Dissecting Rubella Placental Infection in an In Vitro Trophoblast Model
Abstract
1. Introduction
2. Results
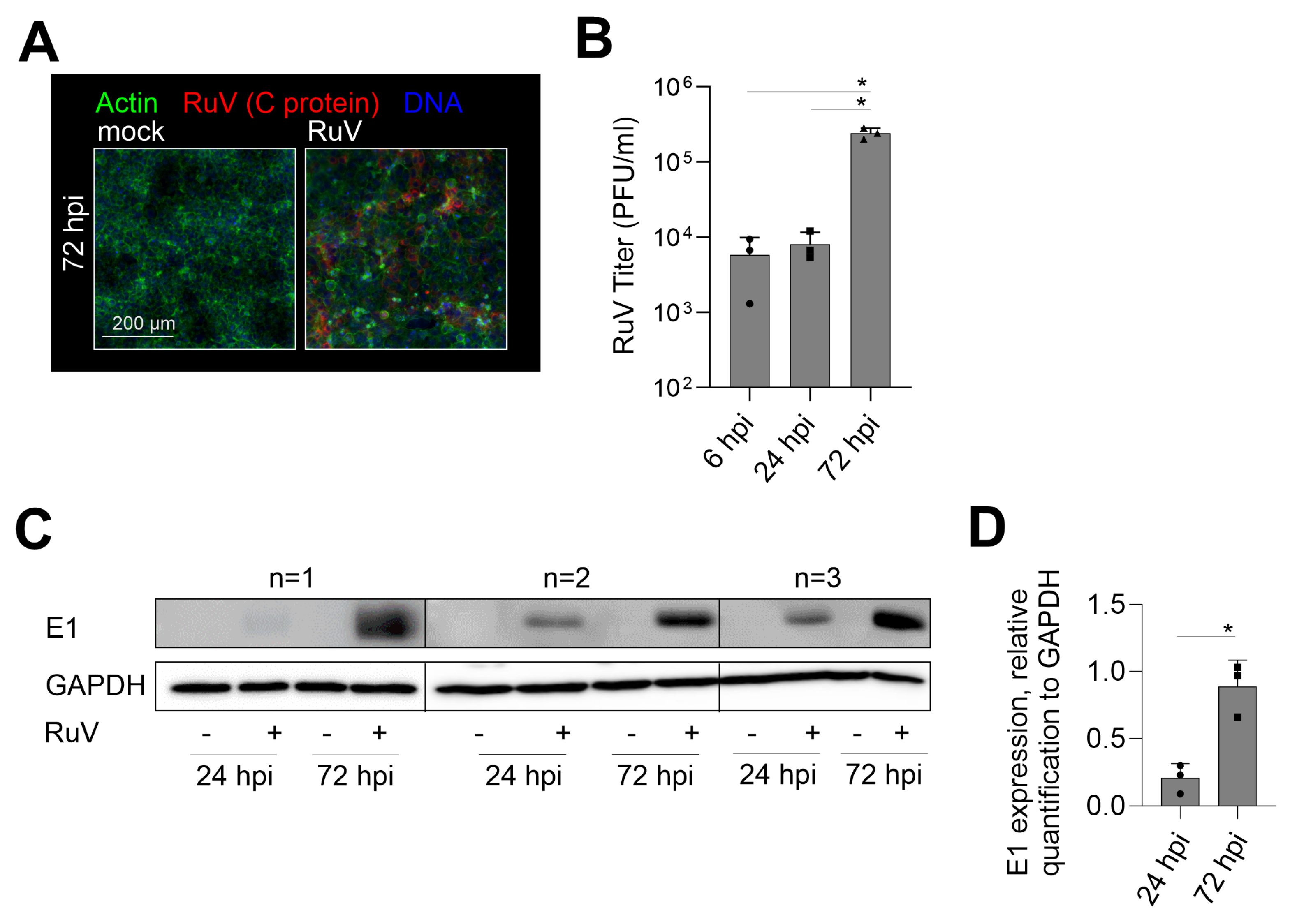
2.1. Productive RuV Infection on BeWo after an Initially Low Infection Rate
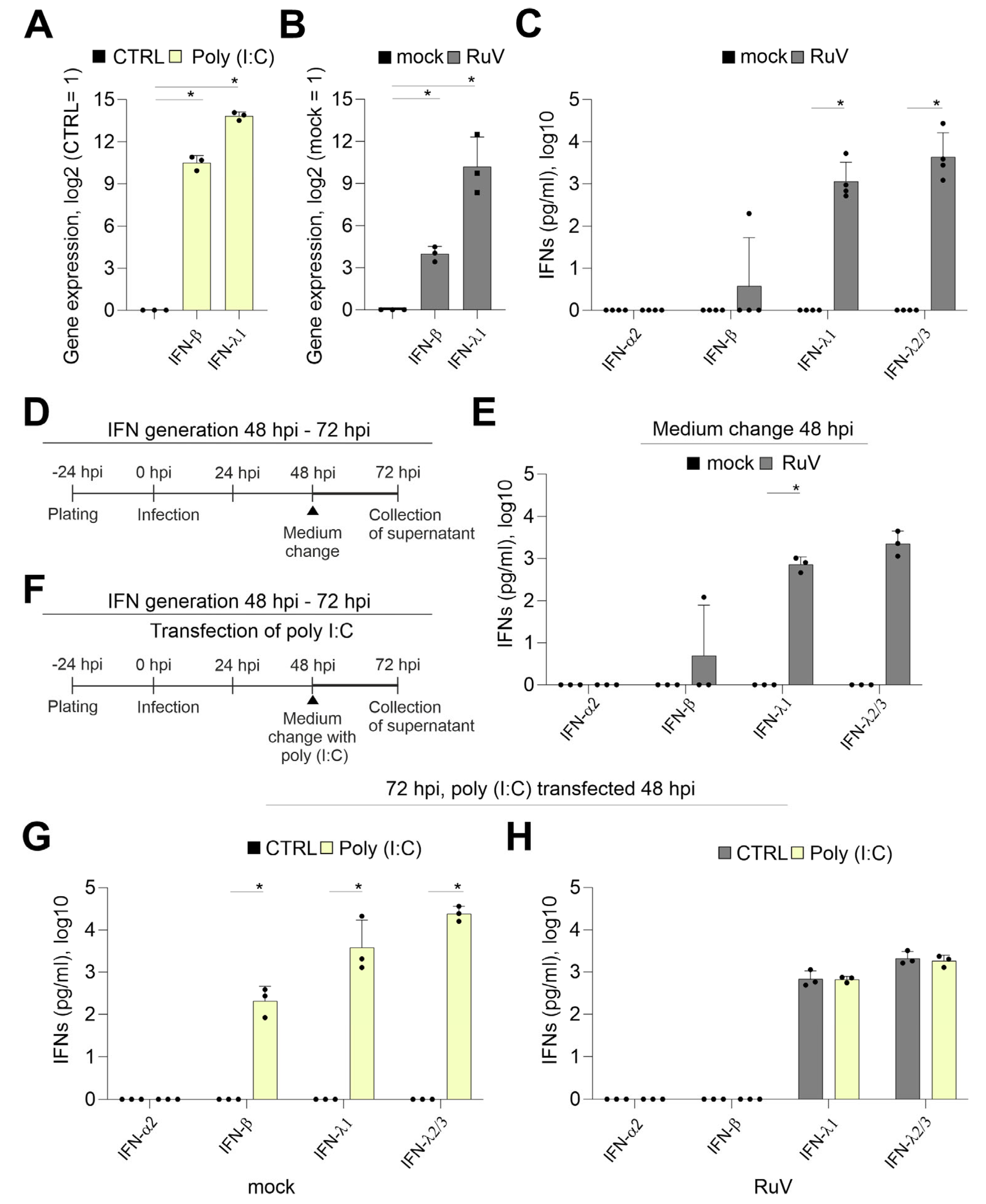
2.2. Generation of IFNs during RuV Infection Differs from the Response of BeWo to dsRNA Analog Poly (I:C)
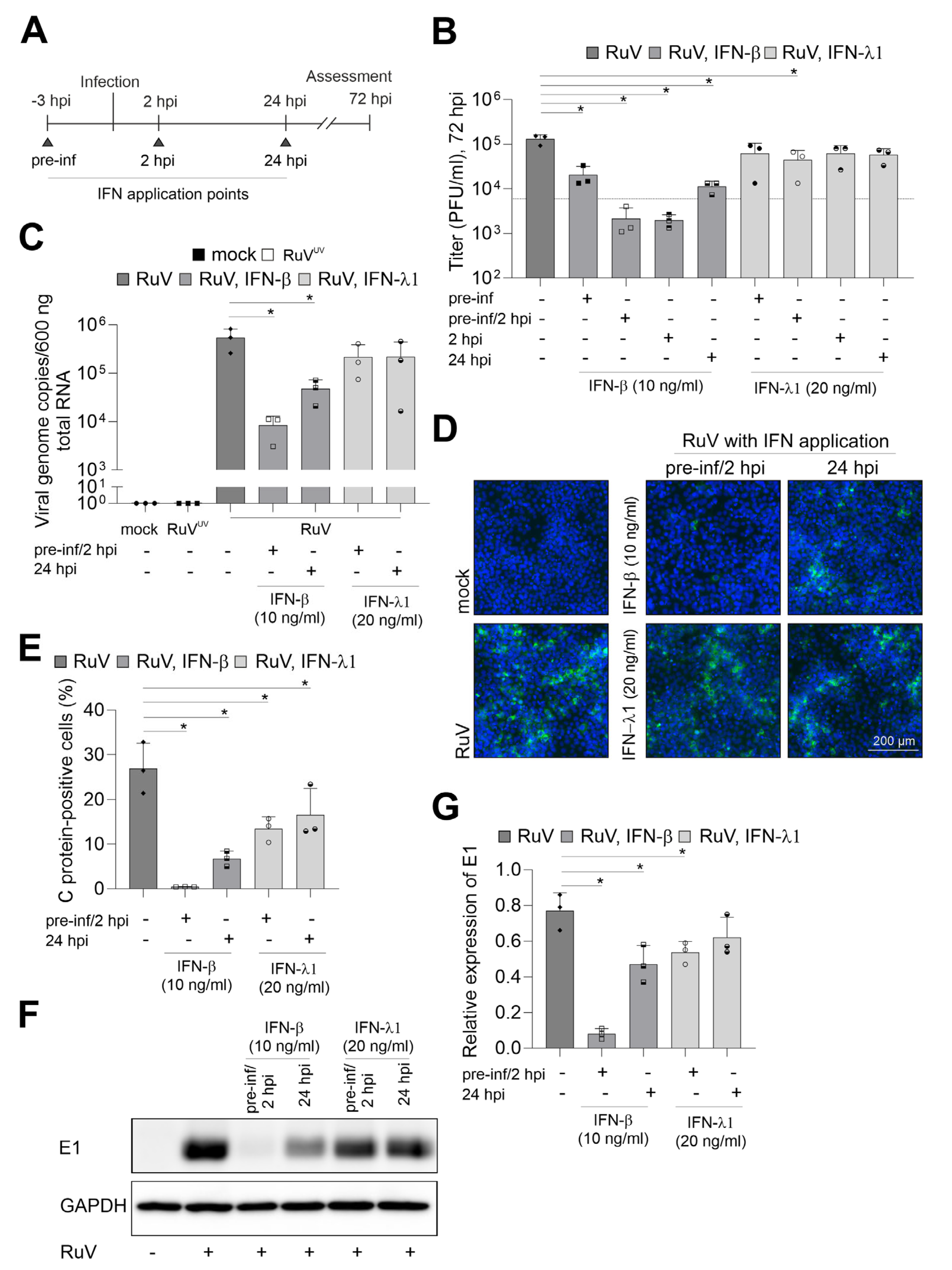
2.3. IFNs Interfere with RuV Infection on BeWo
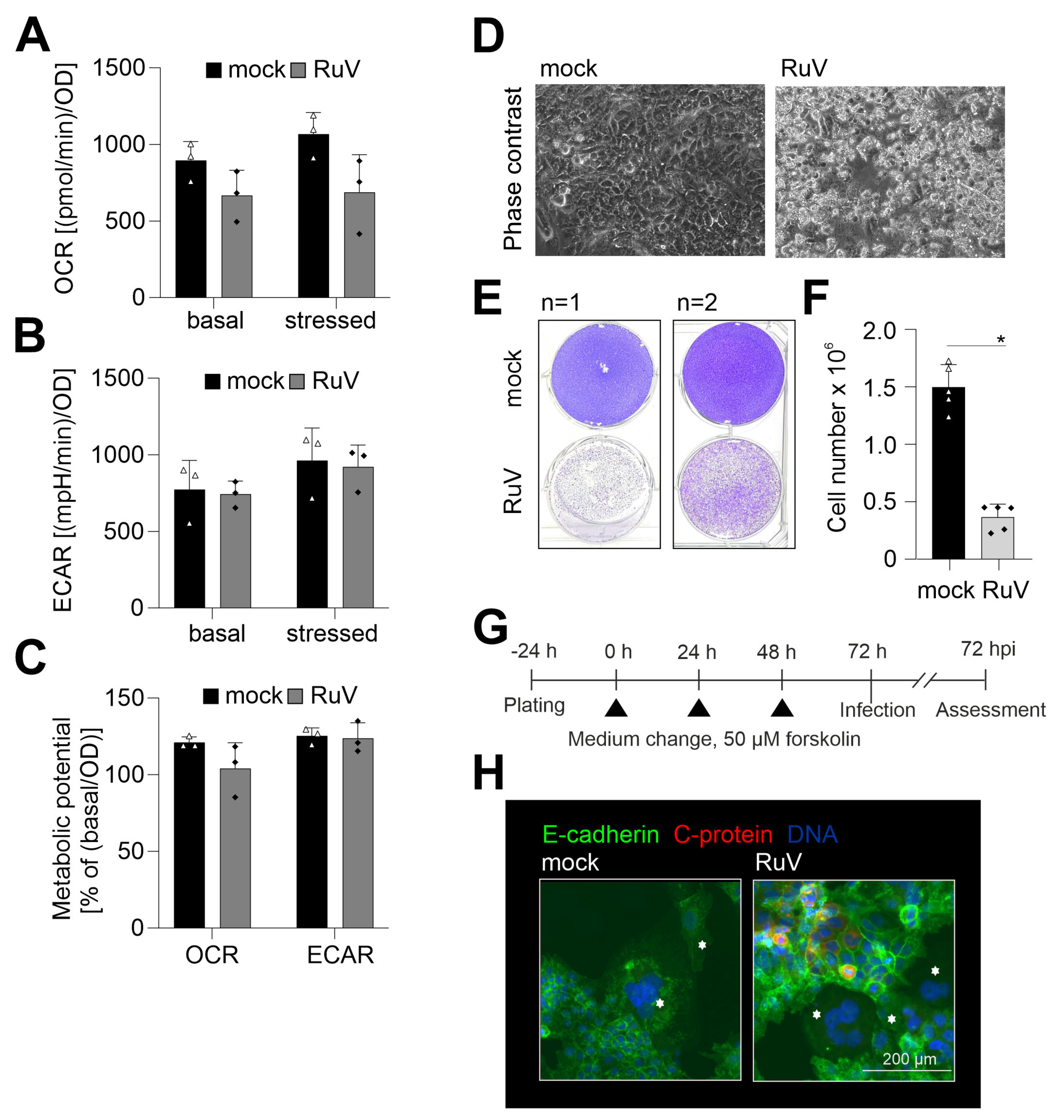
2.4. RuV Infection on BeWo Is Cytopathogenic and Impaired in Syncytialized BeWo
3. Discussion
4. Materials and Methods
4.1. Cell Culture and Virus Infection
4.2. Application of Interferons
4.3. Extracellular Flux Analysis
4.4. Induction of Syncytialization
4.5. RNA Extraction
4.6. Cellular mRNA Expression
4.7. Viral Genome Quantification
4.8. Transfection of dsRNA Analog Poly (I:C)
4.9. Assessment of Adherent Cell Monolayer
4.10. Immunofluorescence Analysis
4.11. Bead-Based Multiplex Assay for IFN Quantification in Cell Culture Supernatants
4.12. Western Blot Analysis
4.13. Statistics
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Delorme-Axford, E.; Sadovsky, Y.; Coyne, C.B. The Placenta as a Barrier to Viral Infections. Annu. Rev. Virol. 2014, 1, 133–146. [Google Scholar] [CrossRef]
- Costa, M.L.; de Moraes Nobrega, G.; Antolini-Tavares, A. Key Infections in the Placenta. Obstet. Gynecol. Clin. N. Am. 2020, 47, 133–146. [Google Scholar] [CrossRef] [PubMed]
