Abstract
The mitochondria play a crucial role in cellular metabolism, reactive oxygen species (ROS) production, and apoptosis. Aberrant mitochondria can cause severe damage to the cells, which have established a tight quality control for the mitochondria. This process avoids the accumulation of damaged mitochondria and can lead to the release of mitochondrial constituents to the extracellular milieu through mitochondrial extracellular vesicles (MitoEVs). These MitoEVs carry mtDNA, rRNA, tRNA, and protein complexes of the respiratory chain, and the largest MitoEVs can even transport whole mitochondria. Macrophages ultimately engulf these MitoEVs to undergo outsourced mitophagy. Recently, it has been reported that MitoEVs can also contain healthy mitochondria, whose function seems to be the rescue of stressed cells by restoring the loss of mitochondrial function. This mitochondrial transfer has opened the field of their use as potential disease biomarkers and therapeutic tools. This review describes this new EVs-mediated transfer of the mitochondria and the current application of MitoEVs in the clinical environment.
1. Introduction
The mitochondria are cellular organelles with a double membrane structure that use aerobic respiration to generate ATP. Apart from their traditional role in oxidative phosphorylation, the mitochondria have key roles in several metabolic pathways, cell proliferation, and differentiation, ROS production and consumption, and apoptosis [1]. The mitochondria also have an important role in the fine modulation of calcium homeostasis, and when compromised, they lead to different pathological conditions [2]. Moreover, it has been shown that the alteration of calcium signals that reach the mitochondria during pathological conditions (such as oxidative stress) is accompanied by a deformation of this organelle structure and function, triggering its clearance [3].
Mammal mitochondria contain several copies of their genome consisting of a circular DNA molecule of 16.6 kb. The mitochondrial genome includes 37 genes encoding 13 proteins for subunits of the respiratory complexes of the electron transport chain, 22 tRNA, and 2 rRNA (12S and 16S rRNA).
The mitochondria are transmitted to subsequent generations through the vertical maternal lineage in mammals. During symmetric cell division, the mitochondria are distributed randomly between daughter cells [4]. Conversely, during asymmetric cell division, the mitochondria are differentially segregated. This was demonstrated using a colored labeling strategy where old mitochondria were labeled 48 to 58 h before cell division, and young mitochondria were labeled 0 to 10 h before division. The authors observed that, after cell division, the old-labeled mitochondria from the mother cells were divided more asymmetrically between daughter cells than the young-labeled mitochondria [5], thus suggesting the existence of active mechanisms that guide mitochondria partitioning between daughter cells.
Recently, the mitochondria have been found to be horizontally transferred between mammalian cells, challenging the current concepts of mitochondria inheritance [6]. Diverse structures mediate intercellular mitochondrial transfer. These include tunneling nanotubes (TNTs) [7], extracellular vesicles (EVs) [8], gap junctions [9], and cell fusion [10]. However, free extracellular mitochondria have also been found in supernatants from cells cultured in vitro, as well as in biological fluids, under both physiological and pathological conditions [11,12].
TNTs, gap junctions, and cell fusion have been extensively described; therefore, in this review, we will focus on mitochondria horizontal transfer through EVs. EVs are double lipid layer-surrounded vesicles that are secreted to the extracellular milieu by almost all cell types and they drive intercellular communication. “EVs” is a general term that englobes several subtypes of cell-released membranous structures, including exosomes, microvesicles, apoptotic bodies, and others, regardless of their biogenesis, size, density, and function [13]. These particles can be isolated from the media of cells in culture and from biological fluids, using different procedures, such as serial ultracentrifugation, ultrafiltration, size-exclusion chromatography, immunoaffinity, or microdevices, among others. The size of EVs ranges from 40 nm to 5 μm [14], and their typical cargo includes proteins, lipids, and nucleic acids. These vesicles are known to be involved in physiological and pathological processes, including the removal of unwanted proteins, antigen presentation, genetic exchange, immune response, inflammation, tumor metastasis, and dissemination of pathogens [15]. Recently, intact organelles such as mitochondria have also been detected in EVs; therefore, EVs are believed to participate in intercellular mitochondrial transfer [16,17].
2. MitoEVs
EVs have different sizes, ranging from 40–150 nm (small EVs) to 500–5000 nm (large EVs). Smaller EVs mostly contain genetic material, such as mtDNA [18,19,20,21,22], mtRNA [23], and mitochondrial proteins [24,25,26,27,28]. Larger EVs may contain entire polarized mitochondria [8,29,30,31]. As several discrepancies have been described in the literature, mostly due to differences in the EV isolation protocol used, in this review, we englobe all these extracellular vesicles containing either intact mitochondrion or mitochondrion components, as MitoEVs.
MitoEVs transfer enables the incorporation of mitochondria or their components into the endogenous mitochondrial network of recipient cells. Mitochondrial transfer likely occurs under normal and physiological conditions between cells, suggesting a regular exchange of mitochondria that ensures a balanced heteroplasmy [32]. As an example, it has been reported that mesenchymal stem cells (MSCs) package intact mitochondria into MitoEVs, which are transferred to chondrocytes in the absence of direct cell−cell interactions or stimulus [8]. In addition, it has been proposed that mitochondria are transferred during mouse development [33]. Since embryonic development requires cells to rely on aerobic glycolysis to support rapid cell proliferation [34], this mitochondria transfer might play a role in mitochondrial respiration-linked remodeling [35]. However, more research is needed in this field to assess the specific involvement of MitoEVs in the control of mtDNA heterogeneity and tissue homeostasis during normal development.
Interestingly, MitoEVs can contain both healthy or damaged mitochondria, with different physiopathological consequences on target cells. Recent research has provided solid evidence to support that mitochondria are released from cells for transcellular degradation or transferred to other cells as metabolic support or regulatory messengers [36,37,38,39].
3. How and Why Do Cells Release MitoEVs?
The intercellular transfer of MitoEVs plays specific roles in different conditions. On the one hand, mitochondria may be released to the extracellular space during developmental processes, inflammatory activation, and in the process of “garbage clearance” of damaged mitochondria [40]. Indeed, MitoEVs are part of the quality control of the mitochondria. When the mitochondria are damaged, cells activate repair mechanisms such as mitochondrial proteostasis, mitochondrial dynamics (fusion/fission), and mitophagy or trans mitophagy [36], that is, sending damaged mitochondria to the surrounding cells (astrocytes or macrophages) to complete the quality control of the mitochondria [41,42].
On the other hand, it is increasingly being reported that MitoEVs released by healthy MSCs promote anti-inflammatory effects and energy metabolism restoration in target cells [43]. It has been suggested that stressed cells send specific signals, such as ROS, leading to the formation of tunneling nanotubes (TNTs) and the shedding of MitoEVs by healthy cells [17,44], resulting in the transfer of healthy mitochondria to stressed cells, thereby restoring their functionality and rescuing them from apoptosis [6]. This was observed in corneal epithelial cells subjected to oxidative stress, which sent environmental cues to MSC, which responded by releasing MitoEVs, that, once internalized, epithelial cells displayed an enhanced survival capacity, elevated mitochondrial respiration, and a wound healing capacity [45]. Similarly, in patients with myoclonus epilepsy with ragged-red fibers (a mitochondrial disease), MSCs donate MitoEVs to rescue injured cells by improving their aerobic respiration, suppressing apoptosis, and decreasing oxidative stress [46].
3.1. MitoEVs for Mitochondria Quality Control
Cells perform mitochondrial quality control through four different pathways (see Figure 1).
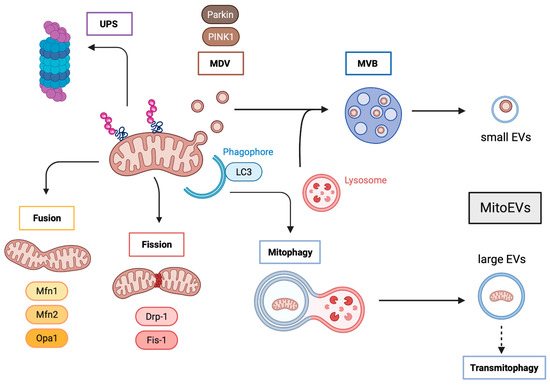
Figure 1.
Mitochondria quality control and MitoEVs formation. Aberrant mitochondrial components can be degraded by the UPS, but also be incorporated in MDV that fuse with lysosomes to form MVB, leading to the formation and delivery of small mitoEVs. Whole damaged mitochondria are subjected to fusion and fission processes, the latter ending in mitophagy and promoting transmitophagy through the formation and release of large mitoEVs. UPS: Ubiquitin proteasome system; MDV: mitochondria-derived vesicles; MVBs: multivesicular bodies; PINK1: PTEN-induced putative protein kinase 1; MVB: multivesicular bodies; EVs: extracellular vesicles; Mfn1: mitofusin 1; Mfn2: mitofusin 2; Opa1: optic atrophy 1; Drp-1: dynamin-related protein 1; FIS1: mitochondrial fission protein 1; LC3: microtubule-associated protein 1 light chain 3.
The proteostasis of mitochondrial proteins includes mitochondria-localized chaperones and proteases that re-fold or degrade individual aberrant proteins, thereby maintaining the quality of proteins functioning within the mitochondria [47]. Mitochondrial proteostasis degrades unfolded or oxidized proteins within the mitochondrial matrix by mitochondrial proteases; although, in some cases, these aberrant proteins can be ubiquitinated and delivered to the cytosolic ubiquitin−proteasome system (UPS) [48,49,50]. It has been reported that ubiquitination occurs at the inner mitochondrial membrane and that some metabolic proteins (such as succinate dehydrogenase subunit A) are UPS-dependent [51]; thereby suggesting that UPS is involved in the regulation of mitochondrial quality control.
