Integrated Metabolomic and Transcriptomic Analyses Reveals Sugar Transport and Starch Accumulation in Two Specific Germplasms of Manihot esculenta Crantz
Abstract
:1. Introduction
2. Results
2.1. Measurement of Total Soluble Hexose and Starch in Different Cassava Storage Roots
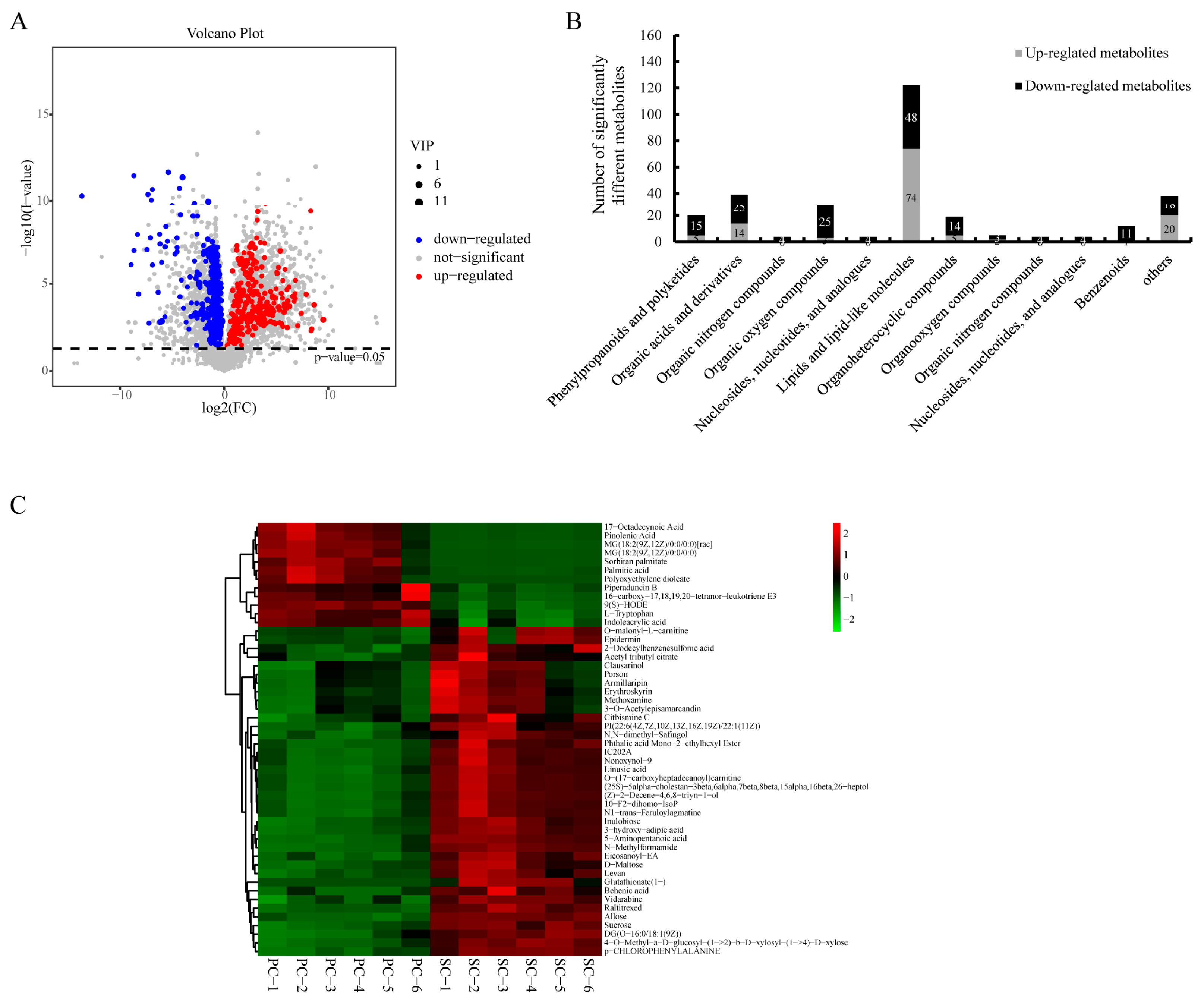
2.2. Metabolomic Analysis of LC-MS Data of All Storage Roots of Cassava
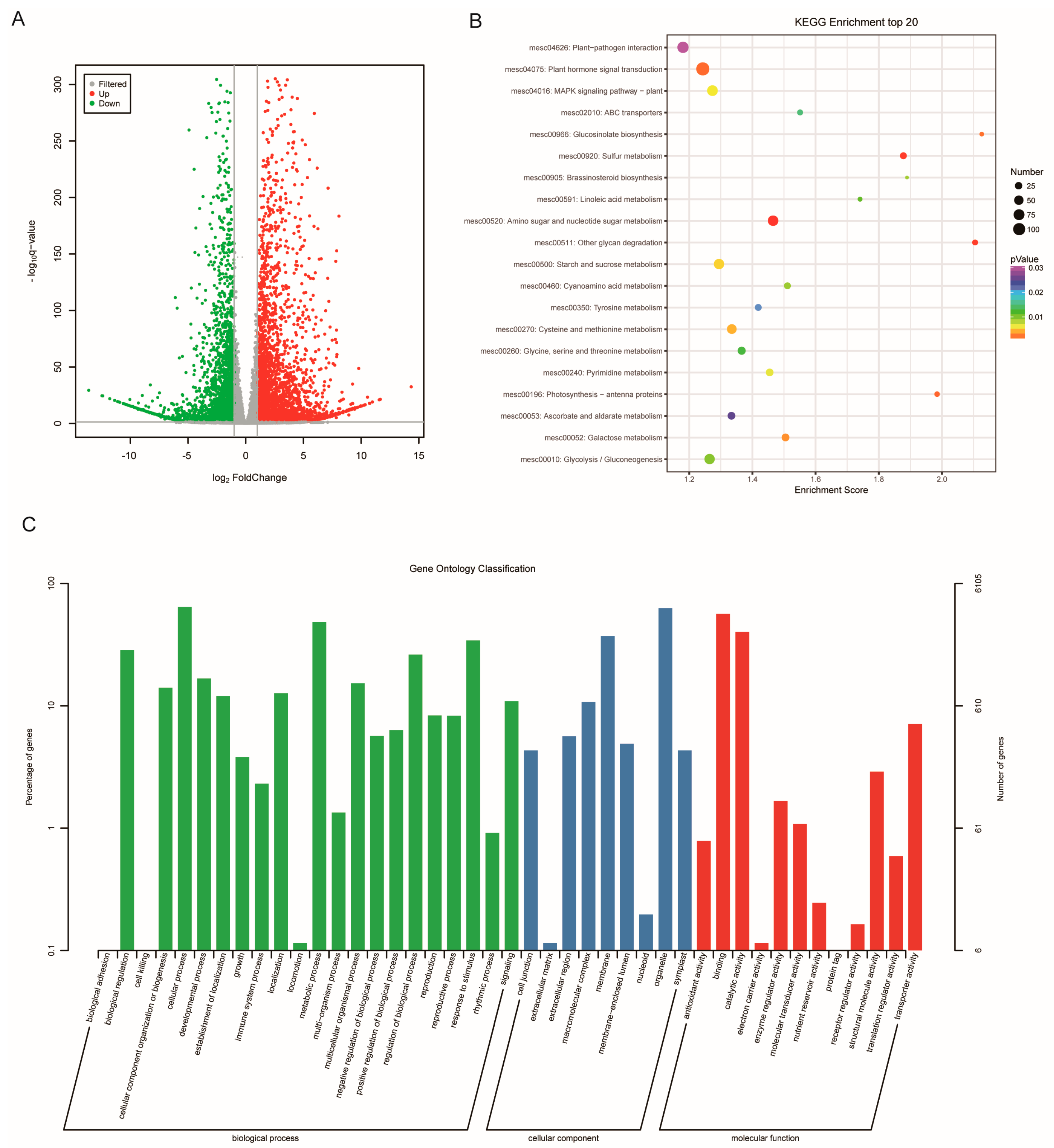
2.3. Differential Expression of Genes between SC and PC
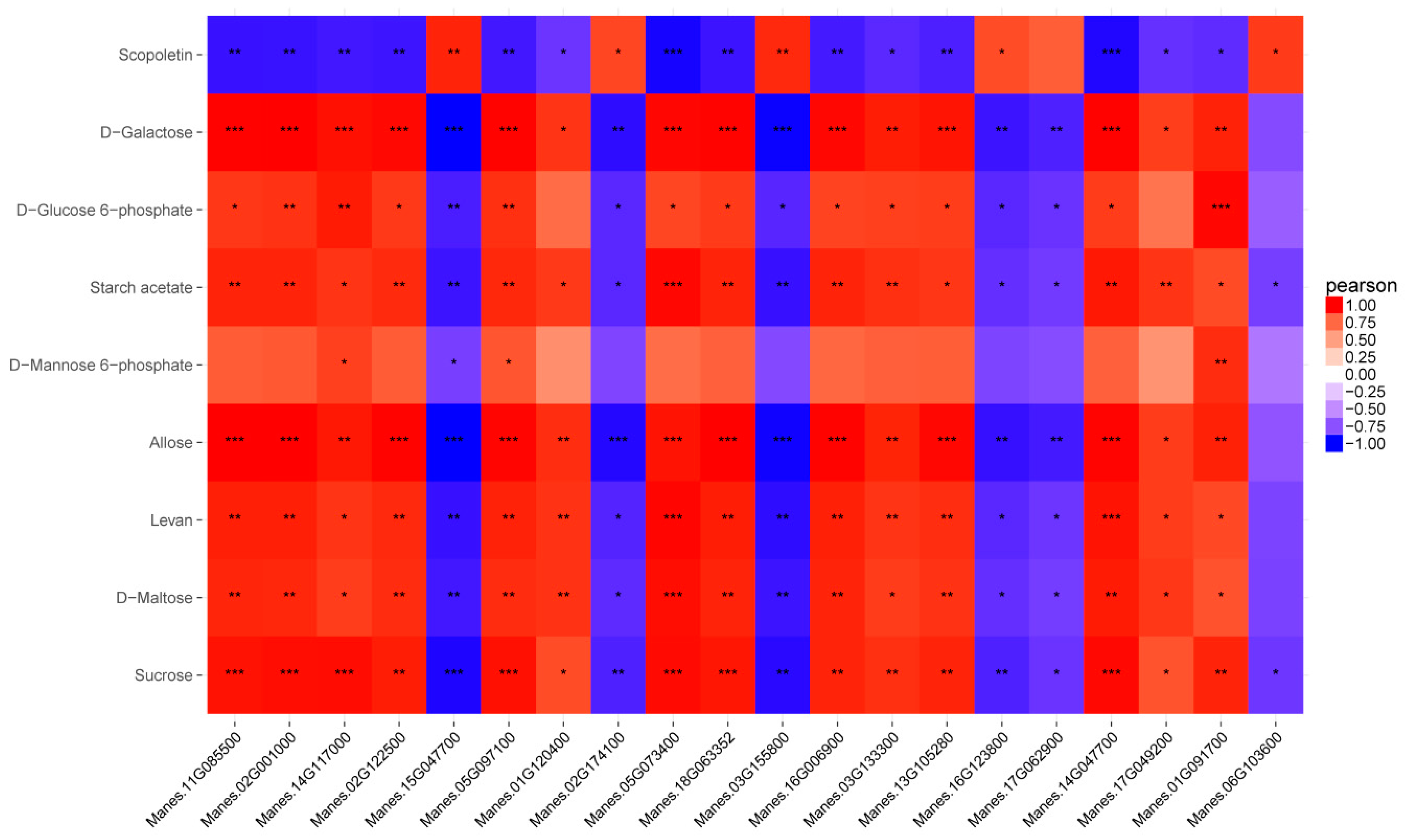
2.4. Correlation Analysis of the Metabolome and Transcriptome
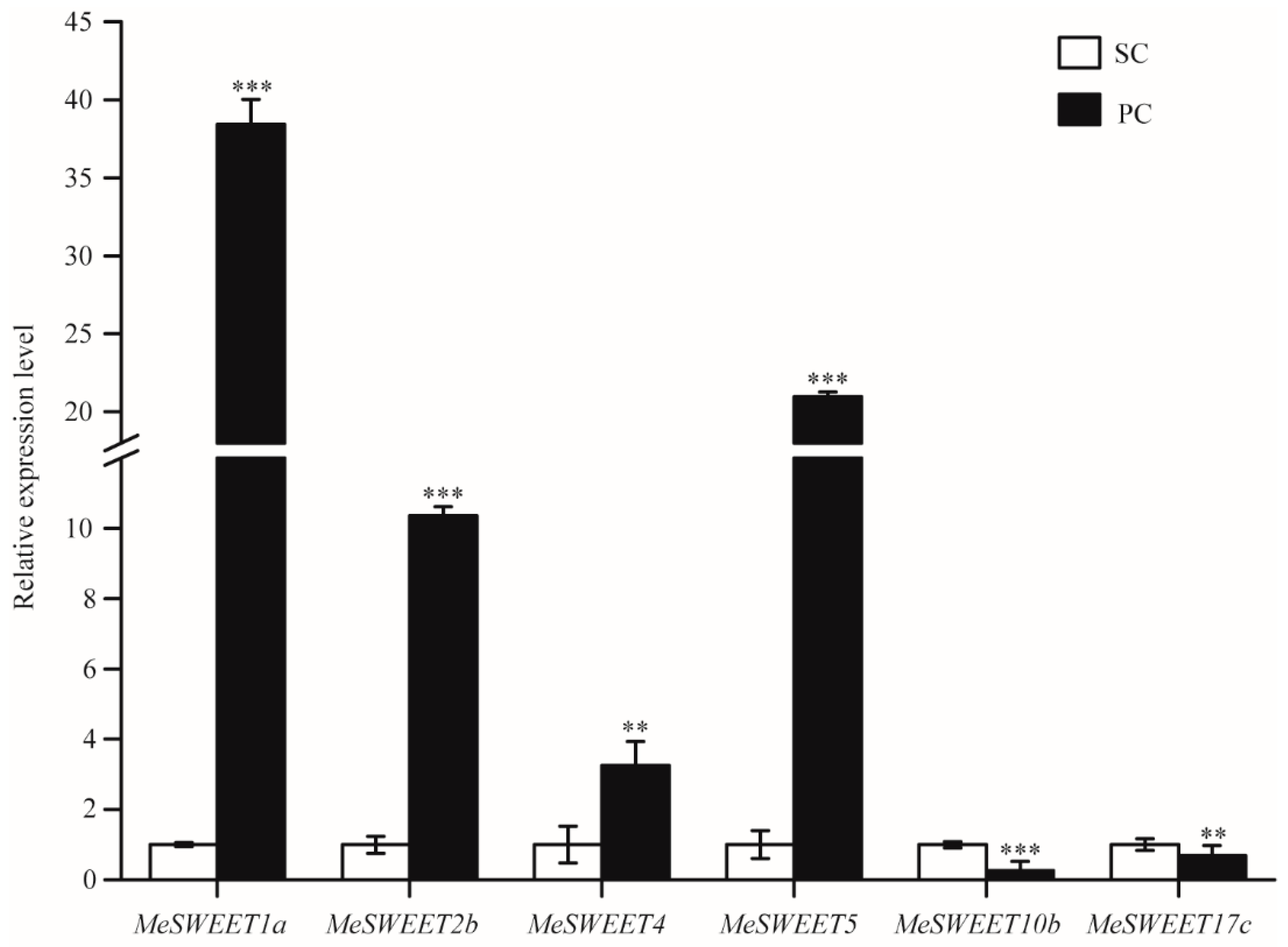
2.5. Alterations of Sugar Transport by SWEETs between SC and PC
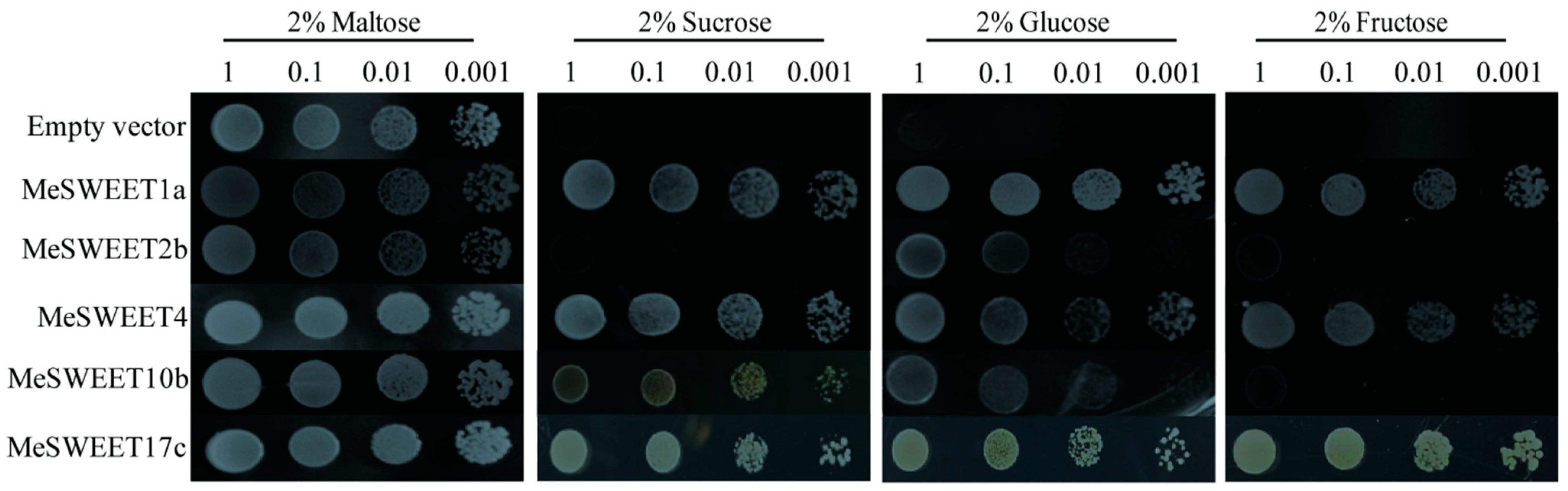
2.6. Sugar Transport Activity by Five SWEETs between SC and PC
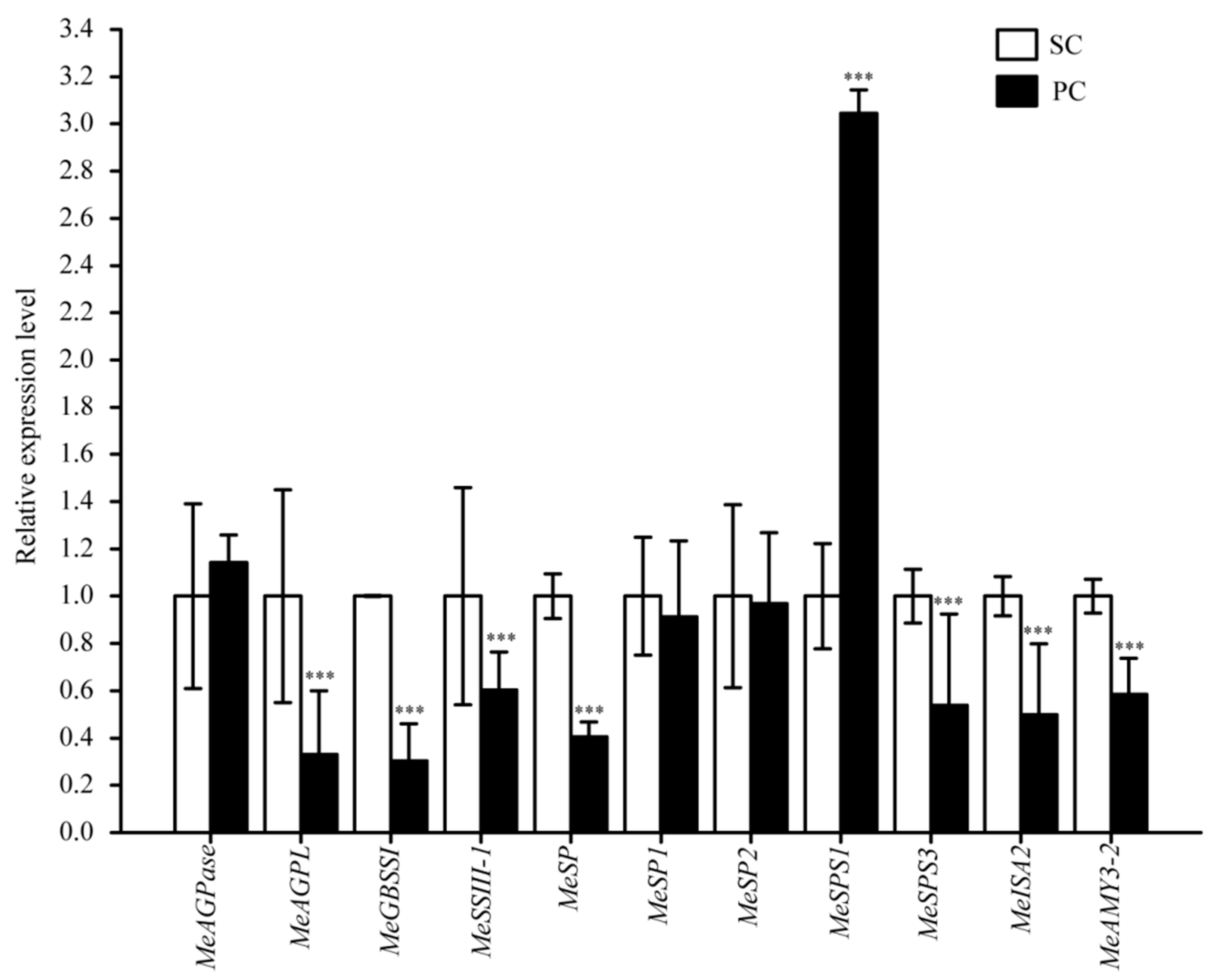
2.7. Alterations in Starch Biosynthesis in Storage Roots of SC and PC
3. Discussion
4. Materials and Methods
4.1. Plant Materials
4.2. Measurement of Soluble Sugar Content
4.3. Measurement of the Total Starch Content
4.4. Metabolite Extraction Procedure
4.5. Detection and Analysis of Metabolites
4.6. RNA Extraction and Illumina Sequencing
4.7. Transcriptome Data Analysis and Annotation
4.8. Correlation Analysis between Transcriptome and Metabolome Data
4.9. qRT-PCR Analysis of Genes Involved in Sugar Transport and Starch Metabolism
4.10. Complementation of Yeast EBY.VW4000
4.11. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhu, F. Composition, structure, physicochemical properties, and modifications of cassava starch. Carbohydr. Polym. 2015, 122, 456–480. [Google Scholar] [CrossRef] [PubMed]
