Relationship between Plasma Lipid Profile and Cognitive Status in Early Alzheimer Disease
Abstract
:1. Introduction
2. Results
2.1. Participants Description
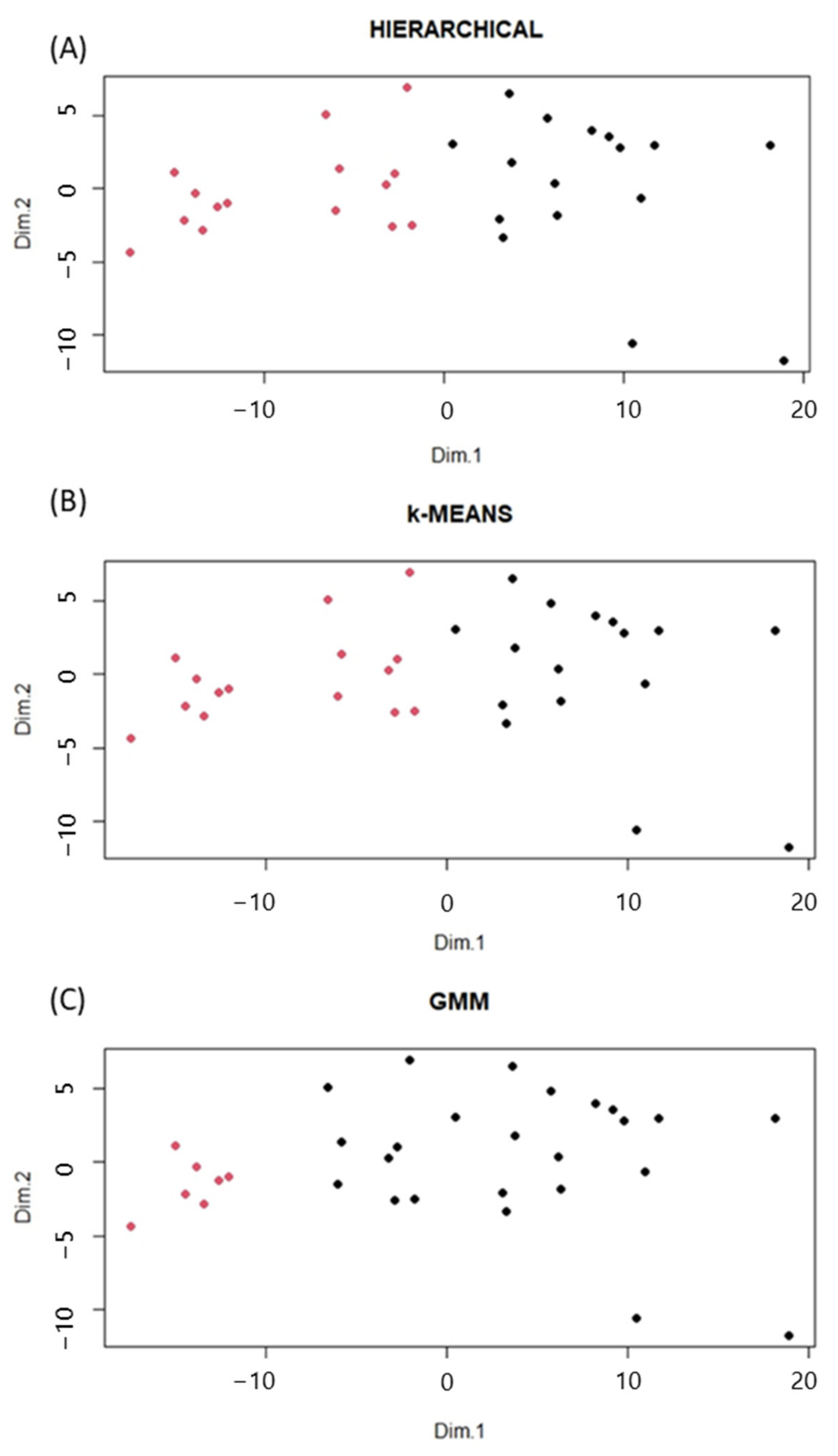
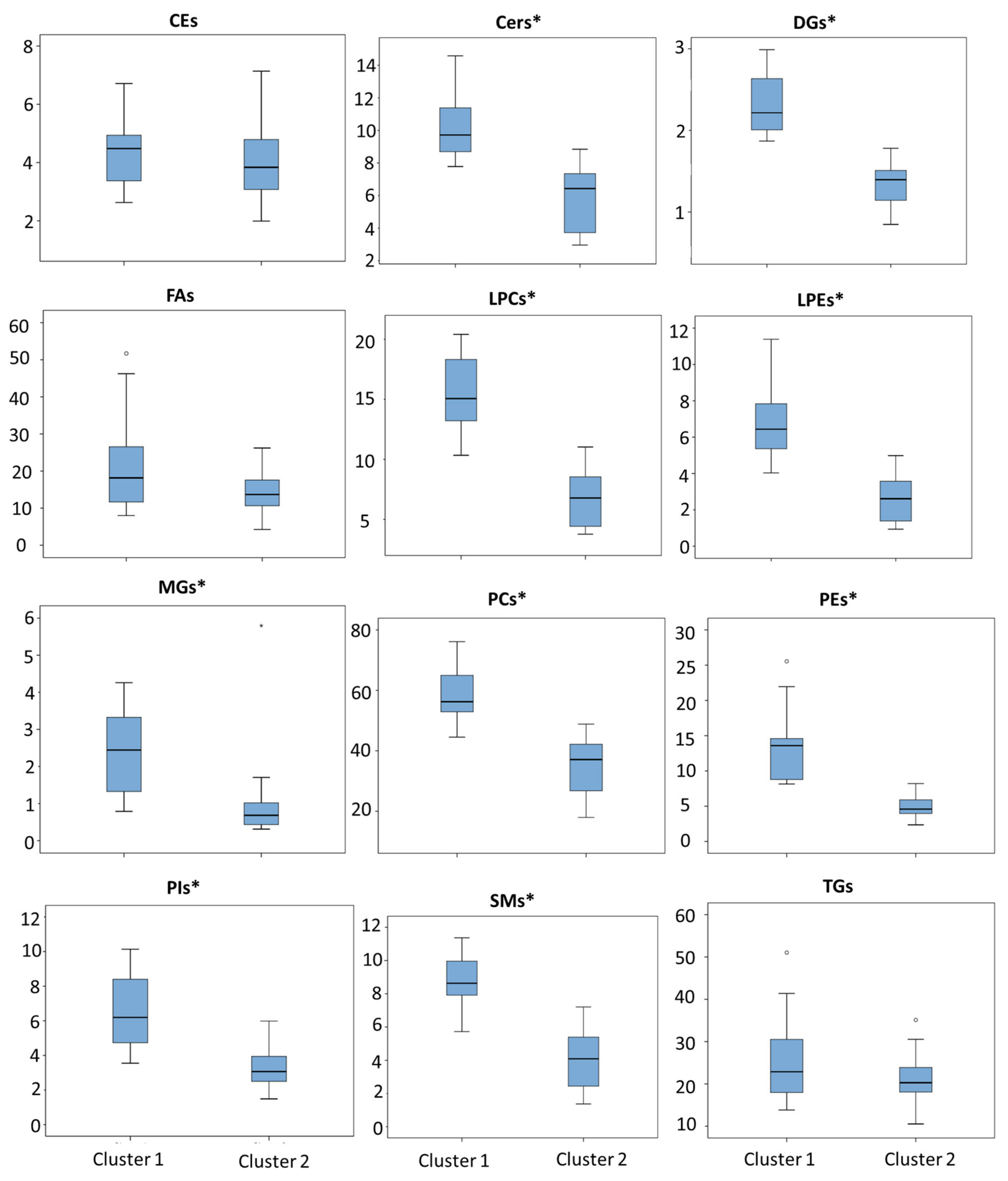
2.2. Clustering Analysis and Lipidomic Profile
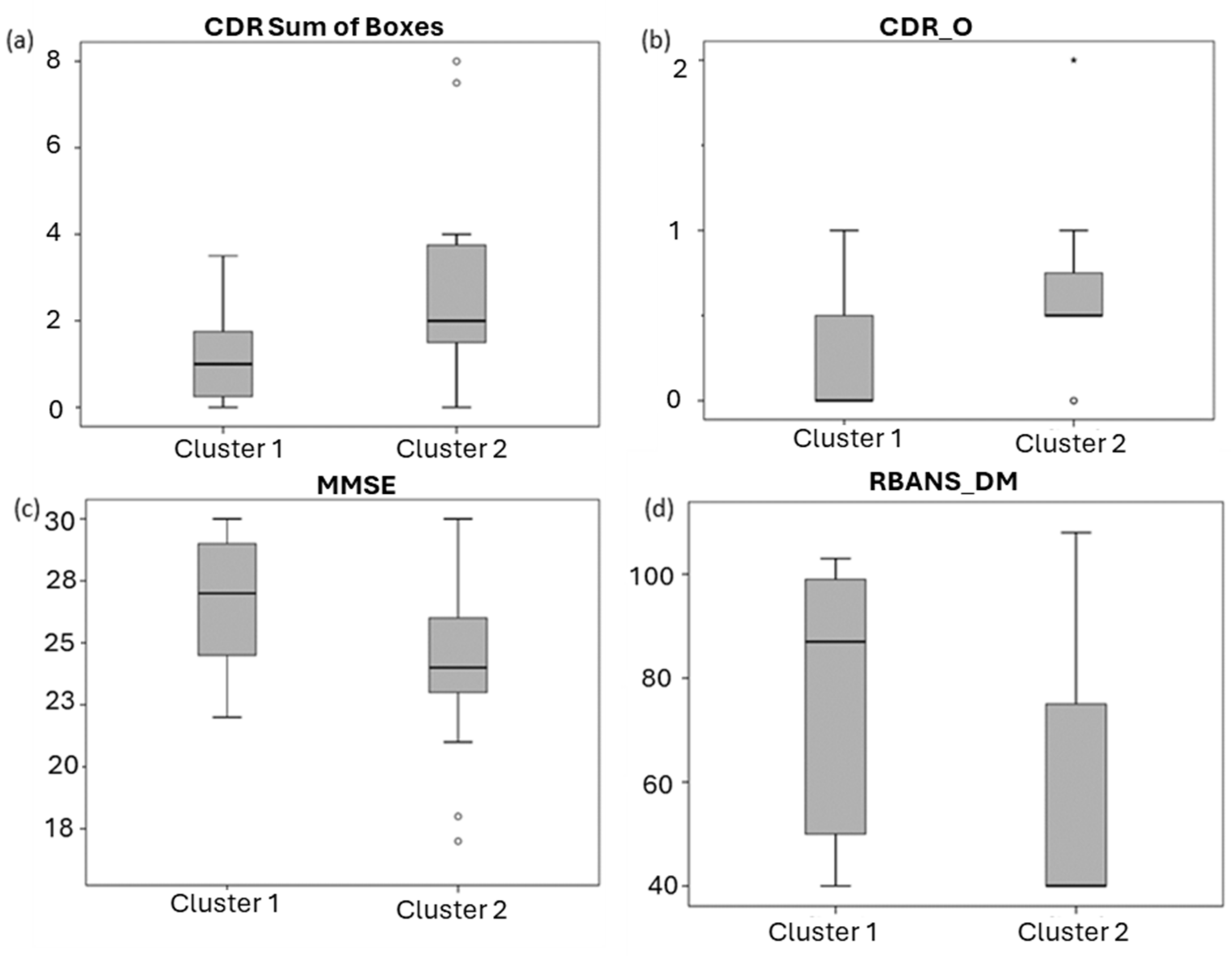
2.3. Clinical Significance of Lipid Profile
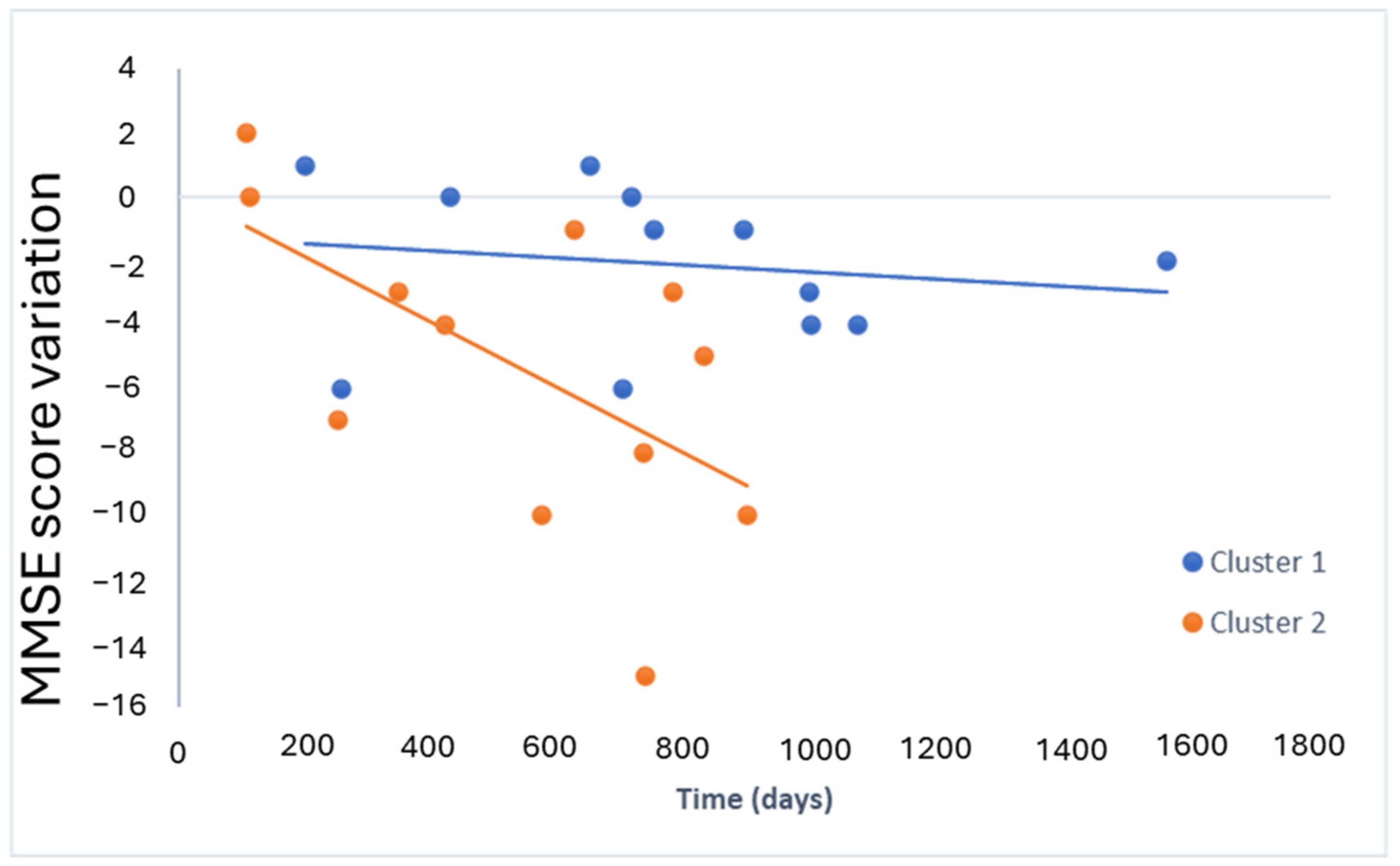
2.4. Lipid Profile for Progression
3. Discussion
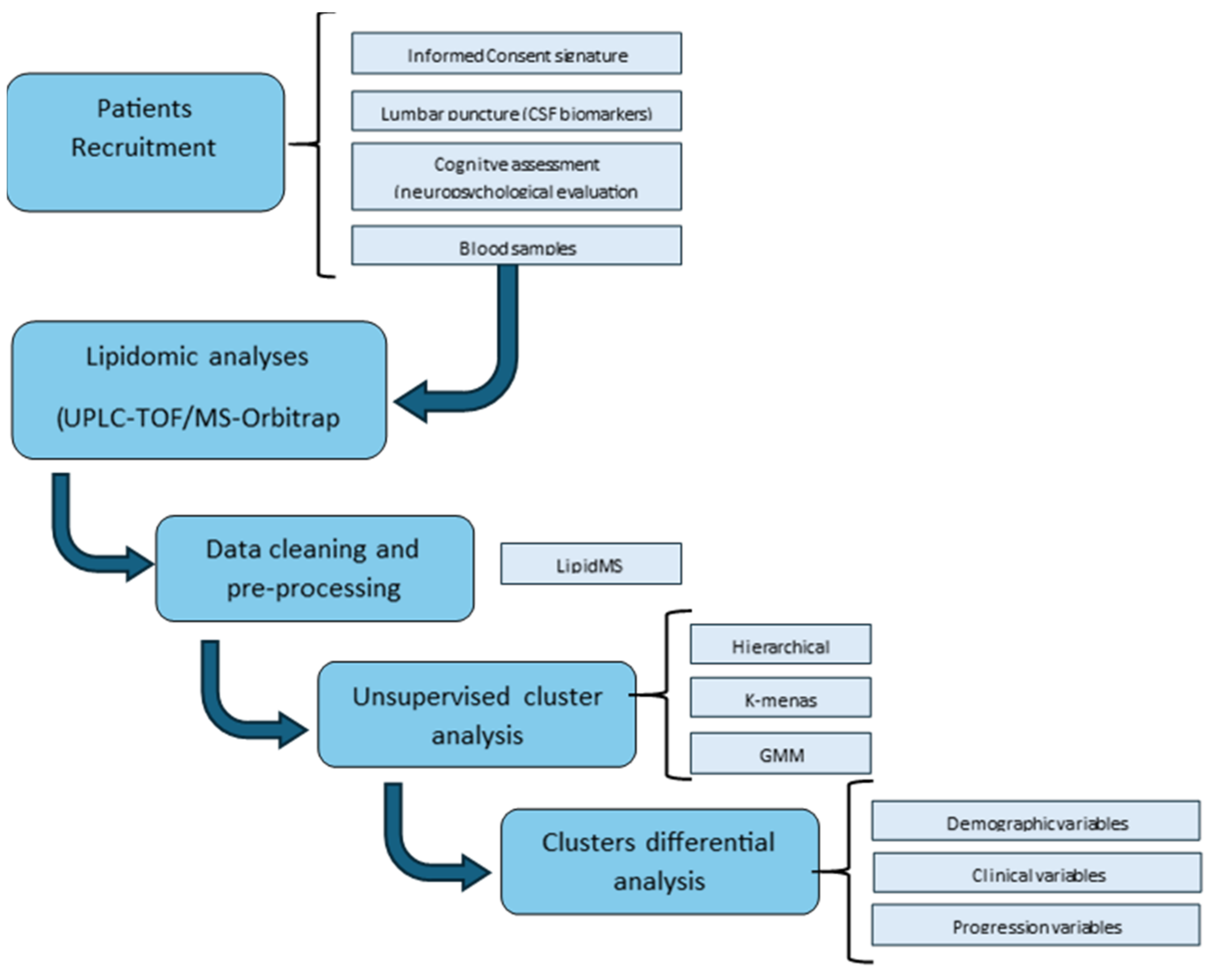
4. Materials and Methods
4.1. Participants and Sample Collection
4.2. Lipidomic Analysis
4.3. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| Aβ42 | Amyloid β42 |
| AD | Alzheimer disease |
| ADCS-MCI-ADL | Alzheimer’s Disease Cooperative Study-Activities of Daily Living for Mild Cognitive Impairment |
| ApoE | Apolipoprotein E |
| APP | Amyloid precursor peptide |
| BACE1 | β-Secretase 1 |
| CDR | Clinical Dementia Rating |
| CE | Cholesterol ester |
| Cer | Ceramide |
| CSF | Cerebrospinal fluid |
| CEIC | Ethics Committee |
| DG | diglycerols |
| DHCer | dihydroceramides |
| DHSM | dihydrosphingomyelin |
| FA | fatty acids |
| FAQ | Functionality Assessment Questionnaire |
| GMM | Gaussian Mixture model |
| HDL | high-density lipoprotein |
| LDL | density lipoprotein |
| LPC | Lysophosphatidylcholines |
| LPE | Lysophosphatidylethanolamines |
| MCI | Mild cognitive impairment |
| MG | Monoglycerides |
| MMSE | Mini-Mental State Examination |
| MTA | Medial temporal lobe atrophy |
| NIA-AA | National Institute on Aging and the Alzheimer’s Association |
| NfL | Neurofilament light chain |
| PC | phosphatidylcholines |
| PE | phosphatidylethanolamines |
| PI | phosphatidylinositols |
| PIsEs | Ethanolamine plasmalogens |
| PSEN | presenilin |
| p-Tau | phosphorylated Tau |
| RBANS | Repeatable Battery for the Assessment of Neuropsychological Status |
| SM | sphingomyelins |
| TG | triglycerides |
| TREM2 | Triggering receptor expressed on myeloid cells 2 |
| t-Tau | total Tau |
| UPLC-TOF/MS | ultra-performance liquid chromatography coupled to time-of-flight mass spectrometry |
References
- Aisen, P.S.; Cummings, J.; Jack, C.R.; Morris, J.C.; Sperling, R.; Frölich, L.; Jones, R.W.; Dowsett, S.A.; Matthews, B.R.; Raskin, J.; et al. On the Path to 2025: Understanding the Alzheimer’s Disease Continuum. Alzheimers Res. Ther. 2017, 9, 60. [Google Scholar] [CrossRef] [PubMed]
- Duara, R.; Barker, W. Heterogeneity in Alzheimer’s Disease Diagnosis and Progression Rates: Implications for Therapeutic Trials. Neurotherapeutics 2022, 19, 8–25. [Google Scholar] [CrossRef]
- Ferrari, C.; Sorbi, S. The Complexity of Alzheimer’s Disease: An Evolving Puzzle. Physiol. Rev. 2021, 101, 1047–1081. [Google Scholar] [CrossRef]
