A Comparative Analysis to Dissect the Histological and Molecular Differences among Lipedema, Lipohypertrophy and Secondary Lymphedema
Abstract
:1. Introduction
2. Results
2.1. Epidermis Thickness Is Increased in Lipedema and Secondary Lymphedema Patients, While Cutaneous Fibrosis Is Reduced in Lipohypertrophy Patients
2.2. Adipocyte Size Is Increased in Lipedema, Secondary Lymphedema and Lipohypertrophy Patients
2.3. Lymphatic Vessel Coverage Is Significantly Lower in Lipohypertrophy Patients and VEGF-D Expression Is Significantly Decreased across All Conditions, in Comparison to Controls
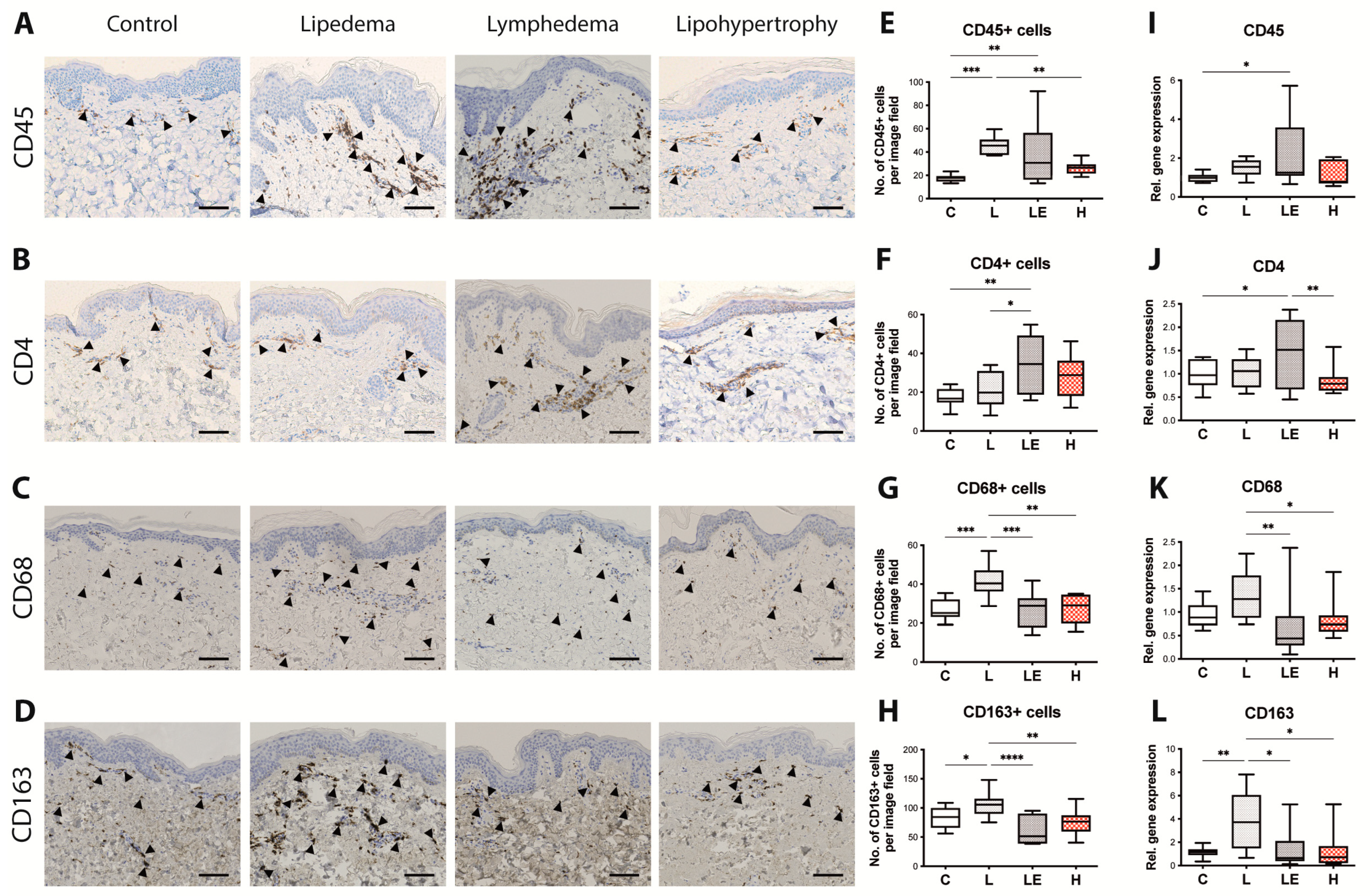
2.4. Immune Cell Infiltrate Changes in Lipedema, Secondary Lymphedema and Lipohypertrophy Patients
3. Discussion
4. Materials and Methods
4.1. Study Population
4.1.1. Diagnostic Criteria
Lymphedema
Lipedema
Lipohypertrophy
4.2. Tissue Collection
4.3. Histology and Immunohistochemistry Staining
4.4. Immunohistochemistry Analysis
4.5. RNA Extraction and Quantitative Polymerase Chain Reaction (qPCR)
4.6. Statistics
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Herpertz, U. Lipedema. Z. Lymphol. 1995, 19, 1–11. [Google Scholar] [PubMed]
- Meier-Vollrath, I.; Schmeller, W. Lipoedema—Current status, new perspectives. J. Dtsch. Dermatol. Ges. 2004, 2, 181–186. [Google Scholar] [CrossRef] [PubMed]
- Stemmer, R. Stemmer’s sign—Possibilities and limits of clinical diagnosis of lymphedema. Wien. Med. Wochenschr. 1999, 149, 85–86. [Google Scholar] [PubMed]
- Felmerer, G.; Stylianaki, A.; Hägerling, R.; Wang, A.; Ströbel, P.; Hollmén, M.; Lindenblatt, N.; Gousopoulos, E. Adipose Tissue Hypertrophy, An Aberrant Biochemical Profile and Distinct Gene Expression in Lipedema. J. Surg. Res. 2020, 253, 294–303. [Google Scholar] [CrossRef] [PubMed]
- Felmerer, G.; Stylianaki, A.; Hollmén, M.; Ströbel, P.; Stepniewski, A.; Wang, A.; Frueh, F.S.; Kim, B.-S.; Giovanoli, P.; Lindenblatt, N.; et al. Increased levels of VEGF-C and macrophage infiltration in lipedema patients without changes in lymphatic vascular morphology. Sci. Rep. 2020, 10, 10947. [Google Scholar] [CrossRef]
- Al-Ghadban, S.; Cromer, W.; Allen, M.; Ussery, C.; Badowski, M.; Harris, D.; Herbst, K.L. Dilated Blood and Lymphatic Microvessels, Angiogenesis, Increased Macrophages, and Adipocyte Hy-pertrophy in Lipedema Thigh Skin and Fat Tissue. J. Obes. 2019, 2019, 8747461. [Google Scholar] [CrossRef] [PubMed]
- Wolf, S.; von Atzigen, J.; Kaiser, B.; Grünherz, L.; Kim, B.-S.; Giovanoli, P.; Lindenblatt, N.; Gousopoulos, E. Is Lymphedema a Systemic Disease? A Paired Molecular and Histological Analysis of the Affected and Unaffected Tissue in Lymphedema Patients. Biomolecules 2022, 12, 1667. [Google Scholar] [CrossRef]