Mitochondrial dynamics consist of the antagonistic and balanced activities of the fusion and fission machinery to shape the mitochondrial compartment. A shift toward fusion favors the generation of interconnected mitochondria, whereas a shift toward fission produces numerous mitochondrial fragments [52]. Large mitochondrial networks generated by fusion are typically observed in metabolically active cells; in contrast, in quiescent cells, the mitochondria are frequently observed as numerous small spheres or short rods. Mitochondrial dynamics are mediated by fusion factors (mitofusin 1 and 2 (MFN1 and MFN2), and optic atrophy 1 (OPA1)) and fission factors (dynamin-related protein 1 (DRP1) and mitochondrial fission protein 1 (FIS1)). Fusion dilutes damaged mitochondria along the network, whereas fission targets dysfunctional mitochondria to their subsequent clearance through mitophagy [53,54]. Depending on the physiological context, MFN2 can either mediate mitochondrial fusion or recruit cytosolic Parkin to initiate mitophagy [55]. Interestingly, alterations in MFN2 can hamper mitochondrial fusion leading to the formation of clumped mitochondrial aggregates [56]. It is likely that in this scenario, clumped mitochondria would also be subjected to degradation through mitophagy.
Severely damaged mitochondria are incorporated into LC3-positive autophagosomes that will eventually fuse with lysosomes or late endosomes for their degradation through mitophagy [57]. This pathway relays on PTEN-induced putative protein kinase 1 (PINK1) and Parkin, which are activated following a loss of mitochondrial membrane potential [58]. Interestingly, it has been reported that autophagy-deficient cells [59,60,61] as well as UPS-deficient cells release an increased number of MitoEVs. Similarly, several stresses increase the number of released MitoEVs. Indeed, under cold stress, brown adipocytes eject MitoEVs containing oxidatively-damaged mitochondria that are cleared by resident macrophages [26,62]. In addition, mesenchymal stem cells (MSC) subjected to oxidative stress package mitochondria into EVs for cellular transfer, which are posteriorly engulfed by macrophages that undergo outsource mitophagy [63].
Mildly damaged mitochondria, not yet completely depolarized, may be also subjected to PINK1 and Parkin action to generate mitochondria-derived vesicles (MDVs) [64,65]. MDVs are generated through the selective incorporation of mitochondrial proteins. These MDVs have a relatively uniform size, between 70 and 150 nm [66]. MDVs have two different fates: they can either fuse with peroxisomes or merge with the endolysosomal system, forming multivesicular bodies (MVBs) that will in turn be released into the extracellular compartment as MitoEVs [67,68]. It is believed that mitochondrial discharge by MitoEVs operates as a first line of defense against partially depolarized mitochondria, before complete depolarization. Moreover, under stress conditions, lysosomal degradation might be exceeded and the MDV containing dysfunctional parts of the damaged mitochondria could then accumulate and act as pro-inflammatory damage-associated molecular patterns (DAMPs) [28,69]. Cells will prevent this chaos by packaging MDVs into multivesicular bodies to be extracellularly discharged as MitoEVs, which are degraded by surrounding macrophages. Very recently, it has been suggested that the elimination of damaged mitochondria via MitoEVs is increased when the lysosomal function is compromised [70], and this mechanism seems to be mediated by the small GTPase Rab7 [71].
In response to stress, it has been published that adipocytes release MitoEVs originating from MDV, which include damaged mitochondria. These MitoEVs are taken up by cardiomyocytes, where they trigger a burst of ROS creating oxidative stress; this results in compensatory antioxidant signaling activation consistent with a metabolic pre-conditioning of the heart [26].
3.2. MitoEVs for Rescuing Damaged Cells
Accumulating shreds of evidence suggest that MSCs play a role in the protection of the surrounding damaged cells by providing their intact mitochondria via MitoEVs. It has been reported that mitochondrial transfer through MitoEVs can rescue stressed cells by restoring the loss of mitochondrial function in recipient cells and increasing their metabolic activity [16,24,72]. In a model of acute respiratory distress syndrome (ARDS), it was shown that MSC-released MitoEVs could restore barrier integrity and normal levels of oxidative phosphorylation, thereby reducing lung injury [73].
Mitochondrial function is involved in maintaining and dictating stem cell fate, which plays a role in metabolic reprogramming during quiescence, activation, self-renewal, proliferation, and differentiation. As the mitochondria produce most of the energy by oxidative phosphorylation, the switch of energy supply from glycolysis to aerobic metabolism is essential for the successful differentiation or reprogramming of recipient cells. Indeed, the transfer of healthy mitochondria can reprogram the differentiated cells [74]. It has been reported that platelets also shed MitoEVs that are integrated by MSCs, activating their pro-angiogenic activity via their metabolic remodeling [75], suggesting that MitoEVs promote tissue repair processes.
The mitochondria have a key role in immune-cell regulation. The mitochondria promote ROS signaling and metabolite availability within immune cells and act as a scaffold for protein interaction. Therefore, the mitochondria are believed to be necessary for immune cells to fulfill their specific role in both innate and adaptive responses [76]. MitoEVs can be integrated by T cells and alter their mitotic processes [29]. Recent studies have shown that macrophages uptake MitoEVs released by MSCs, which stimulates their mitochondrial activity [77]. A study reported that MitoEVs released by healthy MSCs ameliorated acute lung injury because macrophages that engulfed these MitoEVs had enhanced phagocytic capacity and reduced the secretion of TNFα, thereby suppressing lung inflammation [17]. Importantly, macrophages that have engulfed MitoEVs might display either pro- or anti-inflammatory effects. It seems that the pro-inflammatory effects have been described when MitoEVs are released by MSC subjected to pro-inflammatory treatments such as LPS, whereas the anti-inflammatory effects were observed in resting MSC [77,78]. These findings suggest that MitoEVs regulate the immune system.
Interestingly, it has recently been reported that macrophages accumulate in peripheral nervous tissue and donate their mitochondria through EVs to sensory neurons to support pain resolution [79]. This discovery opens a novel set of strategies to resolve chronic pain through the restoration of mitochondrial homeostasis in neurons or by enhancing the transfer of the mitochondria from the macrophages. Moreover, it has been reported that in a model of cerebral ischemia, astrocytes release MitoEVs to protect neurons from hypoxia and glucose deprivation [80].
The following sections are dedicated to review the current use of MitoEVs as therapeutic tools, as well as their role as biomarkers for disease diagnosis.
4. Potential Use of MitoEVs as Diagnostic Markers
Similar to other subtypes of EVs, MitoEVs are altered in several diseases, including cancer, neurodegenerative disorders, and cardiovascular disease [69]. MitoEVs contain a variety of molecular components from releasing cells, including proteins, lipids, and nucleic acids, which may serve as indicators of disease status [69]. Here, we discuss how differences in the content and markers of these vesicles could thus be used as diagnostic tools for distinct conditions.
4.1. Cancer
The search for new methods to diagnose cancer in its early stages and distinguish between states of the disease has led to the development of liquid biopsies. The analysis of body fluids such as blood or urine to gather information about a person’s cancer status has emerged as a powerful tool for cancer diagnosis, prognosis, and treatment monitoring, as it allows for the detection of cancer-related genetic alterations in a minimally invasive manner [81,82].
Cancer cells often release various types of molecules into the bloodstream, including DNA, RNA, and proteins [83]. These molecules can be used to detect cancer cells and track their progression over time. The results of a liquid biopsy can provide important information about the type and stage of cancer, as well as help monitor the effectiveness of the treatment and detect the early signs of cancer recurrence [82].
The analysis of EVs in liquid biopsies has emerged as a novel method to provide new insights into the role of EVs in several diseases, as the content in EVs varies across disease status [84]. Currently, the main application of the analysis of EVs in liquid biopsies is in the detection and characterization of cancer-specific biomarkers [84,85]. This approach offers several advantages over traditional diagnostic methods, such as tissue biopsy or imaging. Firstly, EVs are readily available in the bloodstream, making liquid biopsy with EVs a minimally invasive and convenient option for cancer diagnosis and monitoring. Secondly, EVs contain a wealth of information about cancer cells, including their genetic and epigenetic alterations, which can provide valuable insights into cancer’s biology, progression, and treatment response [86].
The mitochondria, the cellular organelles responsible for energy production, have emerged as crucial players in the development and progression of cancer. Growing evidence links mitochondrial dysfunction to various aspects of cancer biology, including metabolism, apoptosis, and signaling pathways [87]. In this context, it has been shown that cancer cells release EVs that contain specific mitochondria-derived molecules, such as proteins or mtDNA [18,88,89].
mtDNA present in EVs has an important role in cancer biology and progression, making it an interesting source in cancer diagnosis. mtDNA transfer between cancer cells acts as an oncogenic signal, promoting the escape of cells from metabolic quiescence [20]. Similarly, mtDNA contained in metastatic tumor cells is transferred to low-metastatic tumor cells via MitoEVs, enhancing the metastatic potential during tumor progression [22]. In a more recent study, the authors showed that the protein PINK1 mediates the packaging of mtDNA in EVs from cancer cells and that this mtDNA can promote invasiveness through the activation of Toll-like receptor 9 in recipient cells [21].