- Petersen, R.C. Clinical Subtypes of Alzheimer’s Disease. Dement. Geriatr. Cogn. Disord. 1998, 9, 16–24. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, D.; Nordberg, A.; Westman, E. Biological Subtypes of Alzheimer Disease. Neurology 2020, 94, 436–448. [Google Scholar] [CrossRef] [PubMed]
- Tijms, B.M.; Gobom, J.; Reus, L.; Jansen, I.; Hong, S.; Dobricic, V.; Kilpert, F.; ten Kate, M.; Barkhof, F.; Tsolaki, M.; et al. Pathophysiological Subtypes of Alzheimer’s Disease Based on Cerebrospinal Fluid Proteomics. Brain 2020, 143, 3776–3792. [Google Scholar] [CrossRef] [PubMed]
- Neff, R.A.; Wang, M.; Vatansever, S.; Guo, L.; Ming, C.; Wang, Q.; Wang, E.; Horgusluoglu-Moloch, E.; Song, W.; Li, A.; et al. Molecular Subtyping of Alzheimer’s Disease Using RNA Sequencing Data Reveals Novel Mechanisms and Targets. Sci. Adv. 2021, 7, eabb5398. [Google Scholar] [CrossRef]
- Jellinger, K.A. Pathobiological Subtypes of Alzheimer Disease. Dement. Geriatr. Cogn. Disord. 2020, 49, 321–333. [Google Scholar] [CrossRef]
- Calabrò, M.; Rinaldi, C.; Santoro, G.; Crisafulli, C. The Biological Pathways of Alzheimer Disease: A Review. AIMS Neurosci. 2021, 8, 86–132. [Google Scholar] [CrossRef]
- Cummings, J.; Zhou, Y.; Lee, G.; Zhong, K.; Fonseca, J.; Cheng, F. Alzheimer’s Disease Drug Development Pipeline: 2023. Alzheimer’s Dement. Transl. Res. Clin. Interv. 2023, 9, e12385. [Google Scholar] [CrossRef]
- García-Viñuales, S.; Sciacca, M.F.M.; Lanza, V.; Santoro, A.M.; Grasso, G.; Tundo, G.R.; Sbardella, D.; Coletta, M.; Grasso, G.; La Rosa, C.; et al. The Interplay between Lipid and Aβ Amyloid Homeostasis in Alzheimer’s Disease: Risk Factors and Therapeutic Opportunities. Chem. Phys. Lipids 2021, 236, 105072. [Google Scholar] [CrossRef]
- Koutsodendris, N.; Nelson, M.R.; Rao, A.; Huang, Y. Apolipoprotein E and Alzheimer’s Disease: Findings, Hypotheses, and Potential Mechanisms. Annu. Rev. Pathol. Mech. Dis. 2022, 17, 73–99. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Mahley, R.W. Apolipoprotein E: Structure and Function in Lipid Metabolism, Neurobiology, and Alzheimer’s Diseases. Neurobiol. Dis. 2014, 72, 3–12. [Google Scholar] [CrossRef]
- Rudge, J.D. A New Hypothesis for Alzheimer’s Disease: The Lipid Invasion Model. J. Alzheimers Dis. Rep. 2022, 6, 129–161. [Google Scholar] [CrossRef] [PubMed]
- Kao, Y.-C.; Ho, P.-C.; Tu, Y.-K.; Jou, I.-M.; Tsai, K.-J. Lipids and Alzheimer’s Disease. Int. J. Mol. Sci. 2020, 21, 1505. [Google Scholar] [CrossRef]
- Chew, H.; Solomon, V.A.; Fonteh, A.N. Involvement of Lipids in Alzheimer’s Disease Pathology and Potential Therapies. Front. Physiol. 2020, 11, 598. [Google Scholar] [CrossRef]
