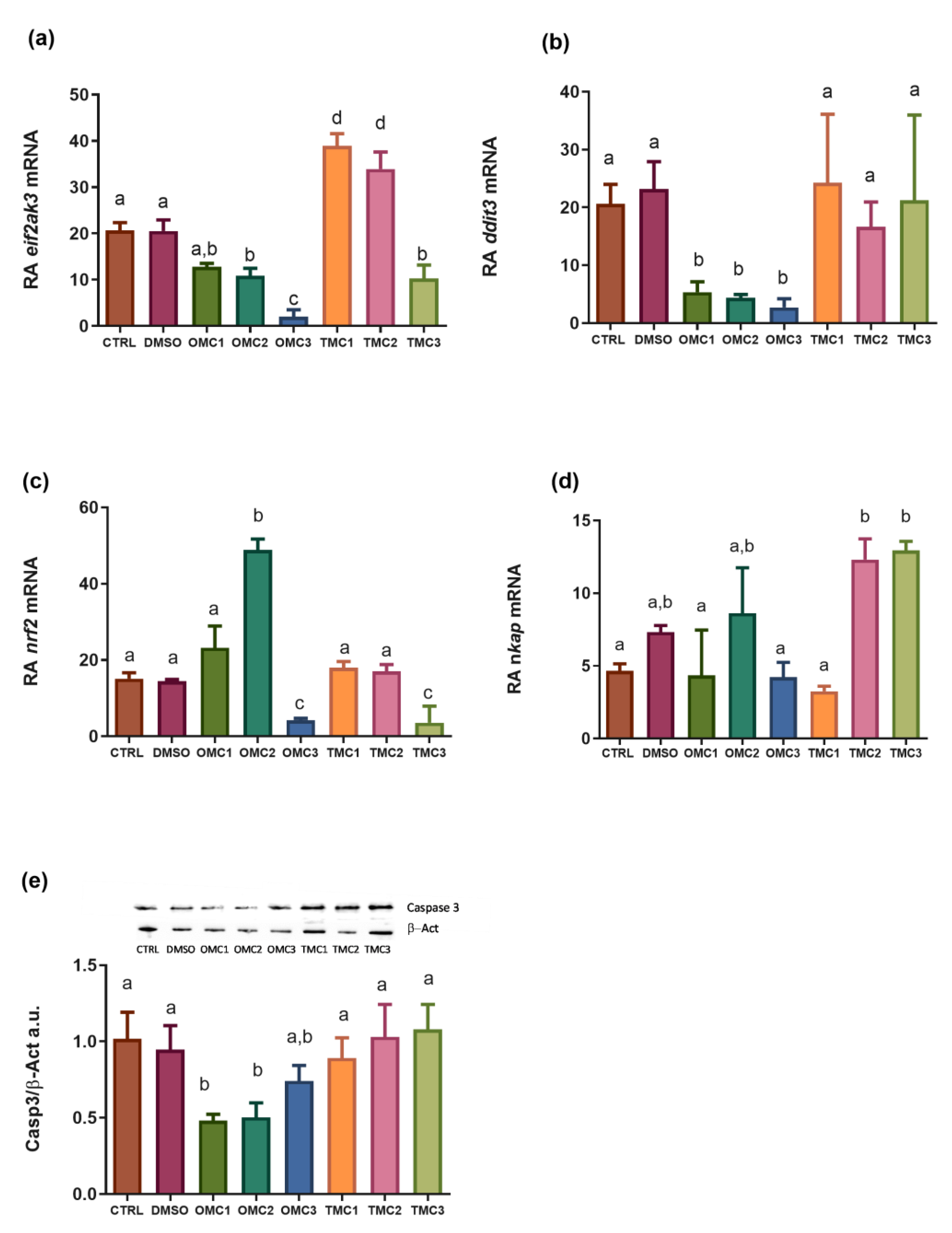
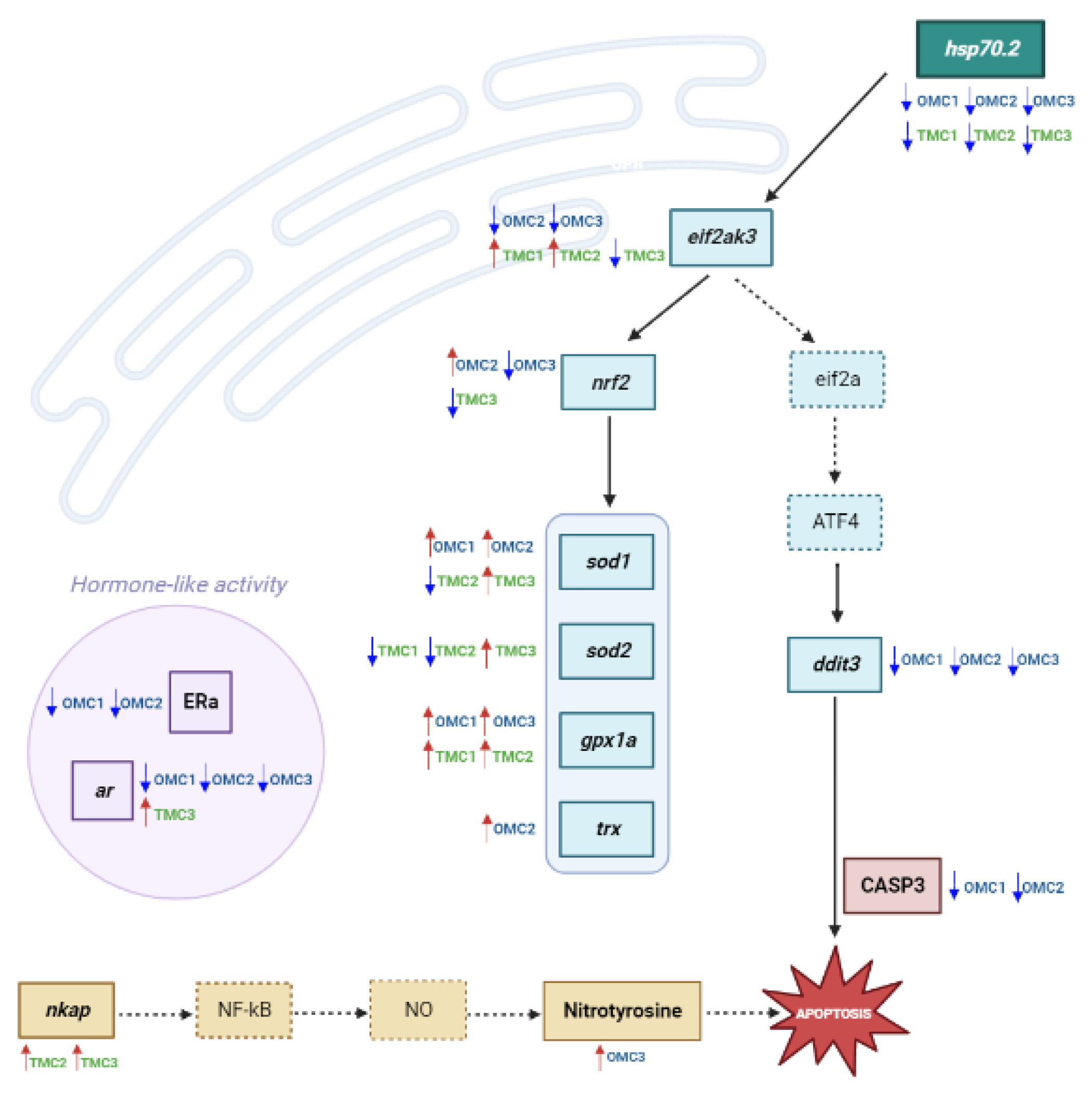
2.2. Analysis of Molecular Targets Involved in Oxidative Stress Response
2.3. Analysis of Molecular Targets Involved in the Endoplasmic Reticulum Stress (ER Stress) Response, Inflammation, and Apoptosis
2.4. Analysis of Androgen Receptor (ar) Transcript and Estrogen Receptor α (ERα) Protein Levels
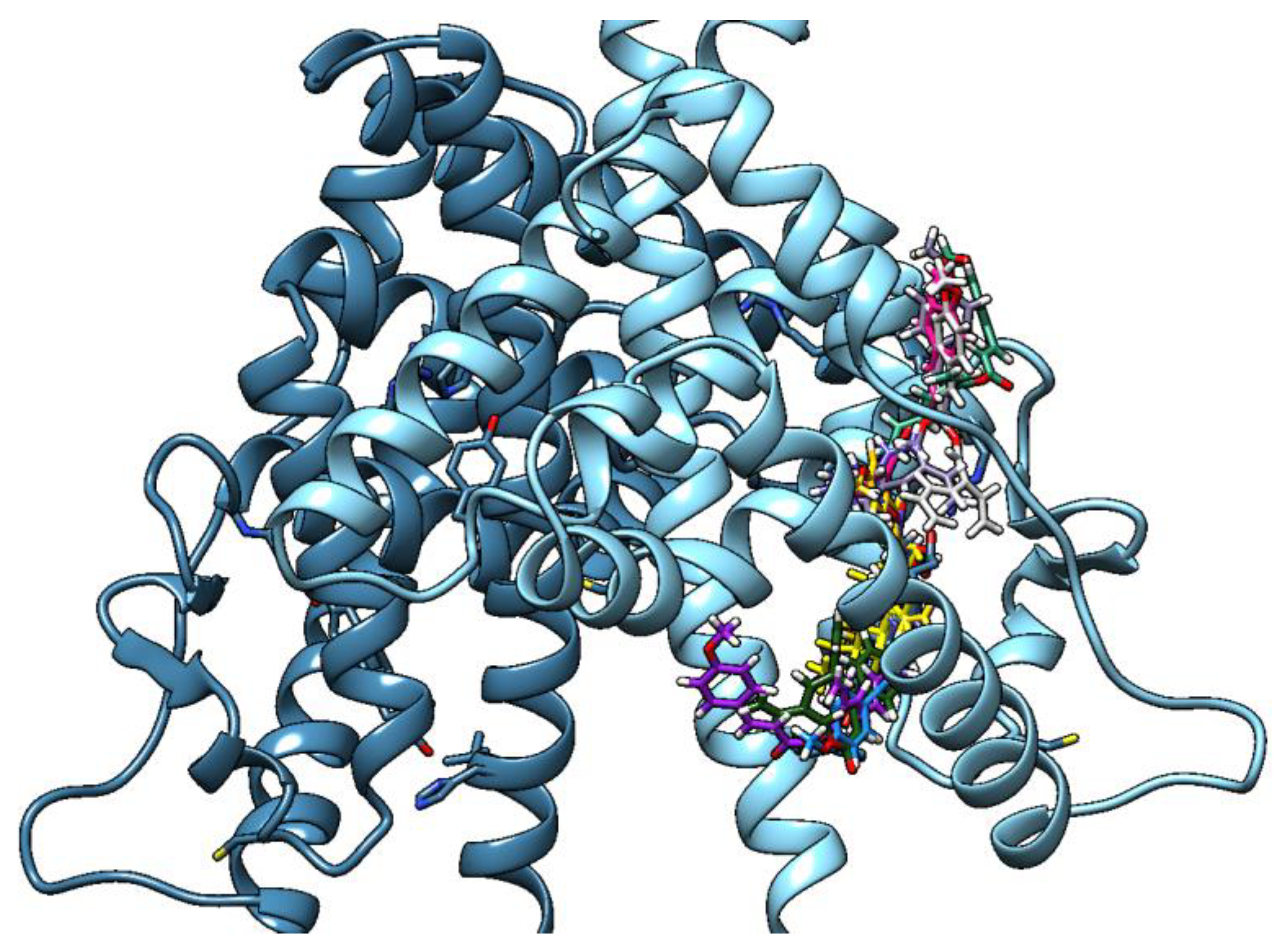
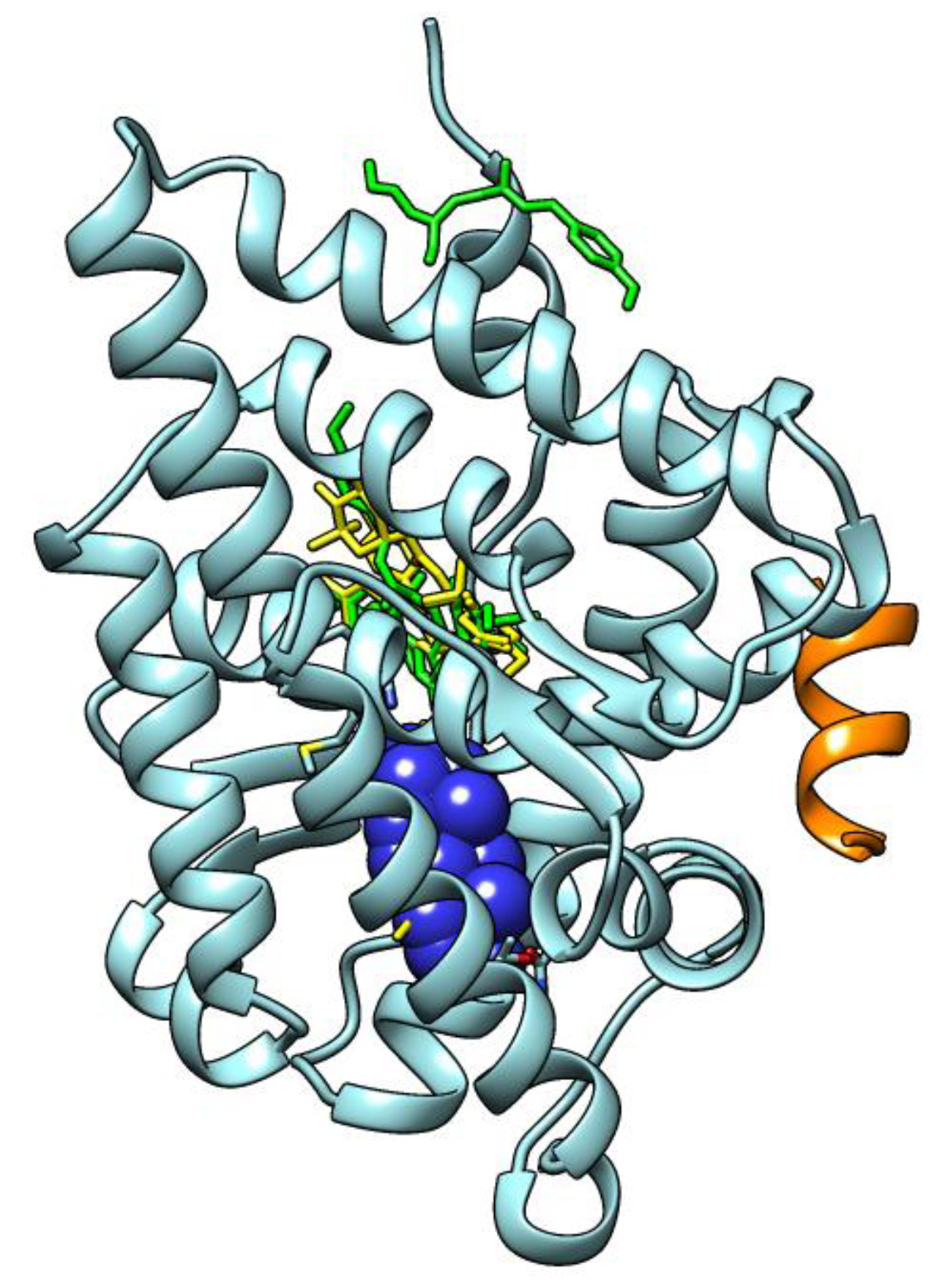
2.5. Molecular Docking Results
3. Discussion
4. Materials and Methods
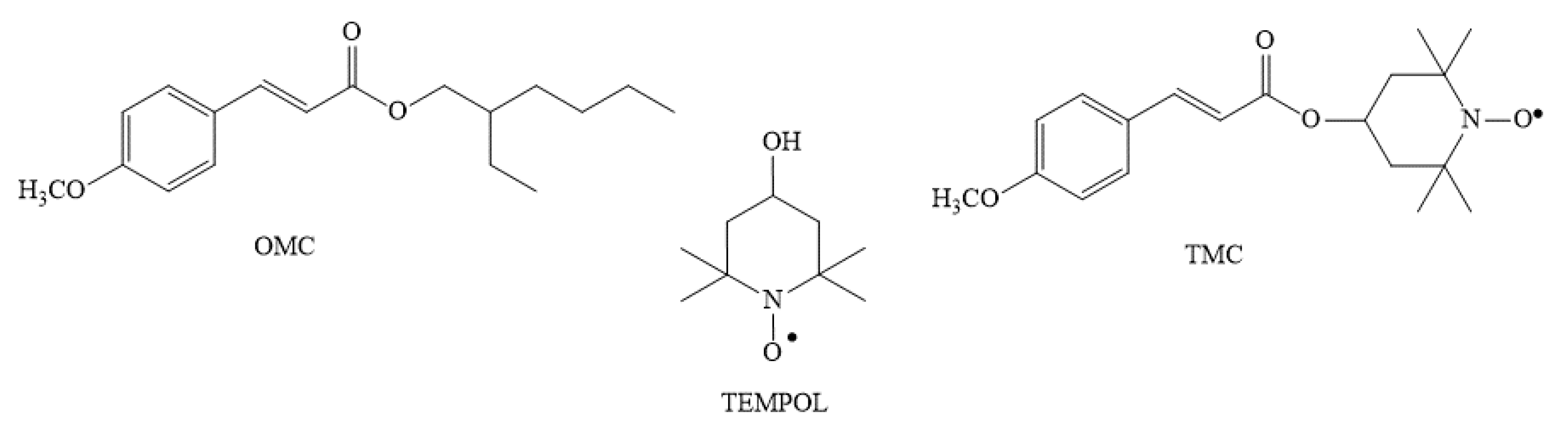
4.1. Chemicals
4.2. Exposure
- Control;
- DMSO: exposed to 0.01% DMSO (vehicle);
- OMC1: exposed to 0.05 μM OMC;
- OMC2: exposed to 0.01 μM OMC;
- OMC3: exposed to 0.005 μM OMC;
- TMC1: exposed to 0.05 μM TMC;
- TMC2: exposed to 0.01 μM TMC;
- TMC3: exposed to 0.005 μM TMC.
4.3. Morphology
4.4. Alcian Blue–Alizarin Red Staining
4.5. RNA Extraction and cDNA Synthesis
4.6. Real-Time PCR
4.7. Western Blot Analysis
4.8. Computational Analysis
4.9. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Carve, M.; Nugegoda, D.; Allinson, G.; Shimeta, J. A Systematic Review and Ecological Risk Assessment for Organic Ultraviolet Fi Lters in Aquatic Environments. Environ. Pollut. 2021, 268, 115894. [Google Scholar] [CrossRef]
- Prakash, V.; Jain, V.; Singh, S.; Parthasarathi, R. Science of the Total Environment Developmental Toxicity Assessment of 4-MBC in Danio Rerio Embryo-Larval Stages. Sci. Total Environ. 2022, 804, 149920. [Google Scholar] [CrossRef]
- Ruszkiewicz, J.A.; Pinkas, A.; Ferrer, B.; Peres, T.V.; Tsatsakis, A.; Aschner, M. Neurotoxic Effect of Active Ingredients in Sunscreen Products, a Contemporary Review. Toxicol. Rep. 2017, 4, 245–259. [Google Scholar] [CrossRef]
- Katiyar, P.; Banerjee, S.; Nathani, S.; Roy, P. Triclosan-Induced Neuroinflammation Develops Caspase-Independent and TNF-α Signaling Pathway Associated Necroptosis in Neuro-2a Cells. Curr. Res. Toxicol. 2022, 3, 100072. [Google Scholar] [CrossRef]
- Huang, C.; Kung, Z.; Wei, C. UV-Filter Octyl Methoxycinnamate Causes Reproductive Toxicity Associated with Germline Apoptosis and Vitellogenin Decrease in Caenorhabditis Elegans. Aquat. Toxicol. 2022, 247, 106149. [Google Scholar] [CrossRef] [PubMed]