Some studies have proposed that MitoEVs could serve as new biomarkers of cancer. Jang et al. discovered that EVs released by melanoma tissue contain higher levels of mitochondrial membrane proteins when compared with non-cancerous cells. In addition, they found that patients with melanoma or other types of cancer such as ovarian or breast cancer have a higher concentration in the plasma of these MitoEVs [25]. Regarding mtDNA, it was recently shown that patients with pancreatic ductal adenocarcinoma have a higher enrichment of mtDNA in circulating EVs, detecting specific mtDNA mutations that could serve as a tool for early cancer detection [90]. Moreover, mtDNA contained in MitoEVs obtained from the plasma exhibit different characteristics among patients with hepatocellular carcinoma, hepatitis, or healthy individuals, indicating a potential role as a diagnostic biomarker in these conditions [91].
4.2. Other Diseases
Although cancer is currently the most studied disease in terms of liquid biopsies and MitoEVs, recent studies have found that the content in MitoEVs can be altered in other diseases, such as neurological or cardiovascular conditions [69].
Multiple lines of evidence suggest that mitochondrial dysfunction plays a key role in the pathogenesis of Parkinson’s disease (PD). Post-mortem studies have shown that there is a reduction in the number and size of mitochondria in the substantia nigra region of PD patients’ brains [92]. Additionally, there is evidence of decreased mitochondrial respiratory chain activity and increased ROS generation in PD patients [93]. Furthermore, mutations in genes that regulate mitochondrial function, such as PINK1 and Parkin, are associated with some forms of PD [94]. Recently, it was shown that these proteins are involved in mitochondrial quality control through the regulation of mitochondria-derived vesicle trafficking [64,95]. Along with these results, a clinical study with PD patients suggested that circulating EVs were altered in the disease; more specifically, they found that a specific mitochondrial signature was present in these patients [96].
Another neurological condition characterized by mitochondrial dysfunction is Down syndrome (DS). Patients have impairments in mitochondrial function, which leads to a decrease in energy production that may contribute to the cognitive impairments seen in individuals with Down syndrome [97]. Additionally, studies have shown that people with Down syndrome have an increased susceptibility to oxidative stress [98]. A recent study that presents a new approach to isolate and separate EV subpopulations from the brain extracellular matrix, identifies a unique subset of EVs of a mitochondrial origin, which they term mitovesicles. The authors found that the number and composition of brain mitovesicles are altered in individuals with DS, indicating their possible role in the neuropathological process [27].
In cardiovascular disease, mitochondrial dysfunction has been linked to the development of key pathological changes such as heart failure or atherosclerosis [99,100]. MitoEVs regulate mitochondrial quality control in the cardiovascular system [101,102] and serve as pro-inflammatory signaling between monocytes and endothelial cells in cardiovascular disease [103]. This particular subtype of vesicles has a crucial role in the maintenance of mitochondrial homeostasis in the heart, as cardiomyocytes release dysfunctional mitochondria taken up by resident macrophages [104].
Thereby, MitoEVs can be detected in biological fluids and seem to play a role in the regulation of mitochondrial biology and intercellular communication, making them an interesting subtype of EVs that could be used as diagnostic markers for several diseases (Figure 2 and Table 1).
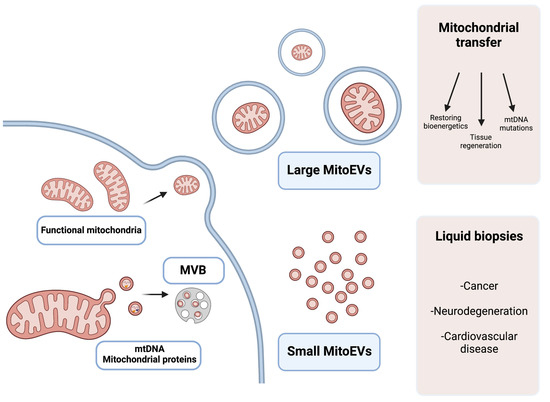
Figure 2.
Graphical explanation of the potential therapeutic and diagnostic use of large and small MitoEVs. Larger MitoEVs refer to EVs that are shed from the plasmatic membrane of the cell and can include whole mitochondria; these EVs are particularly interesting in the field of mitochondrial transfer as therapeutic vehicles. Small MitoEVs are included in MVB previous to their release and contain material from the mitochondrial origin (mtDNA and proteins); its analysis may serve as a diagnostic tool in liquid biopsies.

Table 1.
Summary of applications of MitoEVs in human diseases, regarding the sources of the vesicles and the key findings in different studies.
5. MitoEVs as Therapeutic Tools
EV therapy has shown potential in a variety of applications, including regenerative medicine, cancer treatment, and immune modulation. They play a crucial role in intercellular communication and have been recognized as potential therapeutic agents. One of the major advantages of extracellular vesicle therapy is that it avoids some of the limitations associated with traditional cell-based therapies. EVs have a lower risk of immunogenicity compared with cells, and greater stability and longer half-lives compared with other biological therapeutics [118,119,120,121,122].
As a fairly novel field, the use of MitoEVs as a therapy is not well established; the main challenge that this approach faces is that classic isolation methods for EVs mainly distinguish subtypes of these vesicles by size [121], making it difficult to separate a specific subset that comes from a mitochondrial origin. Currently, the main focus of MitoEVs as therapeutic agents is in the field of mitochondrial transfer (Figure 2 and Table 1). Mitochondrial transfer is a therapeutic strategy that involves transferring healthy mitochondria to cells with dysfunctional mitochondria [123,124]. Mitochondrial transfer offers a promising avenue for the treatment of several diseases, as it addresses the root cause of mitochondrial dysfunction. There are several approaches to mitochondrial transfer, including microinjection, the fusion of cells, and the use of EVs as transfer vehicles [124]. The latter strategy seems to be the more feasible, as EVs are already being studied as therapeutics, and may serve as mitochondria transfer vehicles in a non-invasive manner.
The mitochondrial transfer has been used in preclinical models of several diseases. One of the most studied settings is the use of these healthy mitochondria to improve tissue regeneration. During tissue regeneration, cells must undergo a series of complex processes, including cell proliferation, differentiation, and migration. These processes require high levels of energy, and the mitochondria play a crucial role in providing this energy [125]. They also play a crucial role in regulating cell signaling during tissue regeneration. They are involved in the production of signaling molecules, such as reactive oxygen species, which can activate the signaling pathways that regulate cell proliferation and differentiation [123,126]. The modulation of mitochondrial calcium trafficking has also been highlighted as a potential target in tissue regeneration and other pathophysiological contexts [2]. In addition, studies have shown that mitochondrial dysfunction is a key factor that leads to impaired tissue regeneration in aging [127].
The transfer of healthy mitochondria has been successfully used to improve tissue regeneration in models of myocardial ischemia and reperfusion injury (IRI) [105], brain ischemia [106,107], limb ischemia [109], lung IRI [110], and acute kidney failure [111]. In brain ischemia, transplanted mitochondria are incorporated into various cells, resulting in increased ATP content, complex IV expression, and neurogenesis, while also reducing oxidative stress, apoptosis, and inflammatory responses [106,107,108,128]. In cardiac and limb ischemia, transplanted mitochondria enhance ATP production and synthesis, improve cell viability, and activate proteomic pathways for energy production, mitochondrial function, and cellular respiration, while reducing pro-inflammatory markers and inhibiting endoplasmic reticulum stress and caspase-3 expression [105,109,129]. Further research is needed to determine the effects of these mitochondria on other tissues and damaging agents. Interestingly, researchers recently identified a stress response in adipocytes that prevents oxidative damage in the heart through the release of EVs with mitochondrial content [26]. In the same line, mitochondria-rich EVs from autologous cardiomyocytes derived from stem cells have been shown to improve the bioenergetics of the ischemic heart [31], as well as an increment of the viability of cardiomyocytes in a model of doxorubicin injury [112].
It is interesting to note that many of these pathways affected by the treatment with a mitochondrial transfer, such as decreasing the inflammatory response, lowering oxidative stress, or regulating apoptosis, are also affected by treatment with EVs in models of tissue damage and regeneration [130], suggesting that there may be common mechanisms of action between these two approaches.
Moreover, as stated earlier, neurodegenerative diseases are characterized by a sharp increase in dysfunctional mitochondria. The transfer of healthy mitochondria has demonstrated positive effects in mouse models of Alzheimer’s and Parkinson’s disease [113,114]. These effects include decreases in neuronal loss, reduced gliosis in the hippocampus, and the amelioration of mitochondrial dysfunction in the brain.
One of the most promising domains of mitochondrial transfer may have a curative role in the field of mitochondrial diseases. Mitochondrial diseases are caused by mutations in mtDNA and affect the function of mitochondria and oxidative phosphorylation. These mutations can lead to a wide range of clinical manifestations, including muscle weakness, neurological symptoms, developmental delay, and organ failure. The severity and pattern of symptoms can vary widely depending on the nature and location of the mtDNA mutation and the level of heteroplasmy [131]. The transfer of healthy mitochondria to cells that have defective mitochondria with mtDNA mutations could help to restore cellular function, as small changes in the ranges of heteroplasmy could lead to improved tissue function [132]. This approach has shown beneficial effects in genetic diseases related to mutations in mtDNA, improving bioenergetics in cells that carry mutated mitochondria [115,116,117].
6. Conclusions and Future Perspectives
MitoEVs carry a diversity of mitochondria and mitochondrial components (mtDNA, mtRNA, rRNA, tRNA, and protein complexes). This cargo can be a part of mitochondria quality control, where cells release the trash to the extracellular space. However, this cargo can also be a rescue package for damaged cells uptaking these MitoEVs. Therefore, the exact function of MitoEVs depends on the context of the donor and target cells. To date, the exact mechanism for the selective package of mitochondrial components within MitoEVs is still in its infancy, and more research in the field is needed.
