Short-Term Effect of Nutraceutical Fruit Juices on Lipid Metabolism in Patients with Acquired Hypercholesterolemia
Abstract
1. Introduction
2. Results and Discussion
3. Materials and Methods
3.1. Subjects
3.2. Study Protocol
3.3. The Juice
- (1)
- (2)
- Two grams of phytosterols;
- (3)
- Red yeast rice containing 2.9 mg of monacolins from Monascus purpureus;
- (4)
- 100 mg of berberine complexed with β-cyclodextrin.
Juice Processing and Storage
3.4. Assessments
3.5. Statistical Analyses
4. Conclusions
5. Patents
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Sandesara, P.B.; Virani, S.S.; Fazio, S.; Shapiro, M.D. The Forgotten Lipids: Triglycerides, Remnant Cholesterol, and Athero-sclerotic Cardiovascular Disease Risk. Endocr. Rev. 2019, 40, 537–557. [Google Scholar] [CrossRef] [PubMed]
- Ference, B.A.; Ginsberg, H.N.; Graham, I.; Ray, K.K.; Packard, C.J.; Bruckert, E.; Hegele, R.A.; Krauss, R.M.; Raal, F.J.; Schunkert, H.; et al. Low-density lipoproteins cause atherosclerotic cardiovascular disease. 1. Evidence from genetic, epidemiologic, and clinical studies. A consensus statement from the European Atherosclerosis Society Consensus Panel. Eur. Heart J. 2017, 38, 2459–2472. [Google Scholar] [CrossRef] [PubMed]
- Hobbs, F.D.R.; Banach, M.; Mikhailidis, D.P.; Malhotra, A.; Capewell, S. Is statin-modified reduction in lipids the most important preventive therapy for cardiovascular disease? A pro/con debate. BMC Med. 2016, 14, 4. [Google Scholar] [CrossRef]
- Zeitouni, M.; Sabouret, P.; Kerneis, M.; Silvain, J.; Collet, J.-P.; Bruckert, E.; Montalescot, G. 2019 ESC/EAS Guidelines for management of dyslipidaemia: Strengths and limitations. Eur. Heart J. Cardiovasc. Pharmacother. 2020, 7, 324–333. [Google Scholar] [CrossRef]
- Cicero, A.F.G.; Colletti, A.; Bajraktari, G.; Descamps, O.; Djuric, D.M.; Ezhov, M.; Fras, Z.; Katsiki, N.; Langlois, M.; Latkovskis, G.; et al. Lipid-lowering nutraceuticals in clinical practice: Position paper from an International Lipid Expert Panel. Nutr. Rev. 2017, 75, 731–767. [Google Scholar] [CrossRef]
- Stroes, E.S.; Thompson, P.D.; Corsini, A.; Vladutiu, G.D.; Raal, F.J.; Ray, K.K.; Roden, M.; Stein, E.; Tokgözoğlu, T.; Nordestgaard, B.G.; et al. European Atherosclerosis Society Consensus Panel Statin-associated muscle symptoms: Impact on statin therapy—European Atherosclerosis Society Consensus Panel Statement on Assessment, Aetiology and Management. Eur. Heart J. 2015, 36, 1012–1022. [Google Scholar] [CrossRef]