- Liang, M.; Yan, S.; Chen, R.; Hong, X. 3-(4-Methylbenzylidene) Camphor Induced Reproduction Toxicity and Antiandrogenicity in Japanese Medaka (Oryzias Latipes). Chemosphere 2020, 249, 126224. [Google Scholar] [CrossRef]
- Kumar, V.; Balomajumder, C.; Roy, P. Disruption of LH-Induced Testosterone Biosynthesis in Testicular Leydig Cells by Triclosan: Probable Mechanism of Action. Toxicology 2008, 250, 124–131. [Google Scholar] [CrossRef] [PubMed]
- Mandal, T.K.; Parvin, N.; Joo, S.W.; Roy, P. Risk Assessment of Cosmetics Using Triclosan on Future Generation’s Germ Cell Maturation via Lactating Mother Rats. Int. J. Environ. Res. Public Health 2020, 17, 1143. [Google Scholar] [CrossRef] [Green Version]
- Cuccaro, A.; De Marchi, L.; Oliva, M.; Battaglia, F.; Meucci, V.; Fumagalli, G.; Freitas, R.; Pretti, C. Ecotoxicological Effects of the UV-Filter 4-MBC on Sperms and Adults of the Mussel Mytilus Galloprovincialis. Environ. Res. 2022, 213, 113739. [Google Scholar] [CrossRef]
- Kumar, V.; Chakraborty, A.; Kural, M.R.; Roy, P. Alteration of Testicular Steroidogenesis and Histopathology of Reproductive System in Male Rats Treated with Triclosan. Reprod. Toxicol. 2009, 27, 177–185. [Google Scholar] [CrossRef] [PubMed]
- Chang, H.; Li, J.; Zhang, C.; Qian, W. Octocrylene Exposure Impairs Mouse Oocyte Quality by Inducing Spindle Defects and Mitochondria Dysfunction. Toxicology 2022, 479, 153306. [Google Scholar] [CrossRef]
- Xie, Z.; Zhou, R.; Ding, Z.; Zhou, D.; Jin, Q. Melanin Interference Toxicity or Transgenerational Toxicity of Organic UV Filter Ethylhexyl Salicylate on Zebrafishsh. Sci. Total Environ. 2022, 845, 157365. [Google Scholar] [CrossRef] [PubMed]
- Nataraj, B.; Maharajan, K.; Hemalatha, D.; Rangasamy, B.; Arul, N.; Ramesh, M. Comparative Toxicity of UV-Filter Octyl Methoxycinnamate and Its Photoproducts on Zebrafish Development. Sci. Total Environ. 2020, 718, 134546. [Google Scholar] [CrossRef]
- Ozáez, I.; Martínez-Guitarte, J.L.; Morcillo, G. Effects of in Vivo Exposure to UV Filters (4-MBC, OMC, BP-3, 4-HB, OC, OD-PABA) on Endocrine Signaling Genes in the Insect Chironomus Riparius. Sci. Total Environ. 2013, 456–457, 120–126. [Google Scholar] [CrossRef]
