Ionic Liquid-Supported Photocatalysts: A Reusable Environmentally Friendly Oxidation Reaction System That Uses Air and Light
Abstract
:1. Introduction
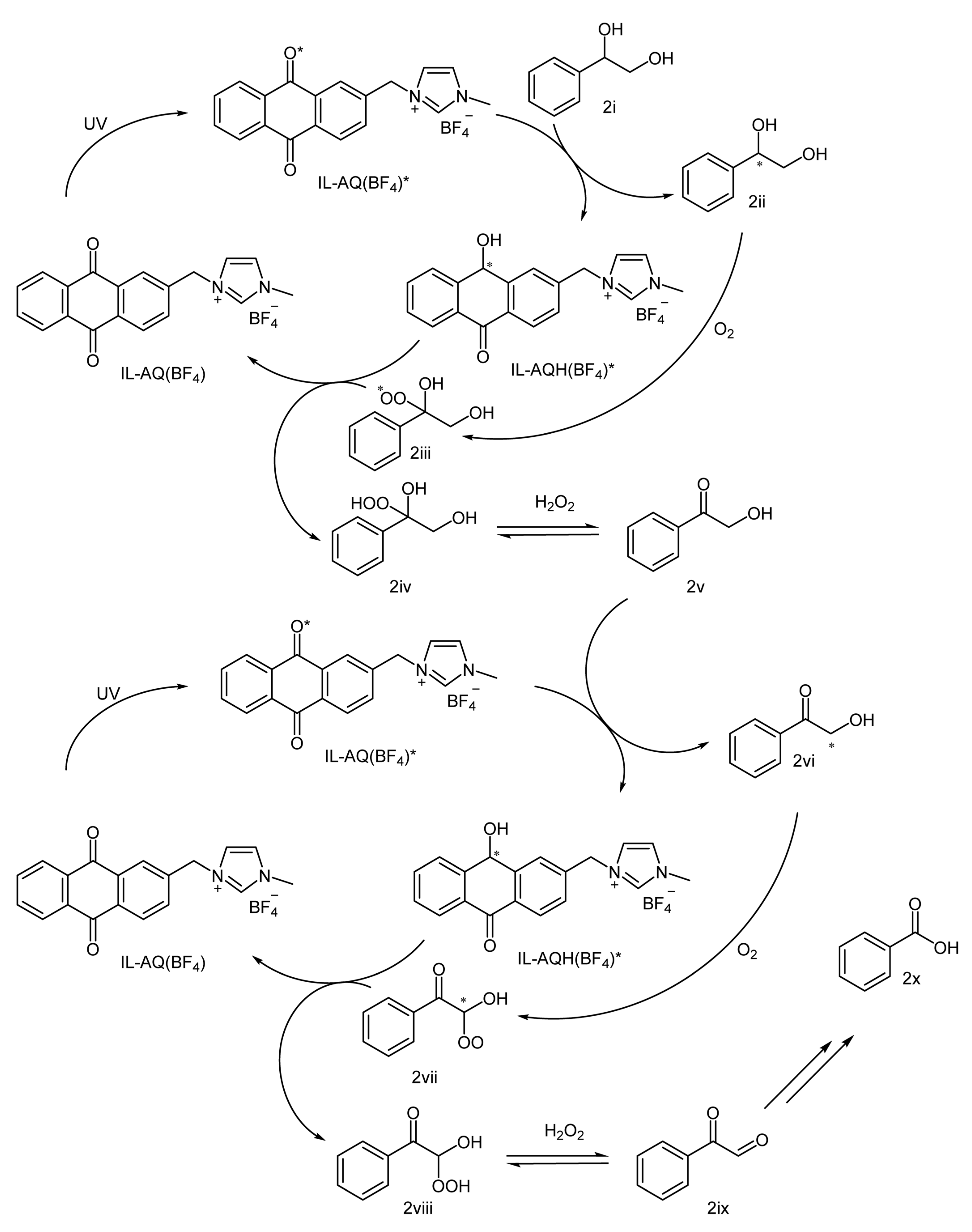
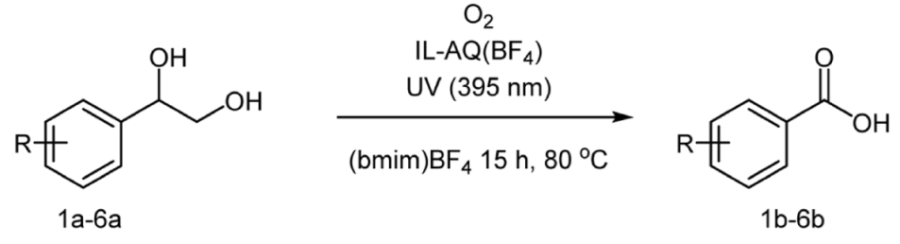
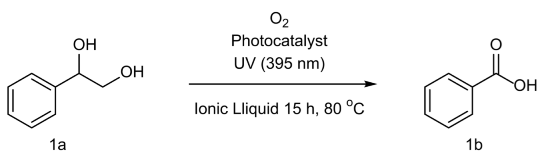
2. Results and Discussion
3. Materials and Methods
3.1. General Information Including Important Notices
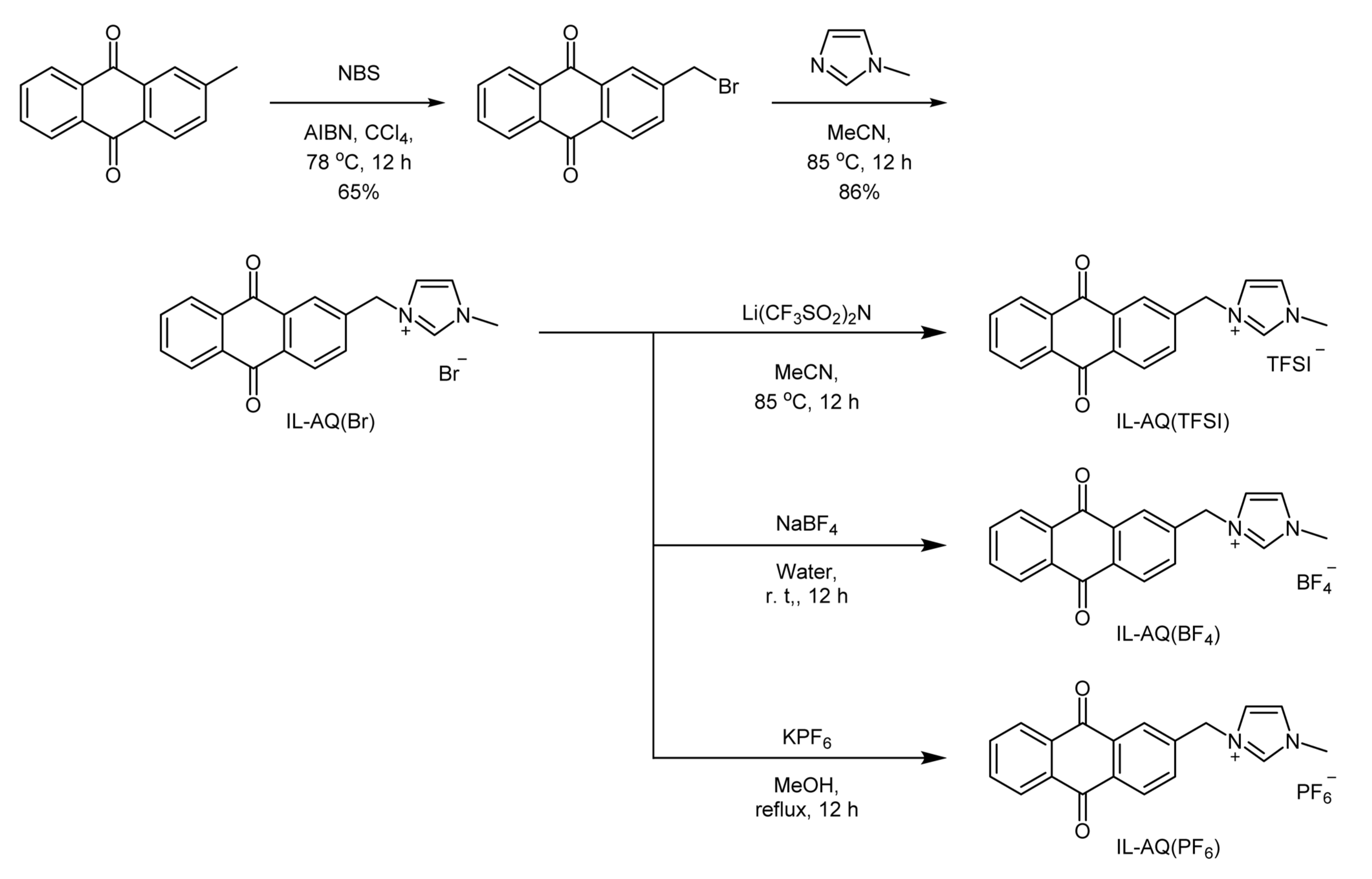
3.2. Synthesis of the Substrates
3.2.1. General Procedure
3.2.2. 2-Bromomethyl-9,10-anthraquinone
3.2.3. 1-((9,10-Anthraquinon-2-yl)methyl)-3-methyl-1H-imidazol-3-ium Bromide IL-AQ(Br)
3.2.4. 1-((9,10-Anthraquinon-2-yl)methyl)-3-methyl-1H-imidazol-3-ium Bis(trifluoromethanesulfonyl) imide IL-AQ (TFSI)
3.2.5. 1-((9,10-Anthraquinon-2-yl)methyl)-3-methyl-1H-imidazol-3-ium Tetrafluoroborate IL-AQ (BF4)
3.2.6. 1-((9,10-Anthraquinon-2-yl)methyl)-3-methyl-1H-imidazol-3-ium Hexafluorophosphate IL-AQ (PF6)
3.2.7. Benzoic Acid
3.2.8. 4-Chlorobenzoic Acid
3.2.9. 4-Methylbenzoic Acid
3.2.10. 4-Acetoxybenzoic Acid
3.2.11. 4-(Tert-butyl) Benzoic Acid
3.2.12. [1,1′-Biphenyl]-4-carboxylic Acid
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Welton, T. Room-Temperature Ionic Liquids. Solvents for Synthesis and Catalysis. Chem. Rev. 1999, 99, 2071–2084. [Google Scholar] [CrossRef] [PubMed]
- Miao, W.; Chan, T.H. Ionic–liquid–supported synthesis: A novel liquid–phase strategy for organic synthesis. Acc. Chem. Res. 2006, 39, 897–908. [Google Scholar] [CrossRef] [PubMed]