- De Souza, A.P.; Massenburg, L.N.; Jaiswal, D.; Cheng, S.; Shekar, R.; Long, S.P. Rooting for cassava: Insights into photosynthesis and associated physiology as a route to improve yield potential. New Phytol. 2019, 213, 50–65. [Google Scholar] [CrossRef] [PubMed]
- An, F.F.; Chen, T.; Li, Q.X.; Qiao, J.J.; Zhang, Z.W.; Carvalho, L.J.C.B.; Li, K.M.; Chen, S.B. Protein cross-interactions for efficient photosynthesis in the cassava cultivar SC205 relative to its wild species. J. Agric. Food Chem. 2019, 67, 87468755. [Google Scholar] [CrossRef] [PubMed]
- Cai, J.; Zhang, J.; Ding, Y.; Yu, S.; Lin, H.X.; Yuan, Z.Q.; Li, K.M.; Ou, W.J.; Chen, S.B. Different fertilizers applied alter fungal community structure in rhizospheric soil of cassava (Manihot esculenta Crantz) and increase crop yield. Front. Microbiol. 2021, 12, 663781. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, L.J.C.B.; Filho, J.F.; Anderson, J.V.; Figueiredo, P.G.; Chen, S.B. Storage Root of Cassava: Morphological Types, Anatomy, Formation, Growth, Development and Harvest Time; IntechOpen: London, UK, 2018; pp. 53–68. [Google Scholar]
- Hillocks, R.J.; Thresh, J.M.; Bellotti, A. Cassava: Biology, Production and Utilization; CABI Publishing: New York, NY, USA, 2002; 308p. [Google Scholar]
- Jia, L.; Hao, K.; Suyala, Q.; Qin, Y.; Yu, J.; Liu, K.; Fan, M. Potato tuber degradation is regulated by carbohydrate metabolism: Results of transcriptomic analysis. Plant Direct 2022, 6, e379. [Google Scholar] [CrossRef] [PubMed]
- Lemoine, R.; Camera, S.L.; Atanassova, R.; Dédaldéchamp, F.; Allario, T.; Pourtau, N.; Bonnemain, J.L.; Laloi, M.; Coutos-Thévenot, P.; Parrilla, J.; et al. Source-to-sink transport of sugar and regulation by environmental factors. Front. Plant Sci. 2013, 4, 272. [Google Scholar] [CrossRef] [Green Version]