MitoEVs carrying damaged mitochondria components are currently being investigated for their usefulness as early disease biomarkers. There has been a particular emphasis on cancer, neurodegenerative disorders, and cardiovascular diseases regarding diagnosis, prognosis, and treatment monitoring. Currently, the main use of MitoEVs as therapeutic agents is mitochondrial transfer, which involves transferring healthy mitochondria to cells with dysfunctional mitochondria, restoring their energetic profile, and improving the tissue’s regenerative potential.
These findings provide a future pathway for MitoEVs-based therapies and the use of this subtype of EVs as biomarkers. Moreover, the ability of MitoEVs to modulate important pathways and processes, such as immune response, has been highlighted. However, there are still many questions that need to be addressed before expanding the use of MitoEVs is expanded, such as how to separate vesicles from the mitochondrial origin and other subtypes of EVs.
Author Contributions
Conceptualization, figure preparation, and writing—original draft preparation, J.S.-R. and C.M.-B.; writing—review and editing, C.B.; visualization and supervision, N.R.-G., J.H.-A. and M.D.; supervision, C.B.; funding acquisition, C.M.-B. and C.B. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the following grants: Grant PID2020–113839RB-I00 funded by MCIN/AEI/10.13039/501100011033, PCIN-2017–117 of the Ministry of Economy and Competitiveness, and the EU Joint Programming Initiative “A Healthy Diet for a Healthy Life” (JPI HDHL INTIMIC-085) to C.B., and CIGE/2021/134 from Conselleria de Innovación, Universidades, Ciencia y Sociedad Digital to C.M.-B. Part of the equipment employed in this work was funded by Generalitat Valenciana and co-financed with ERDF funds (OP ERDF of Comunitat Valenciana 2014–2020).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data sharing not applicable.
Acknowledgments
Figures were created using BioRender.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Brand, M.D.; Orr, A.L.; Perevoshchikova, I.V.; Quinlan, C.L. The role of mitochondrial function and cellular bioenergetics in ageing and disease. Br. J. Dermatol. 2013, 169 (Suppl. S2), 1–8. [Google Scholar] [CrossRef] [PubMed]
- Giorgi, C.; Agnoletto, C.; Bononi, A.; Bonora, M.; De Marchi, E.; Marchi, S.; Missiroli, S.; Patergnani, S.; Poletti, F.; Rimessi, A.; et al. Mitochondrial calcium homeostasis as potential target for mitochondrial medicine. Mitochondrion 2012, 12, 77–85. [Google Scholar] [CrossRef] [PubMed]
- Giorgi, C.; Romagnoli, A.; Pinton, P.; Rizzuto, R. Ca2+ signaling, mitochondria and cell death. Curr. Mol. Med. 2008, 8, 119–130. [Google Scholar] [CrossRef]
- Torralba, D.; Baixauli, F.; Sanchez-Madrid, F. Mitochondria Know No Boundaries: Mechanisms and Functions of Intercellular Mitochondrial Transfer. Front. Cell Dev. Biol. 2016, 4, 107. [Google Scholar] [CrossRef]
- Katajisto, P.; Dohla, J.; Chaffer, C.L.; Pentinmikko, N.; Marjanovic, N.; Iqbal, S.; Zoncu, R.; Chen, W.; Weinberg, R.A.; Sabatini, D.M. Stem cells. Asymmetric apportioning of aged mitochondria between daughter cells is required for stemness. Science 2015, 348, 340–343. [Google Scholar] [CrossRef]
- Qin, Y.; Jiang, X.; Yang, Q.; Zhao, J.; Zhou, Q.; Zhou, Y. The Functions, Methods, and Mobility of Mitochondrial Transfer between Cells. Front. Oncol. 2021, 11, 672781. [Google Scholar] [CrossRef]
- Yang, F.; Zhang, Y.; Liu, S.; Xiao, J.; He, Y.; Shao, Z.; Zhang, Y.; Cai, X.; Xiong, L. Tunneling Nanotube-Mediated Mitochondrial Transfer Rescues Nucleus Pulposus Cells from Mitochondrial Dysfunction and Apoptosis. Oxidative Med. Cell. Longev. 2022, 2022, 3613319. [Google Scholar] [CrossRef]
- Thomas, M.A.; Fahey, M.J.; Pugliese, B.R.; Irwin, R.M.; Antonyak, M.A.; Delco, M.L. Human mesenchymal stromal cells release functional mitochondria in extracellular vesicles. Front. Bioeng. Biotechnol. 2022, 10, 870193. [Google Scholar] [CrossRef] [PubMed]
- Norris, R.P. Transfer of mitochondria and endosomes between cells by gap junction internalization. Traffic 2021, 22, 174–179. [Google Scholar] [CrossRef]
- Wada, K.I.; Hosokawa, K.; Ito, Y.; Maeda, M. Quantitatively Controlled Intercellular Mitochondrial Transfer by Cell Fusion-Based Method Using a Microfluidic Device. Methods Mol. Biol. 2021, 2277, 39–47. [Google Scholar] [CrossRef]
- Chou, S.H.; Lan, J.; Esposito, E.; Ning, M.; Balaj, L.; Ji, X.; Lo, E.H.; Hayakawa, K. Extracellular Mitochondria in Cerebrospinal Fluid and Neurological Recovery After Subarachnoid Hemorrhage. Stroke 2017, 48, 2231–2237. [Google Scholar] [CrossRef] [PubMed]
- Al Amir Dache, Z.; Otandault, A.; Tanos, R.; Pastor, B.; Meddeb, R.; Sanchez, C.; Arena, G.; Lasorsa, L.; Bennett, A.; Grange, T.; et al. Blood contains circulating cell-free respiratory competent mitochondria. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2020, 34, 3616–3630. [Google Scholar] [CrossRef] [PubMed]
- Mas-Bargues, C.; Alique, M. Extracellular Vesicles as “Very Important Particles” (VIPs) in Aging. Int. J. Mol. Sci. 2023, 24, 4250. [Google Scholar] [CrossRef]
- Théry, C.; Witwer, K.W.; Aikawa, E.; Alcaraz, M.J.; Anderson, J.D.; Andriantsitohaina, R.; Antoniou, A.; Arab, T.; Archer, F.; Atkin-Smith, G.K.; et al. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): A position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J. Extracell. Vesicles 2018, 7, 1535750. [Google Scholar] [CrossRef]
- Sanz-Ros, J.; Mas-Bargues, C.; Romero-Garcia, N.; Huete-Acevedo, J.; Dromant, M.; Borras, C. Extracellular Vesicles as Therapeutic Resources in the Clinical Environment. Int. J. Mol. Sci. 2023, 24, 2344. [Google Scholar] [CrossRef] [PubMed]
- Spees, J.L.; Olson, S.D.; Whitney, M.J.; Prockop, D.J. Mitochondrial transfer between cells can rescue aerobic respiration. Proc. Natl. Acad. Sci. USA 2006, 103, 1283–1288. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.N.; Das, S.R.; Emin, M.T.; Wei, M.; Sun, L.; Westphalen, K.; Rowlands, D.J.; Quadri, S.K.; Bhattacharya, S.; Bhattacharya, J. Mitochondrial transfer from bone-marrow-derived stromal cells to pulmonary alveoli protects against acute lung injury. Nat. Med. 2012, 18, 759–765. [Google Scholar] [CrossRef] [PubMed]
- Guescini, M.; Genedani, S.; Stocchi, V.; Agnati, L.F. Astrocytes and Glioblastoma cells release exosomes carrying mtDNA. J. Neural Transm. 2010, 117, 1–4. [Google Scholar] [CrossRef]
- Guescini, M.; Guidolin, D.; Vallorani, L.; Casadei, L.; Gioacchini, A.M.; Tibollo, P.; Battistelli, M.; Falcieri, E.; Battistin, L.; Agnati, L.F.; et al. C2C12 myoblasts release micro-vesicles containing mtDNA and proteins involved in signal transduction. Exp. Cell Res. 2010, 316, 1977–1984. [Google Scholar] [CrossRef]
- Sansone, P.; Savini, C.; Kurelac, I.; Chang, Q.; Amato, L.B.; Strillacci, A.; Stepanova, A.; Iommarini, L.; Mastroleo, C.; Daly, L.; et al. Packaging and transfer of mitochondrial DNA via exosomes regulate escape from dormancy in hormonal therapy-resistant breast cancer. Proc. Natl. Acad. Sci. USA 2017, 114, E9066–E9075. [Google Scholar] [CrossRef]