- Lai, A.Y.; McLaurin, J. Mechanisms of Amyloid-Beta Peptide Uptake by Neurons: The Role of Lipid Rafts and Lipid Raft-Associated Proteins. Int. J. Alzheimers Dis. 2011, 2011, 548380. [Google Scholar] [CrossRef]
- Yin, F. Lipid Metabolism and Alzheimer’s Disease: Clinical Evidence, Mechanistic Link and Therapeutic Promise. FEBS J. 2023, 290, 1420–1453. [Google Scholar] [CrossRef] [PubMed]
- Benseny-Cases, N.; Klementieva, O.; Cotte, M.; Ferrer, I.; Cladera, J. Microspectroscopy (ΜFTIR) Reveals Co-Localization of Lipid Oxidation and Amyloid Plaques in Human Alzheimer Disease Brains. Anal. Chem. 2014, 86, 12047–12054. [Google Scholar] [CrossRef]
- Burns, M.P.; Noble, W.J.; Olm, V.; Gaynor, K.; Casey, E.; LaFrancois, J.; Wang, L.; Duff, K. Co-Localization of Cholesterol, Apolipoprotein E and Fibrillar Aβ in Amyloid Plaques. Mol. Brain Res. 2003, 110, 119–125. [Google Scholar] [CrossRef]
- Byeon, S.K.; Madugundu, A.K.; Jain, A.P.; Bhat, F.A.; Jung, J.H.; Renuse, S.; Darrow, J.; Bakker, A.; Albert, M.; Moghekar, A.; et al. Cerebrospinal Fluid Lipidomics for Biomarkers of Alzheimer’s Disease. Mol. Omics 2021, 17, 454–463. [Google Scholar] [CrossRef]
- Casas-Fernández, E.; Peña-Bautista, C.; Baquero, M.; Cháfer-Pericás, C. Lipids as Early and Minimally Invasive Biomarkers for Alzheimer’s Disease. Curr. Neuropharmacol. 2022, 20, 1613–1631. [Google Scholar] [CrossRef]
- Fiandaca, M.S.; Zhong, X.; Cheema, A.K.; Orquiza, M.H.; Chidambaram, S.; Tan, M.T.; Gresenz, C.R.; FitzGerald, K.T.; Nalls, M.A.; Singleton, A.B.; et al. Plasma 24-Metabolite Panel Predicts Preclinical Transition to Clinical Stages of Alzheimer’s Disease. Front. Neurol. 2015, 6, 237. [Google Scholar] [CrossRef]
- Ma, Y.; Shen, X.; Xu, W.; Huang, Y.; Li, H.; Tan, L.; Tan, C.; Dong, Q.; Tan, L.; Yu, J. A Panel of Blood Lipids Associated with Cognitive Performance, Brain Atrophy, and Alzheimer’s Diagnosis: A Longitudinal Study of Elders without Dementia. Alzheimer’s Dement. Diagn. Assess. Dis. Monit. 2020, 12, e12041. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Liu, W.; Zan, J.; Wu, C.; Tan, W. Untargeted Lipidomics Reveals Progression of Early Alzheimer’s Disease in APP/PS1 Transgenic Mice. Sci. Rep. 2020, 10, 14509. [Google Scholar] [CrossRef]
- Agarwal, M.; Khan, S. Plasma Lipids as Biomarkers for Alzheimer’s Disease: A Systematic Review. Cureus 2020, 12, e12008. [Google Scholar] [CrossRef]
- Lee, J.; Lee, S.; Min, J.; Min, K. Association between Serum Lipid Parameters and Cognitive Performance in Older Adults. J. Clin. Med. 2021, 10, 5405. [Google Scholar] [CrossRef] [PubMed]