- Cahova, J.; Blahova, J.; Marsalek, P.; Doubkova, V.; Franc, A.; Garajová, M.; Tichy, F.; Mares, J.; Svobodova, Z. The Biological Activity of the Organic UV Filter Ethylhexyl Methoxycinnamate in Rainbow Trout (Oncorhynchus Mykiss). Sci. Total Environ. 2021, 774, 145570. [Google Scholar] [CrossRef]
- Cahova, J.; Blahova, J.; Plhalova, L.; Svobodova, Z.; Faggio, C. Do Single-Component and Mixtures Selected Organic UV Filters Induce Embryotoxic Effects in Zebrafish (Danio Rerio)? Water 2021, 13, 2203. [Google Scholar] [CrossRef]
- Nataraj, B.; Maharajan, K.; Malafaia, G.; Hemalatha, D.; Ahmed, M.A.I.; Ramesh, M. Gene Expression Profiling in Liver of Zebrafish Exposed to Ethylhexyl Methoxycinnamate and Its Photoproducts. Sci. Total Environ. 2022, 826, 154046. [Google Scholar] [CrossRef] [PubMed]
- Park, C.B.; Jang, J.; Kim, S.; Kim, Y.J. Single- and Mixture Toxicity of Three Organic UV-Filters, Ethylhexyl Methoxycinnamate, Octocrylene, and Avobenzone on Daphnia Magna. Ecotoxicol. Environ. Saf. 2017, 137, 57–63. [Google Scholar] [CrossRef]
- Danovaro, R.; Bongiorni, L.; Corinaldesi, C.; Giovannelli, D.; Damiani, E.; Astolfi, P.; Greci, L.; Pusceddu, A. Sunscreens Cause Coral Bleaching by Promoting Viral Infections. Environ. Health Perspect. 2008, 116, 441–447. [Google Scholar] [CrossRef] [Green Version]
- Lee, I.; Lee, J.; Jung, D.; Kim, S.; Choi, K. Two-Generation Exposure to 2-Ethylhexyl 4-Methoxycinnamate (EHMC) in Japanese Medaka (Oryzias Latipes) and Its Reproduction and Endocrine Related Effects. Chemosphere 2019, 228, 478–484. [Google Scholar] [CrossRef]
- Zhou, R.; Lu, G.; Yan, Z.; Jiang, R.; Shen, J.; Bao, X. Parental Transfer of Ethylhexyl Methoxy Cinnamate and Induced Biochemical Responses in Zebrafish. Aquat. Toxicol. 2019, 206, 24–32. [Google Scholar] [CrossRef]
- Chu, S.; Reum, B.; Lee, Y.; Zoh, K.; Choi, K. Effects of 2-Ethylhexyl-4-Methoxycinnamate (EHMC) on Thyroid Hormones and Genes Associated with Thyroid, Neurotoxic, and Nephrotoxic Responses in Adult and Larval Zebrafish (Danio Rerio). Chemosphere 2021, 263, 128176. [Google Scholar] [CrossRef]
- Ouchene, L.; Litvinov, I.V.; Netchiporouk, E. Hawaii and Other Jurisdictions Ban Oxybenzone or Octinoxate Sunscreens Based on the Confirmed Adverse Environmental Effects of Sunscreen Ingredients on Aquatic Environments. J. Cutan. Med. Surg. 2019, 23, 648–649. [Google Scholar] [CrossRef]