- Zampell, J.C.; Yan, A.; Elhadad, S.; Avraham, T.; Weitman, E.; Mehrara, B.J. CD4+ Cells Regulate Fibrosis and Lymphangiogenesis in Response to Lymphatic Fluid Stasis. PLoS ONE 2012, 7, e49940. [Google Scholar] [CrossRef]
- Ogata, F.; Fujiu, K.; Matsumoto, S.; Nakayama, Y.; Shibata, M.; Oike, Y.; Koshima, I.; Watabe, T.; Nagai, R.; Manabe, I. Excess Lymphangiogenesis Cooperatively Induced by Macrophages and CD4+ T Cells Drives the Pathogenesis of Lymphedema. J. Investig. Dermatol. 2016, 136, 706–714. [Google Scholar] [CrossRef]
- Gousopoulos, E.; Proulx, S.T.; Scholl, J.; Uecker, M.; Detmar, M. Prominent Lymphatic Vessel Hyperplasia with Progressive Dysfunction and Distinct Immune Cell In-filtration in Lymphedema. Am. J. Pathol. 2016, 186, 2193–2203. [Google Scholar] [CrossRef]
- Avraham, T.; Zampell, J.C.; Yan, A.; Elhadad, S.; Weitman, E.S.; Rockson, S.G.; Bromberg, J.; Mehrara, B.J. Th2 differentiation is necessary for soft tissue fibrosis and lymphatic dysfunction resulting from lymphedema. FASEB J. 2013, 27, 1114–1126. [Google Scholar] [CrossRef] [PubMed]
- Forner-Cordero, I.; Szolnoky, G.; Kemény, L. Lipedema: An overview of its clinical manifestations, diagnosis and treatment of the disproportional fatty deposition syndrome—Systematic review. Clin. Obes. 2012, 2, 86–95. [Google Scholar] [CrossRef] [PubMed]
- Reich-Schupke, S.; Schmeller, W.; Brauer, W.J.; Cornely, M.E.; Faerber, G.; Ludwig, M.; Lulay, G.; Miller, A.; Rapprich, S.; Richter, D.F.; et al. S1 guidelines: Lipedema. JDDG J. Dtsch. Dermatol. Ges. 2017, 15, 758–767. [Google Scholar] [CrossRef] [PubMed]
- Hardy, D.; Williams, A. Best practice guidelines for the management of lipoedema. Br. J. Community Nurs. 2017, 22, S44–S48. [Google Scholar] [CrossRef]
- Halk, A.B.; Damstra, R.J. First Dutch guidelines on lipedema using the international classification of functioning, disability and health. Phlebology 2017, 32, 152–159. [Google Scholar] [CrossRef] [PubMed]
- Herbst, K.L.; Kahn, L.A.; Iker, E.; Ehrlich, C.; Wright, T.; McHutchison, L.; Schwartz, J.; Sleigh, M.; MC Donahue, P.; Lisson, K.H.; et al. Standard of care for lipedema in the United States. Phlebology 2021, 36, 779–796. [Google Scholar] [CrossRef]
- Fu, M.R.; Deng, J.; Armer, J.M. Putting Evidence Into Practice: Cancer-Related Lymphedema. Clin. J. Oncol. Nurs. 2014, 18, 68–79. [Google Scholar] [CrossRef]
- Kruppa, P.; Georgiou, I.; Biermann, N.; Prantl, L.; Klein-Weigel, P.; Ghods, M. Lipedema-Pathogenesis, Diagnosis, and Treatment Options. Dtsch. Arztebl. Int. 2020, 117, 396–403. [Google Scholar] [CrossRef]
- Kamali, P.; Lin, S.J. Lymphedema: Presentation, Diagnosis, and Treatment. Plast. Reconstr. Surg. 2016, 137, 1654–1655. [Google Scholar] [CrossRef]
