Anti-Inflammatory and Antimicrobial Properties of Thyme Oil and Its Main Constituents
Abstract
1. Introduction
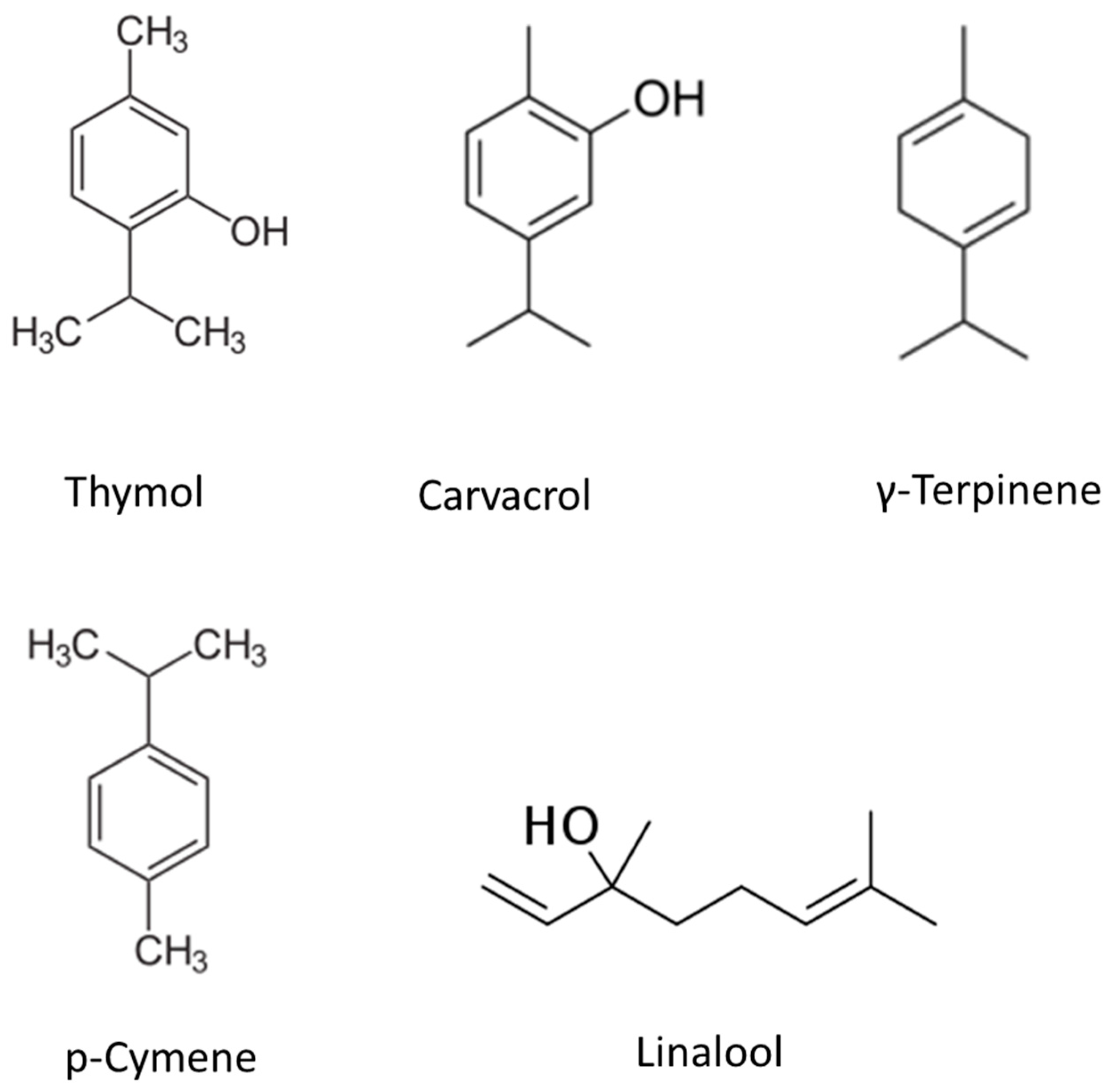
2. Thymol
3. Carvacrol
4. P-Cymene
5. γ-Terpinene
6. Linalool
7. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jalas, J. Notes on Thymus L. (Labiateae) in Europe. Bot. J. Linn. Soc. 1971, 64, 199–215. [Google Scholar]
- Schauer, T. A Field Guide to the Wild Flowers of Britain and Europe; Collins: London, UK, 1978. [Google Scholar]
- Pandur, E.; Micalizzi, G.; Mondello, L.; Horváth, A.; Sipos, K.; Horváth, G. Antioxidant and Anti-Inflammatory Effects of Thyme (Thymus vulgaris L.) Essential Oils Prepared at Different Plant Phenophases on Pseudomonas aeruginosa LPS-Activated THP-1 Macrophages. Antioxidants 2022, 11, 1330. [Google Scholar] [CrossRef] [PubMed]
- Panagiotopoulos, A.; Tseliou, M.; Karakasiliotis, I.; Kotzampasi, D.M.; Daskalakis, V.; Kesesidis, N.; Notas, G.; Lionis, C.; Kampa, M.; Pirintsos, S.; et al. p-cymene impairs SARS-CoV-2 and Influenza A (H1N1) viral replication: In silico predicted interaction with SARS-CoV-2 nucleocapsid protein and H1N1 nucleoprotein. Pharmacol. Res. Perspect. 2021, 9, e00798. [Google Scholar] [CrossRef] [PubMed]
- Pujante-Galián, M.A.; Pérez, S.A.; Montalbán, M.G.; Carissimi, G.; Fuster, M.G.; Víllora, G.; García, G. p-Cymene Complexes of Ruthenium (II) as Antitumor Agents. Molecules 2020, 25, 5063. [Google Scholar] [CrossRef] [PubMed]
- Cao, X.L.; Sparling, M.; Dabeka, R. p-Cymene, a natural antioxidant, in Canadian total diet foods: Occurrence and dietary exposures. J. Sci. Food Agric. 2019, 99, 5606–5609. [Google Scholar] [CrossRef] [PubMed]
- Salimi, A.; Khodaparast, F.; Bohlooli, S.; Hashemidanesh, N.; Baghal, E.; Rezagholizadeh, L. Linalool reverses benzene-induced cytotoxicity, oxidative stress and lysosomal/mitochondrial damages in human lymphocytes. Drug Chem. Toxicol. 2022, 45, 2454–2462. [Google Scholar] [CrossRef]
- Lam, N.S.; Long, X.; Su, X.Z.; Lu, F. Melaleuca alternifolia (tea tree) oil and its monoterpene constituents in treating protozoan and helminthic infections. Biomed. Pharmacother. 2020, 130, 110624. [Google Scholar] [CrossRef]
- Demirci, F.; Karaca, N.; Tekin, M.; Demirci, B. Anti-inflammatory and antibacterial evaluation of Thymus sipyleus Boiss. subsp. sipyleus var. sipyleus essential oil against rhinosinusitis pathogens. Microb. Pathog. 2018, 122, 117–121. [Google Scholar] [CrossRef]
- Zhang, J.; Ma, S.; Du, S.; Chen, S.; Sun, H. Antifungal activity of thymol and carvacrol against postharvest pathogens Botrytis cinerea. J. Food Sci. Technol. 2019, 56, 2611–2620. [Google Scholar] [CrossRef]