- Damiani, E.; Astolfi, P.; Cionna, L.; Ippoliti, F.; Greci, L. Synthesis and Application of a Novel Sunscreen-Antioxidant. Free Radic. Res. 2006, 40, 485–494. [Google Scholar] [CrossRef]
- Damiani, E.; Astolfi, P.; Greci, L. Synthesis of a Nitroxide Antioxidant and Methods of Use in Cosmetic and Dermatological Compositions 2006. U.S. Patent 7,833,540, 16 November 2010. [Google Scholar]
- Barbazuk, W.B.; Korf, I.; Kadavi, C.; Heyen, J.; Tate, S.; Wun, E.; Bedell, J.A.; McPherson, J.D.; Johnson, S.L. The Syntenic Relationship of the Zebrafish and Human Genomes [1]. Genome Res. 2000, 10, 1351–1358. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boix, N.; Teixido, E.; Pique, E.; Llobet, J.M.; Gomez-Catalan, J. Modulation and Protection Effects of Antioxidant Compounds against Oxidant Induced Developmental Toxicity in Zebrafish. Antioxidants 2020, 9, 721. [Google Scholar] [CrossRef]
- Zambrano, D.; Millán, D.; Guevara-Pulido, J. In Silico Design, Synthesis and Evaluation of a Less Toxic Octinoxate Alternative with Suitable Photoprotection Properties. Eur. J. Pharm. Sci. 2023, 180, 106332. [Google Scholar] [CrossRef] [PubMed]
- Zennaro, C.; Mariotti, M.; Carraro, M.; Pasqualetti, S.; Corbelli, A.; Armelloni, S.; Li, M.; Ikehata, M.; Clai, M.; Artero, M.; et al. Podocyte Developmental Defects Caused by Adriamycin in Zebrafish Embryos and Larvae: A Novel Model of Glomerular Damage. PLoS ONE 2014, 9, e98131. [Google Scholar] [CrossRef] [Green Version]
- Huang, W.; Wu, T.; Au, W.W.; Wu, K. Impact of Environmental Chemicals on Craniofacial Skeletal Development: Insights from Investigations Using Zebrafish Embryos. Environ. Pollut. 2021, 286, 117–541. [Google Scholar] [CrossRef]
- Maradonna, F.; Gioacchini, G.; Falcinelli, S.; Bertotto, D.; Radaelli, G.; Olivotto, I.; Carnevali, O. Probiotic Supplementation Promotes Calcification in Danio Rerio Larvae: A Molecular Study. PLoS ONE 2013, 8, e83155. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sojan, J.M.; Gundappa, M.K.; Carletti, A.; Gaspar, V.; Gavaia, P.; Maradonna, F.; Carnevali, O. Zebrafish as a Model to Unveil the Pro-Osteogenic Effects of Boron-Vitamin D3 Synergism. Front. Nutr. 2022, 9, 868805. [Google Scholar] [CrossRef]