- Rabas, N.; Palmer, S.; Mitchell, L.; Ismail, S.; Gohlke, A.; Riley, J.S.; Tait, S.W.G.; Gammage, P.; Soares, L.L.; Macpherson, I.R.; et al. PINK1 drives production of mtDNA-containing extracellular vesicles to promote invasiveness. J. Cell Biol. 2021, 220, e202006049. [Google Scholar] [CrossRef] [PubMed]
- Takenaga, K.; Koshikawa, N.; Nagase, H. Intercellular transfer of mitochondrial DNA carrying metastasis-enhancing pathogenic mutations from high- to low-metastatic tumor cells and stromal cells via extracellular vesicles. BMC Mol. Cell Biol. 2021, 22, 52. [Google Scholar] [CrossRef] [PubMed]
- Dluzen, D.F.; Noren Hooten, N.; De, S.; Wood, W.H., 3rd; Zhang, Y.; Becker, K.G.; Zonderman, A.B.; Tanaka, T.; Ferrucci, L.; Evans, M.K. Extracellular RNA profiles with human age. Aging Cell 2018, 17, e12785. [Google Scholar] [CrossRef] [PubMed]
- Peruzzotti-Jametti, L.; Bernstock, J.D.; Willis, C.M.; Manferrari, G.; Rogall, R.; Fernandez-Vizarra, E.; Williamson, J.C.; Braga, A.; van den Bosch, A.; Leonardi, T.; et al. Neural stem cells traffic functional mitochondria via extracellular vesicles. PLoS Biol. 2021, 19, e3001166. [Google Scholar] [CrossRef]
- Jang, S.C.; Crescitelli, R.; Cvjetkovic, A.; Belgrano, V.; Olofsson Bagge, R.; Sundfeldt, K.; Ochiya, T.; Kalluri, R.; Lotvall, J. Mitochondrial protein enriched extracellular vesicles discovered in human melanoma tissues can be detected in patient plasma. J. Extracell. Vesicles 2019, 8, 1635420. [Google Scholar] [CrossRef]
- Crewe, C.; Funcke, J.B.; Li, S.; Joffin, N.; Gliniak, C.M.; Ghaben, A.L.; An, Y.A.; Sadek, H.A.; Gordillo, R.; Akgul, Y.; et al. Extracellular vesicle-based interorgan transport of mitochondria from energetically stressed adipocytes. Cell Metab. 2021, 33, 1853–1868.e11. [Google Scholar] [CrossRef]
- D’Acunzo, P.; Perez-Gonzalez, R.; Kim, Y.; Hargash, T.; Miller, C.; Alldred, M.J.; Erdjument-Bromage, H.; Penikalapati, S.C.; Pawlik, M.; Saito, M.; et al. Mitovesicles are a novel population of extracellular vesicles of mitochondrial origin altered in Down syndrome. Sci. Adv. 2021, 7, eabe5085. [Google Scholar] [CrossRef]
- Todkar, K.; Chikhi, L.; Desjardins, V.; El-Mortada, F.; Pepin, G.; Germain, M. Selective packaging of mitochondrial proteins into extracellular vesicles prevents the release of mitochondrial DAMPs. Nat. Commun. 2021, 12, 1971. [Google Scholar] [CrossRef]
- Hough, K.P.; Trevor, J.L.; Strenkowski, J.G.; Wang, Y.; Chacko, B.K.; Tousif, S.; Chanda, D.; Steele, C.; Antony, V.B.; Dokland, T.; et al. Exosomal transfer of mitochondria from airway myeloid-derived regulatory cells to T cells. Redox Biol. 2018, 18, 54–64. [Google Scholar] [CrossRef]
- D’Souza, A.; Burch, A.; Dave, K.M.; Sreeram, A.; Reynolds, M.J.; Dobbins, D.X.; Kamte, Y.S.; Zhao, W.; Sabatelle, C.; Joy, G.M.; et al. Microvesicles transfer mitochondria and increase mitochondrial function in brain endothelial cells. J. Control. Release Off. J. Control. Release Soc. 2021, 338, 505–526. [Google Scholar] [CrossRef]
- Ikeda, G.; Santoso, M.R.; Tada, Y.; Li, A.M.; Vaskova, E.; Jung, J.H.; O’Brien, C.; Egan, E.; Ye, J.; Yang, P.C. Mitochondria-Rich Extracellular Vesicles From Autologous Stem Cell-Derived Cardiomyocytes Restore Energetics of Ischemic Myocardium. J. Am. Coll. Cardiol. 2021, 77, 1073–1088. [Google Scholar] [CrossRef] [PubMed]
- Jayaprakash, A.D.; Benson, E.K.; Gone, S.; Liang, R.; Shim, J.; Lambertini, L.; Toloue, M.M.; Wigler, M.; Aaronson, S.A.; Sachidanandam, R. Stable heteroplasmy at the single-cell level is facilitated by intercellular exchange of mtDNA. Nucleic Acids Res. 2015, 43, 2177–2187. [Google Scholar] [CrossRef] [PubMed]
- Marti Gutierrez, N.; Mikhalchenko, A.; Ma, H.; Koski, A.; Li, Y.; Van Dyken, C.; Tippner-Hedges, R.; Yoon, D.; Liang, D.; Hayama, T.; et al. Horizontal mtDNA transfer between cells is common during mouse development. iScience 2022, 25, 103901. [Google Scholar] [CrossRef]
- Krisher, R.L.; Prather, R.S. A role for the Warburg effect in preimplantation embryo development: Metabolic modification to support rapid cell proliferation. Mol. Reprod. Dev. 2012, 79, 311–320. [Google Scholar] [CrossRef]
- Bennett, C.F.; Latorre-Muro, P.; Puigserver, P. Mechanisms of mitochondrial respiratory adaptation. Nat. Rev. Mol. Cell Biol. 2022, 23, 817–835. [Google Scholar] [CrossRef] [PubMed]
- Davis, C.H.; Marsh-Armstrong, N. Discovery and implications of transcellular mitophagy. Autophagy 2014, 10, 2383–2384. [Google Scholar] [CrossRef]
- Dong, W.; Zhang, W.; Yuan, L.; Xie, Y.; Li, Y.; Li, K.; Zhu, W. Rescuers from the Other Shore: Intercellular Mitochondrial Transfer and Its Implications in Central Nervous System Injury and Diseases. Cell. Mol. Neurobiol. 2023. [Google Scholar] [CrossRef]
- Cai, W.; Zhang, J.; Yu, Y.; Ni, Y.; Wei, Y.; Cheng, Y.; Han, L.; Xiao, L.; Ma, X.; Wei, H.; et al. Mitochondrial Transfer Regulates Cell Fate Through Metabolic Remodeling in Osteoporosis. Adv. Sci. 2023, 10, e2204871. [Google Scholar] [CrossRef]
- Fairley, L.H.; Grimm, A.; Eckert, A. Mitochondria Transfer in Brain Injury and Disease. Cells 2022, 11, 3603. [Google Scholar] [CrossRef]
- Stephens, O.R.; Grant, D.; Frimel, M.; Wanner, N.; Yin, M.; Willard, B.; Erzurum, S.C.; Asosingh, K. Characterization and origins of cell-free mitochondria in healthy murine and human blood. Mitochondrion 2020, 54, 102–112. [Google Scholar] [CrossRef]
- Kiriyama, Y.; Nochi, H. Intra- and Intercellular Quality Control Mechanisms of Mitochondria. Cells 2017, 7, 1. [Google Scholar] [CrossRef] [PubMed]
- Davis, C.H.; Kim, K.Y.; Bushong, E.A.; Mills, E.A.; Boassa, D.; Shih, T.; Kinebuchi, M.; Phan, S.; Zhou, Y.; Bihlmeyer, N.A.; et al. Transcellular degradation of axonal mitochondria. Proc. Natl. Acad. Sci. USA 2014, 111, 9633–9638. [Google Scholar] [CrossRef]
- Gomzikova, M.O.; James, V.; Rizvanov, A.A. Mitochondria Donation by Mesenchymal Stem Cells: Current Understanding and Mitochondria Transplantation Strategies. Front. Cell Dev. Biol. 2021, 9, 653322. [Google Scholar] [CrossRef]
- Ahmad, T.; Mukherjee, S.; Pattnaik, B.; Kumar, M.; Singh, S.; Kumar, M.; Rehman, R.; Tiwari, B.K.; Jha, K.A.; Barhanpurkar, A.P.; et al. Miro1 regulates intercellular mitochondrial transport & enhances mesenchymal stem cell rescue efficacy. EMBO J. 2014, 33, 994–1010. [Google Scholar] [CrossRef]
- Jiang, D.; Gao, F.; Zhang, Y.; Wong, D.S.; Li, Q.; Tse, H.F.; Xu, G.; Yu, Z.; Lian, Q. Mitochondrial transfer of mesenchymal stem cells effectively protects corneal epithelial cells from mitochondrial damage. Cell Death Dis. 2016, 7, e2467. [Google Scholar] [CrossRef] [PubMed]
- Chuang, Y.C.; Liou, C.W.; Chen, S.D.; Wang, P.W.; Chuang, J.H.; Tiao, M.M.; Hsu, T.Y.; Lin, H.Y.; Lin, T.K. Mitochondrial Transfer from Wharton’s Jelly Mesenchymal Stem Cell to MERRF Cybrid Reduces Oxidative Stress and Improves Mitochondrial Bioenergetics. Oxidative Med. Cell. Longev. 2017, 2017, 5691215. [Google Scholar] [CrossRef] [PubMed]
- Moehle, E.A.; Shen, K.; Dillin, A. Mitochondrial proteostasis in the context of cellular and organismal health and aging. J. Biol. Chem. 2019, 294, 5396–5407. [Google Scholar] [CrossRef]