- McFarlane, O.; Kozakiewicz, M.; Kędziora-Kornatowska, K.; Gębka, D.; Szybalska, A.; Szwed, M.; Klich-Rączka, A. Blood Lipids and Cognitive Performance of Aging Polish Adults: A Case-Control Study Based on the PolSenior Project. Front. Aging Neurosci. 2020, 12, 590546. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Yan, P.; Cheng, G.; Liu, D.; Xu, L.; Yang, M.; Xu, H.; Cheng, X.; Lian, P.; Zeng, Y. Correlation between Serum Lipid Profiles and Cognitive Impairment in Old Age: A Cross-Sectional Study. Gen. Psychiatr. 2023, 36, e101009. [Google Scholar] [CrossRef]
- Peña-Bautista, C.; Álvarez-Sánchez, L.; Roca, M.; García-Vallés, L.; Baquero, M.; Cháfer-Pericás, C. Plasma Lipidomics Approach in Early and Specific Alzheimer’s Disease Diagnosis. J. Clin. Med. 2022, 11, 5030. [Google Scholar] [CrossRef]
- Wood, P.L.; Locke, V.A.; Herling, P.; Passaro, A.; Vigna, G.B.; Volpato, S.; Valacchi, G.; Cervellati, C.; Zuliani, G. Targeted Lipidomics Distinguishes Patient Subgroups in Mild Cognitive Impairment (MCI) and Late Onset Alzheimer’s Disease (LOAD). BBA Clin. 2016, 5, 25–28. [Google Scholar] [CrossRef] [PubMed]
- Sabbagh, M.; Zahiri, H.R.; Ceimo, J.; Cooper, K.; Gaul, W.; Connor, D.; Sparks, D.L. Is There a Characteristic Lipid Profile in Alzheimer’s Disease? J. Alzheimer’s Dis. 2005, 6, 585–589. [Google Scholar] [CrossRef] [PubMed]
- Ancelin, M.-L.; Ripoche, E.; Dupuy, A.-M.; Barberger-Gateau, P.; Auriacombe, S.; Rouaud, O.; Berr, C.; Carrière, I.; Ritchie, K. Sex Differences in the Associations Between Lipid Levels and Incident Dementia. J. Alzheimer’s Dis. 2013, 34, 519–528. [Google Scholar] [CrossRef] [PubMed]
- Ma, R.M.; Li, G.G.; Ding, Y.W.; Lyu, J.; Shao, C.Q.; Liu, J.Z.; Liu, J.; Zhang, G.J. Correlation of Serum Lipids Levels of Alzheimer’s Disease Patients with Sex, Age and Apolipoprotein E Gene Polymorphism. Zhonghua Yu Fang Yi Xue Za Zhi 2022, 56, 280–286. [Google Scholar] [CrossRef] [PubMed]
- Wong, M.W.K.; Braidy, N.; Pickford, R.; Vafaee, F.; Crawford, J.; Muenchhoff, J.; Schofield, P.; Attia, J.; Brodaty, H.; Sachdev, P.; et al. Plasma Lipidome Variation during the Second Half of the Human Lifespan Is Associated with Age and Sex but Minimally with BMI. PLoS ONE 2019, 14, e0214141. [Google Scholar] [CrossRef] [PubMed]
- Lim, W.L.F.; Huynh, K.; Chatterjee, P.; Martins, I.; Jayawardana, K.S.; Giles, C.; Mellett, N.A.; Laws, S.M.; Bush, A.I.; Rowe, C.C.; et al. Relationships Between Plasma Lipids Species, Gender, Risk Factors, and Alzheimer’s Disease. J. Alzheimer’s Dis. 2020, 76, 303–315. [Google Scholar] [CrossRef] [PubMed]
- Hu, N.; Gao, L.; Jiang, Y.; Wei, S.; Shang, S.; Chen, C.; Dang, L.; Wang, J.; Huo, K.; Deng, M.; et al. The Relationship between Blood Lipids and Plasma Amyloid Beta Is Depend on Blood Pressure: A Population-Based Cross-Sectional Study. Lipids Health Dis. 2020, 19, 8. [Google Scholar] [CrossRef] [PubMed]