- Sojan, J.M.; Gioacchini, G.; Giorgini, E.; Orlando, P.; Tiano, L.; Maradonna, F.; Carnevali, O. Zebrafish Caudal Fin as a Model to Investigate the Role of Probiotics in Bone Regeneration. Sci. Rep. 2022, 12. [Google Scholar] [CrossRef]
- Lorigo, M.; Cairrao, E. Antioxidants as Stabilizers of UV Filters: An Example for the UV-B Filter Octylmethoxycinnamate. Biomed. Dermatol. 2019, 3, 11. [Google Scholar] [CrossRef]
- Carnevali, O.; Maradonna, F. Exposure to Xenobiotic Compounds: Looking for New Biomarkers. Gen. Comp. Endocrinol. 2003, 131, 203–209. [Google Scholar] [CrossRef]
- Maradonna, F.; Carnevali, O. Vitellogenin, Zona Radiata Protein, Cathepsin D and Heat Shock Protein 70 as Biomarkers of Exposure to Xenobiotics. Biomarkers 2007, 12, 240–255. [Google Scholar] [CrossRef] [PubMed]
- Frydman, J. Folding of Newly Translated Proteins in Vivo: The Role of Molecular Chaperones. Annu. Rev. Biochem. 2001, 70, 603–647. [Google Scholar] [CrossRef]
- Fernández-Fernández, M.R.; Gragera, M.; Ochoa-Ibarrola, L.; Quintana-Gallardo, L.; Valpuesta, J.M. Hsp70—A Master Regulator in Protein Degradation. FEBS Lett. 2017, 591, 2648–2660. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Doyle, S.M.; Genest, O.; Wickner, S. Protein Rescue from Aggregates by Powerful Molecular Chaperone Machines. Nat. Rev. Mol. Cell Biol. 2013, 14, 617–629. [Google Scholar] [CrossRef]
- Arndt, V.; Rogon, C.; Höhfeld, J. To Be, or Not to Be—Molecular Chaperones in Protein Degradation. Cell. Mol. Life Sci. 2007, 64, 2525–2541. [Google Scholar] [CrossRef] [PubMed]
- Ohoka, N.; Yoshii, S.; Hattori, T.; Onozaki, K.; Hayashi, H. TRB3, a Novel ER Stress-Inducible Gene, Is Induced via ATF4-CHOP Pathway and Is Involved in Cell Death. EMBO J. 2005, 24, 1243–1255. [Google Scholar] [CrossRef]
- Walter, P.; Ron, D. The Unfolded Protein Response: From Stress Pathway to Homeostatic Regulation. Sci. (80-) 2011, 334, 1081–1086. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yoshida, H.; Haze, K.; Yanagi, H.; Yura, T.; Mori, K. Identification of the Cis-Acting Endoplasmic Reticulum Stress Response Element Responsible for Transcriptional Induction of Mammalian Glucose- Regulated Proteins: Involvement of Basic Leucine Zipper Transcription Factors. J. Biol. Chem. 1998, 273, 33741–33749. [Google Scholar] [CrossRef] [Green Version]