- Bangalore, S.; Fayyad, R.; Hovingh, G.K.; Laskey, R.; Vogt, L.; DeMicco, D.A.; Waters, D.D.; Treating to New Targets Steering Committee and Investigators. Treating to New Targets Steering Committee and Investigators Statin and the risk of renal-related serious adverse events: Analysis from the IDEAL, TNT, CARDS, ASPEN, SPARCL, and other placebo-controlled trials. Am. J. Cardiol. 2014, 113, 2018–2020. [Google Scholar] [CrossRef]
- Patel, J.; Martin, S.S.; Banach, M. Expert opinion: The therapeutic challenges faced by statin intolerance. Expert Opin. Pharmacother. 2016, 17, 1497–1507. [Google Scholar] [CrossRef]
- Banach, M.; Rizzo, M.; Toth, P.P.; Farnier, M.; Davidson, M.H.; Al-Rasadi, K.; Aronow, W.S.; Athyros, V.; Djuric, D.M.; Ezhov, M.V.; et al. Statin intolerance—An attempt at a unified definition. Position paper from an Interna-tional Lipid Expert Panel. Arch. Med. Sci. 2015, 11, 1–23. [Google Scholar] [CrossRef]
- Cicero, A.F.G.; Fogacci, F.; Colletti, A. Food and plant bioactives for reducing cardiometabolic disease risk: An evidence based approach. Food Funct. 2017, 8, 2076–2088. [Google Scholar] [CrossRef]
- Catapano, A.L.; Graham, I.; De Backer, G.; Wiklund, O.; Chapman, M.J.; Drexel, H.; Hoes, A.W.; Jennings, C.S.; Landmesser, U.; Pedersen, T.R.; et al. 2016 ESC/EAS Guidelines for the Management of Dyslipidaemias: The Task Force for the Management of Dyslipidaemias of the European Society of Cardiology (ESC) and Eu-ropean Atherosclerosis Society (EAS) Developed with the special contribution of the European Assocciation for Cardiovascular Prevention & Rehabilitation (EACPR). Atherosclerosis 2016, 253, 281–344. [Google Scholar]
- Cholesterol Treatment Trialists’ (CTT) Collaboration. Efficacy and safety of more intensive lowering of LDL cholesterol: A meta-analysis of data from 170,000 participants in 26 randomised trials. Lancet 2010, 376, 1670–1681. [Google Scholar] [CrossRef] [PubMed]
- Derosa, G.; Colletti, A.; Maffioli, P.; D’angelo, A.; Lupi, A.; Zito, G.B.; Mureddu, G.F.; Raddino, R.; Fedele, F.; Cicero, A.F. Lipid-lowering nutraceuticals update on scientific evidence. J. Cardiovasc. Med. 2020, 21, 845–859. [Google Scholar] [CrossRef] [PubMed]
- Shepherd, J.; Blauw, G.J.; Murphy, M.B.; Bollen, E.L.; Buckley, B.M.; Cobbe, S.M.; Ford, I.; Gaw, A.; Hyland, M.; Jukema, J.W.; et al. PROSPER Study Group Pravastatin in elderly individuals at risk of vascular disease (PROSPER): A randomised controlled trial. Lancet 2002, 360, 1623–1630. [Google Scholar] [CrossRef] [PubMed]
- Heart Protection Study Collaborative Group. MRC/BHF Heart Protection Study of cholesterol lowering with simvastatin in 20,536 high-risk individuals: A randomised placebo-controlled trial. Lancet 2002, 360, 7–22. [Google Scholar] [CrossRef]