- Dahham, S.S.; Tabana, Y.; Asif, M.; Ahmed, M.; Babu, D.; Hassan, L.E.; Ahamed, M.B.K.; Sandai, D.; Barakat, K.; Siraki, A.; et al. β-Caryophyllene Induces Apoptosis and Inhibits Angiogenesis in Colorectal Cancer Models. Int. J. Mol. Sci. 2021, 22, 10550. [Google Scholar] [CrossRef]
- Woo, H.J.; Yang, J.Y.; Lee, M.H.; Kim, H.W.; Kwon, H.J.; Park, M.; Kim, S.K.; Park, S.Y.; Kim, S.H.; Kim, J.B. Inhibitory Effects of β-Caryophyllene on Helicobacter pylori Infection In Vitro and In Vivo. Int. J. Mol. Sci. 2020, 21, 1008. [Google Scholar] [CrossRef] [PubMed]
- Elbe, H.; Ozturk, F.; Yigitturk, G.; Baygar, T.; Cavusoglu, T. Anticancer activity of linalool: Comparative investigation of ultrastructural changes and apoptosis in breast cancer cells. Ultrastruct. Pathol. 2022, 46, 348–358. [Google Scholar] [CrossRef] [PubMed]
- Casiglia, S.; Bruno, M.; Scandolera, E.; Senatore, F.; Senatore, F. Influence of harvesting time on composition of the essential oil of Thymus capitatus (L.) Hoffmanns. & Link. growing wild in northern Sicily and its activity on microorganisms affecting historical art crafts. Arab. J. Chem. 2019, 12, 2704–2712. [Google Scholar]
- Kryvtsova, M.; Hrytsyna, M.; Salamon, I.; Skybitska, M.; Novykevuch, O. Chemotypes of Species of the Genus Thymus L. in Carpathians Region of Ukraine—Their Essential Oil Qualitative and Quantitative Characteristics and Antimicrobial Activity. Horticulturae 2022, 8, 1218. [Google Scholar] [CrossRef]
- Trendafilova, A.; Todorova, M.; Ivanova, V.; Zhelev, P.; Aneva, I. Essential Oil Composition of Five Thymus Species from Bulgaria. Chem. Biodivers. 2021, 18, e2100498. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.H.; Lee, J.C.; Choi, Y.H. Essential oils of Thymus quinquecostatus celakov. and Thymus magnus Nakai. Korean J. Med. Crop Sci. 1994, 2, 234–240. [Google Scholar]
- Wang, Q.; Cheng, F.; Xu, Y.; Zhang, J.; Qi, J.; Liu, X.; Wang, R. Thymol alleviates lipopolysaccharide-stimulated inflammatory response via downregulation of RhoA-mediated NF-κB signalling pathway in human peritoneal mesothelial cells. Eur. J. Pharmacol. 2018, 833, 210–220. [Google Scholar] [CrossRef]
- Kwon, H.I.; Jeong, N.H.; Kim, S.Y.; Kim, M.H.; Son, J.H.; Jun, S.H.; Kim, S.; Jeon, H.; Kang, S.C.; Kim, S.H.; et al. Inhibitory effects of thymol on the cytotoxicity and inflammatory responses induced by Staphylococcus aureus extracellular vesicles in cultured keratinocytes. Microb. Pathog. 2019, 134, 103603. [Google Scholar] [CrossRef]
- Sköld, K.; Twetman, S.; Hallgren, A.; Yucel-Lindberg, T.; Modéer, T. Effect of a chlorhexidine/thymol-containing varnish on prostaglandin E2 levels in gingival crevicular fluid. Eur. J. Oral. Sci. 1998, 106, 571–575. [Google Scholar] [CrossRef]