- Kling, M.A.; Goodenowe, D.B.; Senanayake, V.; MahmoudianDehkordi, S.; Arnold, M.; Massaro, T.J.; Baillie, R.; Han, X.; Leung, Y.; Saykin, A.J.; et al. Circulating Ethanolamine Plasmalogen Indices in Alzheimer’s Disease: Relation to Diagnosis, Cognition, and CSF Tau. Alzheimer’s Dement. 2020, 16, 1234–1247. [Google Scholar] [CrossRef] [PubMed]
- Sakr, F.; Dyrba, M.; Bräuer, A.; Teipel, S. Association of Lipidomics Signatures in Blood with Clinical Progression in Preclinical and Prodromal Alzheimer’s Disease. J. Alzheimer’s Dis. 2022, 85, 1115–1127. [Google Scholar] [CrossRef]
- Wong, M.W.K.; Braidy, N.; Crawford, J.; Pickford, R.; Song, F.; Mather, K.A.; Attia, J.; Brodaty, H.; Sachdev, P.; Poljak, A. APOE Genotype Differentially Modulates Plasma Lipids in Healthy Older Individuals, with Relevance to Brain Health. J. Alzheimer’s Dis. 2019, 72, 703–716. [Google Scholar] [CrossRef]
- Sharman, M.J.; Shui, G.; Fernandis, A.Z.; Lim, W.L.F.; Berger, T.; Hone, E.; Taddei, K.; Martins, I.J.; Ghiso, J.; Buxbaum, J.D.; et al. Profiling Brain and Plasma Lipids in Human APOE Ε2, Ε3, and Ε4 Knock-in Mice Using Electrospray Ionization Mass Spectrometry. J. Alzheimer’s Dis. 2010, 20, 105–111. [Google Scholar] [CrossRef]
- Novotny, B.C.; Fernandez, M.V.; Wang, C.; Budde, J.P.; Bergmann, K.; Eteleeb, A.M.; Bradley, J.; Webster, C.; Ebl, C.; Norton, J.; et al. Metabolomic and Lipidomic Signatures in Autosomal Dominant and Late-onset Alzheimer’s Disease Brains. Alzheimer’s Dement. 2023, 19, 1785–1799. [Google Scholar] [CrossRef]
- Chakraborty, A.; Praharaj, S.K.; Prabhu, R.V.K.; Prabhu, M.M. Lipidomics and Cognitive Dysfunction—A Narrative Review. Turk. J. Biochem. 2020, 45, 109–119. [Google Scholar] [CrossRef]
- Mielke, M.M.; Bandaru, V.V.R.; Haughey, N.J.; Rabins, P.V.; Lyketsos, C.G.; Carlson, M.C. Serum Sphingomyelins and Ceramides Are Early Predictors of Memory Impairment. Neurobiol. Aging 2010, 31, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Mielke, M.M.; Haughey, N.J.; Han, D.; An, Y.; Bandaru, V.V.R.; Lyketsos, C.G.; Ferrucci, L.; Resnick, S.M. The Association Between Plasma Ceramides and Sphingomyelins and Risk of Alzheimer’s Disease Differs by Sex and APOE in the Baltimore Longitudinal Study of Aging. J. Alzheimer’s Dis. 2017, 60, 819–828. [Google Scholar] [CrossRef]
- Wood, P.L.; Medicherla, S.; Sheikh, N.; Terry, B.; Phillipps, A.; Kaye, J.A.; Quinn, J.F.; Woltjer, R.L. Targeted Lipidomics of Fontal Cortex and Plasma Diacylglycerols (DAG) in Mild Cognitive Impairment and Alzheimer’s Disease: Validation of DAG Accumulation Early in the Pathophysiology of Alzheimer’s Disease. J. Alzheimer’s Dis. 2015, 48, 537–546. [Google Scholar] [CrossRef]