- Hallett, J.; Welton, T. Room-Temperature Ionic Liquids: Solvents for Synthesis and Catalysis. 2. Chem. Rev. 2011, 111, 3508–3576. [Google Scholar] [CrossRef] [PubMed]
- Wasserscheid, P.; Keim, W. Ionic Liquids-New “Solutions” for Transition Metal Catalysis. Angew. Chem. Int. Ed. 2000, 39, 3772–3789. [Google Scholar]
- Koguchi, S. An ionic–liquid–supported 18-crown-6 ether: Recyclable catalyst for acetylation and fluorination in anionic liquid. Trans. Mater. Res. Soc. Jpn. 2013, 38, 35–36. [Google Scholar] [CrossRef] [Green Version]
- Koguchi, S.; Nakamura, K. Ascorbic acid based ionic liquid: Recyclable and efficient catalytic systems for the Huisgen cycloaddition. Synlett 2013, 24, 2305–2309. [Google Scholar] [CrossRef]
- Koguchi, S.; Izawa, K. Ionic liquid-phase synthesis of 1,5-disubstituted 1,2,3-triazoles. ACS Comb. Sci. 2014, 16, 381–385. [Google Scholar] [CrossRef] [PubMed]
- Koguchi, S.; Mihoya, A.; Mimura, M. Alcohol oxidation via recyclable hydrophobic ionic liquid-supported IBX. Tetrahedron 2016, 72, 7633–7637. [Google Scholar] [CrossRef]
- Koguchi, S.; Shibuya, Y.; Igarashi, Y.; Takemura, H. Ionic liquid–supported 1,3-dimethylimidazolidin-2-one: Application as a reusable halogenation reagent. Synlett 2019, 30, 943–946. [Google Scholar] [CrossRef]