- Khosravi, A.R.; Erle, D.J. Chitin-Induced Airway Epithelial Cell Innate Immune Responses are Inhibited by Carvacrol/Thymol. PLoS ONE 2016, 11, e0159459. [Google Scholar] [CrossRef]
- Qoorchi Moheb Seraj, F.; Heravi-Faz, N.; Soltani, A.; Ahmadi, S.S.; Shahbeiki, F.; Talebpour, A.; Afshari, A.R.; Ferns, G.A.; Bahrami, A. Thymol has anticancer effects in U-87 human malignant glioblastoma cells. Mol. Biol. Rep. 2022, 49, 9623–9632. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wen, J.M.; Du, C.J.; Hu, S.M.; Chen, J.X.; Zhang, S.G.; Zhang, N.; Gao, F.; Li, S.J.; Mao, X.W.; et al. Thymol inhibits bladder cancer cell proliferation via inducing cell cycle arrest and apoptosis. Biochem. Biophys. Res. Commun. 2017, 491, 530–536. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Q.; Che, Y.; Zhang, Y.; Chen, M.; Guo, Q.; Zhang, W. Thymol Isolated from Thymus vulgaris L. Inhibits Colorectal Cancer Cell Growth and Metastasis by Suppressing the Wnt/β-Catenin Pathway. Drug Des. Devel. Ther. 2020, 14, 2535–2547. [Google Scholar] [CrossRef] [PubMed]
- Deb, D.D.; Parimala, G.; Saravana Devi, S.; Chakraborty, T. Effect of thymol on peripheral blood mononuclear cell PBMC and acute promyelotic cancer cell line HL-60. Chem. Biol. Interact. 2011, 193, 97–106. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.T.; Khan, M.; Ahmad, J.; Wahab, R.; Abd-Elkader, O.H.; Musarrat, J.; Alkhathlan, H.Z.; Al-Kedhairy, A.A. Thymol and carvacrol induce autolysis, stress, growth inhibition and reduce the biofilm formation by Streptococcus mutans. AMB Express 2017, 7, 49. [Google Scholar] [CrossRef]
- Kowalczyk, A.; Przychodna, M.; Sopata, S.; Bodalska, A.; Fecka, I. Thymol and Thyme Essential Oil-New Insights into Selected Therapeutic Applications. Molecules 2020, 25, 4125. [Google Scholar] [CrossRef] [PubMed]
- Thosar, N.; Basak, S.; Bahadure, R.N.; Rajurkar, M. Antimicrobial efficacy of five essential oils against oral pathogens: An in vitro study. Eur. J. Dent. 2013, 7, S071–S077. [Google Scholar] [CrossRef]
- Perez, A.P.; Perez, N.; Lozano, C.M.S.; Altube, M.J.; de Farias, M.A.; Portugal, R.V.; Buzzola, F.; Morilla, M.J.; Romero, E.L. The anti MRSA biofilm activity of Thymus vulgaris essential oil in nanovesicles. Phytomedicine 2019, 57, 339–351. [Google Scholar] [CrossRef]
- Kryvtsova, M.V.; Salamon, I.; Koscova, J.; Bucko, D.; Spivak, M. Antimicrobial, antibiofilm and biochemichal properties of Thymus vulgaris essential oil against clinical isolates of opportunistic infections. Biosyst. Divers. 2019, 27, 270–275. [Google Scholar] [CrossRef]
- Tohidpour, A.; Sattari, M.; Omidbaigi, R.; Yadegar, A.; Nazemi, J. Antibacterial effect of essential oils from two medicinal plants against Methicillin-resistant Staphylococcus aureus (MRSA). Phytomedicine 2010, 17, 142–145. [Google Scholar] [CrossRef]