- Ravanelli, S.; den Brave, F.; Hoppe, T. Mitochondrial Quality Control Governed by Ubiquitin. Front. Cell Dev. Biol. 2020, 8, 270. [Google Scholar] [CrossRef]
- Bragoszewski, P.; Turek, M.; Chacinska, A. Control of mitochondrial biogenesis and function by the ubiquitin-proteasome system. Open Biol. 2017, 7, 170007. [Google Scholar] [CrossRef]
- Song, J.; Herrmann, J.M.; Becker, T. Quality control of the mitochondrial proteome. Nat. Rev. Mol. Cell Biol. 2021, 22, 54–70. [Google Scholar] [CrossRef]
- Lavie, J.; De Belvalet, H.; Sonon, S.; Ion, A.M.; Dumon, E.; Melser, S.; Lacombe, D.; Dupuy, J.W.; Lalou, C.; Benard, G. Ubiquitin-Dependent Degradation of Mitochondrial Proteins Regulates Energy Metabolism. Cell Rep. 2018, 23, 2852–2863. [Google Scholar] [CrossRef] [PubMed]
- Westermann, B. Mitochondrial fusion and fission in cell life and death. Nat. Rev. Mol. Cell Biol. 2010, 11, 872–884. [Google Scholar] [CrossRef] [PubMed]
- Ni, H.M.; Williams, J.A.; Ding, W.X. Mitochondrial dynamics and mitochondrial quality control. Redox Biol. 2015, 4, 6–13. [Google Scholar] [CrossRef]
- Suarez-Rivero, J.M.; Villanueva-Paz, M.; de la Cruz-Ojeda, P.; de la Mata, M.; Cotan, D.; Oropesa-Avila, M.; de Lavera, I.; Alvarez-Cordoba, M.; Luzon-Hidalgo, R.; Sanchez-Alcazar, J.A. Mitochondrial Dynamics in Mitochondrial Diseases. Diseases 2016, 5, 1. [Google Scholar] [CrossRef]
- Li, J.; Dang, X.; Franco, A.; Dorn, G.W., 2nd. Reciprocal Regulation of Mitofusin 2-Mediated Mitophagy and Mitochondrial Fusion by Different PINK1 Phosphorylation Events. Front. Cell Dev. Biol. 2022, 10, 868465. [Google Scholar] [CrossRef] [PubMed]
- Franco, A.; Walton, C.E.; Dang, X. Mitochondria Clumping vs. Mitochondria Fusion in CMT2A Diseases. Life 2022, 12, 2110. [Google Scholar] [CrossRef] [PubMed]
- Pickles, S.; Vigie, P.; Youle, R.J. Mitophagy and Quality Control Mechanisms in Mitochondrial Maintenance. Curr. Biol. 2018, 28, R170–R185. [Google Scholar] [CrossRef] [PubMed]
- Ashrafi, G.; Schwarz, T.L. The pathways of mitophagy for quality control and clearance of mitochondria. Cell Death Differ. 2013, 20, 31–42. [Google Scholar] [CrossRef]
- Mondal, P.; Towers, C. Beyond mitophagy: Mitochondrial-derived vesicles can get the job done! Autophagy 2022, 18, 449–451. [Google Scholar] [CrossRef]
- Towers, C.G.; Wodetzki, D.K.; Thorburn, J.; Smith, K.R.; Caino, M.C.; Thorburn, A. Mitochondrial-derived vesicles compensate for loss of LC3-mediated mitophagy. Dev. Cell 2021, 56, 2029–2042.e5. [Google Scholar] [CrossRef]
- Poillet-Perez, L.; White, E. MDVs to the rescue: How autophagy-deficient cancer cells adapt to defective mitophagy. Dev. Cell 2021, 56, 2010–2012. [Google Scholar] [CrossRef]
- Rosina, M.; Ceci, V.; Turchi, R.; Chuan, L.; Borcherding, N.; Sciarretta, F.; Sanchez-Diaz, M.; Tortolici, F.; Karlinsey, K.; Chiurchiu, V.; et al. Ejection of damaged mitochondria and their removal by macrophages ensure efficient thermogenesis in brown adipose tissue. Cell Metab. 2022, 34, 533–548.e12. [Google Scholar] [CrossRef] [PubMed]
- Phinney, D.G.; Di Giuseppe, M.; Njah, J.; Sala, E.; Shiva, S.; St Croix, C.M.; Stolz, D.B.; Watkins, S.C.; Di, Y.P.; Leikauf, G.D.; et al. Mesenchymal stem cells use extracellular vesicles to outsource mitophagy and shuttle microRNAs. Nat. Commun. 2015, 6, 8472. [Google Scholar] [CrossRef] [PubMed]
- McLelland, G.L.; Soubannier, V.; Chen, C.X.; McBride, H.M.; Fon, E.A. Parkin and PINK1 function in a vesicular trafficking pathway regulating mitochondrial quality control. EMBO J. 2014, 33, 282–295. [Google Scholar] [CrossRef] [PubMed]
- McLelland, G.L.; Lee, S.A.; McBride, H.M.; Fon, E.A. Syntaxin-17 delivers PINK1/parkin-dependent mitochondrial vesicles to the endolysosomal system. J. Cell Biol. 2016, 214, 275–291. [Google Scholar] [CrossRef]
- Sugiura, A.; McLelland, G.L.; Fon, E.A.; McBride, H.M. A new pathway for mitochondrial quality control: Mitochondrial-derived vesicles. EMBO J. 2014, 33, 2142–2156. [Google Scholar] [CrossRef] [PubMed]
- Picca, A.; Guerra, F.; Calvani, R.; Bucci, C.; Lo Monaco, M.R.; Bentivoglio, A.R.; Coelho-Júnior, H.J.; Landi, F.; Bernabei, R.; Marzetti, E. Mitochondrial Dysfunction and Aging: Insights from the Analysis of Extracellular Vesicles. Int. J. Mol. Sci. 2019, 20, 805. [Google Scholar] [CrossRef]
- Picca, A.; Guerra, F.; Calvani, R.; Coelho-Junior, H.J.; Bossola, M.; Landi, F.; Bernabei, R.; Bucci, C.; Marzetti, E. Generation and Release of Mitochondrial-Derived Vesicles in Health, Aging and Disease. J. Clin. Med. 2020, 9, 1440. [Google Scholar] [CrossRef]
- Popov, L.D. Mitochondrial-derived vesicles: Recent insights. J. Cell. Mol. Med. 2022, 26, 3323–3328. [Google Scholar] [CrossRef]
- Sharma, A. Mitochondrial cargo export in exosomes: Possible pathways and implication in disease biology. J. Cell. Physiol. 2023. [Google Scholar] [CrossRef]
- Liang, W.; Diao, R.Y.; Quiles, J.M.; Najor, R.H.; Chi, L.; Woodall, B.P.; Leon, L.J.; Duran, J.; Cauvi, D.M.; De Maio, A.; et al. The Small GTPase Rab7 Regulates Release of Mitochondria in Extracellular Vesicles in Response to Lysosomal Dysfunction. bioRxiv 2023. [Google Scholar] [CrossRef]
- Cho, Y.M.; Kim, J.H.; Kim, M.; Park, S.J.; Koh, S.H.; Ahn, H.S.; Kang, G.H.; Lee, J.B.; Park, K.S.; Lee, H.K. Mesenchymal stem cells transfer mitochondria to the cells with virtually no mitochondrial function but not with pathogenic mtDNA mutations. PLoS ONE 2012, 7, e32778. [Google Scholar] [CrossRef] [PubMed]
- Dutra Silva, J.; Su, Y.; Calfee, C.S.; Delucchi, K.L.; Weiss, D.; McAuley, D.F.; O’Kane, C.; Krasnodembskaya, A.D. Mesenchymal stromal cell extracellular vesicles rescue mitochondrial dysfunction and improve barrier integrity in clinically relevant models of ARDS. Eur. Respir. J. 2021, 58, 2002978. [Google Scholar] [CrossRef] [PubMed]
- Acquistapace, A.; Bru, T.; Lesault, P.F.; Figeac, F.; Coudert, A.E.; le Coz, O.; Christov, C.; Baudin, X.; Auber, F.; Yiou, R.; et al. Human mesenchymal stem cells reprogram adult cardiomyocytes toward a progenitor-like state through partial cell fusion and mitochondria transfer. Stem Cells 2011, 29, 812–824. [Google Scholar] [CrossRef] [PubMed]
- Levoux, J.; Prola, A.; Lafuste, P.; Gervais, M.; Chevallier, N.; Koumaiha, Z.; Kefi, K.; Braud, L.; Schmitt, A.; Yacia, A.; et al. Platelets Facilitate the Wound-Healing Capability of Mesenchymal Stem Cells by Mitochondrial Transfer and Metabolic Reprogramming. Cell Metab. 2021, 33, 283–299.e9. [Google Scholar] [CrossRef]
- Weinberg, S.E.; Sena, L.A.; Chandel, N.S. Mitochondria in the regulation of innate and adaptive immunity. Immunity 2015, 42, 406–417. [Google Scholar] [CrossRef]
- Morrison, T.J.; Jackson, M.V.; Cunningham, E.K.; Kissenpfennig, A.; McAuley, D.F.; O’Kane, C.M.; Krasnodembskaya, A.D. Mesenchymal Stromal Cells Modulate Macrophages in Clinically Relevant Lung Injury Models by Extracellular Vesicle Mitochondrial Transfer. Am. J. Respir. Crit. Care Med. 2017, 196, 1275–1286. [Google Scholar] [CrossRef]
- Lo Sicco, C.; Reverberi, D.; Balbi, C.; Ulivi, V.; Principi, E.; Pascucci, L.; Becherini, P.; Bosco, M.C.; Varesio, L.; Franzin, C.; et al. Mesenchymal Stem Cell-Derived Extracellular Vesicles as Mediators of Anti-Inflammatory Effects: Endorsement of Macrophage Polarization. Stem Cells Transl. Med. 2017, 6, 1018–1028. [Google Scholar] [CrossRef]