- Chen, L.Q.; Cheung, L.S.; Feng, L.; Tanner, W.; Frommer, W.B. Transport of sugars. Annu. Rev. Biochem. 2015, 84, 865–894. [Google Scholar] [CrossRef]
- Ji, J.L.; Yang, L.M.; Fang, Z.Y.; Zhang, Y.Y.; Zhuang, M.; Lv, H.H.; Wang, Y. Plant SWEET family of sugar transporters: Structure, evolution and biological functions. Biomolecules 2022, 12, 205. [Google Scholar] [CrossRef]
- Lin, W.I.; Sosso, D.; Chen, L.Q.; Gase, K.; Kim, S.G.; Kessker, D.; Klinkenberg, P.M.; Gorder, M.K.; Hou, B.H.; Qu, X.Q.; et al. Nectar secretion requires sucrose phosphate synthases and the sugar transporter SWEET9. Nature 2014, 508, 546–549. [Google Scholar] [CrossRef]
- Chen, L.Q.; Qu, X.Q.; Hou, B.H.; Sosso, D.; Osorio, S.; Fernie, A.R.; Frommer, W.B. Sucrose efflux mediated by SWEET proteins as a key step for phloem transport. Science 2012, 335, 207–211. [Google Scholar] [CrossRef]
- Li, P.; Wang, L.; Liu, H.; Yuan, M. Impaired SWEET-mediated sugar transportation impacts starch metabolism in developing rice seeds. Crop J. 2022, 10, 98–108. [Google Scholar] [CrossRef]
- Sosso, D.; Luo, D.; Li, Q.B.; Sasse, J.; Yang, J.; Gendrot, G.; Suzuki, M.; Koch, K.E.; McCarty, D.R.; Chourey, P.S.; et al. Seed filling in domesticated maize and rice depends on SWEET-mediated hexose transport. Nat. Genet. 2015, 47, 1489–1493. [Google Scholar] [CrossRef] [PubMed]
- Sameeullah, M.; Demiral, T.; Aslam, N.; Baloch, F.S.; Gurel, E. In silico functional analyses of SWEETs reveal cues for their role in AMF symbiosis. Plant Soil Microbes 2016, 2, 45–58. [Google Scholar]
- Wang, S.; Yokosho, K.; Guo, R.; Whelan, J.; Ruan, Y.L.; Ma, J.F.; Shou, H. The soybean sugar transporter GmSWEET15 mediates sucrose export from endosperm to early embryo. Plant Physiol. 2019, 180, 2133–2141. [Google Scholar] [CrossRef] [Green Version]