- Kumari, P.; Mishra, R.; Arora, N.; Chatrath, A.; Gangwar, R.; Roy, P.; Prasad, R. Antifungal and Anti-Biofilm Activity of Essential Oil Active Components against Cryptococcus neoformans and Cryptococcus laurentii. Front. Microbiol. 2017, 8, 2161. [Google Scholar] [CrossRef] [PubMed]
- Liggri, P.G.V.; Tsitsanou, K.E.; Stamati, E.C.V.; Saitta, F.; Drakou, C.E.; Leonidas, D.D.; Fessas, D.; Zographos, S.E. The structure of AgamOBP5 in complex with the natural insect repellents Carvacrol and Thymol: Crystallographic, fluorescence and thermodynamic binding studies. Int. J. Biol. Macromol. 2023, 237, 124009. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, J.C.; de Meneses, D.A.; de Vasconcelos, A.P.; Piauilino, C.A.; Almeida, F.R.; Napoli, E.M.; Ruberto, G.; de Araújo, D.A. Essential oil composition and antinociceptive activity of Thymus capitatus. Pharm. Biol. 2017, 55, 782–786. [Google Scholar] [CrossRef]
- Daldal, H.; Nazıroğlu, M. Carvacrol protects the ARPE19 retinal pigment epithelial cells against high glucose-induced oxidative stress, apoptosis, and inflammation by suppressing the TRPM2 channel signaling pathways. Graefe’s Arch. Clin. Exp. Ophthalmol. 2022, 260, 2567–2583. [Google Scholar] [CrossRef]
- Wijesundara, N.M.; Lee, S.F.; Davidson, R.; Cheng, Z.; Rupasinghe, H.P.V. Carvacrol Suppresses Inflammatory Biomarkers Production by Lipoteichoic Acid- and Peptidoglycan-Stimulated Human Tonsil Epithelial Cells. Nutrients 2022, 14, 503. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Xu, X.; Wu, R.; Bi, L.; Zhang, C.; Chen, H.; Yang, Y. Antioral Squamous Cell Carcinoma Effects of Carvacrol via Inhibiting Inflammation, Proliferation, and Migration Related to Nrf2/Keap1 Pathway. Biomed. Res. Int. 2021, 2021, 6616547. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; He, L.; Shang, J.; Chen, L.; Xu, Y.; Chen, X.; Li, X.; Jiao, Q.; Jin, S.; Hu, X.; et al. Carvacrol Suppresses Human Osteosarcoma Cells via the Wnt/β-Catenin Signaling Pathway. Anti-Cancer Agents Med. Chem. 2022, 22, 1714–1722. [Google Scholar] [CrossRef]
- Li, L.; He, L.; Wu, Y.; Zhang, Y. Carvacrol affects breast cancer cells through TRPM7 mediated cell cycle regulation. Life Sci. 2021, 266, 118894. [Google Scholar] [CrossRef]
- Alanazi, R.; Nakatogawa, H.; Wang, H.; Ji, D.; Luo, Z.; Golbourn, B.; Feng, Z.P.; Rutka, J.T.; Sun, H.S. Inhibition of TRPM7 with carvacrol suppresses glioblastoma functions in vivo. Eur. J. Neurosci. 2022, 55, 1483–1491. [Google Scholar] [CrossRef]
- Nazıroğlu, M. A novel antagonist of TRPM2 and TRPV4 channels: Carvacrol. Metab. Brain Dis. 2022, 37, 711–728. [Google Scholar] [CrossRef]