- Cicero, A.F.G.; Colletti, A. An update on the safety of nutraceuticals and effects on lipid parameters. Expert Opin. Drug Saf. 2018, 17, 303–313. [Google Scholar] [CrossRef]
- Faustino, M.; Veiga, M.; Sousa, P.; Costa, E.M.; Silva, S.; Pintado, M. Agro-Food Byproducts as a New Source of Natural Food Additives. Molecules 2019, 24, 1056. [Google Scholar] [CrossRef]
- Campoy-Muñoz, P.; Cardenete, M.A.; Delgado, M.D.C.; Sancho, F. Food Losses and Waste: A Needed Assessment for Future Policies. Int. J. Environ. Res. Public Health 2021, 18, 11586. [Google Scholar] [CrossRef]
- Ballistreri, G.; Amenta, M.; Fabroni, S.; Consoli, V.; Grosso, S.; Vanella, L.; Sorrenti, V.; Rapisarda, P. Evaluation of lipid and cholesterol-lowering effect of bioflavonoids from bergamot extract. Nat. Prod. Res. 2020, 35, 5378–5383. [Google Scholar] [CrossRef]
- Yubero, N.; Sanz-Buenhombre, M.; Guadarrama, A.; Villanueva, S.; Carrión, J.M.; Larrarte, E.; Moro, C. LDL cholesterol-lowering effects of grape extract used as a dietary supplement on healthy volunteers. Int. J. Food Sci. Nutr. 2012, 64, 400–406. [Google Scholar] [CrossRef]
- Gammon, C.S.; Kruger, R.; Minihane, A.M.; Conlon, C.A.; von Hurst, P.R.; Stonehouse, W. Kiwifruit consumption favourably affects plasma lipids in a randomised controlled trial in hypercholesterolaemic men. Br. J. Nutr. 2012, 109, 2208–2218. [Google Scholar] [CrossRef] [PubMed]
- Tenore, G.C.; Caruso, D.; Buonomo, G.; D’Avino, M.; Campiglia, P.; Marinelli, L.; Novellino, E. A Healthy Balance of Plasma Cholesterol by a Novel Annurca Apple-Based Nutraceutical Formulation: Results of a Randomized Trial. J. Med. Food 2017, 20, 288–300. [Google Scholar] [CrossRef] [PubMed]
- Ras, R.T.; Hiemstra, H.; Lin, Y.; Vermeer, M.A.; Duchateau, G.S.; Trautwein, E.A. Consumption of plant sterol-enriched foods and effects on plasma plant sterol concentrations—A meta-analysis of randomized controlled studies. Atherosclerosis 2013, 230, 336–346. [Google Scholar] [CrossRef] [PubMed]
- Piepoli, M.F.; Hoes, A.W.; Agewall, S.; Albus, C.; Brotons, C.; Catapano, A.L.; Cooney, M.-T.; Corrà, U.; Cosyns, B.; Deaton, C.; et al. 2016 European Guidelines on cardiovascular disease prevention in clinical practice: The Sixth Joint Task Force of the European Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practice (constituted by representatives of 10 societies and by invited experts): Developed with the special contribution of the European Association for Cardiovascular Prevention & Rehabilitation (EACPR). Eur. J. Prev. Cardiol. 2016, 23, NP1-96. [Google Scholar]