- Pall, M.L.; Levine, S. Nrf2, a Master Regulator of Detoxification and Also Antioxidant, Anti-Inflammatory and Other Cytoprotective Mechanisms, Is Raised by Health Promoting Factors. Sheng Li Xue Bao 2015, 67, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Touyz, R.M.; Chignalia, A.; Sedeek, M. Reactive Oxygen Species, Oxidative Stress, and Hypertension. In Studies on Cardiovascular Disorders; Sauer, H., Shah, A.M., Laurindo, F.R.M., Eds.; Humana Press: Totowa, NJ, USA, 2010; pp. 281–315. ISBN 978-1-60761-600-9. [Google Scholar]
- Rodríguez-Fuentes, G.; Sandoval-Gío, J.J.; Arroyo-Silva, A.; Noreña-Barroso, E.; Escalante-Herrera, K.S.; Olvera-Espinosa, F. Evaluation of the Estrogenic and Oxidative Stress Effects of the UV Filter 3-Benzophenone in Zebrafish (Danio Rerio) Eleuthero-Embryos. Ecotoxicol. Environ. Saf. 2015, 115, 14–18. [Google Scholar] [CrossRef] [PubMed]
- José, J.; Gío, S.; Noreña, E.; Karla, B.; Herrera, E.; Rodríguez, G. Effect of Benzophenone—3 to Acetylcholinesterase and Antioxidant System in Zebrafish (Danio Rerio) Embryos. Bull. Environ. Contam. Toxicol. 2021, 107, 814–819. [Google Scholar] [CrossRef]
- Bai, L.; Zhang, S.; Zhou, X.; Li, Y.; Bai, J. Brain-Derived Neurotrophic Factor Induces Thioredoxin-1 Expression through TrkB/Akt/CREB Pathway in SH-SY5Y Cells. Biochimie 2019, 160, 55–60. [Google Scholar] [CrossRef]
- Lu, J.; Holmgren, A. The Thioredoxin Antioxidant System. Free Radic. Biol. Med. 2014, 66, 75–87. [Google Scholar] [CrossRef]
- Yang, L.; Zeng, C.; Zhang, Y.; Wang, F.; Takamiya, M.; Strähle, U. Functions of Thioredoxin1 in Brain Development and in Response to Environmental Chemicals in Zebrafish Embryos. Toxicol. Lett. 2019, 314, 43–52. [Google Scholar] [CrossRef]
- Zhang, J.; Cui, X.; Wang, L.; Liu, F.; Jiang, T.; Li, C.; Li, D.; Huang, M.; Liao, S.; Wang, J.; et al. The Mitochondrial Thioredoxin Is Required for Liver Development in Zebrafish. Curr. Mol. Med. 2014, 14, 772–782. [Google Scholar] [CrossRef]
- Meng, Q.T.; Chen, R.; Chen, C.; Su, K.; Li, W.; Tang, L.H.; Liu, H.M.; Xue, R.; Sun, Q.; Leng, Y.; et al. Transcription Factors Nrf2 and NF-ΚB Contribute to Inflammation and Apoptosis Induced by Intestinal Ischemia-Reperfusion in Mice. Int. J. Mol. Med. 2017, 40, 1731–1740. [Google Scholar] [CrossRef] [Green Version]
- Song, T.; JY, L.; Jj, Y. NKAP Plays an Oncogenic Function Partly through AKT Signaling Pathway in Hepatocellular Carcinoma. Neoplasma 2019, 60, 792–800. [Google Scholar] [CrossRef]
- Kunz, P.Y.; Galicia, H.F.; Fent, K. Comparison of in Vitro and in Vivo Estrogenic Activity of UV Filters in Fish. Toxicol. Sci. 2006, 90, 349–361. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schlumpf, M.; Schmid, P.; Durrer, S.; Conscience, M.; Maerkel, K.; Henseler, M.; Gruetter, M.; Herzog, I.; Reolon, S.; Ceccatelli, R.; et al. Endocrine Activity and Developmental Toxicity of Cosmetic UV Filters—An Update. Toxicology 2004, 205, 113–122. [Google Scholar] [CrossRef] [PubMed]
- Maradonna, F.; Fontana, C.M.; Sella, F.; Giommi, C.; Facchinello, N.; Rampazzo, C.; Caichiolo, M.; Hoseinifar, S.H.; Dalla Valle, L.; Van Doan, H.; et al. A Zebrafish HCT116 Xenograft Model to Predict Anandamide Outcomes on Colorectal Cancer. Cell Death Dis. 2022, 13, 1069. [Google Scholar] [CrossRef] [PubMed]
- Morris, G.M.; Huey, R.; Lindstrom, W.; Sanner, M.F.; Belew, R.K.; Goodsell, D.S.; Olson, A.J. AutoDock4 and AutoDockTools4: Automated Docking with Selective Receptor Flexibility. J. Comput. Chem. 2009, 30, 2785–2791. [Google Scholar] [CrossRef] [Green Version]





| 48 hpf | Rappresentative Image | D1 | D2 | E.D. | D.B.E. |
|---|---|---|---|---|---|
| CTRL |  | 1198.36 ± 84.77 | 1183.15 ± 91.31 | 412.09 ± 58.31 | 85.33 ± 23.15 |
| DMSO |  | 1168.3 ± 40.30 | 1141.65 ± 42.22 | 324 ± 30.48 | 60 ± 28.28 |
| OMC1 |  | 1195.83 ± 44.59 | 1167.75 ± 49.60 | 368.33 ± 73.54 | 98 ± 47.73 |
| OMC2 |  | 1196.08 ± 56.72 | 1151 ± 56.67 | 318 ± 65.49 | 72.23 ± 18.32 |
| OMC3 |  | 1185.53 ± 46.44 | 1164.8 6± 48.39 | 342.5 ± 29.89 | 48.33 ± 18.58 |
| TMC1 |  | 1199.91 ± 85.44 | 1167.58 ± 74.16 | 299.14 ± 51.19 | 47.66 ± 7.57 |
| TMC2 |  | 1167 ± 40.12 | 1130.23 ± 60.33 | 305.22 ± 36.71 | 65.66 ± 27.06 |
| TMC3 |  | 1196.07 ± 58.46 | 1214.21 ± 223.72 | 345.07 ± 37.13 | 49 ± 11.04 |
| 72 hpf | Rappresentative Image | L | E.L. | Y.A. | Y.P. |
|---|---|---|---|---|---|
| CTRL |  | 3611.66 ± 260.67 | 271 ± 32.03 | 174,437 ± 28,116.37 | 1774.88 ± 140.17 |
| DMSO |  | 3490.11 ± 298.73 | 249.11 ± 40.58 | 193,376.44 ± 29,491.53 | 1859 ± 127.52 |
| OMC1 |  | 3571.55 ± 146.17 | 261 ± 19.41 | 210,217.66 ± 31,725.03 | 1763.77 ± 586.06 |
| OMC2 |  | 3576.33 ± 121.05 | 254.22 ± 20.85 | 172,466.88 ± 26,570.47 | 1757.66 ± 128.10 |
| OMC3 |  | 3422.88 ± 253.89 | 248.66 ± 30.62 | 199,967.77 ± 34,357.39 | 1876.44 ± 139.70 |
| TMC1 |  | 3385.88 ± 339.38 | 258.33 ± 36.77 | 207,813.22 ± 38,902.59 | 1928.55 ± 184.00 |
| TMC2 |  | 3442.33 ± 244.32 | 259.33 ± 23.61 | 212,495.33 ± 41,906.682 | 1947 ± 192.24 |
| TMC3 |  | 3549.22 ± 244.46 | 251.33 ± 43.82 | 185,205.77 ± 28,941.01 | 1882.55 ±178.39 |
| 96 h | Rappresentative Image | L. | E.L | Y.A. | Y.P. |
|---|---|---|---|---|---|
| CTRL |  | 3735.66 ± 473.19 | 283.4 ± 41.01 | 189,534.14 ± 3420.9 | 1861.14 ± 141.42 |
| DMSO |  | 3751.07 ± 218.15 | 295 ± 20.91 | 183,176.21 ± 34,651.49 | 1865.57 ± 180.09 |
| OMC1 |  | 3713.06 ± 324.75 | 282.4 ± 23.46 | 189,039.33 ± 29,766.13 | 1916.53 ± 98.61 |
| OMC2 |  | 3712.73 ± 287.94 | 291.93 ± 37.82 | 204,543.13 ± 34,860.92 | 1965.6 ± 136.70 |
| OMC3 |  | 3733.6 ± 263.70 | 287.53 ± 32.42 | 196,972.66 ± 26,756.82 | 1932.86 ± 120.59 |
| TMC1 |  | 3714.2 ± 359.77 | 279.66 ± 41.19 | 216,675.4 ± 42,217.47 | 2019.86 ± 186.53 |
| TMC2 |  | 3767.4 ± 271.57 | 280.46 ± 18.65 | 187,298.93 ± 29,537.75 | 1892.8 ± 125.72 |
| TMC3 |  | 3771.4 ± 291.18 | 285.73 ± 23.15 | 206,535.73 ± 24,249.74 | 1995.86 ± 112.04 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Damiani, E.; Sella, F.; Astolfi, P.; Galeazzi, R.; Carnevali, O.; Maradonna, F. First In Vivo Insights on the Effects of Tempol-Methoxycinnamate, a New UV Filter, as Alternative to Octyl Methoxycinnamate, on Zebrafish Early Development. Int. J. Mol. Sci. 2023, 24, 6767. https://doi.org/10.3390/ijms24076767
Damiani E, Sella F, Astolfi P, Galeazzi R, Carnevali O, Maradonna F. First In Vivo Insights on the Effects of Tempol-Methoxycinnamate, a New UV Filter, as Alternative to Octyl Methoxycinnamate, on Zebrafish Early Development. International Journal of Molecular Sciences. 2023; 24(7):6767. https://doi.org/10.3390/ijms24076767
Chicago/Turabian StyleDamiani, Elisabetta, Fiorenza Sella, Paola Astolfi, Roberta Galeazzi, Oliana Carnevali, and Francesca Maradonna. 2023. "First In Vivo Insights on the Effects of Tempol-Methoxycinnamate, a New UV Filter, as Alternative to Octyl Methoxycinnamate, on Zebrafish Early Development" International Journal of Molecular Sciences 24, no. 7: 6767. https://doi.org/10.3390/ijms24076767