- Celik Topkara, K.; Kilinc, E.; Cetinkaya, A.; Saylan, A.; Demir, S. Therapeutic effects of carvacrol on beta-amyloid-induced impairments in in vitro and in vivo models of Alzheimer’s disease. Eur. J. Neurosci. 2022, 56, 5714–5726. [Google Scholar] [CrossRef] [PubMed]
- Ghorani, V.; Alavinezhad, A.; Rajabi, O.; Boskabady, M.H. Carvacrol improves pulmonary function tests, oxidant/antioxidant parameters and cytokine levels in asthmatic patients: A randomized, double-blind, clinical trial. Phytomedicine 2021, 85, 153539. [Google Scholar] [CrossRef]
- Soković, M.; Glamočlija, J.; Marin, P.D.; Brkić, D.; van Griensven, L.J. Antibacterial effects of the essential oils of commonly consumed medicinal herbs using an in vitro model. Molecules 2010, 15, 7532–7546. [Google Scholar] [CrossRef] [PubMed]
- Gedikoğlu, A.; Sökmen, M.; Çivit, A. Evaluation of Thymus vulgaris and Thymbra spicata essential oils and plant extracts for chemical composition, antioxidant, and antimicrobial properties. Food Sci. Nutr. 2019, 7, 1704–1714. [Google Scholar] [CrossRef]
- Magi, G.; Marini, E.; Facinelli, B. Antimicrobial activity of essential oils and carvacrol, and synergy of carvacrol and erythromycin, against clinical, erythromycin-resistant Group A Streptococci. Front. Microbiol. 2015, 6, 165. [Google Scholar] [CrossRef] [PubMed]
- Wijesundara, N.M.; Lee, S.F.; Cheng, Z.; Davidson, R.; Rupasinghe, H.P.V. Carvacrol exhibits rapid bactericidal activity against Streptococcus pyogenes through cell membrane damage. Sci. Rep. 2021, 11, 1487. [Google Scholar] [CrossRef] [PubMed]
- Boukhatem, M.N.; Darwish, N.H.E.; Sudha, T.; Bahlouli, S.; Kellou, D.; Benelmouffok, A.B.; Chader, H.; Rajabi, M.; Benali, Y.; Mousa, S.A. In Vitro Antifungal and Topical Anti-Inflammatory Properties of Essential Oil from Wild-Growing Thymus vulgaris (Lamiaceae) Used for Medicinal Purposes in Algeria: A New Source of Carvacrol. Sci. Pharm. 2020, 88, 33. [Google Scholar] [CrossRef]
- Satyal, P.; Murray, B.L.; McFeeters, R.L.; Setzer, W.N. Essential Oil Characterization of Thymus vulgaris from Various Geographical Locations. Foods 2016, 5, 70. [Google Scholar] [CrossRef]
- Nazzaro, F.; Fratianni, F.; Coppola, R.; Feo, V. Essential Oils and Antifungal Activity. Pharmaceuticals 2017, 10, 86. [Google Scholar] [CrossRef]
- Giordani, R.; Regli, P.; Kaloustian, J.; Mikaïl, C.; Abou, L.; Portugal, H. Antifungal effect of various essential oils against Candidaalbicans. Potentiation of antifungal action of amphotericin B by essential oil from Thymus vulgaris. Phytother. Res. 2004, 18, 990–995. [Google Scholar] [CrossRef]