- Mielke, M.M.; Haughey, N.J.; Bandaru, V.V.R.; Weinberg, D.D.; Darby, E.; Zaidi, N.; Pavlik, V.; Doody, R.S.; Lyketsos, C.G. Plasma Sphingomyelins Are Associated with Cognitive Progression in Alzheimer’s Disease. J. Alzheimer’s Dis. 2011, 27, 259–269. [Google Scholar] [CrossRef] [PubMed]
- Dakterzada, F.; Jové, M.; Huerto, R.; Carnes, A.; Sol, J.; Pamplona, R.; Piñol-Ripoll, G. Changes in Plasma Neutral and Ether-Linked Lipids Are Associated with The Pathology and Progression of Alzheimer’s Disease. Aging Dis. 2023, 14, 1728. [Google Scholar] [CrossRef] [PubMed]
- Tremblay-Franco, M.; Canlet, C.; Carriere, A.; Nakhle, J.; Galinier, A.; Portais, J.-C.; Yart, A.; Dray, C.; Lu, W.-H.; Bertrand Michel, J.; et al. Integrative Multimodal Metabolomics to Early Predict Cognitive Decline Among Amyloid Positive Community-Dwelling Older Adults. J. Gerontol. A Biol. Sci. Med. Sci. 2024, 79, glae077. [Google Scholar] [CrossRef]
- Bredesen, D.E. Metabolic Profiling Distinguishes Three Subtypes of Alzheimer’s Disease. Aging 2015, 7, 595–600. [Google Scholar] [CrossRef]
- Jack, C.R.; Bennett, D.A.; Blennow, K.; Carrillo, M.C.; Dunn, B.; Haeberlein, S.B.; Holtzman, D.M.; Jagust, W.; Jessen, F.; Karlawish, J.; et al. NIA-AA Research Framework: Toward a Biological Definition of Alzheimer’s Disease. Alzheimer’s Dement. 2018, 14, 535–562. [Google Scholar] [CrossRef]
- Randolph, C.; Tierney, M.C.; Mohr, E.; Chase, T.N. The Repeatable Battery for the Assessment of Neuropsychological Status (RBANS): Preliminary Clinical Validity. J. Clin. Exp. Neuropsychol. 1998, 20, 310–319. [Google Scholar] [CrossRef] [PubMed]
- Folstein, M.F.; Folstein, S.E.; McHugh, P.R. “Mini-Mental State”: A practical method for grading the cognitive state of patients for the clinician. J. Psychiatr. Res. 1975, 12, 189–198. [Google Scholar] [CrossRef] [PubMed]
- Pfeffer, R.I.; Kurosaki, T.T.; Harrah, C.H.; Chance, J.M.; Filos, S. Measurement of Functional Activities in Older Adults in the Community. J. Gerontol. 1982, 37, 323–329. [Google Scholar] [CrossRef] [PubMed]
- Galasko, D.; Bennett, D.; Sano, M.; Ernesto, C.; Thomas, R.; Grundman, M.; Ferris, S. An Inventory to Assess Activities of Daily Living for Clinical Trials in Alzheimer’s Disease. Alzheimer Dis. Assoc. Disord. 1997, 11, 33–39. [Google Scholar] [CrossRef]
- Alcoriza-Balaguer, M.I.; García-Cañaveras, J.C.; López, A.; Conde, I.; Juan, O.; Carretero, J.; Lahoz, A. LipidMS: An R Package for Lipid Annotation in Untargeted Liquid Chromatography-Data Independent Acquisition-Mass Spectrometry Lipidomics. Anal. Chem. 2019, 91, 836–845. [Google Scholar] [CrossRef]