- Mihoya, A.; Koguchi, S.; Shibuya, Y.; Mimura, M.; Oba, M. Oxidation of Thiol Using Ionic Liquid-Supported Organotelluride as a Recyclable Catalyst. Catalysts 2020, 10, 398. [Google Scholar] [CrossRef] [Green Version]
- Mihoya, A.; Shibuya, Y.; Ito, A.; Toyoda, A.; Oba, M.; Koguchi, S. Aerobic Oxidation of Phosphite Esters to Phosphate Esters by Using an Ionic-Liquid-Supported Organotelluride Reusable Catalyst. Synlett 2020, 31, 2043–2045. [Google Scholar]
- Mastsusaki, Y.; Yamaguchi, T.; Tada, N.; Miura, T.; Ito, A. Aerobic Photooxidative Cleavage of Vicinal Diols to Carboxylic Acids Using 2-Chloroanthraquinone. Synlett 2012, 23, 2059–2062. [Google Scholar]
 | |||
|---|---|---|---|
| Entry | Photocatalysts | Ionic Liquid | Yield b (%) |
| 1 | IL-AQ(PF6) | (bmim)PF6 | 96 |
| 2 | IL-AQ(TFSI) | (bmim)TFSI | 98 |
| 3 | IL-AQ(BF4) | (bmim)BF4 | quant. |
| 4 | IL-AQ(Br) | (bmim)Br | 99 |
| 5 | IL-AQ(PF6) | (bmim)BF4 | 88 |
| 6 | IL-AQ(TFSI) | (bmim)BF4 | 91 |
| 7 | IL-AQ(Br) | (bmim)BF4 | 60 |
| 8 | anthraquinone | (bmim)BF4 | 98 |
| 9 c | IL-AQ(BF4) | (bmim)BF4 | n. r. |
| 10 | - | (bmim)BF4 | 40 |
| 11 d | IL-AQ(BF4) | (bmim)BF4 | 65 |
 | ||
 | 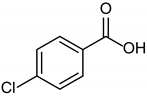 |  |
| 1b quant. | 2b 98% | 3b quant. |
 | 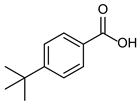 | 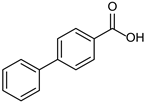 |
| 4b 95% | 5b quant. | 6b 99% |
 | ||||||
|---|---|---|---|---|---|---|
| Entry | Condition | 1 Cycle | 2 Cycles | 3 Cycles | 4 Cycles | 5 Cycles |