- Pina-Vaz, C.; Gonçalves Rodrigues, A.; Pinto, E.; Costa-de-Oliveira, S.; Tavares, C.; Salgueiro, L.; Cavaleiro, C.; Gonçalves, M.J.; Martinez-de-Oliveira, J. Antifungal activity of Thymus oils and their major compounds. J. Eur. Acad. Dermatol. Venereol. 2004, 18, 73–78. [Google Scholar] [CrossRef] [PubMed]
- Pinto, E.; Pina-Vaz, C.; Salgueiro, L.; Gonçalves, M.J.; Costa-de-Oliveira, S.; Cavaleiro, C.; Palmeira, A.; Rodrigues, A.; Martinez-de-Oliveira, J. Antifungal activity of the essential oil of Thymus pulegioides on Candida, Aspergillus and dermatophyte species. J. Med. Microbiol. 2006, 55, 1367–1373. [Google Scholar] [CrossRef] [PubMed]
- Baser, K.H. Biological and pharmacological activities of carvacrol and carvacrol bearing essential oils. Curr. Pharm. Des. 2008, 14, 3106–3119. [Google Scholar] [CrossRef] [PubMed]
- Bouyahya, A.; Abrini, J.; Dakka, N.; Bakri, Y. Essential oils of Origanum compactum increase membrane permeability, disturb cell membrane integrity, and suppress quorum-sensing phenotype in bacteria. J. Pharm. Anal. 2019, 9, 301–311. [Google Scholar] [CrossRef]
- Altun, M.; Yapici, B.M. Determination of chemical compositions and antibacterial effects of selected essential oils against human pathogenic strains. An. Acad. Bras. Ciências 2022, 94, e20210074. [Google Scholar] [CrossRef] [PubMed]
- Ose, R.; Tu, J.; Schink, A.; Maxeiner, J.; Schuster, P.; Lucas, K.; Saloga, J.; Bellinghausen, I. Cinnamon extract inhibits allergen-specific immune responses in human and murine allergy models. Clin. Exp. Allergy 2020, 50, 41–50. [Google Scholar] [CrossRef] [PubMed]
- Schink, A.; Naumoska, K.; Kitanovski, Z.; Kampf, C.J.; Fröhlich-Nowoisky, J.; Thines, E.; Pöschl, U.; Schuppan, D.; Lucas, K. Anti-inflammatory effects of cinnamon extract and identification of active compounds influencing the TLR2 and TLR4 signaling pathways. Food Funct. 2018, 9, 5950–5964. [Google Scholar] [CrossRef]
- Formiga, R.O.; Alves Júnior, E.B.; Vasconcelos, R.C.; Guerra, G.C.B.; Antunes de Araújo, A.; Carvalho, T.G.; Garcia, V.B.; de Araújo Junior, R.F.; Gadelha, F.; Vieira, G.C.; et al. p-Cymene and Rosmarinic Acid Ameliorate TNBS-Induced Intestinal Inflammation Upkeeping ZO-1 and MUC-2: Role of Antioxidant System and Immunomodulation. Int. J. Mol. Sci. 2020, 21, 5870. [Google Scholar] [CrossRef]
- Jin, H.; Leng, Q.; Zhang, C.; Zhu, Y.; Wang, J. P-cymene prevent high-fat diet-associated colorectal cancer by improving the structure of intestinal flora. J. Cancer 2021, 12, 4355–4361. [Google Scholar] [CrossRef]
- Wang, S.; Wang, X.; Wang, Y.U.; Leng, Q.; Sun, Y.U.; Hoffman, R.M.; Jin, H. The Anti-oxidant Monoterpene p-Cymene Reduced the Occurrence of Colorectal Cancer in a Hyperlipidemia Rat Model by Reducing Oxidative Stress and Expression of Inflammatory Cytokines. Anticancer Res. 2021, 41, 1213–1218. [Google Scholar] [CrossRef]