| Participants (n = 31) | ||
|---|---|---|
| Age (years) (median (IQR)) | 71 (68,74) | |
| Sex (Female, n (%)) | 16 (48%) | |
| Educational level (n, %) | Primary | 11 (36.7%) |
| Secondary | 12 (38.7%) | |
| Universitary | 7 (22.6%) | |
| Drugs (n, %) | Statins | 15 (48,4) |
| Fibrates | 4 (12.9) | |
| Benzodiazepines | 5 (16.1%) | |
| Antidepressants | 2 (6.5%) | |
| Antihypertensives | 11 (35.5%) | |
| Comorbidities (n, %) | Dislipidemia | 14 (45.2%) |
| Diabetes | 2 (6.5%) | |
| Hypertension | 11 (35.5%) | |
| Heart Disease | 0 (0%) | |
| Cerebrovascular disease | 0 (0%) | |
| Smoke status (Yes) (n, %) | 4 (12.9%) | |
| Alcohol (n, %) | 2 (6.5%) | |
| Depression (n, %) | 7 (22.6%) | |
| Anxiety (n, %) | 4 (12.9%) | |
| ApoE4 carrier (n, %) | 13 (76%) * | |
| CSF Amyloid-β42 (Aβ42) (pg mL−1) (median (IQR)) | 508 (436,675) | |
| CSF t-Tau (pg mL−1) (median (IQR)) | 526 (341,733) | |
| CSF p-Tau (pg mL−1) (median (IQR)) | 76 (57,105) | |
| CSF Amyloid-β40 (Aβ40) (pg mL−1) | 10292 (5959,12464) | |
| Aβ42/Aβ40 (median (IQR)) | 0.06 (0.05,0.10) | |
| Neurofilament light chain (NfL) (pg mL−1) (median (IQR)) | 818 (550,1442) | |
| t-Tau/Aβ42 (median (IQR)) | 0.94 (0.68,1.30) | |
| CDR (score) (median (IQR)) | 0.5 (0.5,0.5) | |
| MMSE (score) (median (IQR)) | 26 (24,28) | |
| RBANS.DM (score) (median (IQR)) | 60 (40,91) | |
| FAQ (score) (median (IQR)) | 5 (1,8) | |
| Lipid Family | Cluster 1 (n = 16) | Cluster 2 (n = 15) | p Value (Mann–Whitney) | ||
|---|---|---|---|---|---|
| Mean (SD) (a.u.) | Median (IQR) (a.u.) | Mean (SD) (a.u.) | Median (IQR) (a.u.) | ||
| CEs | 4.33 (1.08) | 4.48 (3.37,4.95) | 3.94 (1.38) | 3.84 (3.05,5.17) | 0.318 |
| Cers | 10.28 (2.05) | 9.72 (8.65,11.649) | 5.71 (2.15) | 6.43 (3.59,7.77) | 0.000 * |
| DGs | 2.33 (0.38) | 2.22 (2.00,2.69) | 1.34 (0.28) | 1.40 (1.06,1.55) | 0.000 * |
| FAs | 22.05 (13.68) | 18.16 (11.00,27.23) | 14.26 (5.84) | 13.68 (10.65,19.50) | 0.188 |
| LPCs | 15.53 (3.03) | 15.06 (12.82,18.40) | 6.80 (2.57) | 6.78 (4.35,9.79) | 0.000 * |
| LPEs | 6.76 (1.86) | 6.44 (5.31,7.97) | 2.65 (1.33) | 2.61 (1.36,4.00) | 0.000 * |
| MGs | 2.37 (1.13) | 2.44 (1.19,3.44) | 1.06 (1.36) | 0.68 (0.40,1,12) | 0.000 * |
| PCs | 58.29 (8.17) | 56.25 (52.65,65.79) | 34.80 (9.23) | 37.11 (25.56,42.34) | 0.000 * |
| PEs | 13.21 (4.99) | 13.60 (8.64,14.59) | 4.89 (1.59) | 4.59 (3.82,6.40) | 0.000 * |
| PIs | 6.63 (2.03) | 6.20 (4.70,8.55) | 3.33 (1.34) | 3.07 (2.48,3.98) | 0.000 * |
| SMs | 8.75 (1.43) | 8.63 (7.89,10.02) | 4.05 (1.85) | 4.09 (2.37,6.01) | 0.000 * |
| TGs | 25.60 (10.16) | 22.87 (17.90,31.90) | 21.36 (6.24) | 20.29 (17.84,26.15) | 0.338 |
| SUMBOX CDR | CDRM | CDRADA | MMSE | RBANS.MI | RBANS.A | RBANS.DM | |
|---|---|---|---|---|---|---|---|
| DGs (PCC (p value)) | −0.312 (0.14) | −0.330 (0.12) | −0.161 (0.45) | 0.378 (0.039) * | 0.298 (0.11) | −0.002 (0.99) | 0.391 (0.033) * |
| FAs (PCC (p value)) | −0.211 (0.32) | −0.190 (0.38) | −0.221 (0.30) | 0.011 (0.96) | 0.069 (0.72) | −0.387 (0.034) * | 0.059 (0.76) |
| LPCs (PCC (p value)) | −0.407 (0.049) * | −0.410 (0.047) * | −0.251 (0.24) | 0.328 (0.08) | 0.248 (0.19) | −0.043 (0.82) | 0.213 (0.26) |
| MGs (PCC (p value)) | −0.427 (0.037) * | −0.423 (0.039) * | −0.463 (0.023) * | 0.350 (0.06) | 0.393 (0.032) * | 0.077 (0.69) | 0.431 (0.018) * |
| SMs (PCC (p value)) | −0.437 (0.033) * | −0.449 (0.028) * | −0.275 (0.19) | 0.420 (0.021) * | 0.324 (0.08) | 0.108 (0.57) | 0.371 (0.044) * |
| Estimate | Standard Error | p-Value | |
|---|---|---|---|
| Intercept (model 1) | −1.2 | 1.8 | 0.4990 |
| Slope (model 1) | −0.001 | 0.002 | 0.6040 |
| Intercept (model 2) | 0 | 3 | 0.9591 |
| Slope (model 2) | −0.010 | 0.004 | 0.0436 * |
| Estimate | Standard Error | p-Value | |
|---|---|---|---|
| Intercept | −0.5 | 1.6 | 0.7494 |
| Slope (x) | −0.002 | 0.002 | 0.3666 |
| Interaction Coefficient (x:factor) | −0.007 | 0.002 | 0.0021 ** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peña-Bautista, C.; Álvarez-Sánchez, L.; García-Lluch, G.; Raga, L.; Quevedo, P.; Peretó, M.; Balaguer, A.; Baquero, M.; Cháfer-Pericás, C. Relationship between Plasma Lipid Profile and Cognitive Status in Early Alzheimer Disease. Int. J. Mol. Sci. 2024, 25, 5317. https://doi.org/10.3390/ijms25105317
Peña-Bautista C, Álvarez-Sánchez L, García-Lluch G, Raga L, Quevedo P, Peretó M, Balaguer A, Baquero M, Cháfer-Pericás C. Relationship between Plasma Lipid Profile and Cognitive Status in Early Alzheimer Disease. International Journal of Molecular Sciences. 2024; 25(10):5317. https://doi.org/10.3390/ijms25105317
Chicago/Turabian StylePeña-Bautista, Carmen, Lourdes Álvarez-Sánchez, Gemma García-Lluch, Luis Raga, Paola Quevedo, Mar Peretó, Angel Balaguer, Miguel Baquero, and Consuelo Cháfer-Pericás. 2024. "Relationship between Plasma Lipid Profile and Cognitive Status in Early Alzheimer Disease" International Journal of Molecular Sciences 25, no. 10: 5317. https://doi.org/10.3390/ijms25105317
APA StylePeña-Bautista, C., Álvarez-Sánchez, L., García-Lluch, G., Raga, L., Quevedo, P., Peretó, M., Balaguer, A., Baquero, M., & Cháfer-Pericás, C. (2024). Relationship between Plasma Lipid Profile and Cognitive Status in Early Alzheimer Disease. International Journal of Molecular Sciences, 25(10), 5317. https://doi.org/10.3390/ijms25105317