- Sahebkar, A.; Serban, M.-C.; Gluba-Brzózka, A.; Mikhailidis, D.P.; Cicero, A.F.; Rysz, J.; Banach, M. Lipid-modifying effects of nutraceuticals: An evidence-based approach. Nutrition 2016, 32, 1179–1192. [Google Scholar] [CrossRef] [PubMed]
- Vinogradova, Y.; Coupland, C.; Brindle, P.; Hippisley-Cox, J. Discontinuation and restarting in patients on statin treatment: Prospective open cohort study using a primary care database. BMJ 2016, 353, i3305. [Google Scholar] [CrossRef]
- Martín, M.; Ramos, S. Impact of Dietary Flavanols on Microbiota, Immunity and Inflammation in Metabolic Diseases. Nutrients 2021, 13, 850. [Google Scholar] [CrossRef]
- Colletti, A.; Fratter, A.; Pellizzato, M.; Cravotto, G. Nutraceutical Approaches to Dyslipidaemia: The Main Formulative Issues Preventing Efficacy. Nutrients 2022, 14, 4769. [Google Scholar] [CrossRef]
- Recio-Rodriguez, J.I.; EVIDENT Group; Gomez-Marcos, M.A.; Patino-Alonso, M.C.; Puigdomenech, E.; Notario-Pacheco, B.; Mendizabal-Gallastegui, N.; Fuente, A.D.L.C.D.L.; Otegui-Ilarduya, L.; Maderuelo-Fernandez, J.A.; et al. Effects of kiwi consumption on plasma lipids, fibrinogen and insulin resistance in the context of a normal diet. Nutr. J. 2015, 14, 97. [Google Scholar] [CrossRef]
- Sadeghi-Dehsahraei, H.; Ghaleh, H.E.G.; Mirnejad, R.; Parastouei, K. The effect of bergamot (KoksalGarry) supplementation on lipid profiles: A systematic review and meta-analysis of randomized controlled trials. Phytotherapy Res. 2022, 36, 4409–4424. [Google Scholar] [CrossRef]
- Lupoli, R.; Ciciola, P.; Costabile, G.; Giacco, R.; Di Minno, M.N.D.; Capaldo, B. Impact of Grape Products on Lipid Profile: A Meta-Analysis of Randomized Controlled Studies. J. Clin. Med. 2020, 9, 313. [Google Scholar] [CrossRef] [PubMed]
- Wu, A.H.; Spicer, D.; Stanczyk, F.Z.; Tseng, C.-C.; Yang, C.S.; Pike, M.C. Effect of 2-Month Controlled Green Tea Intervention on Lipoprotein Cholesterol, Glucose, and Hormone Levels in Healthy Postmenopausal Women. Cancer Prev. Res. 2012, 5, 393–402. [Google Scholar] [CrossRef] [PubMed]
| Baseline | 4 Weeks | 12 Weeks | p Baseline vs. 4 Weeks | p Baseline vs. 12 Weeks | p 4 Weeks vs. 12 Weeks | |
|---|---|---|---|---|---|---|
| Total cholesterol (mg/dL) | 276.9 ± 60.8 | 233.6 ± 50.6 | 246.1 ± 63.1 | <0.001 | <0.001 | NS * |
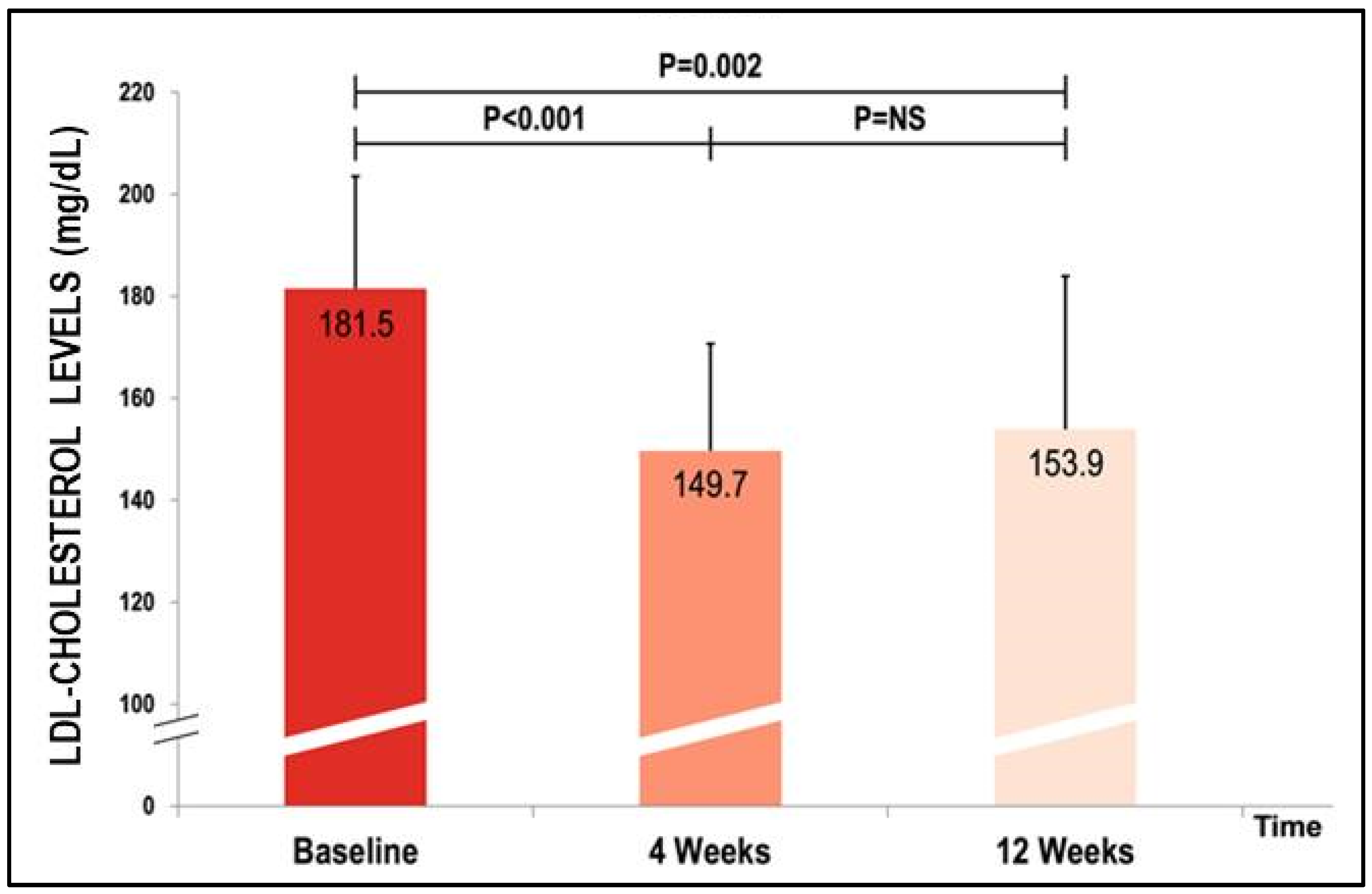
| LDL-cholesterol (mg/dL) | 181.5 ± 44.4 | 149.7 ± 42.2 | 153.9 ± 60.5 | <0.001 | 0.002 | NS |