- Coyne, C.B.; Lazear, H.M. Zika virus-reigniting the TORCH. Nat. Rev. Microbiol. 2016, 14, 707–715. [Google Scholar] [CrossRef] [PubMed]
- Holder, B.; Aplin, J.D.; Gomez-Lopez, N.; Heazell, A.E.P.; James, J.L.; Jones, C.J.P.; Jones, H.; Lewis, R.M.; Mor, G.; Roberts, C.T.; et al. ‘Fetal side’ of the placenta: Anatomical mis-annotation of carbon particle ‘transfer’ across the human placenta. Nat. Commun. 2021, 12, 7049. [Google Scholar] [CrossRef] [PubMed]
- Reagan-Steiner, S.; Bhatnagar, J.; Martines, R.B.; Milligan, N.S.; Gisondo, C.; Williams, F.B.; Lee, E.; Estetter, L.; Bullock, H.; Goldsmith, C.S.; et al. Detection of SARS-CoV-2 in Neonatal Autopsy Tissues and Placenta. Emerg. Infect. Dis. 2022, 28. [Google Scholar] [CrossRef] [PubMed]
- Pomorski, M.; Trzeszcz, M.; Matera-Witkiewicz, A.; Krupinska, M.; Fuchs, T.; Zimmer, M.; Zimmer-Stelmach, A.; Rosner-Tenerowicz, A.; Budny-Winska, J.; Tarczynska-Podraza, A.; et al. SARS-CoV-2 Infection and Pregnancy: Maternal and Neonatal Outcomes and Placental Pathology Correlations. Viruses 2022, 14, 2043. [Google Scholar] [CrossRef]
- Arora, N.; Sadovsky, Y.; Dermody, T.S.; Coyne, C.B. Microbial Vertical Transmission during Human Pregnancy. Cell Host Microbe 2017, 21, 561–567. [Google Scholar] [CrossRef]