- Van der Vlist, M.; Raoof, R.; Willemen, H.; Prado, J.; Versteeg, S.; Martin Gil, C.; Vos, M.; Lokhorst, R.E.; Pasterkamp, R.J.; Kojima, T.; et al. Macrophages transfer mitochondria to sensory neurons to resolve inflammatory pain. Neuron 2022, 110, 613–626.e9. [Google Scholar] [CrossRef]
- Berridge, M.V.; Schneider, R.T.; McConnell, M.J. Mitochondrial Transfer from Astrocytes to Neurons following Ischemic Insult: Guilt by Association? Cell Metab. 2016, 24, 376–378. [Google Scholar] [CrossRef]
- Lone, S.N.; Nisar, S.; Masoodi, T.; Singh, M.; Rizwan, A.; Hashem, S.; El-Rifai, W.; Bedognetti, D.; Batra, S.K.; Haris, M.; et al. Liquid biopsy: A step closer to transform diagnosis, prognosis and future of cancer treatments. Mol. Cancer 2022, 21, 79. [Google Scholar] [CrossRef] [PubMed]
- Ignatiadis, M.; Sledge, G.W.; Jeffrey, S.S. Liquid biopsy enters the clinic—Implementation issues and future challenges. Nat. Rev. Clin. Oncol. 2021, 18, 297–312. [Google Scholar] [CrossRef] [PubMed]
- Corcoran, R.B.; Chabner, B.A. Application of Cell-free DNA Analysis to Cancer Treatment. N. Engl. J. Med. 2018, 379, 1754–1765. [Google Scholar] [CrossRef]
- Zhou, B.; Xu, K.; Zheng, X.; Chen, T.; Wang, J.; Song, Y.; Shao, Y.; Zheng, S. Application of exosomes as liquid biopsy in clinical diagnosis. Signal Transduct. Target. Ther. 2020, 5, 144. [Google Scholar] [CrossRef]
- Ciferri, M.C.; Quarto, R.; Tasso, R. Extracellular Vesicles as Biomarkers and Therapeutic Tools: From Pre-Clinical to Clinical Applications. Biology 2021, 10, 359. [Google Scholar] [CrossRef] [PubMed]
- Yu, D.; Li, Y.; Wang, M.; Gu, J.; Xu, W.; Cai, H.; Fang, X.; Zhang, X. Exosomes as a new frontier of cancer liquid biopsy. Mol. Cancer 2022, 21, 56. [Google Scholar] [CrossRef]
- Zong, W.X.; Rabinowitz, J.D.; White, E. Mitochondria and Cancer. Mol. Cell 2016, 61, 667–676. [Google Scholar] [CrossRef]
- Mears, R.; Craven, R.A.; Hanrahan, S.; Totty, N.; Upton, C.; Young, S.L.; Patel, P.; Selby, P.J.; Banks, R.E. Proteomic analysis of melanoma-derived exosomes by two-dimensional polyacrylamide gel electrophoresis and mass spectrometry. Proteomics 2004, 4, 4019–4031. [Google Scholar] [CrossRef]
- Staubach, S.; Razawi, H.; Hanisch, F.G. Proteomics of MUC1-containing lipid rafts from plasma membranes and exosomes of human breast carcinoma cells MCF-7. Proteomics 2009, 9, 2820–2835. [Google Scholar] [CrossRef]
- Vikramdeo, K.S.; Anand, S.; Khan, M.A.; Khushman, M.; Heslin, M.J.; Singh, S.; Singh, A.P.; Dasgupta, S. Detection of mitochondrial DNA mutations in circulating mitochondria-originated extracellular vesicles for potential diagnostic applications in pancreatic adenocarcinoma. Sci. Rep. 2022, 12, 18455. [Google Scholar] [CrossRef]
- Li, Y.; Guo, X.; Guo, S.; Wang, Y.; Chen, L.; Liu, Y.; Jia, M.; An, J.; Tao, K.; Xing, J. Next generation sequencing-based analysis of mitochondrial DNA characteristics in plasma extracellular vesicles of patients with hepatocellular carcinoma. Oncol. Lett. 2020, 20, 2820–2828. [Google Scholar] [CrossRef] [PubMed]
- Perier, C.; Vila, M. Mitochondrial biology and Parkinson’s disease. Cold Spring Harb. Perspect. Med. 2012, 2, a009332. [Google Scholar] [CrossRef] [PubMed]
- Dias, V.; Junn, E.; Mouradian, M.M. The role of oxidative stress in Parkinson’s disease. J. Parkinsons Dis. 2013, 3, 461–491. [Google Scholar] [CrossRef] [PubMed]
- Ge, P.; Dawson, V.L.; Dawson, T.M. PINK1 and Parkin mitochondrial quality control: A source of regional vulnerability in Parkinson’s disease. Mol. Neurodegener. 2020, 15, 20. [Google Scholar] [CrossRef]
- Ryan, T.A.; Phillips, E.O.; Collier, C.L.; Robinson, A.J.; Routledge, D.; Wood, R.E.; Assar, E.A.; Tumbarello, D.A. Tollip coordinates Parkin-dependent trafficking of mitochondrial-derived vesicles. EMBO J. 2020, 39, e102539. [Google Scholar] [CrossRef]
- Picca, A.; Guerra, F.; Calvani, R.; Marini, F.; Biancolillo, A.; Landi, G.; Beli, R.; Landi, F.; Bernabei, R.; Bentivoglio, A.R.; et al. Mitochondrial Signatures in Circulating Extracellular Vesicles of Older Adults with Parkinson’s Disease: Results from the EXosomes in PArkiNson’s Disease (EXPAND) Study. J. Clin. Med. 2020, 9, 504. [Google Scholar] [CrossRef]
- Izzo, A.; Mollo, N.; Nitti, M.; Paladino, S.; Calì, G.; Genesio, R.; Bonfiglio, F.; Cicatiello, R.; Barbato, M.; Sarnataro, V.; et al. Mitochondrial dysfunction in down syndrome: Molecular mechanisms and therapeutic targets. Mol. Med. 2018, 24, 2. [Google Scholar] [CrossRef]
- Coskun, P.E.; Busciglio, J. Oxidative Stress and Mitochondrial Dysfunction in Down’s Syndrome: Relevance to Aging and Dementia. Curr. Gerontol. Geriatr. Res. 2012, 2012, 383170. [Google Scholar] [CrossRef]
- Chistiakov, D.A.; Shkurat, T.P.; Melnichenko, A.A.; Grechko, A.V.; Orekhov, A.N. The role of mitochondrial dysfunction in cardiovascular disease: A brief review. Ann. Med. 2018, 50, 121–127. [Google Scholar] [CrossRef]
- Ballinger, S.W. Mitochondrial dysfunction in cardiovascular disease. Free Radic. Biol. Med. 2005, 38, 1278–1295. [Google Scholar] [CrossRef]
- Heyn, J.; Heuschkel, M.A.; Goettsch, C. Mitochondrial-Derived Vesicles-Link to Extracellular Vesicles and Implications in Cardiovascular Disease. Int. J. Mol. Sci. 2023, 24, 2637. [Google Scholar] [CrossRef] [PubMed]
- Cadete, V.J.; Deschênes, S.; Cuillerier, A.; Brisebois, F.; Sugiura, A.; Vincent, A.; Turnbull, D.; Picard, M.; McBride, H.M.; Burelle, Y. Formation of mitochondrial-derived vesicles is an active and physiologically relevant mitochondrial quality control process in the cardiac system. J. Physiol. 2016, 594, 5343–5362. [Google Scholar] [CrossRef] [PubMed]
- Puhm, F.; Afonyushkin, T.; Resch, U.; Obermayer, G.; Rohde, M.; Penz, T.; Schuster, M.; Wagner, G.; Rendeiro, A.F.; Melki, I.; et al. Mitochondria Are a Subset of Extracellular Vesicles Released by Activated Monocytes and Induce Type I IFN and TNF Responses in Endothelial Cells. Circ. Res. 2019, 125, 43–52. [Google Scholar] [CrossRef] [PubMed]
- Nicolás-Ávila, J.A.; Lechuga-Vieco, A.V.; Esteban-Martínez, L.; Sánchez-Díaz, M.; Díaz-García, E.; Santiago, D.J.; Rubio-Ponce, A.; Li, J.L.; Balachander, A.; Quintana, J.A.; et al. A Network of Macrophages Supports Mitochondrial Homeostasis in the Heart. Cell 2020, 183, 94–109.e23. [Google Scholar] [CrossRef]
- Shin, B.; Cowan, D.B.; Emani, S.M.; Del Nido, P.J.; McCully, J.D. Mitochondrial Transplantation in Myocardial Ischemia and Reperfusion Injury. Adv. Exp. Med. Biol. 2017, 982, 595–619. [Google Scholar] [CrossRef] [PubMed]
- Huang, P.J.; Kuo, C.C.; Lee, H.C.; Shen, C.I.; Cheng, F.C.; Wu, S.F.; Chang, J.C.; Pan, H.C.; Lin, S.Z.; Liu, C.S.; et al. Transferring Xenogenic Mitochondria Provides Neural Protection Against Ischemic Stress in Ischemic Rat Brains. Cell Transplant. 2016, 25, 913–927. [Google Scholar] [CrossRef]
- Zhang, Z.; Ma, Z.; Yan, C.; Pu, K.; Wu, M.; Bai, J.; Li, Y.; Wang, Q. Muscle-derived autologous mitochondrial transplantation: A novel strategy for treating cerebral ischemic injury. Behav. Brain Res. 2019, 356, 322–331. [Google Scholar] [CrossRef]