| HDL-cholesterol (mg/dL) | 60.7 ± 15.7 | 62.1 ± 16.6 | 60.7 ± 16.0 | NS | NS | NS |
| Triglycerides (mg/dL) | 167.9 ± 102.5 | 122.4 ± 59 | 168.6 ± 163.1 | 0.011 | NS | NS |
| Non-HDL-cholesterol (mg/dL) | 216.2 ± 57.7 | 171.4 ± 48.9 | 185.4 ± 65.7 | <0.001 | <0.001 | NS |
| Apolipoprotein A1 (mg/dL) | 155.8 ± 24.0 | 163.4 ± 27.6 | 163.8 ± 31.4 | NS | NS | NS |
| Apolipoprotein B (mg/dL) | 131.0 ± 33.6 | 113.9 ± 27.3 | 118.8 ± 33.0 | <0.001 | 0.009 | NS |
| HbA1c (%) | 5.6 ± 0.4 | 5.6 ± 0.5 | 5.7 ± 0.4 | NS | NS | NS |
| Baseline | 4 Weeks | 12 Weeks | |
|---|---|---|---|
| Age (years) | 65.5 ± 9.4 | - | - |
| Males/females (n) | 5/9 | - | - |
| Family history of cardiovascular diseases (n) | 12 | - | - |
| History of hypertension (n) | 6 | - | - |
| Obesity (n) | 1 | - | - |
| Smokers (n) | 0 | - | - |
| Heart rate (beats per minute) | 62.7 ± 9.4 | 63.1 ± 8.9 | 62.6 ± 9.1 |
| Systolic blood pressure (mmHg) | 128.9 ± 17.7 | 125.9 ± 19.7 | 127.9 ± 18.7 |
| Diastolic blood pressure (mmHg) | 80.3 ± 11.1 | 79.7.1 ± 9.9 | 80.6 ± 12.1 |
| Height (cm) | 171.3 ± 22.7 | 172.3 ± 21.4 | 171.8 ± 21.7 |
| Weight (Kg) | 73.3 ± 8.7 | 74.2 ± 10.1 | 73.0 ± 8.9 |
| BMI (kg/m2) | 24.7 ± 1.7 | 24.6 ± 1.9 | 24.7 ± 1.8 |
| Waist circumference (cm) | 88.3 ± 7.9 | 89.1 ± 7.7 | 88.9 ± 8.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ardissino, D.; Colletti, A.; Pellizzato, M.; Pagliari, G.; Di Pierro, F.; Cravotto, G. Short-Term Effect of Nutraceutical Fruit Juices on Lipid Metabolism in Patients with Acquired Hypercholesterolemia. Int. J. Mol. Sci. 2023, 24, 7358. https://doi.org/10.3390/ijms24087358
Ardissino D, Colletti A, Pellizzato M, Pagliari G, Di Pierro F, Cravotto G. Short-Term Effect of Nutraceutical Fruit Juices on Lipid Metabolism in Patients with Acquired Hypercholesterolemia. International Journal of Molecular Sciences. 2023; 24(8):7358. https://doi.org/10.3390/ijms24087358
Chicago/Turabian StyleArdissino, Diego, Alessandro Colletti, Marzia Pellizzato, Gianna Pagliari, Francesco Di Pierro, and Giancarlo Cravotto. 2023. "Short-Term Effect of Nutraceutical Fruit Juices on Lipid Metabolism in Patients with Acquired Hypercholesterolemia" International Journal of Molecular Sciences 24, no. 8: 7358. https://doi.org/10.3390/ijms24087358
APA StyleArdissino, D., Colletti, A., Pellizzato, M., Pagliari, G., Di Pierro, F., & Cravotto, G. (2023). Short-Term Effect of Nutraceutical Fruit Juices on Lipid Metabolism in Patients with Acquired Hypercholesterolemia. International Journal of Molecular Sciences, 24(8), 7358. https://doi.org/10.3390/ijms24087358