- Robbins, J.R.; Skrzypczynska, K.M.; Zeldovich, V.B.; Kapidzic, M.; Bakardjiev, A.I. Placental syncytiotrophoblast constitutes a major barrier to vertical transmission of Listeria monocytogenes. PLoS Pathog. 2010, 6, e1000732. [Google Scholar] [CrossRef]
- Zeldovich, V.B.; Robbins, J.R.; Kapidzic, M.; Lauer, P.; Bakardjiev, A.I. Invasive extravillous trophoblasts restrict intracellular growth and spread of Listeria monocytogenes. PLoS Pathog. 2011, 7, e1002005. [Google Scholar] [CrossRef]
- Maidji, E.; McDonagh, S.; Genbacev, O.; Tabata, T.; Pereira, L. Maternal antibodies enhance or prevent cytomegalovirus infection in the placenta by neonatal Fc receptor-mediated transcytosis. Am. J. Pathol. 2006, 168, 1210–1226. [Google Scholar] [CrossRef]
- Garcia, A.G.; Marques, R.L.; Lobato, Y.Y.; Fonseca, M.E.; Wigg, M.D. Placental pathology in congenital rubella. Placenta 1985, 6, 281–295. [Google Scholar] [CrossRef]
- Lazar, M.; Perelygina, L.; Martines, R.; Greer, P.; Paddock, C.D.; Peltecu, G.; Lupulescu, E.; Icenogle, J.; Zaki, S.R. Immunolocalization and Distribution of Rubella Antigen in Fatal Congenital Rubella Syndrome. EBioMedicine 2016, 3, 86–92. [Google Scholar] [CrossRef]
- Tondury, G.; Smith, D.W. Fetal rubella pathology. J. Pediatr. 1966, 68, 867–879. [Google Scholar] [CrossRef] [PubMed]
- Pham, N.T.K.; Trinh, Q.D.; Takada, K.; Komine-Aizawa, S.; Hayakawa, S. Low Susceptibility of Rubella Virus in First-Trimester Trophoblast Cell Lines. Viruses 2022, 14, 1169. [Google Scholar] [CrossRef] [PubMed]