- Abelenda, J.A.; Bergonzi, S.; Oortwijn, M.; Sonnewald, S.; Du, M.; Visser, R.G.F.; Sonnewald, U.; Bachem, C.W. Source-sink regulation is mediated by interaction of an FT homolog with a SWEET protein in potato. Curr. Biol. 2019, 29, 1178–1186. [Google Scholar] [CrossRef] [Green Version]
- Liu, Q.; Ma, C.; Feng, S.P.; Tang, Z.J.; Chen, Y.H.; Luo, L.J.; Niu, X.L. Molecular cloning, subcellular localization and function analysis of a MeSWEET1 gene from Manihot esculenta. Mol. Plant Breed. 2017, 15, 2502–2509. [Google Scholar]
- Xue, B.B.; Qin, L.F.; Dong, M.Y.; Li, Y.Z.; Fan, X.W. Bioinformatics analysis and expressional characteristics of cassava SWEETs gene family. Genom. Appl. Biol. 2019, 38, 260–268. [Google Scholar]
- Zhu, B.G.; Li, C.; Zhang, X.J.; Xie, Q.B.; Tao, J.; Luo, K.; Chen, Y.H.; Niu, X.L. Molecular cloning and function analysis of a MeSWEET3b gene from Manihot esculenta. Mol. Plant Breed. 2022, 20, 733–741. [Google Scholar]
- Fan, X.W.; Sun, J.L.; Cai, Z.; Zhang, F.; Li, Y.Z.; Palta, J.A. MeSWEET15a/b genes play a role in the resistance of cassava (Manihot esculenta Crantz) to water and salt stress by modulating sugar distribution. Plant Physiol. Biochem. 2023, 194, 394–405. [Google Scholar] [CrossRef]
- Cohn, M.; Bart, R.S.; Shybut, M.; Dahlbeck, D.; Gomez, M.; Morbitzer, R.; Hou, B.H.; Frommer, W.B.; Lahaye, T.; Staskawicz, B.J. Xanthomonas axonopodis virulence is promoted by a transcription activator-like effector-mediated induction of a SWEET sugar transporter in cassava. Mol. Plant-Microbe Interact. 2014, 27, 1186–1198. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.J.; Geng, M.T.; Pan, R.R.; Zhang, T.; Lu, X.H.; Zhen, X.H.; Che, Y.N.; Li, R.M.; Liu, J.; Chen, Y.H.; et al. Engineering bacterial blight-resistant plants through CRISPR/Cas9-targeted editing of the MeSWEET10a promoter in cassava. bioRxiv 2022. [Google Scholar] [CrossRef]
- Drapal, M.; de Carvalho, E.B.; Ovalle Rivera, T.M.; Lopze-Lavalle, L.A.B.; Fraser, P.D. Capturing biochemical diversity in cassava (Manihot esculenta Crantz) through the application of metabolite profiling. J. Agric. Food Chem. 2018, 67, 986–993. [Google Scholar] [CrossRef]
- Perez-Fons, L.; Bohorquez-Chaux, A.; Irigoyen, M.L.; Garceau, D.C.; Morreel, K.; Boerjan, W.; Walling, L.L.; Lopze-Lavalle, L.A.B.; Fraser, P.D. A metabolomics characterisation of natural variation in the resistance of cassava to whitefly. BMC Plant Biol. 2019, 19, 518. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Montagnac, J.A.; Davis, C.; Tanumihardjo, S.A. Nutritional value of cassava for use as a staple food and recent advances for improvement. Compr. Rev. Food Sci. F 2010, 8, 181–194. [Google Scholar] [CrossRef] [PubMed]
- Fu, L.; Ding, Z.; Tie, W.; Yang, J.; Yan, Y.; Hu, W. Integrated metabolomic and transcriptomic analyses reveal novel insights of anthocyanin biosynthesis on color formation in Cassava tuberous roots. Front. Nutr. 2022, 9, 842693. [Google Scholar] [CrossRef]
- Morante, N.; Sánchez, T.; Ceballos, H.; Pérez, C.J.; Egesi, C.; Cuambe, C.E.; Escobar, A.F.; Ortiz, A.L.; Chávez, A.L.; Fregene, M. Tolerance to postharvest physiological deterioration in cassava roots. Crop Sci. 2010, 50, 1333–1338. [Google Scholar] [CrossRef]
- Qin, Y.; Djabou, A.S.M.; An, F.F.; Li, K.M.; Li, Z.G.; Yang, L.; Wang, X.J.; Chen, S.B. Proteomic analysis of injured storage roots in cassava (Manihot esculenta Crantz) under postharvest physiological deterioration. PLoS ONE 2017, 12, e0174238. [Google Scholar] [CrossRef] [Green Version]
