Prominent Roles and Conflicted Attitudes of Eumelanin in the Living World
Abstract
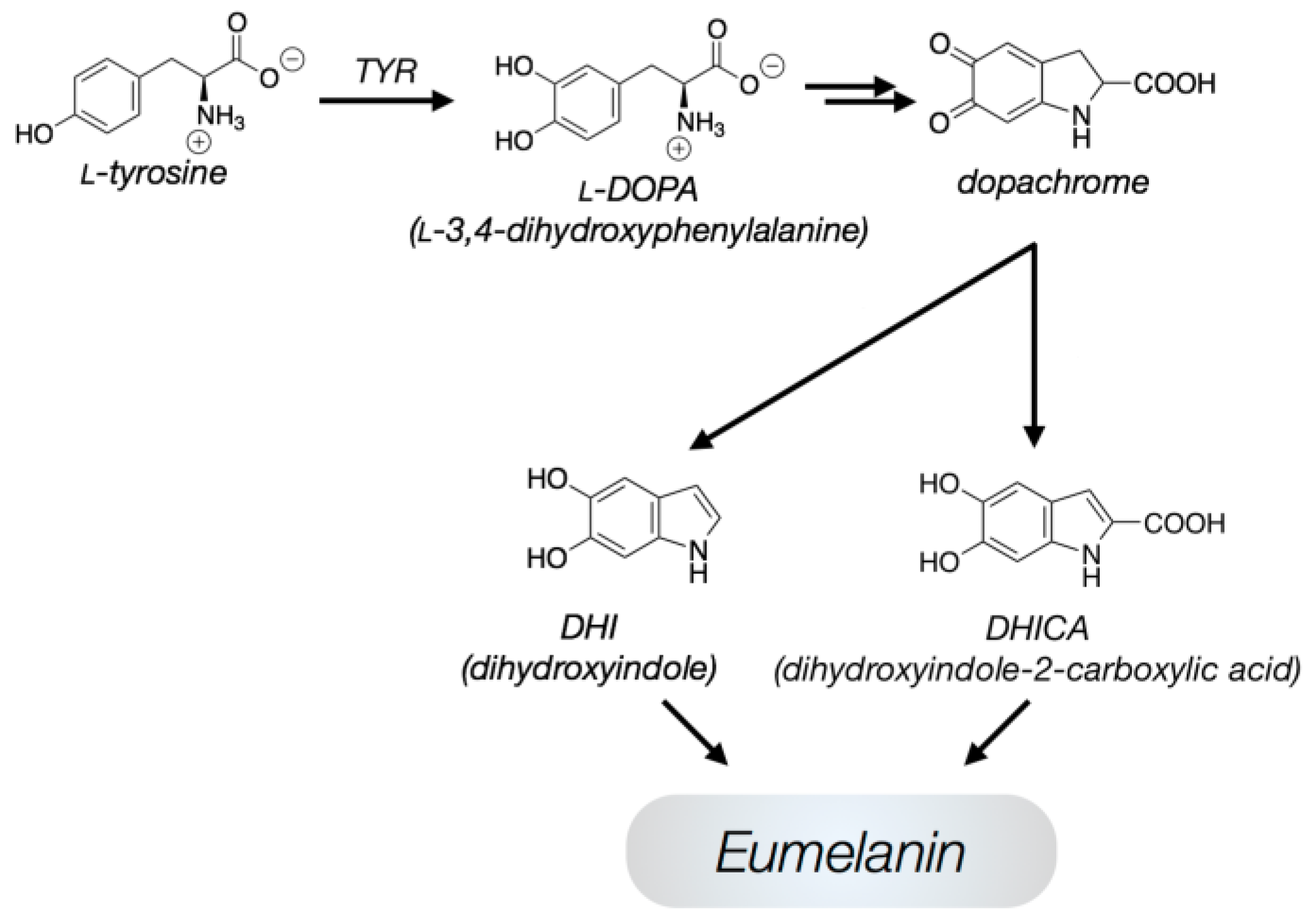
:1. Introduction
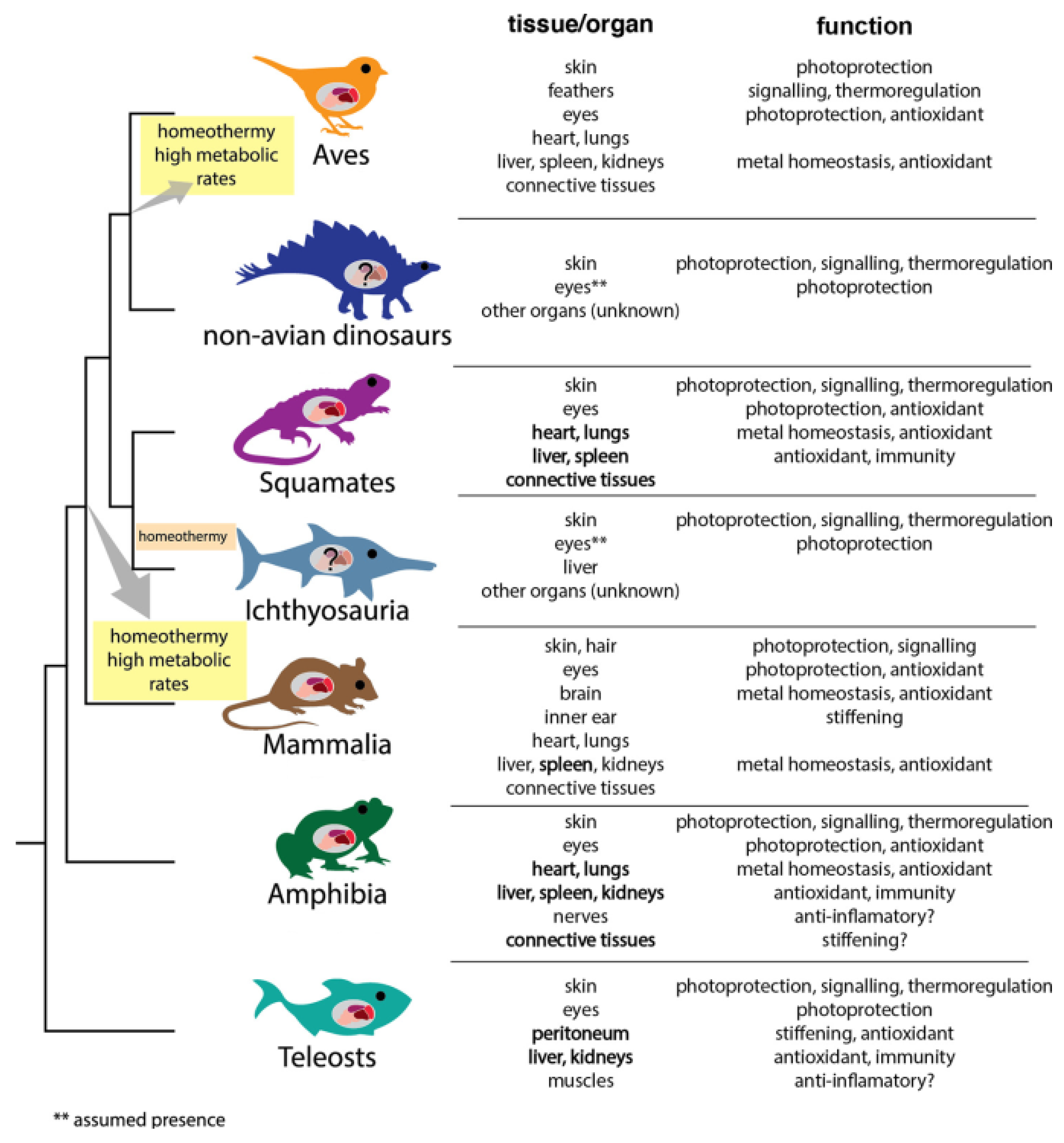
2. The Ubiquity in the Living World
3. The Keeping over Times of Chemical/Physical Features
4. The Ability to Withstand Extreme Conditions
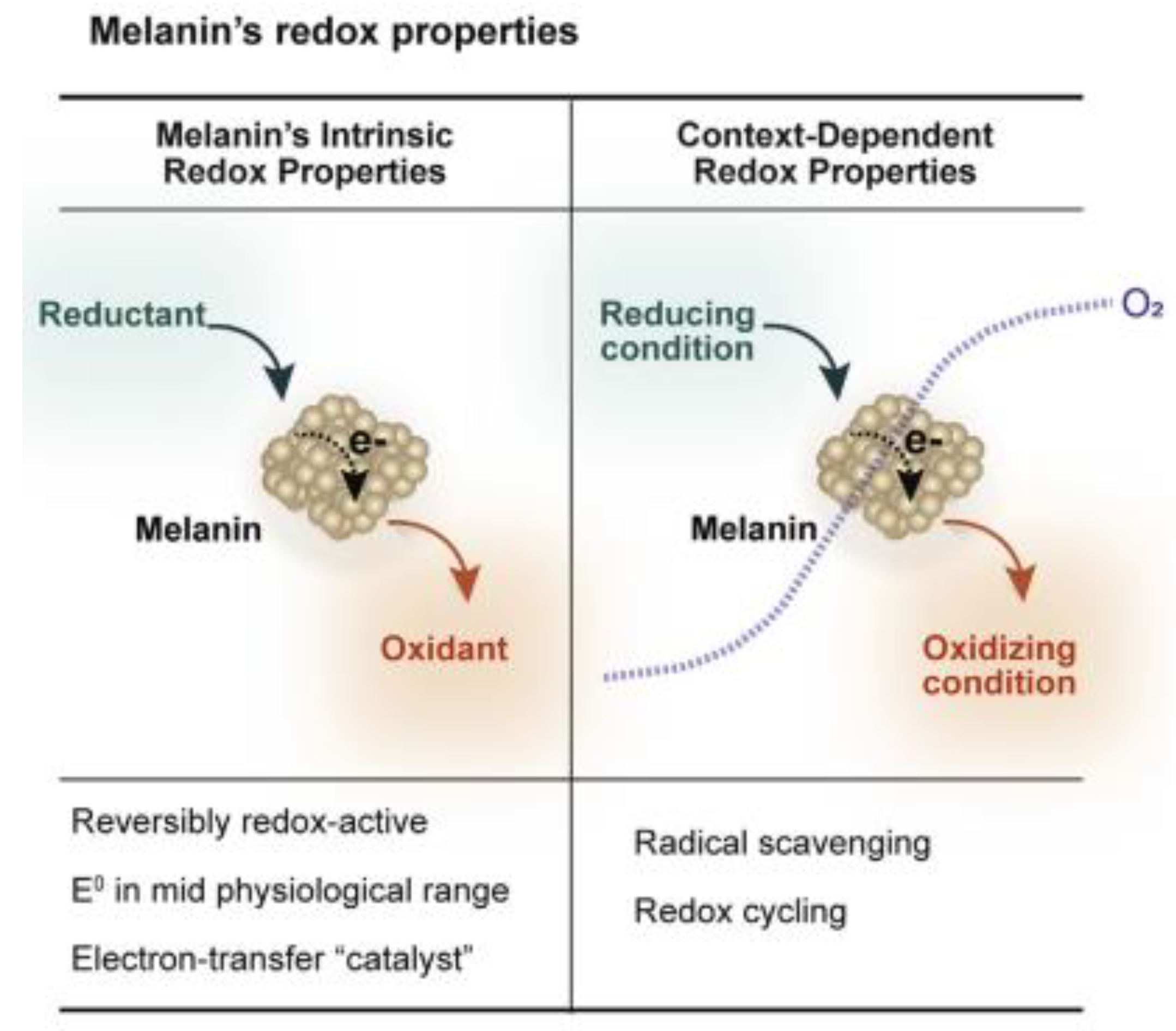
5. The Good and the Bad: Playing Conflicting Roles
6. Last Considerations: Still Open Questions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Büngeler, A.; Hämisch, B.; Strube, O.I. The supramolecular buildup of eumelanin: Structures, mechanisms, controllability. Int. J. Mol. Sci. 2017, 18, 1901. [Google Scholar] [CrossRef]
- Meredith, P.; Sarna, T. The physical and chemical properties of eumelanin. Pigment. Cell Res. 2006, 19, 572–594. [Google Scholar] [CrossRef]
- Kaxiras, E.; Tsolakidis, A.; Zonios, G.; Meng, S. Structural model of eumelanin. Phys. Rev. Lett. 2006, 97, 218102. [Google Scholar] [CrossRef]
- Solano, F. Melanins: Skin pigments and much more—Types, structural models, biological function and formation routes. New J. Sci. 2014, 6, 848–866. [Google Scholar] [CrossRef]
- Panzella, L.; Ebato, A.; Napolitano, A.; Koike, K. The Late Stages of Melanogenesis: Exploring the Chemical Facets and the Application Opportunities. Int. J. Mol. Sci. 2018, 19, 1753. [Google Scholar] [CrossRef]
- Ming, X.; Wei, C.; Weiyao, L.; Jiuzhou, Z.; You-lee, H.; Yusuke, N.; Toshikazu, M.; Matthew, D.S.; Ali, D. Elucidation of the hierarchical structure of natural eumelanins. J. R. Soc. Interface 2018, 15, 2180045. [Google Scholar] [CrossRef]
- Pralea, I.E.; Moldovan, R.-C.; Petrache, A.-M.; Ilieș, M.; Hegheș, S.-C.; Ielciu, I.; Nicoara, R.; Moldovan, M.; Ene, M.; Radu, M.; et al. From extraction to advanced analytical methods: The challenges of melanin analysis. Int. J. Mol. Sci. 2019, 20, 3943. [Google Scholar] [CrossRef] [PubMed]
- Affenzeller, S.; Frauendorf, H.; Licha, T.; Jackson, D.J.; Wolkenstein, K. Quantitation of eumelanin and pheomelanin markers in diverse biological samples by HPLCUV-MS following solid-phase extraction. PLoS ONE 2019, 14, e0223552. [Google Scholar] [CrossRef] [PubMed]
- Ito, S.; Sugumaran, M.; Wakamatsu, K. Chemical Reactivities of ortho-Quinones Produced in Living Organisms: Fate of Quinonoid Products Formed by Tyrosinase and Phenoloxidase Action on Phenols and Catechols. Int. J. Mol. Sci. 2020, 21, 6080. [Google Scholar] [CrossRef] [PubMed]
- Büngeler, A.; Hämisch, B.; Huber, K.; Bremser, W.; Strube, O.I. Insight into the Final Step of the Supramolecular Buildup of Eumelanin. Langmuir 2017, 33, 6895–6901. [Google Scholar] [CrossRef]
- Reali, M.; Gouda, A.; Bellemare, J.; Ménard, D.; Nunzi, J.-M.; Soavi, F.; Santato, C. Electronic transport in the Biopigment Sepia Melanin. ACS Appl. Bio Mater. 2020, 3, 5244–5252. [Google Scholar] [CrossRef]
- Batagin-Neto, A.; Bronze-Uhle, E.S.; Graeff, C.F.D.O. Electronic structure calculations of ESR parameters of melanin. Phys. Chem. Chem. Phys. 2015, 17, 7264–7274. [Google Scholar] [CrossRef] [PubMed]