- Sakata, M.; Tani, H.; Anraku, M.; Kataoka, M.; Nagata, N.; Seki, F.; Tahara, M.; Otsuki, N.; Okamoto, K.; Takeda, M.; et al. Analysis of VSV pseudotype virus infection mediated by rubella virus envelope proteins. Sci. Rep. 2017, 7, 11607. [Google Scholar] [CrossRef] [PubMed]
- Mori, Y.; Sakata, M.; Sakai, S.; Okamoto, T.; Nakatsu, Y.; Taguwa, S.; Otsuki, N.; Maeda, Y.; Hanada, K.; Matsuura, Y.; et al. Membrane Sphingomyelin in Host Cells Is Essential for Nucleocapsid Penetration into the Cytoplasm after Hemifusion during Rubella Virus Entry. mBio 2022, 13, e0169822. [Google Scholar] [CrossRef]
- Aldo, P.B.; Mulla, M.J.; Romero, R.; Mor, G.; Abrahams, V.M. Viral ssRNA induces first trimester trophoblast apoptosis through an inflammatory mechanism. Am. J. Reprod. Immunol. 2010, 64, 27–37. [Google Scholar] [CrossRef]
- Abrahams, V.M.; Schaefer, T.M.; Fahey, J.V.; Visintin, I.; Wright, J.A.; Aldo, P.B.; Romero, R.; Wira, C.R.; Mor, G. Expression and secretion of antiviral factors by trophoblast cells following stimulation by the TLR-3 agonist, Poly(I:C). Hum. Reprod. 2006, 21, 2432–2439. [Google Scholar] [CrossRef]
- Broggi, A.; Granucci, F.; Zanoni, I. Type III interferons: Balancing tissue tolerance and resistance to pathogen invasion. J. Exp. Med. 2020, 217, e20190295. [Google Scholar] [CrossRef]