- Santos, W.B.R.; Melo, M.A.O.; Alves, R.S.; de Brito, R.G.; Rabelo, T.K.; Prado, L.D.S.; Silva, V.; Bezerra, D.P.; de Menezes-Filho, J.E.R.; Souza, D.S.; et al. p-Cymene attenuates cancer pain via inhibitory pathways and modulation of calcium currents. Phytomedicine 2019, 61, 152836. [Google Scholar] [CrossRef] [PubMed]
- Rudolph, K.; Parthier, C.; Egerer-Sieber, C.; Geiger, D.; Muller, Y.A.; Kreis, W.; Müller-Uri, F. Expression, crystallization and structure elucidation of γ-terpinene synthase from Thymus vulgaris. Acta Crystallogr. Sect. F Struct. Biol. Commun. 2016, 72, 16–23. [Google Scholar] [CrossRef]
- Sousa, L.G.V.; Castro, J.; Cavaleiro, C.; Salgueiro, L.; Tomás, M.; Palmeira-Oliveira, R.; Martinez-Oliveira, J.; Cerca, N. Synergistic effects of carvacrol, α-terpinene, γ-terpinene, ρ-cymene and linalool against Gardnerella species. Sci. Rep. 2022, 12, 4417. [Google Scholar] [CrossRef]
- Ramalho, T.R.; Filgueiras, L.R.; Pacheco de Oliveira, M.T.; Lima, A.L.; Bezerra-Santos, C.R.; Jancar, S.; Piuvezam, M.R. Gamma-Terpinene Modulation of LPS-Stimulated Macrophages is Dependent on the PGE2/IL-10 Axis. Planta Med. 2016, 82, 1341–1345. [Google Scholar] [CrossRef] [PubMed]
- National Center for Biotechnology Information. PubChem Compound Summary for CID 6549, L.R.M. 2023. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Linalool (accessed on 19 March 2023).
- Letizia, C.S.; Cocchiara, J.; Lalko, J.; Api, A.M. Fragrance material review on linalool. Food Chem. Toxicol. 2003, 41, 943–964. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Xu, H.; Wu, J.; Qu, C.; Sun, F.; Xu, S. Linalool inhibits cigarette smoke-induced lung inflammation by inhibiting NF-κB activation. Int. Immunopharmacol. 2015, 29, 708–713. [Google Scholar] [CrossRef]
- Huo, M.; Cui, X.; Xue, J.; Chi, G.; Gao, R.; Deng, X.; Guan, S.; Wei, J.; Soromou, L.W.; Feng, H.; et al. Anti-inflammatory effects of linalool in RAW 264.7 macrophages and lipopolysaccharide-induced lung injury model. J. Surg. Res. 2013, 180, e47–e54. [Google Scholar] [CrossRef]
- Sabogal-Guáqueta, A.M.; Osorio, E.; Cardona-Gómez, G.P. Linalool reverses neuropathological and behavioral impairments in old triple transgenic Alzheimer’s mice. Neuropharmacology 2016, 102, 111–120. [Google Scholar] [CrossRef]
- Kim, M.G.; Kim, S.M.; Min, J.H.; Kwon, O.K.; Park, M.H.; Park, J.W.; Ahn, H.I.; Hwang, J.Y.; Oh, S.R.; Lee, J.W.; et al. Anti-inflammatory effects of linalool on ovalbumin-induced pulmonary inflammation. Int. Immunopharmacol. 2019, 74, 105706. [Google Scholar] [CrossRef]
- Altinoz, E.; Oner, Z.; Elbe, H.; Uremis, N.; Uremis, M. Linalool exhibits therapeutic and protective effects in a rat model of doxorubicin-induced kidney injury by modulating oxidative stress. Drug Chem. Toxicol. 2022, 45, 2024–2030. [Google Scholar] [CrossRef]
- Gunaseelan, S.; Balupillai, A.; Govindasamy, K.; Muthusamy, G.; Ramasamy, K.; Shanmugam, M.; Prasad, N.R. The preventive effect of linalool on acute and chronic UVB-mediated skin carcinogenesis in Swiss albino mice. Photochem. Photobiol. Sci. 2016, 15, 851–860. [Google Scholar] [CrossRef] [PubMed]
- Sabogal-Guáqueta, A.M.; Hobbie, F.; Keerthi, A.; Oun, A.; Kortholt, A.; Boddeke, E.; Dolga, A. Linalool attenuates oxidative stress and mitochondrial dysfunction mediated by glutamate and NMDA toxicity. Biomed. Pharmacother. 2019, 118, 109295. [Google Scholar] [CrossRef] [PubMed]

| Thymus spp. (T.) | Thymol (%) | Carvacrol (%) | Linalool (%) | γ-Terpinene (%) | P-Cymene (%) | Reference |
|---|---|---|---|---|---|---|
| T. capitatus (L.) Hofmgg. Link | 8–61 | 14.2–81.2 | 0–0.4 | 2.6–33.4 | 5–22.8 | [14] |
| T. serpyllum | 0.6–0.8 | 6–20 | 0.4–63 | 2.5–4.0 | 2–9.1 | [15] |
| T. pulegoides | 0.2–2.2 | 9.5–18 | 0.6–13 | 1.5–8 | 1.6–6 | [15] |
| T. marschtallianus | 2–2.2 | 6.5–9 | 3.5–4.5 | 4.0 | 1.0 | [15] |
| T. vulgaris L. | 54.2–55.8 | 2.3–2.9 | 1.46–2.1 | 0.82–1.4 | 12.89–20.6 | [3] |
| T. atticus | 0.7 | 0.3 | 1.0 | Trace | 0.2 | [16] |
| T. leucotrichus | 2.7 | 0.6 | 1.8 | Trace | 0.2 | [16] |
| T. striatus | 1.9 | 4.3 | 0.9 | Trace | 0.2 | [16] |
| T. perinicus | 20.9 | 1.1 | 4.6 | 0.5 | 4.8 | [16] |
| T. zygioides | 51.2 | 2.9 | 0.1 | 1.1 | 6.5 | [16] |
| T. quinquecostatus Celak. | 39.8 | 2.6 | 0.1 | 10 | 9.2 | [16] |
| T. magnus Nakai | 54.7 | 3.2 | Trace | 6.4–15.0 | 3.5–6.7 | [17] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vassiliou, E.; Awoleye, O.; Davis, A.; Mishra, S. Anti-Inflammatory and Antimicrobial Properties of Thyme Oil and Its Main Constituents. Int. J. Mol. Sci. 2023, 24, 6936. https://doi.org/10.3390/ijms24086936
Vassiliou E, Awoleye O, Davis A, Mishra S. Anti-Inflammatory and Antimicrobial Properties of Thyme Oil and Its Main Constituents. International Journal of Molecular Sciences. 2023; 24(8):6936. https://doi.org/10.3390/ijms24086936
Chicago/Turabian StyleVassiliou, Evros, Oreoluwa Awoleye, Amanda Davis, and Sasmita Mishra. 2023. "Anti-Inflammatory and Antimicrobial Properties of Thyme Oil and Its Main Constituents" International Journal of Molecular Sciences 24, no. 8: 6936. https://doi.org/10.3390/ijms24086936
APA StyleVassiliou, E., Awoleye, O., Davis, A., & Mishra, S. (2023). Anti-Inflammatory and Antimicrobial Properties of Thyme Oil and Its Main Constituents. International Journal of Molecular Sciences, 24(8), 6936. https://doi.org/10.3390/ijms24086936