| 1 | Photocatalyst; IL-AQ(BF4) Ionic Liquid; (bmim)BF4 | quant. | quant. | quant. | 99% | quant. |
| 2 | Photocatalyst; IL-AQ(PF6) Ionic Liquid; (bmim) PF6 | 96% | 96% | 90% | 95% | 97% |
| 3 | Photocatalyst; IL-AQ(TFSI) Ionic Liquid; (bmim) TFSI | 98% | 98% | 72% | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Koguchi, S.; Fujita, H.; Shibuya, Y. Ionic Liquid-Supported Photocatalysts: A Reusable Environmentally Friendly Oxidation Reaction System That Uses Air and Light. Int. J. Mol. Sci. 2023, 24, 7141. https://doi.org/10.3390/ijms24087141
Koguchi S, Fujita H, Shibuya Y. Ionic Liquid-Supported Photocatalysts: A Reusable Environmentally Friendly Oxidation Reaction System That Uses Air and Light. International Journal of Molecular Sciences. 2023; 24(8):7141. https://doi.org/10.3390/ijms24087141
Chicago/Turabian StyleKoguchi, Shinichi, Haruto Fujita, and Yuga Shibuya. 2023. "Ionic Liquid-Supported Photocatalysts: A Reusable Environmentally Friendly Oxidation Reaction System That Uses Air and Light" International Journal of Molecular Sciences 24, no. 8: 7141. https://doi.org/10.3390/ijms24087141
APA StyleKoguchi, S., Fujita, H., & Shibuya, Y. (2023). Ionic Liquid-Supported Photocatalysts: A Reusable Environmentally Friendly Oxidation Reaction System That Uses Air and Light. International Journal of Molecular Sciences, 24(8), 7141. https://doi.org/10.3390/ijms24087141