- Bilz, N.C.; Jahn, K.; Lorenz, M.; Ludtke, A.; Hubschen, J.M.; Geyer, H.; Mankertz, A.; Hubner, D.; Liebert, U.G.; Claus, C. Rubella Viruses Shift Cellular Bioenergetics to a More Oxidative and Glycolytic Phenotype with a Strain-Specific Requirement for Glutamine. J. Virol. 2018, 92, e00934-18. [Google Scholar] [CrossRef]
- Zhang, J.; Nuebel, E.; Wisidagama, D.R.; Setoguchi, K.; Hong, J.S.; Van Horn, C.M.; Imam, S.S.; Vergnes, L.; Malone, C.S.; Koehler, C.M.; et al. Measuring energy metabolism in cultured cells, including human pluripotent stem cells and differentiated cells. Nat. Protoc. 2012, 7, 1068–1085. [Google Scholar] [CrossRef] [PubMed]
- Wice, B.; Menton, D.; Geuze, H.; Schwartz, A.L. Modulators of cyclic AMP metabolism induce syncytiotrophoblast formation in vitro. Exp. Cell Res. 1990, 186, 306–316. [Google Scholar] [CrossRef] [PubMed]
- Keryer, G.; Yassenko, M.; Labbe, J.C.; Castro, A.; Lohmann, S.M.; Evain-Brion, D.; Tasken, K. Mitosis-specific phosphorylation and subcellular redistribution of the RIIalpha regulatory subunit of cAMP-dependent protein kinase. J. Biol. Chem. 1998, 273, 34594–34602. [Google Scholar] [CrossRef] [PubMed]
- Delidaki, M.; Gu, M.; Hein, A.; Vatish, M.; Grammatopoulos, D.K. Interplay of cAMP and MAPK pathways in hCG secretion and fusogenic gene expression in a trophoblast cell line. Mol. Cell Endocrinol. 2011, 332, 213–220. [Google Scholar] [CrossRef] [PubMed]
- Carrera, J.; Trenerry, A.M.; Simmons, C.P.; Mackenzie, J.M. Flavivirus replication kinetics in early-term placental cell lines with different differentiation pathways. Virol. J. 2021, 18, 251. [Google Scholar] [CrossRef]
- Charlier, C.; Beaudoin, M.C.; Couderc, T.; Lortholary, O.; Lecuit, M. Arboviruses and pregnancy: Maternal, fetal, and neonatal effects. Lancet Child Adolesc. Health 2017, 1, 134–146. [Google Scholar] [CrossRef]
- McMillen, C.M.; Arora, N.; Boyles, D.A.; Albe, J.R.; Kujawa, M.R.; Bonadio, J.F.; Coyne, C.B.; Hartman, A.L. Rift Valley fever virus induces fetal demise in Sprague-Dawley rats through direct placental infection. Sci. Adv. 2018, 4, eaau9812. [Google Scholar] [CrossRef]
- McMillen, C.M.; Boyles, D.A.; Kostadinov, S.G.; Hoehl, R.M.; Schwarz, M.M.; Albe, J.R.; Demers, M.J.; Hartman, A.L. Congenital Rift Valley fever in Sprague Dawley rats is associated with diffuse infection and pathology of the placenta. PLoS Negl. Trop. Dis. 2022, 16, e0010898. [Google Scholar] [CrossRef]
- Tallarek, A.C.; Urbschat, C.; Fonseca Brito, L.; Stanelle-Bertram, S.; Krasemann, S.; Frascaroli, G.; Thiele, K.; Wieczorek, A.; Felber, N.; Lutgehetmann, M.; et al. Inefficient Placental Virus Replication and Absence of Neonatal Cell-Specific Immunity Upon SARS-CoV-2 Infection During Pregnancy. Front. Immunol. 2021, 12, 698578. [Google Scholar] [CrossRef]