- Pourmohammadi-Bejarpasi, Z.; Roushandeh, A.M.; Saberi, A.; Rostami, M.K.; Toosi, S.M.R.; Jahanian-Najafabadi, A.; Tomita, K.; Kuwahara, Y.; Sato, T.; Roudkenar, M.H. Mesenchymal stem cells-derived mitochondria transplantation mitigates I/R-induced injury, abolishes I/R-induced apoptosis, and restores motor function in acute ischemia stroke rat model. Brain Res. Bull. 2020, 165, 70–80. [Google Scholar] [CrossRef]
- Orfany, A.; Arriola, C.G.; Doulamis, I.P.; Guariento, A.; Ramirez-Barbieri, G.; Moskowitzova, K.; Shin, B.; Blitzer, D.; Rogers, C.; Del Nido, P.J.; et al. Mitochondrial transplantation ameliorates acute limb ischemia. J. Vasc. Surg. 2020, 71, 1014–1026. [Google Scholar] [CrossRef]
- Moskowitzova, K.; Orfany, A.; Liu, K.; Ramirez-Barbieri, G.; Thedsanamoorthy, J.K.; Yao, R.; Guariento, A.; Doulamis, I.P.; Blitzer, D.; Shin, B.; et al. Mitochondrial transplantation enhances murine lung viability and recovery after ischemia-reperfusion injury. Am. J. Physiol. Lung Cell. Mol. Physiol. 2020, 318, L78–L88. [Google Scholar] [CrossRef]
- Doulamis, I.P.; Guariento, A.; Duignan, T.; Kido, T.; Orfany, A.; Saeed, M.Y.; Weixler, V.H.; Blitzer, D.; Shin, B.; Snay, E.R.; et al. Mitochondrial transplantation by intra-arterial injection for acute kidney injury. Am. J. Physiol. Renal. Physiol. 2020, 319, F403–F413. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, C.G.; Ozen, M.O.; Ikeda, G.; Vaskova, E.; Jung, J.H.; Bayardo, N.; Santoso, M.R.; Shi, L.; Wahlquist, C.; Jiang, Z.; et al. Mitochondria-Rich Extracellular Vesicles Rescue Patient-Specific Cardiomyocytes From Doxorubicin Injury: Insights Into the SENECA Trial. JACC CardioOncol 2021, 3, 428–440. [Google Scholar] [CrossRef] [PubMed]
- Nitzan, K.; Benhamron, S.; Valitsky, M.; Kesner, E.E.; Lichtenstein, M.; Ben-Zvi, A.; Ella, E.; Segalstein, Y.; Saada, A.; Lorberboum-Galski, H.; et al. Mitochondrial Transfer Ameliorates Cognitive Deficits, Neuronal Loss, and Gliosis in Alzheimer’s Disease Mice. J. Alzheimers Dis. 2019, 72, 587–604. [Google Scholar] [CrossRef]
- Chang, J.C.; Wu, S.L.; Liu, K.H.; Chen, Y.H.; Chuang, C.S.; Cheng, F.C.; Su, H.L.; Wei, Y.H.; Kuo, S.J.; Liu, C.S. Allogeneic/xenogeneic transplantation of peptide-labeled mitochondria in Parkinson’s disease: Restoration of mitochondria functions and attenuation of 6-hydroxydopamine-induced neurotoxicity. Transl. Res. 2016, 170, 40–56.e3. [Google Scholar] [CrossRef] [PubMed]
- Lin, T.K.; Chen, S.D.; Chuang, Y.C.; Lan, M.Y.; Chuang, J.H.; Wang, P.W.; Hsu, T.Y.; Wang, F.S.; Tsai, M.H.; Huang, S.T.; et al. Mitochondrial Transfer of Wharton’s Jelly Mesenchymal Stem Cells Eliminates Mutation Burden and Rescues Mitochondrial Bioenergetics in Rotenone-Stressed MELAS Fibroblasts. Oxid. Med. Cell Longev. 2019, 2019, 9537504. [Google Scholar] [CrossRef]
- Lin, H.Y.; Liou, C.W.; Chen, S.D.; Hsu, T.Y.; Chuang, J.H.; Wang, P.W.; Huang, S.T.; Tiao, M.M.; Chen, J.B.; Lin, T.K.; et al. Mitochondrial transfer from Wharton’s jelly-derived mesenchymal stem cells to mitochondria-defective cells recaptures impaired mitochondrial function. Mitochondrion 2015, 22, 31–44. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.C.; Liu, K.H.; Li, Y.C.; Kou, S.J.; Wei, Y.H.; Chuang, C.S.; Hsieh, M.; Liu, C.S. Functional recovery of human cells harbouring the mitochondrial DNA mutation MERRF A8344G via peptide-mediated mitochondrial delivery. Neurosignals 2013, 21, 160–173. [Google Scholar] [CrossRef] [PubMed]
- De Jong, O.G.; Van Balkom, B.W.; Schiffelers, R.M.; Bouten, C.V.; Verhaar, M.C. Extracellular vesicles: Potential roles in regenerative medicine. Front. Immunol. 2014, 5, 608. [Google Scholar] [CrossRef]
- Buzas, E.I.; György, B.; Nagy, G.; Falus, A.; Gay, S. Emerging role of extracellular vesicles in inflammatory diseases. Nat. Rev. Rheumatol. 2014, 10, 356–364. [Google Scholar] [CrossRef]
- Lener, T.; Gimona, M.; Aigner, L.; Börger, V.; Buzas, E.; Camussi, G.; Chaput, N.; Chatterjee, D.; Court, F.A.; Del Portillo, H.A.; et al. Applying extracellular vesicles based therapeutics in clinical trials—An ISEV position paper. J. Extracell. Vesicles 2015, 4, 30087. [Google Scholar] [CrossRef]
- Gimona, M.; Pachler, K.; Laner-Plamberger, S.; Schallmoser, K.; Rohde, E. Manufacturing of Human Extracellular Vesicle-Based Therapeutics for Clinical Use. Int. J. Mol. Sci. 2017, 18, 1190. [Google Scholar] [CrossRef]
- Barile, L.; Vassalli, G. Exosomes: Therapy delivery tools and biomarkers of diseases. Pharmacol. Ther. 2017, 174, 63–78. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Gao, Y.; Liu, J.; Huang, Y.; Yin, J.; Feng, Y.; Shi, L.; Meloni, B.P.; Zhang, C.; Zheng, M.; et al. Intercellular mitochondrial transfer as a means of tissue revitalization. Signal Transduct. Target. Ther. 2021, 6, 65. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Sun, Y.; Qi, Z.; Cao, L.; Ding, S. Mitochondrial transfer/transplantation: An emerging therapeutic approach for multiple diseases. Cell Biosci. 2022, 12, 66. [Google Scholar] [CrossRef] [PubMed]
- Ahlqvist, K.J.; Suomalainen, A.; Hämäläinen, R.H. Stem cells, mitochondria and aging. Biochim. Biophys. Acta 2015, 1847, 1380–1386. [Google Scholar] [CrossRef] [PubMed]
- Hong, X.; Isern, J.; Campanario, S.; Perdiguero, E.; Ramírez-Pardo, I.; Segalés, J.; Hernansanz-Agustín, P.; Curtabbi, A.; Deryagin, O.; Pollán, A.; et al. Mitochondrial dynamics maintain muscle stem cell regenerative competence throughout adult life by regulating metabolism and mitophagy. Cell Stem Cell 2022, 29, 1298–1314.e10. [Google Scholar] [CrossRef] [PubMed]
- López-Otín, C.; Blasco, M.A.; Partridge, L.; Serrano, M.; Kroemer, G. The hallmarks of aging. Cell 2013, 153, 1194–1217. [Google Scholar] [CrossRef]
- Fang, S.Y.; Roan, J.N.; Lee, J.S.; Chiu, M.H.; Lin, M.W.; Liu, C.C.; Lam, C.F. Transplantation of viable mitochondria attenuates neurologic injury after spinal cord ischemia. J. Thorac. Cardiovasc. Surg. 2021, 161, e337–e347. [Google Scholar] [CrossRef]
- Masuzawa, A.; Black, K.M.; Pacak, C.A.; Ericsson, M.; Barnett, R.J.; Drumm, C.; Seth, P.; Bloch, D.B.; Levitsky, S.; Cowan, D.B.; et al. Transplantation of autologously derived mitochondria protects the heart from ischemia-reperfusion injury. Am. J. Physiol. Heart Circ. Physiol. 2013, 304, H966–H982. [Google Scholar] [CrossRef]
- Nagelkerke, A.; Ojansivu, M.; van der Koog, L.; Whittaker, T.E.; Cunnane, E.M.; Silva, A.M.; Dekker, N.; Stevens, M.M. Extracellular vesicles for tissue repair and regeneration: Evidence, challenges and opportunities. Adv. Drug Deliv. Rev. 2021, 175, 113775. [Google Scholar] [CrossRef]
- Gorman, G.S.; Chinnery, P.F.; DiMauro, S.; Hirano, M.; Koga, Y.; McFarland, R.; Suomalainen, A.; Thorburn, D.R.; Zeviani, M.; Turnbull, D.M. Mitochondrial diseases. Nat. Rev. Dis. Prim. 2016, 2, 16080. [Google Scholar] [CrossRef] [PubMed]
- Wallace, D.C.; Chalkia, D. Mitochondrial DNA genetics and the heteroplasmy conundrum in evolution and disease. Cold Spring Harb. Perspect. Biol. 2013, 5, a021220. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).