- Mostert, A.B.; Hanson, G.R.; Sarna, T.; Gentle, I.R.; Powell, B.J.; Meredith, P. Hydration-controlled X-band EPR spectroscopy: A tool for unravelling the complexities of the solid-state free radical in eumelanin. J. Phys. Chem. B 2013, 117, 4965–4972. [Google Scholar] [CrossRef]
- Mostert, A.B.; Rienecker, S.B.; Noble, C.; Hanson, G.R.; Meredith, P. The Photoreactive Free Radical in Eumelanin. Sci. Adv. 2018, 4, eaaq1293. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.T.; Chuang, C.; Cao, J.; Ball, V.; Ruch, D.; Buehler, M.J. Excitonic Effects from Geometric Order and Disorder Explain Broadband Optical Absorption in Eumelanin. Nat. Commun. 2014, 5, 3859. [Google Scholar] [CrossRef] [PubMed]
- Ball, V. Determination of the extinction coefficient of “polydopamine” films obtained by using NaIO4 as the oxidant. Mater. Chem. Phys. 2017, 186, 546–551. [Google Scholar] [CrossRef]
- Kohl, F.R.; Grieco, C.; Kohler, B. Ultrafast spectral hole burning reveals the distinct chromophores in eumelanin and their common photoresponse. Chem. Sci. 2020, 11, 1248–1259. [Google Scholar] [CrossRef]
- Kim, E.; Kang, M.; Tschirhart, T.; Malo, M.; Dadachova, E.; Cao, G.; Yin, J.J.; Bentley, W.E.; Wang, Z.; Payne, G.F. Spectroelectrochemical reverse engineering demonstrates that melanin’s redox and radical scavenging activities are linked. Biomacromolecules 2017, 18, 4084–4098. [Google Scholar] [CrossRef]
- Hong, L.; Simon, J.D. Current understanding of the binding sites, capacity, affinity, and biological significance of metals in melanin. J. Phys. Chem. B 2007, 111, 7938–7947. [Google Scholar] [CrossRef]
- Di Mauro, E.; Xu, R.; Soliveri, G.; Santato, C. Natural melanin pigments and their interfaces with metal ions and oxides: Emerging concepts and technologies. MRS Commun. 2017, 7, 141–151. [Google Scholar] [CrossRef]
- Sarna, T.; Swartz, H.M.; Zadlo, A. Interaction of Melanin with Metal Ions Modulates Their Cytotoxic Potential. Appl. Magn. Reson. 2022, 53, 105–121. [Google Scholar] [CrossRef]
- Reali, M.; Saini, P.; Santato, C. Electronic and protonic transport in bio-sourced materials: A new perspective on semiconductivity. Mater. Adv. 2021, 2, 15. [Google Scholar] [CrossRef]
- Dadachova, E.; Bryan, R.A.; Huang, X.; Moadel, T.; Schweitzer, A.D.; Aisen, P.; Nosanchuk, J.D.; Casadevall, A. Ionizing radiation changes the electronic properties of melanin and enhances the growth of melanized fungi. PLoS ONE 2007, 2, e457. [Google Scholar] [CrossRef] [PubMed]
- Barra, M.; Bonadies, I.; Carfagna, C.; Cassinese, A.; Cimino, F.; Crescenzi, O.; Criscuolo, V.; d’Ischia, M.; Maglione, M.G.; Manini, P.; et al. Eumelanin-Based Organic Bioelectronics: Myth or Reality? MRS Adv. 2016, 1, 3801–3810. [Google Scholar] [CrossRef]
- Vahidzadeh, E.; Kalra, A.P.; Shankar, K. Melanin-based electronics: From proton conductors to photovoltaics and beyond. Biosens. Bioelectron. 2018, 122, 127–139. [Google Scholar] [CrossRef] [PubMed]