- Perelygina, L.; Zheng, Q.; Metcalfe, M.; Icenogle, J. Persistent infection of human fetal endothelial cells with rubella virus. PLoS ONE 2013, 8, e73014. [Google Scholar] [CrossRef]
- Hemphill, M.L.; Forng, R.Y.; Abernathy, E.S.; Frey, T.K. Time course of virus-specific macromolecular synthesis during rubella virus infection in Vero cells. Virology 1988, 162, 65–75. [Google Scholar] [CrossRef] [PubMed]
- Duncan, R.; Muller, J.; Lee, N.; Esmaili, A.; Nakhasi, H.L. Rubella virus-induced apoptosis varies among cell lines and is modulated by Bcl-XL and caspase inhibitors. Virology 1999, 255, 117–128. [Google Scholar] [CrossRef]
- Adamo, M.P.; Zapata, M.; Frey, T.K. Analysis of gene expression in fetal and adult cells infected with rubella virus. Virology 2008, 370, 1–11. [Google Scholar] [CrossRef]
- Chen, M.H.; Zhu, Z.; Zhang, Y.; Favors, S.; Xu, W.B.; Featherstone, D.A.; Icenogle, J.P. An indirect immunocolorimetric assay to detect rubella virus infected cells. J. Virol. Methods 2007, 146, 414–418. [Google Scholar] [CrossRef]
- Zobel, S.; Lorenz, M.; Frascaroli, G.; Bohnke, J.; Bilz, N.C.; Stanifer, M.L.; Boulant, S.; Bergs, S.; Liebert, U.G.; Claus, C. Rubella Virus Strain-Associated Differences in the Induction of Oxidative Stress Are Independent of Their Interferon Activation. Viruses 2018, 10, 540. [Google Scholar] [CrossRef] [PubMed]
- Schilling, E.; Grahnert, A.; Pfeiffer, L.; Koehl, U.; Claus, C.; