Evidence of the Dysbiotic Effect of Psychotropics on Gut Microbiota and Capacity of Probiotics to Alleviate Related Dysbiosis in a Model of the Human Colon
Abstract
:1. Introduction
2. Results
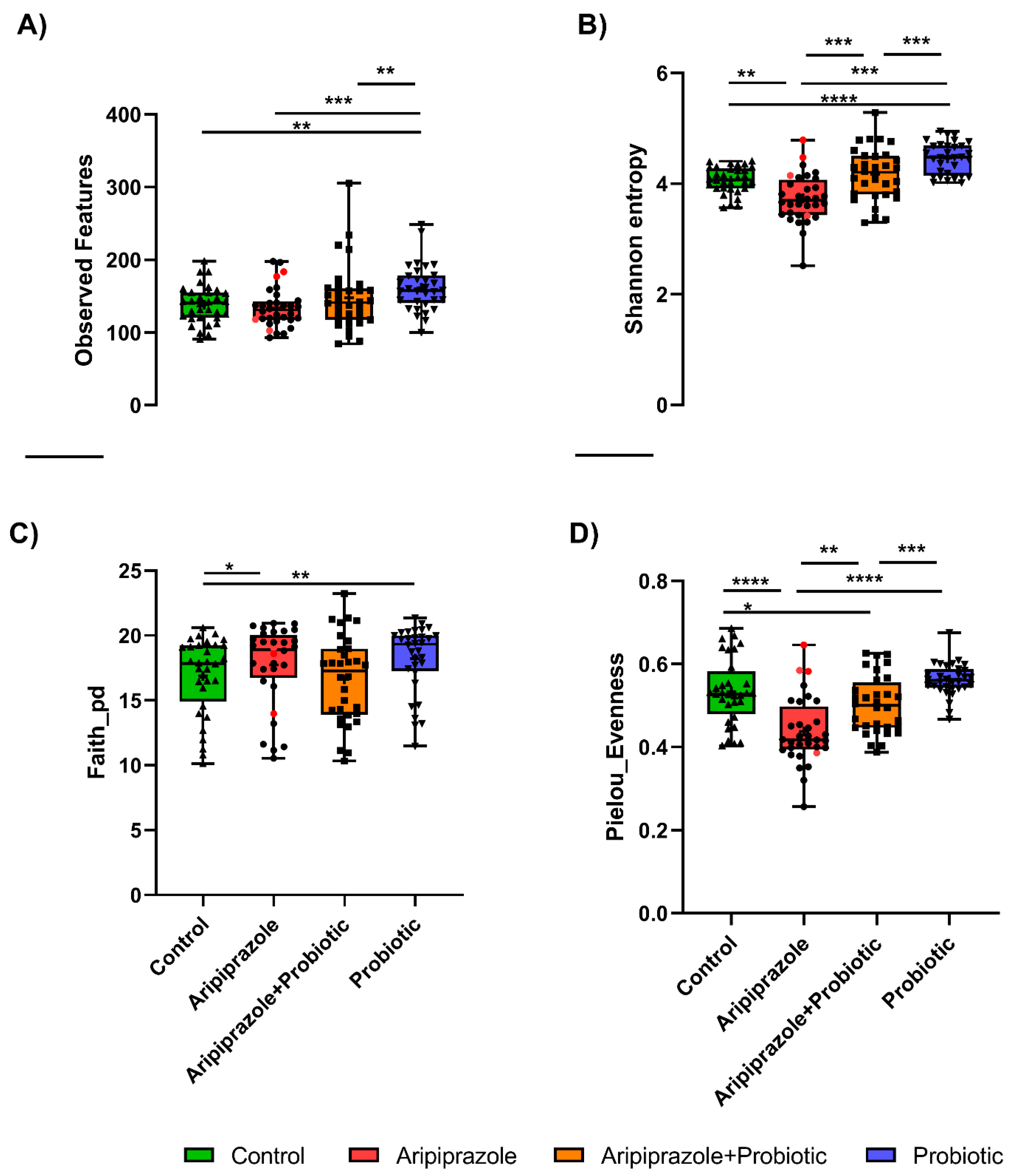
2.1. Psychotropics Alter the Diversity of the Gut Microbiome
2.2. Effect of Psychotropics Alone or in Combination with Probiotics on Gut Microbiome Composition
2.3. Probiotic Strains Resist the Psychotropics’ Antimicrobial Effect as Illustrated by qPCR Analysis
2.4. Effect of Psychotropics Alone or in Combination with Probiotics on Microbiota SCFAs Metabolism
3. Discussion
4. Materials and Methods
4.1. Psychotropics and Probiotics
4.2. Feces Collection and Microbiota Immobilization
4.3. Experimental Set-Up and Fermentation Procedure
- CR bioreactor: served as non-treatment control
- TR1 bioreactor: challenged with aripiprazole or (S)-citalopram at a final concentration of 400 µg/mL, simulating an estimated single daily dose of psychotropics as discussed before in our previous study [6].
- TR2 bioreactor: challenged with the probiotic mixture (L. rhamnosus and B. longum) added at a final concentration of 109 CFU/mL each.
- TR3 bioreactor: challenged with aripiprazole or (S)-citalopram at 400 µg/mL and the probiotic mixture.
4.4. Microbial Community Analyses
4.4.1. DNA Extraction
4.4.2. 16S rRNA Gene Sequencing
4.4.3. Detection and Quantification of Viable Probiotics with PMA-qPCR
4.4.4. Determination of SCFAs Content
4.5. Statistical Analyses
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cryan, J.F.; Dinan, T.G. Mind-Altering Microorganisms: The Impact of the Gut Microbiota on Brain and Behaviour. Nat. Rev. Neurosci. 2012, 13, 701–712. [Google Scholar] [CrossRef]
- Szyszkowicz, J.K.; Wong, A.; Anisman, H.; Merali, Z.; Audet, M.-C. Implications of the Gut Microbiota in Vulnerability to the Social Avoidance Effects of Chronic Social Defeat in Male Mice. Brain Behav. Immun. 2017, 66, 45–55. [Google Scholar] [CrossRef]
- Barandouzi, Z.A.; Starkweather, A.R.; Henderson, W.A.; Gyamfi, A.; Cong, X.S. Altered Composition of Gut Microbiota in Depression: A Systematic Review. Front. Psychiatry 2020, 11, 541. [Google Scholar] [CrossRef] [PubMed]
- Nikolova, V.L.; Hall, M.R.B.; Hall, L.J.; Cleare, A.J.; Stone, J.M.; Young, A.H. Perturbations in Gut Microbiota Composition in Psychiatric Disorders: A Review and Meta-Analysis. JAMA Psychiatry 2021, 78, 1343. [Google Scholar] [CrossRef] [PubMed]
- Mason, B.L.; Li, Q.; Minhajuddin, A.; Czysz, A.H.; Coughlin, L.A.; Hussain, S.K.; Koh, A.Y.; Trivedi, M.H. Reduced Anti-Inflammatory Gut Microbiota Are Associated with Depression and Anhedonia. J. Affect. Disord. 2020, 266, 394–401. [Google Scholar] [CrossRef] [PubMed]
- Ait Chait, Y.; Mottawea, W.; Tompkins, T.A.; Hammami, R. Unravelling the Antimicrobial Action of Antidepressants on Gut Commensal Microbes. Sci. Rep. 2020, 10, 17878. [Google Scholar] [CrossRef]
- Cussotto, S.; Strain, C.R.; Fouhy, F.; Strain, R.G.; Peterson, V.L.; Clarke, G.; Stanton, C.; Dinan, T.G.; Cryan, J.F. Differential Effects of Psychotropic Drugs on Microbiome Composition and Gastrointestinal Function. Psychopharmacology 2019, 236, 1671–1685. [Google Scholar] [CrossRef]
- Flowers, S.A.; Evans, S.J.; Ward, K.M.; McInnis, M.G.; Ellingrod, V.L. Interaction Between Atypical Antipsychotics and the Gut Microbiome in a Bipolar Disease Cohort. Pharmacotherapy 2017, 37, 261–267. [Google Scholar] [CrossRef]
- Tanner, S.A.; Zihler Berner, A.; Rigozzi, E.; Grattepanche, F.; Chassard, C.; Lacroix, C. In Vitro Continuous Fermentation Model (PolyFermS) of the Swine Proximal Colon for Simultaneous Testing on the Same Gut Microbiota. PLoS ONE 2014, 9, e94123. [Google Scholar] [CrossRef] [Green Version]
- Mottawea, W.; Sultan, S.; Landau, K.; Bordenave, N.; Hammami, R. Evaluation of the Prebiotic Potential of a Commercial Synbiotic Food Ingredient on Gut Microbiota in an Ex Vivo Model of the Human Colon. Nutrients 2020, 12, 2669. [Google Scholar] [CrossRef]
- Zihler Berner, A.; Fuentes, S.; Dostal, A.; Payne, A.N.; Vazquez Gutierrez, P.; Chassard, C.; Grattepanche, F.; de Vos, W.M.; Lacroix, C. Novel Polyfermentor Intestinal Model (PolyFermS) for Controlled Ecological Studies: Validation and Effect of PH. PLoS ONE 2013, 8, e77772. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ait Chait, Y.; Mottawea, W.; Tompkins, T.A.; Hammami, R. Nutritional and Therapeutic Approaches for Protecting Human Gut Microbiota from Psychotropic Treatments. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2021, 108, 110182. [Google Scholar] [CrossRef] [PubMed]
- Barker, A.K.; Duster, M.; Valentine, S.; Hess, T.; Archbald-Pannone, L.; Guerrant, R.; Safdar, N. A Randomized Controlled Trial of Probiotics for Clostridium Difficile Infection in Adults (PICO). J. Antimicrob. Chemother. 2017, 72, 3177–3180. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grazul, H.; Kanda, L.L.; Gondek, D. Impact of Probiotic Supplements on Microbiome Diversity Following Antibiotic Treatment of Mice. Gut Microbes 2016, 7, 101–114. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, X.; Shin, Y.-J.; Jang, H.-M.; Joo, M.-K.; Yoo, J.-W.; Kim, D.-H. Lactobacillus Rhamnosus and Bifidobacterium Longum Alleviate Colitis and Cognitive Impairment in Mice by Regulating IFN-γ to IL-10 and TNF-α to IL-10 Expression Ratios. Sci. Rep. 2021, 11, 20659. [Google Scholar] [CrossRef]
- Deb, S.; Farmah, B.K.; Arshad, E.; Deb, T.; Roy, M.; Unwin, G.L. The Effectiveness of Aripiprazole in the Management of Problem Behaviour in People with Intellectual Disabilities, Developmental Disabilities and/or Autistic Spectrum Disorder—A Systematic Review. Res. Dev. Disabil. 2014, 35, 711–725. [Google Scholar] [CrossRef] [PubMed]
- Maier, L.; Pruteanu, M.; Kuhn, M.; Zeller, G.; Telzerow, A.; Anderson, E.E.; Brochado, A.R.; Fernandez, K.C.; Dose, H.; Mori, H.; et al. Extensive Impact of Non-Antibiotic Drugs on Human Gut Bacteria. Nature 2018, 555, 623–628. [Google Scholar] [CrossRef]
- Shin, N.-R.; Whon, T.W.; Bae, J.-W. Proteobacteria: Microbial Signature of Dysbiosis in Gut Microbiota. Trends Biotechnol. 2015, 33, 496–503. [Google Scholar] [CrossRef]
- Garrett, W.S.; Gallini, C.A.; Yatsunenko, T.; Michaud, M.; DuBois, A.; Delaney, M.L.; Punit, S.; Karlsson, M.; Bry, L.; Glickman, J.N.; et al. Enterobacteriaceae Act in Concert with the Gut Microbiota to Induce Spontaneous and Maternally Transmitted Colitis. Cell Host Microbe 2010, 8, 292–300. [Google Scholar] [CrossRef] [Green Version]
- La Rosa, S.L.; Leth, M.L.; Michalak, L.; Hansen, M.E.; Pudlo, N.A.; Glowacki, R.; Pereira, G.; Workman, C.T.; Arntzen, M.Ø.; Pope, P.B.; et al. The Human Gut Firmicute Roseburia Intestinalis Is a Primary Degrader of Dietary β-Mannans. Nat. Commun. 2019, 10, 905. [Google Scholar] [CrossRef] [Green Version]
- Kasahara, K.; Krautkramer, K.A.; Org, E.; Romano, K.A.; Kerby, R.L.; Vivas, E.I.; Mehrabian, M.; Denu, J.M.; Bäckhed, F.; Lusis, A.J.; et al. Interactions between Roseburia Intestinalis and Diet Modulate Atherogenesis in a Murine Model. Nat. Microbiol. 2018, 3, 1461–1471. [Google Scholar] [CrossRef] [PubMed]
- Zheng, P.; Zeng, B.; Zhou, C.; Liu, M.; Fang, Z.; Xu, X.; Zeng, L.; Chen, J.; Fan, S.; Du, X.; et al. Gut Microbiome Remodeling Induces Depressive-like Behaviors through a Pathway Mediated by the Host’s Metabolism. Mol. Psychiatry 2016, 21, 786–796. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Ling, Z.; Zhang, Y.; Mao, H.; Ma, Z.; Yin, Y.; Wang, W.; Tang, W.; Tan, Z.; Shi, J.; et al. Altered Fecal Microbiota Composition in Patients with Major Depressive Disorder. Brain Behav. Immun. 2015, 48, 186–194. [Google Scholar] [CrossRef] [Green Version]
- Lai, F.; Jiang, R.; Xie, W.; Liu, X.; Tang, Y.; Xiao, H.; Gao, J.; Jia, Y.; Bai, Q. Intestinal Pathology and Gut Microbiota Alterations in a Methyl-4-Phenyl-1,2,3,6-Tetrahydropyridine (MPTP) Mouse Model of Parkinson’s Disease. Neurochem. Res. 2018, 43, 1986–1999. [Google Scholar] [CrossRef] [PubMed]
- Liśkiewicz, P.; Pełka-Wysiecka, J.; Kaczmarczyk, M.; Łoniewski, I.; Wroński, M.; Bąba-Kubiś, A.; Skonieczna-Żydecka, K.; Marlicz, W.; Misiak, B.; Samochowiec, J. Fecal Microbiota Analysis in Patients Going through a Depressive Episode during Treatment in a Psychiatric Hospital Setting. J. Clin. Med. 2019, 8, 164. [Google Scholar] [CrossRef] [Green Version]
- Ducatelle, R.; Eeckhaut, V.; Haesebrouck, F.; Van Immerseel, F. A Review on Prebiotics and Probiotics for the Control of Dysbiosis: Present Status and Future Perspectives. Animal 2015, 9, 43–48. [Google Scholar] [CrossRef]
- Marzorati, M.; Van den Abbeele, P.; Bubeck, S.S.; Bayne, T.; Krishnan, K.; Young, A.; Mehta, D.; DeSouza, A. Bacillus Subtilis HU58 and Bacillus Coagulans SC208 Probiotics Reduced the Effects of Antibiotic-Induced Gut Microbiome Dysbiosis in An M-SHIME® Model. Microorganisms 2020, 8, E1028. [Google Scholar] [CrossRef]
- Arslanova, A.; Tarasova, A.; Alexandrova, A.; Novoselova, V.; Shaidullov, I.; Khusnutdinova, D.; Grigoryeva, T.; Yarullina, D.; Yakovleva, O.; Sitdikova, G. Protective Effects of Probiotics on Cognitive and Motor Functions, Anxiety Level, Visceral Sensitivity, Oxidative Stress and Microbiota in Mice with Antibiotic-Induced Dysbiosis. Life 2021, 11, 764. [Google Scholar] [CrossRef]
- Berni Canani, R.; Sangwan, N.; Stefka, A.T.; Nocerino, R.; Paparo, L.; Aitoro, R.; Calignano, A.; Khan, A.A.; Gilbert, J.A.; Nagler, C.R. Lactobacillus Rhamnosus GG-Supplemented Formula Expands Butyrate-Producing Bacterial Strains in Food Allergic Infants. ISME J. 2016, 10, 742–750. [Google Scholar] [CrossRef] [Green Version]
- Morrison, D.J.; Preston, T. Formation of Short Chain Fatty Acids by the Gut Microbiota and Their Impact on Human Metabolism. Gut Microbes 2016, 7, 189–200. [Google Scholar] [CrossRef] [Green Version]
- Smith, P.M.; Howitt, M.R.; Panikov, N.; Michaud, M.; Gallini, C.A.; Bohlooly-Y, M.; Glickman, J.N.; Garrett, W.S. The Microbial Metabolites, Short Chain Fatty Acids, Regulate Colonic Treg Cell Homeostasis. Science 2013, 341, 569–573. [Google Scholar] [CrossRef] [Green Version]
- Liu, R.T.; Rowan-Nash, A.D.; Sheehan, A.E.; Walsh, R.F.L.; Sanzari, C.M.; Korry, B.J.; Belenky, P. Reductions in Anti-Inflammatory Gut Bacteria Are Associated with Depression in a Sample of Young Adults. Brain Behav. Immun. 2020, 88, 308–324. [Google Scholar] [CrossRef] [PubMed]
- Gevers, D.; Kugathasan, S.; Denson, L.A.; Vázquez-Baeza, Y.; Van Treuren, W.; Ren, B.; Schwager, E.; Knights, D.; Song, S.J.; Yassour, M.; et al. The Treatment-Naive Microbiome in New-Onset Crohn’s Disease. Cell Host Microbe 2014, 15, 382–392. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Quévrain, E.; Maubert, M.A.; Michon, C.; Chain, F.; Marquant, R.; Tailhades, J.; Miquel, S.; Carlier, L.; Bermúdez-Humarán, L.G.; Pigneur, B.; et al. Identification of an Anti-Inflammatory Protein from Faecalibacterium Prausnitzii, a Commensal Bacterium Deficient in Crohn’s Disease. Gut 2016, 65, 415–425. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schulthess, J.; Pandey, S.; Capitani, M.; Rue-Albrecht, K.C.; Arnold, I.; Franchini, F.; Chomka, A.; Ilott, N.E.; Johnston, D.G.W.; Pires, E.; et al. The Short Chain Fatty Acid Butyrate Imprints an Antimicrobial Program in Macrophages. Immunity 2019, 50, 432–445.e7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qu, Y.; Yang, C.; Ren, Q.; Ma, M.; Dong, C.; Hashimoto, K. Comparison of (R)-Ketamine and Lanicemine on Depression-like Phenotype and Abnormal Composition of Gut Microbiota in a Social Defeat Stress Model. Sci. Rep. 2017, 7, 15725. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Macedo, D.; Filho, A.J.M.C.; Soares de Sousa, C.N.; Quevedo, J.; Barichello, T.; Júnior, H.V.N.; Freitas de Lucena, D. Antidepressants, Antimicrobials or Both? Gut Microbiota Dysbiosis in Depression and Possible Implications of the Antimicrobial Effects of Antidepressant Drugs for Antidepressant Effectiveness. J. Affect. Disord. 2017, 208, 22–32. [Google Scholar] [CrossRef]
- Nikolova, V.L.; Cleare, A.J.; Young, A.H.; Stone, J.M. Updated Review and Meta-Analysis of Probiotics for the Treatment of Clinical Depression: Adjunctive vs. Stand-Alone Treatment. JCM 2021, 10, 647. [Google Scholar] [CrossRef]
- Le Blay, G.; Hammami, R.; Lacroix, C.; Fliss, I. Stability and Inhibitory Activity of Pediocin PA-1 Against Listeria Sp. in Simulated Physiological Conditions of the Human Terminal Ileum. Probiotics Antimicrob. Proteins 2012, 4, 250–258. [Google Scholar] [CrossRef]
- Macfarlane, G.T.; Macfarlane, S.; Gibson, G.R. Validation of a Three-Stage Compound Continuous Culture System for Investigating the Effect of Retention Time on the Ecology and Metabolism of Bacteria in the Human Colon. Microb. Ecol. 1998, 35, 180–187. [Google Scholar] [CrossRef]
- Bolyen, E.; Rideout, J.R.; Dillon, M.R.; Bokulich, N.A.; Abnet, C.C.; Al-Ghalith, G.A.; Alexander, H.; Alm, E.J.; Arumugam, M.; Asnicar, F.; et al. Reproducible, Interactive, Scalable and Extensible Microbiome Data Science Using QIIME 2. Nat. Biotechnol. 2019, 37, 852–857. [Google Scholar] [CrossRef] [PubMed]
- Segata, N.; Izard, J.; Waldron, L.; Gevers, D.; Miropolsky, L.; Garrett, W.S.; Huttenhower, C. Metagenomic Biomarker Discovery and Explanation. Genome Biol. 2011, 12, R60. [Google Scholar] [CrossRef] [PubMed] [Green Version]






Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ait Chait, Y.; Mottawea, W.; Tompkins, T.A.; Hammami, R. Evidence of the Dysbiotic Effect of Psychotropics on Gut Microbiota and Capacity of Probiotics to Alleviate Related Dysbiosis in a Model of the Human Colon. Int. J. Mol. Sci. 2023, 24, 7326. https://doi.org/10.3390/ijms24087326
Ait Chait Y, Mottawea W, Tompkins TA, Hammami R. Evidence of the Dysbiotic Effect of Psychotropics on Gut Microbiota and Capacity of Probiotics to Alleviate Related Dysbiosis in a Model of the Human Colon. International Journal of Molecular Sciences. 2023; 24(8):7326. https://doi.org/10.3390/ijms24087326
Chicago/Turabian StyleAit Chait, Yasmina, Walid Mottawea, Thomas A. Tompkins, and Riadh Hammami. 2023. "Evidence of the Dysbiotic Effect of Psychotropics on Gut Microbiota and Capacity of Probiotics to Alleviate Related Dysbiosis in a Model of the Human Colon" International Journal of Molecular Sciences 24, no. 8: 7326. https://doi.org/10.3390/ijms24087326
APA StyleAit Chait, Y., Mottawea, W., Tompkins, T. A., & Hammami, R. (2023). Evidence of the Dysbiotic Effect of Psychotropics on Gut Microbiota and Capacity of Probiotics to Alleviate Related Dysbiosis in a Model of the Human Colon. International Journal of Molecular Sciences, 24(8), 7326. https://doi.org/10.3390/ijms24087326