- Xie, W.; Pakdel, E.; Liang, Y.; Kim, Y.J.; Liu, D.; Sun, L.; Wang, X. Natural eumelanin and its derivatives as multifunctional materials for bioinspired applications: A review. Biomacromolecules 2019, 20, 4312–4331. [Google Scholar] [CrossRef]
- Battistella, C.; McCallum, N.C.; Gnanasekaran, K.; Zhou, X.; Caponetti, V.; Montalti, M.; Gianneschi, N.C. Mimicking Natural Human Hair Pigmentation with Synthetic Melanin. ACS Cent. Sci. 2020, 6, 1179–1188. [Google Scholar] [CrossRef] [PubMed]
- Caldas, M.; Santos, A.C.; Veiga, F.; Rebelo, R.; Reis, R.L.; Correlo, V.M. Melanin nanoparticles as a promising tool for biomedical applications—A review. Acta Biomater. 2020, 105, 26–43. [Google Scholar] [CrossRef]
- Mostert, A.B. Melanin, the What, the Why and the How: An Introductory Review for Materials Scientists Interested in Flexible and Versatile Polymers. Polymers 2021, 13, 1670. [Google Scholar] [CrossRef]
- Terranova, M.L. Radioactivity to Rethink the Earth’s Energy Balance. Glob. Chall. 2021, 5, 2000094. [Google Scholar] [CrossRef]
- Terranova, M.L.; Tamburri, E. Understanding the way eumelanin works: A unique example of properties and skills driven by molecular heterogeneity. Polymer 2021, 229, 123952. [Google Scholar] [CrossRef]
- d’Ischia, M.; Napolitano, A.; Pezzella, A.; Meredith, P.; Buehler, M. Melanin Biopolymers: Tailoring Chemical Complexity for Materials Design. Angew. Chem. Int Ed. Engl. 2020, 59, 11196–11205. [Google Scholar] [CrossRef] [PubMed]
- Ameta, S.; Blokhuis, A.; Jeancolas, C.; Nghe, P. Chapter 13: Toward evolution in chemical reaction networks. Prebiotic Chemistry and Life’s Origin, Royal Society of Chemistry. Chem. Biol. 2022, 379–423. [Google Scholar] [CrossRef]
- Benner, S.A. Defining life. Astrobiology 2010, 10, 1021–1030. [Google Scholar] [CrossRef]
- Borovansky, J. Melanins and Melanosomes: Biosynthesis, Structure, Physiological and Pathological Functions; Patrick, J.B., Riley, A., Eds.; Wiley-VCH Verlag & Co.: Weinheim, Germany, 2011. [Google Scholar]
- Zecca, L.; Bellei, C.; Costi, P.; Albertini, A.; Monzani, E.; Casella, L.; Gallorini, M.; Bergamaschi, L.; Moscatelli, A.; Turro, N.J.; et al. New Melanic Pigments in the Human Brain That Accumulate in Aging and Block Environmental Toxic Metals. Proc. Natl. Acad. Sci. USA 2008, 105, 17567–17572. [Google Scholar] [CrossRef] [PubMed]
- Glass, K.; Ito, S.; Wilby, P.R.; Sota, T.; Nakamura, A.; Bowers, C.R.; Miller, K.E.; Dutta, S.; Summons, R.E.; Briggs, D.E.G.; et al. Impact of diagenesis and maturation on the survival of eumelanin in the fossil record. Org. Geochem. 2013, 64, 29–37. [Google Scholar] [CrossRef]
- Herrera, J.; Sistiaga, A.; Vinther, J.; Brown, C.M.; Henderson, D.M.; Summons, R.E. Molecular characterization and effect of diagenesis and maduration of melanin in the fossil record. In Proceedings of the 29th International Meeting on Organic Geochemistry, Gothenburg, Sweden, 1–6 September 2019; p. 152636. [Google Scholar]
- Schweitzer, M.H. Soft tissue preservation in terrestrial mesozoic vertebrates. Annu. Rev. Earth Planet Sci. 2011, 39, 187–216. [Google Scholar] [CrossRef]