- Felmerer, G.; Sattler, T.; Lohrmann, C.; Tobbia, D. Treatment of various secondary lymphedemas by microsurgical lymph vessel transplantation. Microsurgery 2012, 32, 171–177. [Google Scholar] [CrossRef]
- Baglivo, M.; Martelli, F.; Paolacci, S.; Manara, E.; Michelini, S.; Bertelli, M. Electrical Stimulation in the Treatment of Lymphedema and Associated Skin Ulcers. Lymphat. Res. Biol. 2020, 18, 270–276. [Google Scholar] [CrossRef] [PubMed]
- Bellini, E.; Grieco, M.P.; Raposio, E. A journey through liposuction and liposculture: Review. Ann. Med. Surg. 2017, 24, 53–60. [Google Scholar] [CrossRef] [PubMed]
- Mellor, R.H.; Bush, N.L.; Stanton, A.W.B.; Bamber, J.C.; Levick, J.R.; Mortimer, P.S. Dual-Frequency Ultrasound Examination of Skin and Subcutis Thickness in Breast Cancer-Related Lymphedema. Breast J. 2004, 10, 496–503. [Google Scholar] [CrossRef] [PubMed]
- Rockson, S.G.; Tian, W.; Jiang, X.; Kuznetsova, T.; Haddad, F.; Zampell, J.; Mehrara, B.; Sampson, J.P.; Roche, L.; Kim, J.; et al. Pilot studies demonstrate the potential benefits of antiinflammatory therapy in human lymphedema. J. Clin. Investig. 2018, 3, 20. [Google Scholar] [CrossRef]
- Mehrara, B.J.; Park, H.J.; Kataru, R.P.; Bromberg, J.; Coriddi, M.; Baik, J.E.; Shin, J.; Li, C.; Cavalli, M.R.; Encarnacion, E.M.; et al. Pilot Study of Anti-Th2 Immunotherapy for the Treatment of Breast Cancer-Related Upper Extremity Lymphedema. Biology 2021, 10, 934. [Google Scholar] [CrossRef]
- Zhang, J.; Hoffner, M.; Brorson, H. Adipocytes are larger in lymphedematous extremities than in controls. J. Plast. Surg. Hand Surg. 2022, 56, 172–179. [Google Scholar] [CrossRef]
- Duhon, B.H.; Phan, T.T.; Taylor, S.L.; Crescenzi, R.L.; Rutkowski, J.M. Current Mechanistic Understandings of Lymphedema and Lipedema: Tales of Fluid, Fat, and Fibrosis. Int. J. Mol. Sci. 2022, 23, 6621. [Google Scholar] [CrossRef]
- O’Donnell, J.J., 3rd; Birukova, A.A.; Beyer, E.C.; Birukov, K.G. Gap junction protein connexin43 exacerbates lung vascular permeability. PLoS ONE 2014, 9, e100931. [Google Scholar] [CrossRef]
- Mehta, D.; Malik, A.B. Signaling Mechanisms Regulating Endothelial Permeability. Physiol. Rev. 2006, 86, 279–367. [Google Scholar] [CrossRef]
- Brice, G.; Ostergaard, P.; Jeffery, S.; Gordon, K.; Mortimer, P.; Mansour, S. A novel mutation in GJA1 causing oculodentodigital syndrome and primary lymphoedema in a three generation family. Clin. Genet. 2013, 84, 378–381. [Google Scholar] [CrossRef]
- Castorena-Gonzalez, J.A.; Zawieja, S.D.; Li, M.; Srinivasan, R.S.; Simon, A.M.; de Wit, C.; de la Torre, R.; Martinez-Lemus, L.A.; Hennig, G.W.; Davis, M.J. Mechanisms of Connexin-Related Lymphedema. Circ. Res. 2018, 123, 964–985. [Google Scholar] [CrossRef] [PubMed]
- Kanady, J.D.; Dellinger, M.T.; Munger, S.J.; Witte, M.H.; Simon, A.M. Connexin37 and Connexin43 deficiencies in mice disrupt lymphatic valve development and result in lymphatic disorders including lymphedema and chylothorax. Dev. Biol. 2011, 354, 253–266. [Google Scholar] [CrossRef] [PubMed]
- Günzel, D.; Yu, A.S.L. Claudins and the Modulation of Tight Junction Permeability. Physiol. Rev. 2013, 93, 525–569. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.; Xu, J.-T.; Jin, H.-N.; Zhao, R.; Zhao, D.; Du, S.-H.; Xue, Y.; Xie, X.-L.; Wang, Q. Increased cerebral expressions of MMPs, CLDN5, OCLN, ZO1 and AQPs are associated with brain edema fol-lowing fatal heat stroke. Sci. Rep. 2017, 7, 1691. [Google Scholar] [CrossRef]
- Heinolainen, K.; Karaman, S.; D’Amico, G.; Tammela, T.; Sormunen, R.; Eklund, L.; Alitalo, K.; Zarkada, G. VEGFR3 Modulates Vascular Permeability by Controlling VEGF/VEGFR2 Signaling. Circ. Res. 2017, 120, 1414–1425. [Google Scholar] [CrossRef]
- Mendola, A.; Schlögel, M.; Ghalamkarpour, A.; Irrthum, A.; Nguyen, H.; Fastré, E.; Bygum, A.; van der Vleuten, C.; Fagerberg, C.; Baselga, E.; et al. Mutations in the VEGFR3 Signaling Pathway Explain 36% of Familial Lymphedema. Mol. Syndr. 2013, 4, 257–266. [Google Scholar] [CrossRef]
- Zhang, L.; Zhou, F.; Han, W.; Shen, B.; Luo, J.; Shibuya, M.; He, Y. VEGFR-3 ligand-binding and kinase activity are required for lymphangiogenesis but not for angiogenesis. Cell Res. 2010, 20, 1319–1331. [Google Scholar] [CrossRef]
- Honkanen, H.-K.; Izzi, V.; Petäistö, T.; Holopainen, T.; Harjunen, V.; Pihlajaniemi, T.; Alitalo, K.; Heljasvaara, R. Elevated VEGF-D Modulates Tumor Inflammation and Reduces the Growth of Carcinogen-Induced Skin Tumors. Neoplasia 2016, 18, 436–446. [Google Scholar] [CrossRef]
- Gousopoulos, E.; Proulx, S.T.; Bachmann, S.B.; Scholl, J.; Dionyssiou, D.; Demiri, E.; Halin, C.; Dieterich, L.C.; Detmar, M. Regulatory T cell transfer ameliorates lymphedema and promotes lymphatic vessel function. J. Clin. Investig. 2016, 1, e89081. [Google Scholar] [CrossRef]
- Domoto, R.; Sekiguchi, F.; Tsubota, M.; Kawabata, A. Macrophage as a Peripheral Pain Regulator. Cells 2021, 10, 1881. [Google Scholar] [CrossRef]
- Chen, O.; Donnelly, C.R.; Ji, R.-R. Regulation of pain by neuro-immune interactions between macrophages and nociceptor sensory neurons. Curr. Opin. Neurobiol. 2020, 62, 17–25. [Google Scholar] [CrossRef] [PubMed]
- Wold, L.E.; Hines, E.A., Jr.; Allen, E.V. Lipedema of the legs; a syndrome characterized by fat legs and edema. Ann. Intern. Med. 1951, 34, 1243–1250. [Google Scholar] [CrossRef] [PubMed]



| Lipedema | Secondary Lymphedema | Lipohypertrophy | |
|---|---|---|---|
| Sex | Women | Women and Men | Women |
| Family History | + | - | + |
| Bilateral Swelling | + | - | + |
| Symmetric Swelling | + | - | + |
| Disproportion | + | + | + |
| Edema | -/(+) * | +++ | - |
| Inclusion of Feet | - | + | - |
| Pain | + | + | - |
| Bruising Tendency | + | - | - |
| Tissue Turgor | soft | firm | soft |
| Affinity to Infection | - | + | - |
| Epidermal Thickness | ++ | ++ | (+) |
| Adipocyte Size | +++ | + | +++ |
| Lymphatic system | unchanged phenotype | lymphatic impairment | decreased lymphatic coverage |
| Fibrosis | + | +++ | - |
| CD45+ cells | +++ | ++ | - |
| CD4+ T helper cells | - | +++ | - |
| CD68+ Macrophages | +++ | - | - |
| CD163+ Macrophages | +++ | - | - |
| Control | Lipedema | Lipohypertrophy | Lymphedema | |
|---|---|---|---|---|
| Number of cases | 10 | 10 | 10 | 10 |
| Gender | ||||
| Female | 10 | 10 | 10 | 10 |
| Male | 0 | 0 | 0 | 0 |
| Average BMI | 27.86 ± 4.478 | 29.10 ± 3.816 | 27.62 ± 4.943 | 26.55 ± 4.815 |
| Average Age | 48.45 ± 9.501 | 50.56 ± 11.41 | 46.20 ± 16.36 | 60.70 ± 11.15 |
| Lymphedema Stage | ||||
| Stage 1 | ||||
| Stage 2 | 7 | |||
| Stage 3 | 3 | |||
| Lipedema Stage | ||||
| Stage 1 | ||||
| Stage 2 | 6 | |||
| Stage 3 | 4 | |||
| Harvesting location Lower extremity | 10 | 10 | 10 | 9 |
| Upper extremity | 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
von Atzigen, J.; Burger, A.; Grünherz, L.; Barbon, C.; Felmerer, G.; Giovanoli, P.; Lindenblatt, N.; Wolf, S.; Gousopoulos, E. A Comparative Analysis to Dissect the Histological and Molecular Differences among Lipedema, Lipohypertrophy and Secondary Lymphedema. Int. J. Mol. Sci. 2023, 24, 7591. https://doi.org/10.3390/ijms24087591
von Atzigen J, Burger A, Grünherz L, Barbon C, Felmerer G, Giovanoli P, Lindenblatt N, Wolf S, Gousopoulos E. A Comparative Analysis to Dissect the Histological and Molecular Differences among Lipedema, Lipohypertrophy and Secondary Lymphedema. International Journal of Molecular Sciences. 2023; 24(8):7591. https://doi.org/10.3390/ijms24087591
Chicago/Turabian Stylevon Atzigen, Julia, Anna Burger, Lisanne Grünherz, Carlotta Barbon, Gunther Felmerer, Pietro Giovanoli, Nicole Lindenblatt, Stefan Wolf, and Epameinondas Gousopoulos. 2023. "A Comparative Analysis to Dissect the Histological and Molecular Differences among Lipedema, Lipohypertrophy and Secondary Lymphedema" International Journal of Molecular Sciences 24, no. 8: 7591. https://doi.org/10.3390/ijms24087591
APA Stylevon Atzigen, J., Burger, A., Grünherz, L., Barbon, C., Felmerer, G., Giovanoli, P., Lindenblatt, N., Wolf, S., & Gousopoulos, E. (2023). A Comparative Analysis to Dissect the Histological and Molecular Differences among Lipedema, Lipohypertrophy and Secondary Lymphedema. International Journal of Molecular Sciences, 24(8), 7591. https://doi.org/10.3390/ijms24087591