- Cui, M.J. Non-Targeted Metabolomics Study on Postharvest Physiological Deterioration of Cassava Tuberous Roots. Master’s Thesis, Nanjing Agricultural University, Nanjing, China, 2020. [Google Scholar]
- Knoblauch, M.; Knoblauch, J.; Mullendore, D.L.; Savage, J.A.; Babst, B.A.; Beecher, S.D.; Dodgen, A.C.; Jensen, K.H.; Holbrook, N.M. Testing the Münch hypothesis of long distance phloem transport in plants. eLife 2016, 5, e15341. [Google Scholar] [CrossRef] [Green Version]
- Julius, B.T.; Leach, K.A.; Tran, T.M.; Mertz, R.A.; Braun, D.M. Sugar transporters in plants: New insights and discoveries. Plant Cell Physiol. 2017, 58, 1442–1460. [Google Scholar] [CrossRef] [Green Version]
- Dhungana, S.R.; Braun, D.M. Sugar transporters in grasses: Function and modulation in source and storage tissues. J. Plant Physiol. 2021, 266, 153541. [Google Scholar] [CrossRef]
- Alves, A.A.C. Cassava botany and physiology. In Cassava: Biology, Production and Utilization; Hillocks, R.J., Thresh, J.M., Bellotti, A., Eds.; CABI: Wallingford, UK, 2002; pp. 67–89. [Google Scholar]
- Yan, W.; Wu, X.Y.; Li, Y.N.; Liu, G.H.; Cui, Z.F.; Jiang, T.L.; Ma, Q.X.; Luo, L.J.; Zhang, P. Cell wall invertase 3 affects cassava productivity via regulating sugar allocation from source to sink. Front. Plant Sci. 2019, 10, 541. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sui, J.L.; Xiao, X.H.; Qi, J.Y.; Fang, Y.J.; Tang, C.R. The SWEET gene family in Hevea brasiliensis-its evolution and expression compared with four other plant species. FEBS Open Biol. 2017, 7, 1943–1959. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.Q.; Hou, B.H.; Lalonde, S.; Takanaga, H.; Hartung, M.L.; Qu, X.Q.; Guo, W.J.; Kin, J.G.; Underwood, W.; Chaudhuri, B.; et al. Sugar transporters for intercellular exchange and nutrition of pathogens. Nature 2010, 468, 527–532. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kryvoruchko, I.S.; Sinharoy, S.; Torres-Jerez, I.; Sosso, D.; Pislariu, C.I.; Guan, D.; Murray, J.; Benedito, V.A.; Frommer, W.B.; Udvardi, M.K. MtSWEET11, a nodule-specific sucrose transporter of Medicago truncatula. Plant Physiol. 2016, 171, 554–565. [Google Scholar] [CrossRef] [Green Version]
- Yao, L.; Ding, C.; Hao, X.; Zeng, J.; Yang, Y.; Wang, X.; Wang, L. CsSWEET1a and CsSWEET17 mediate growth and freezing tolerance by promoting sugar transport across the plasma membrane. Plant Cell Physiol. 2020, 61, 1669–1682. [Google Scholar] [CrossRef]
- Ko, H.Y.; Ho, L.H.; Neuhaus, H.E.; Guo, W.J. Transporter SlSWEET15 unloads sucrose from phloem and seed coat for fruit and seed development in tomato. Plant Physiol. 2021, 187, 2230–2245. [Google Scholar] [CrossRef]
- Li, X.; Guo, W.; Li, J.; Yue, P.; Bu, H.; Jiang, J.; Liu, W.T.; Xu, Y.X.; Yuan, H.; Li, T.; et al. Histone acetylation at the promoter for the transcription factor PuWRKY31 affects sucrose accumulation in pear fruit. Plant Physiol. 2020, 182, 2035–2046. [Google Scholar] [CrossRef] [Green Version]
- Jaramillo, A.M.; Sierra, S.; Chavarriaga-Aguirre, P.; Castillo, D.K.; Gkanogiannis, A.; López-Lavalle, L.A.B.; Arciniegas, J.P.; Sun, T.H.; Li, L.; Welsh, R.; et al. Characterization of cassava ORANGE proteins and their capability to increase provitamin A carotenoids accumulation. PLoS ONE 2022, 17, e0262412. [Google Scholar] [CrossRef]
- Wieczorke, R.; Krampe, S.; Weierstall, T.; Freidel, K.; Hollenberg, C.; Boles, E. Concurrent knock-out of at least 20 transporter genes is required to block uptake of hexoses in Saccharomyces cerevisiae. FEBS Lett. 1999, 464, 123–128. [Google Scholar] [CrossRef] [Green Version]
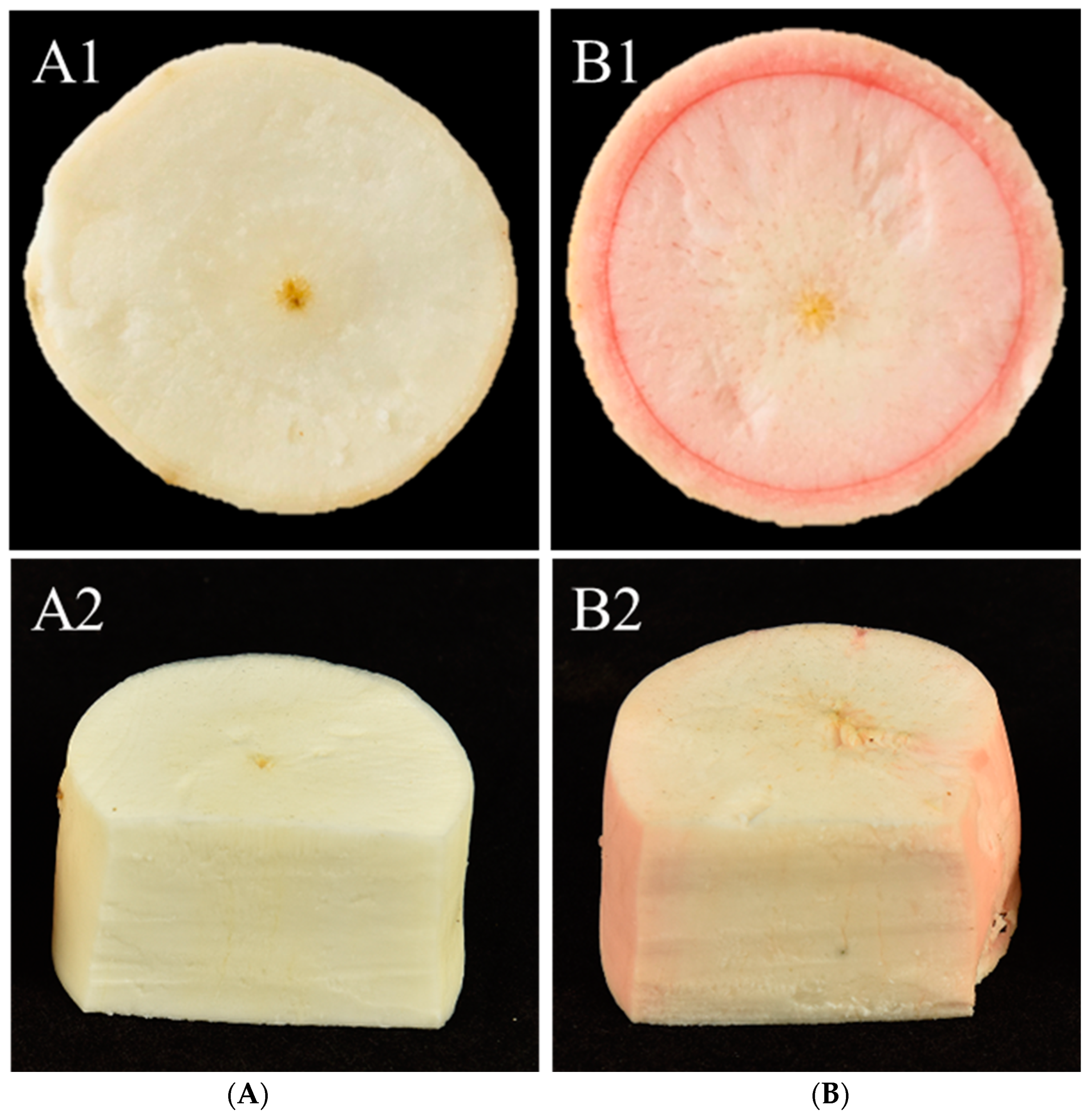
| Cassava Germplasms | The Content of Total Starch (%) | The Content of Sucrose (mg/kg) | The Content of Glucose (mg/kg) | The Content of Fructose (mg/kg) |
|---|---|---|---|---|
| SC | 11.03 ± 0.05 b | 12,850.65 ± 452.95 b | 8725.70 ± 157.66 a | 9874.10 ± 271.01 a |
| PC | 29.04 ± 0.18 a | 16,049.00 ± 115.97 a | 1707.52 ± 54.79 b | 1756.78 ± 36.99 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cai, J.; Xue, J.; Zhu, W.; Luo, X.; Lu, X.; Xue, M.; Wei, Z.; Cai, Y.; Ou, W.; Li, K.; et al. Integrated Metabolomic and Transcriptomic Analyses Reveals Sugar Transport and Starch Accumulation in Two Specific Germplasms of Manihot esculenta Crantz. Int. J. Mol. Sci. 2023, 24, 7236. https://doi.org/10.3390/ijms24087236
Cai J, Xue J, Zhu W, Luo X, Lu X, Xue M, Wei Z, Cai Y, Ou W, Li K, et al. Integrated Metabolomic and Transcriptomic Analyses Reveals Sugar Transport and Starch Accumulation in Two Specific Germplasms of Manihot esculenta Crantz. International Journal of Molecular Sciences. 2023; 24(8):7236. https://doi.org/10.3390/ijms24087236
Chicago/Turabian StyleCai, Jie, Jingjing Xue, Wenli Zhu, Xiuqin Luo, Xiaohua Lu, Maofu Xue, Zhuowen Wei, Yuqi Cai, Wenjun Ou, Kaimian Li, and et al. 2023. "Integrated Metabolomic and Transcriptomic Analyses Reveals Sugar Transport and Starch Accumulation in Two Specific Germplasms of Manihot esculenta Crantz" International Journal of Molecular Sciences 24, no. 8: 7236. https://doi.org/10.3390/ijms24087236