- Briggs, D.E.G.; Evershed, R.P.; Lockheart, M.J. The biomolecular paleontology of continental fossils. Paleobiology 2000, 26, 169–193. [Google Scholar] [CrossRef]
- Wilby, P.R.; Hudson, J.D.; Clements, R.G.; Hollingworth, N.T.J. Taphonomy and origin of an accumulate of soft-bodied cephalopods in the Oxford Clay Formation (Jurassic, England). Palaeontology 2004, 47, 1159–1180. [Google Scholar] [CrossRef]
- Glass, K.; Ito, S.; Wilby, P.R.; Sota, T.; Nakamura, A.; CRussell Bowers, C.R.; Vinther, J.; Dutta, S.; Summons, R.E.; Briggs, D.E.G.; et al. Direct chemical evidence for eumelanin pigment from the Jurassic period. Proc. Natl. Acad. Sci. USA 2012, 109, 10218–10223. [Google Scholar] [CrossRef]
- Gabbott, S.E.; Donoghue, P.C.J.; Sansom, R.S.; Vinther, J.; Dolocan, A.; Purnell, M.A. Pigmented anatomy in Carboniferous cyclostomes and the evolution of the vertebrate eye. Proc. R. Soc. 2016, 283, 20161151. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.Y.; Fisher, D.E. Melanocyte biology and skin pigmentation. Nature 2007, 445, 843–850. [Google Scholar] [CrossRef]
- Vinther, J.; Briggs, D.E.; Prum, R.O.; Saranathan, V.T. The colour of fossil feathers. Biol. Lett. 2008, 4, 522–525. [Google Scholar] [CrossRef] [PubMed]
- Roy, A.; Pittman, M.; Saitta, E.T.; Kaye, T.G.; Xu, X. Recent advances in amniote palaeocolour reconstruction and a framework for future research. Biol. Rev. Camb. Philos. Soc. 2020, 95, 22–50. [Google Scholar] [CrossRef] [PubMed]
- Fabbri, M.; Wiemann, J.; Manucci, F.; Briggs, D.E.G. Three dimensional soft tissue preservation revealed in the skin of a non-avian dinosaur. Palaeontology 2020, 63, 185–193. [Google Scholar] [CrossRef]
- D’Alba, L.; Shawkey, M.-D. Melanosomes: Biogenesis, Properties, and Evolution of an Ancient Organelle. Physiol. Rev. 2019, 99, 1–19. [Google Scholar] [CrossRef]
- Liu, Y.; Simon, J.D. The effect of preparation procedures on the morphology of melanin from the ink sac of Sepia officinalis. Pigment. Cell Res. 2003, 16, 72–80. [Google Scholar] [CrossRef]
- Rossi, V.; McNamara, M.E.; Webb, S.M.; Ito, S.; Wakamatsu, K. Tissue-specific geometry and chemistry of modern and fossilized melanosomes reveal internal anatomy of extinct vertebrates. Proc. Natl. Acad. Sci. USA 2019, 116, 17880–17889. [Google Scholar] [CrossRef]
- Dubey, S.; Roulin, A. Evolutionary and biomedical consequences of internal melanins. Pigment. Cell Melanoma Res. 2014, 27, 327–338. [Google Scholar] [CrossRef]
- Wakamatsu, K.; Ito, S. Melanins in Vertebrates. In Pigments, Pigment Cells and Pigment Patterns; Hashimoto, H., Goda, M., Futahashi, R., Kelsh, R., Akiyama, T., Eds.; Springer: Singapore, 2021. [Google Scholar] [CrossRef]
- McNamara, M.E.; Rossi, V.; Slater, T.S.; Rogers, C.S.; Ducrest, A.-L.; Dubey, S.; Roulin, A. Decoding the Evolution of Melanin in Vertebrates Trends. Ecol. Evol. 2021, 36, 430–443. [Google Scholar] [CrossRef]
- d’Ischia, M.; Manini, P.; Martins, Z.; Remusat, L.; O’D Alexander, C.M.; Puzzarini, C.; Barone, V.; Saladino, R. Insoluble organic matter in chondrites: Archetypal melanin-like PAH-based multifunctionality at the origin of life? Phys. Life Rev. 2021, 37, 65–93. [Google Scholar] [CrossRef] [PubMed]
- Tesei, D. Black Fungi Research: Out-of-This-World Implications. Encyclopedia 2022, 2, 212–229. [Google Scholar] [CrossRef]
- Tugay, T.I.; Zheltonozhskaya, M.V.; Sadovnikov, L.V.; Tugay, A.V.; Farfán, E.B. Effects of ionizing radiation on the antioxidant system of microscopic fungi with radioadaptive properties found in the Chernobyl exclusion zone. Health Phys. 2011, 101, 375–382. [Google Scholar] [CrossRef] [PubMed]
- Langfelder, K.; Streibel, M.; Jahn, B.; Haase, G.; Brakhage, A. Biosynthesis of fungal melanins and their importance for human pathogenic fungi. Fungal Genet. Biol. 2003, 38, 143–158. [Google Scholar] [CrossRef]
- Sarna, T.; Menon, I.A.; Sealy, R.C. Photosensitization of melanins: A comparative study. Photochem. Photobiol. 1985, 42, 529–532. [Google Scholar] [CrossRef] [PubMed]
- Wróbel, D.; Planner, A.; Hanyz, I.; Wielgus, A.; Sarna, T. Melaninporphyrin interaction monitored by delayed luminescence and photoacoustics. J. Photochem. Photobiol. B 1997, 41, 45–52. [Google Scholar] [CrossRef]
- Burke, J.M.; Kaczara, P.; Skumatz, C.M.; Zareba, M.; Raciti, M.W.; Sarna, T. Dynamic analyses reveal cytoprotection by RPE melanosomes against non-photic stress. Mol. Vis. 2011, 17, 2864–2877. [Google Scholar]
- Hong, L.; Liu, Y.; Simon, J.D. Binding of metal ions to melanin and their effects on the aerobic reactivity. Photochem. Photobiol. 2007, 80, 477–481. [Google Scholar] [CrossRef]
- Scalia, M.; Geremia, E.; Corsaro, C.; Santoro, C.; Baratta, D.; Sichel, G. Lipid peroxidation in pigmented and unpigmented liver tissues: Protective role of melanin. Pigment. Cell Res. 1990, 3, 115–119. [Google Scholar] [CrossRef]
- Porebska-Budny, M.; Sakina, N.L.; Stepien, K.B.; Dontsov, A.E.; Wilczok, T. Antioxidative activity of synthetic melanins. Cardiolipin liposome model. Biochim. Biophys. Acta 1992, 1116, 11–16. [Google Scholar] [CrossRef]
- Jacobson, E.S.; Ikeda, R. Effect of melanization upon porosity of the cryptococcal cell wall. Med. Mycol. 2005, 43, 327–333. [Google Scholar] [CrossRef]
- Plemenitaš, A.; Vaupotic, T.; Lenassi, M.; Kogej, T. Adaptation of extremely halotolerant black yeast Hortaea werneckii to increased osmolarity: A molecular perspective at a glance. Stud. Mycol. 2008, 61, 67–75. [Google Scholar] [CrossRef]
- Cassaro, A.; Pacelli, C.; Onofri, S. Survival, metabolic activity, and ultrastructural damages of Antarctic black fungus in perchlorates media. Front. Microbiol. 2022, 13, 992077. [Google Scholar] [CrossRef] [PubMed]
- Shields, L.M.; Drouet, F. Distribution of Terrestrial Algae within the Nevada Test Site. Am. J. Bot. 1962, 49, 547–554. [Google Scholar] [CrossRef]
- Osburn, W.S. Ecological Concentration of Nuclear Fallout in a Colorado Mountain Watershed. In Radioecological Concentration Processes; Hungate, P., Frank, B.Å., Eds.; Pergamon: New York, NY, USA, 1967; pp. 675–709. Available online: https://www.elsevier.com/books/radioecological-concentration-processes/aberg/978-0-08-012122-2 (accessed on 20 January 2023).
- Sinilova, N.G.; Pershina, Z.G.; Duplitseva, A.P.; Pavlova, I.B. A radioresistant pigmented bacterial culture isolated from atomic reactor water. Zh. Mikrobiol. Epidemiol. Immunobiol. 1969, 46, 94–99. (In Russian) [Google Scholar]
- Zhadanova, N.N.; Vasilevskaya, A.I.; Artyshkova, L.V.; Sadovnikov, Y.S.; Lashko, T.N.; Gavrilyuk, V.I.; Dighton, J. Changes in micromycete communities in soil in response to pollution by long-lived radionuclides emitted in the Chernobyl accident. Mycol. Res. 1994, 98, 789–795. [Google Scholar] [CrossRef]
- Mironenko, N.V.; Alekhina, I.A.; Zhdanova, N.N.; Bulat, S.A. Intraspecific variation in gamma radiation resistance and genomic structure in the filamentous fungus Alternaria alternata: A case study of strains inhabiting Chernobyl reactor no. 4. Ecotoxicol. Environ. Saf. 2000, 45, 177–187. [Google Scholar] [CrossRef]
- Dadachova, E.; Casadevall, A. Ionizing radiation: How fungi cope, adapt, and exploit with the help of melanin. Curr. Opin. Microbiol. 2008, 11, 525–531. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.; Panzella, L.; Napolitano, A.; Payne, G.F. Redox activities of melanins investigated by electrochemical reverse engineering: Implications for their roles in oxidative stress. J. Investig. Dermatol. 2020, 140, 537–543. [Google Scholar] [CrossRef]
- Schweitzer, A.D.; Howell, R.C.; Jiang, Z.; Bryan, R.A.; Gerfen, G.; Chen, C.-C.; Mah, D.; Cahill, S.; Casadevall, A.; Dadachova, E. Physico-Chemical Evaluation of Rationally Designed Melanins as Novel Nature-Inspired Radioprotectors. PLoS ONE 2009, 4, e7229. [Google Scholar] [CrossRef]
- Turick, C.E.; Ekechukwu, A.A.; Milliken, C.E.; Casadevall, A.; Dadachova, E. Gamma radiation interacts with melanin to alter its oxidation-reduction potential and results in electric current production. Bioelectrochemistry 2011, 82, 69–73. [Google Scholar] [CrossRef]
- Dadachova, E.; Casadevall, A. Melanin and Resistance to Ionizing Radiation in Fungi. In Extremophiles Handbook; Horikoshi, K., Ed.; Springer: Berlin/Heidelberg, Germany, 2011. [Google Scholar] [CrossRef]
- Malo, M.E.; Dadachova, E. Melanin as an Energy Transducer and a Radioprotector in Black Fungi. In Fungi in Extreme Environments: Ecological Role and Biotechnological Significance; Tiquia-Arashiro, S., Grube, M., Eds.; Springer: Berlin/Heidelberg, Germany, 2019. [Google Scholar] [CrossRef]
- Compton, A.H. A quantum theory of the scattering of X-rays by light elements. Phys. Rev. 1923, 21, 483–502. [Google Scholar] [CrossRef]
- Revskaya, E.; Chu, P.; Howell, R.C.; Schweitzer, A.D.; Bryan, R.A.; Harris, M.; Gerfen, G.; Zewei, J.; Thomas, J.; Kami, K.; et al. Compton scattering by internal shields based on melanin-containing mushrooms provides protection of gastrointestinal tract from ionizing radiation. Cancer Biother. Radiopharm. 2012, 27, 570–576. [Google Scholar] [CrossRef] [PubMed]
- Pacelli, C.; Bryan, R.A.; Onofri, S.; Selbmann, L.; Shuryak, I.; Dadachova, E. Melanin is effective in protecting fast and slow growing fungi from various types of ionizing radiation. Environ. Microbiol. 2017, 19, 1612–1624. [Google Scholar] [CrossRef] [PubMed]
- Vasileiou, T.; Summerer, L. A biomimetic approach to shielding from ionizing radiation: The case of melanized fungi. PLoS ONE 2020, 15, e0229921, Erratum in PLoS ONE 2021, 16, e0257068. [Google Scholar] [CrossRef]
- Symonds, R.; Mills, J.; Duxbury, A. (Eds.) Walter and Miller’s Textbook of Radiotherapy: Radiation Physics, Therapy, and Oncology, 8th ed.; Elsevier: Amsterdam, The Netherlands, 2020; ISBN 9780702074844. [Google Scholar]
- Pacelli, C.; Selbmann, L.; Zucconi, L.; Raguse, M.; Moeller, R.; Shuryak, I.; Onofri, S. Survival, DNA integrity, and ultrastructural damage in antarctic cryptoendolithic eukaryotic microorganisms exposed to ionizing radiation. Astrobiology 2017, 17, 126–135. [Google Scholar] [CrossRef]
- Malo, M.E.; Bryan, R.A.; Shuryak, I.; Dadachova, E. Morphological changes in melanized and non-melanized Cryptococcus neoformans cells post exposure to sparsely and densely ionizing radiation demonstrate protective effect of melanin. Fungal Biol. 2018, 122, 449–456. [Google Scholar] [CrossRef]
- Pacelli, C.; Cassaro, A.; Aureli, L.; Moeller, R.; Fujimori, A.; Onofri, S. The Responses of the Black Fungus Cryomyces Antarcticus to High Doses of Accelerated Helium Ions Radiation within Martian Regolith Simulants and Their Relevance for Mars. Life 2020, 10, 130. [Google Scholar] [CrossRef]
- Pacelli, C.; Cassaro, A.; Siong, L.M.; Aureli, L.; Moeller, R.; Fujimor, A.; Shuryak, I.; Onofri, S. Insights into the Survival Capabilities of Cryomyces antarcticus Hydrated Colonies after Exposure to Fe Particle Radiation. J. Fungi 2021, 7, 495. [Google Scholar] [CrossRef]
- Pacelli, C.; Bryan, R.A.; Onofri, S.; Selbmann, L.; Zucconi, L.; Shuryak, I.; Dadachova, E. Survival and redox activity of Friedmanniomyces endolithicus, an Antarctic endemic black meristematic fungus, after gamma rays exposure. Fungal Biol. 2018, 122, 1222–1227. [Google Scholar] [CrossRef]
- Zhdanova, N.N.; Tugay, T.; Dighton, J.; Zheltonozhsky, V.; Mcdermott, P. Ionizing radiation attracts soil fungi. Mycol. Res. 2004, 108, 1089–1096. [Google Scholar] [CrossRef] [PubMed]
- Bryan, R.; Jiang, Z.; Friedman, M.; Dadachova, E. The effects of gamma radiation, UV and visible light on ATP levels in yeast cells depend on cellular melanization. Fungal Biol. 2011, 115, 945–949. [Google Scholar] [CrossRef]
- Robertson, K.L.; Mostaghim, A.; Cuomo, C.A.; Soto, C.M.; Lebedev, N.; Bailey, R.F.; Wang, Z. Adaptation of the black yeast Wangiella dermatitidis to ionizing radiation: Molecular and cellular mechanisms. PLoS ONE 2012, 7, e48674. [Google Scholar] [CrossRef] [PubMed]
- Casadeval, A.; Cordero, R.J.B.; Bryan, R.; Nosanchuk, J.; Dadachova, E. Melanin, Radiation, and Energy Transduction in Fungi. In The Fungal Kingdom; ASM Press: Washington, DC, USA, 2017; pp. 509–514. [Google Scholar] [CrossRef]
- Kothamasi, D.; Wannijn, J.; Van Hees, M.; Nauts, R.; Van Gompel, A.; Vanhoudt, N.; Vandenhove, H. Exposure to ionizing radiation affects the growth of ectomycorrhizal fungi and induces increased melanin production and increased capacities of reactive oxygen species scavenging enzymes. J. Environ. Radioact. 2019, 197, 16–22. [Google Scholar] [CrossRef]
- Cecchi, T.; Pezzella, A.; Di Mauro, E.; Cestola, S.; Ginsburg, D.; Luzi, M.; Rigucci, A.; Santato, C. On the antioxidant activity of eumelanin biopigments: A quantitative comparison between free radical scavenging and redox properties. Nat. Prod. Res. 2020, 34, 2465–2473. [Google Scholar] [CrossRef] [PubMed]
- Schweitzer, A.D.; Revskaya, E.; Chu, P.; Pazo, V.; Friedman, M.; Nosanchuk, J.D.; Cahill, V.S.; Frases, S.; Casadevall, A.; Dadachova, E. Melanin-covered nanoparticles for protection of bone marrow during radiation therapy of cancer. Int. J. Radiat. Oncol. Biol. Phys. 2010, 78, 1494–1502. [Google Scholar] [CrossRef] [PubMed]
- Kunwar, A.; Adhikary, B.; Jayakumar, S.; Barik, A.; Chattopadhyay, S.; Raghukumar, S.; Priyadarsini, K.I. Melanin, a promising radioprotector: Mechanisms of actions in a mice model. Toxicol. Appl. Pharmacol. 2012, 264, 202–211. [Google Scholar] [CrossRef]
- Rageh, M.M.; El-Gebaly, R.H.; Abou-Shady, H.; Amin, D.G. Melanin nanoparticles (MNPs)] provide protection against whole-body γ-irradiation in mice via restoration of hematopoietic tissues. Mol. Cell. Biochem. 2015, 399, 59–69. [Google Scholar] [CrossRef]
- Hulot, G.; Gallet, Y. Do superchrons occur without any palaeomagnetic warning? Earth Planet. Sci. Lett. 2003, 210, 191–201. [Google Scholar] [CrossRef]
- Casadevall, A. Fungal virulence, vertebrate endothermy, and dinosaur extinction: Is there a connection? Fungal Genet. Biol. 2005, 42, 98–106. [Google Scholar] [CrossRef]
- Wolbarsht, M.L.; Walsh, A.W.; George, G. Melanin, a unique biological absorber. Appl. Opt. 1981, 20, 2184–2186. [Google Scholar] [CrossRef] [PubMed]
- Cassaro, A.; Pacelli, C.; Baqué, M.; de Vera, J.-P.P.; Böttger, U.; Botta, L.; Saladino, R.; Rabbow, E.; Onofri, S. Fungal Biomarkers Stability in Mars Regolith Analogues after Simulated Space and Mars-Like Conditions. J. Fungi 2021, 7, 859. [Google Scholar] [CrossRef]
- Gevi, F.; Leo, P.; Cassaro, A.; Pacelli, C.; de Vera, J.-P.P.; Rabbow, E.; Timperio, A.M.; Onofri, S. Metabolomic Profile of the Fungus Cryomyces antarcticus Under Simulated Martian and Space Conditions as Support for Life-Detection Missions on Mars. Front. Microbiol. 2022, 13, 749396. [Google Scholar] [CrossRef] [PubMed]
- Sheliakina, M.; Mostert, A.B.; Meredith, P. Decoupling Ionic and Electronic Currents in Melanin. Adv. Funct. Mater. 2018, 28, 1805514. [Google Scholar] [CrossRef]
- Simon, J.D.; Peles, D.N. The Red and the Black. Acc. Chem. Res. 2010, 43, 1452–1460. [Google Scholar] [CrossRef]
- Crippa, P.R.; Cristofoletti, V.; Romeo, N. A band model for melanin deduced from optical absorption and photoconductivity experiments. Biochim. Et Biophys. Acta (BBA) Gen. Subj. 1978, 538, 164–170. [Google Scholar] [CrossRef]
- Meredith, P.; Riesz, J. Radiative relaxation quantum yields for synthetic eumelanin. Photochem. Photobiol. 2004, 79, 211–216. [Google Scholar] [CrossRef] [PubMed]
- Gauden, M.; Pezzella, A.; Pçanzella, L.; Napolitano, A.; d’Ischia, M.; Sundstrom, V. Ultrafast Excited State Dynamics of DHI. J. Phys. Chem. B 2009, 113, 12575–12580. [Google Scholar] [CrossRef]
- Zareba, M.; Sarna, T.; Szewczyk, G.; Burke, J.M. Photobleaching of melanosomes from retinal pigment epithelium: II. Effects on the response of living cells to photic stress. Photochem. Photobiol. 2007, 83, 925–930. [Google Scholar] [CrossRef]
- Gray-Schopfer, V.; Wellbrock, C.; Marais, R. Melanoma biology and new targeted therapy. Nature 2007, 445, 851–857. [Google Scholar] [CrossRef]
- Noonan, F.P.; Zaidi, M.R.; Wolnicka-Glubisz, A.; Anver, M.R.; Bahn, J.; Wielgus, A.; Cadet, J.; Douki, T.; Mouret, S.; Tucker, M.A.; et al. Melanoma induction by ultraviolet A but not ultraviolet B radiation requires melanin pigment. Nat. Commun. 2012, 3, 884. [Google Scholar] [CrossRef]
- Premi, S.; Wallisch, S.; Mano, C.M.; Weiner, A.B.; Bacchiocchi, A.; Wakamatsu, K.; Bechara, E.J.H.; Halaban, R.; Douki, T.; Brash, D.E. Chemiexcitation of melanin derivatives induces DNA photoproducts long after UV exposure. Science 2015, 347, 842–847. [Google Scholar] [CrossRef] [PubMed]
- Olchawa, M.M.; Szewczyk, G.M.; Zadlo, A.C.; Krzysztynska-Kuleta, O.I.; Sarna, T.J. The effect of aging and antioxidants on photoreactivity and phototoxicity of human melanosomes: An in vitro study. Pigment. Cell Melanoma Res. 2021, 34, 670–682. [Google Scholar] [CrossRef]
- Nosanchuk, J.D.; Casadevall, A. The contribution of melanin to microbial pathogenesis. Cell. Microbiol. 2003, 5, 203–223. [Google Scholar] [CrossRef]
- Kaczara, P.; Zaręba, M.; Herrnreiter, A.; Skumatz, C.M.B.; Żądło, A.; Sarna, T.; Burke, J.M. Melanosome–iron interactions within retinal pigment epithelium-derived cells. Pigment. Cell Melanoma Res. 2012, 25, 804–814. [Google Scholar] [CrossRef]
- Zucca, F.A.; Segura-Aguilar, J.; Ferrari, E.; Munoz, P.; Paris, I.; Sulzer, D.; Sarna, T.; Casella, L.; Zecca, L. Interactions of iron, dopamine and neuromelanin pathways in brain aging and Parkinson’s disease. Prog. Neurobiol. 2017, 155, 96–119. [Google Scholar] [CrossRef]
- Wakamatsu, K.; Zippin, J.H.; Ito, S. Chemical and biochemical control of skin pigmentation with special emphasis on mixed melanogenesis. Pigment. Cell Melanoma Res. 2021, 34, 730–747. [Google Scholar] [CrossRef] [PubMed]
- Cordero, J.B.; Vij, R.; Casadevall, A. Microbial melanins for radioprotection and bioremediation. Microb. Biotechnol. 2017, 10, 1186–1190. [Google Scholar] [CrossRef]
- Operational Radiation Safety Program (Supersedes Report No. 127); U.S. National Council Radiation Protection and Measurements (NCRP) No. 187; AAPM: Alexandria, VA, USA, 2022.
- Cordero, J.B. Melanin for space travel radioprotection. Environ. Microbiol. 2017, 19, 2529–2532. [Google Scholar] [CrossRef] [PubMed]
- Cortese, F.; Klokov, D.; Osipov, A.; Stefaniak, J.; Moskalev, A.; Schastnaya, J.; Cantor, C.; Aliper, A.; Mamoshina, P.; Ushakov, I.; et al. Vive la radiorésistance!: Converging research in radiobiology and biogerontology to enhance human radioresistance for deep space exploration and colonization. Oncotarget 2018, 9, 14692–14722. [Google Scholar] [CrossRef]
- NASA Technology Transfer Program Spinoff 2023—NASA Helps Serve Yellowstone Fungi for Breakfast; NASA: Washington, DC, USA, 2022; pp. 34–37.
- Revankar, S.G.; Sutton, D.A. Melanized fungi in human disease. Clin. Microbiol. Rev. 2010, 23, 884–928. [Google Scholar] [CrossRef] [PubMed]
- Eisenman, H.C.; Nosanchuk, J.D.; Webber, J.B.W.; Emerson, R.J.; Camesano, T.A.; Casadevall, A. Microstructure of cell wall-associated melanin in the human pathogenic fungus Cryptococcus neoformans. Biochemistry 2005, 44, 3683–3693. [Google Scholar] [CrossRef]
- Nosanchuk, J.D.; Casadevall, A. Impact of melanin on microbial virulence and clinical resistance to antimicrobial compounds. Antimicrob. Agents Chemother. 2006, 50, 3519–3528. [Google Scholar] [CrossRef]
- Cordero, R.J.; Casadevall, A. Functions of fungal melanin beyond virulence. Fungal Biol. Rev. 2017, 31, 99–112. [Google Scholar] [CrossRef] [PubMed]
- Lalla, R.V.; Latortue, M.C.; Hong, C.H.; Ariyawardana, A.; D’Amato-Palumbo, S.; Fischer, D.J.; Martof, A.; Nicolatou-Galitis, O.; Patton, L.L.; Elting, L.S.; et al. A systematic review of oral fungal infections in patients receiving cancer therapy. Fungal Infect. Sect. Oral Care Study Group Multinatl. Assoc. Support. Care Cancer (MASCC)/Int. Soc. Oral Oncol. (ISOO) 2010, 18, 985–992. [Google Scholar] [CrossRef]
- Washington, M.A. Melanized fungi and military medical operations in the nuclear environment. Mil. Med. 2014, 179, 1181–1183. [Google Scholar] [CrossRef]
- Alekhova, T.A.; Aleksandrova, A.A.; Novozhilova TYu Lysak, L.V.; Zagustina, N.A.; Bezborodov, A.M. Monitoring of microbial degraders in manned space stations. Appl. Biochem. Microbiol. 2005, 41, 382–389. [Google Scholar] [CrossRef]
- Simone, A.; Balagna, C. Nano-Base Coating for Spacecraft: Antibacterial Film for Manned Applications. In Nanotechnology in Space; Terranova, M.L., Tamburri, E., Eds.; Jenny Stanford Publishing Ltd.: Singapore, 2022; Chapter 5; pp. 169–190. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Terranova, M.L. Prominent Roles and Conflicted Attitudes of Eumelanin in the Living World. Int. J. Mol. Sci. 2023, 24, 7783. https://doi.org/10.3390/ijms24097783
Terranova ML. Prominent Roles and Conflicted Attitudes of Eumelanin in the Living World. International Journal of Molecular Sciences. 2023; 24(9):7783. https://doi.org/10.3390/ijms24097783
Chicago/Turabian StyleTerranova, Maria Letizia. 2023. "Prominent Roles and Conflicted Attitudes of Eumelanin in the Living World" International Journal of Molecular Sciences 24, no. 9: 7783. https://doi.org/10.3390/ijms24097783




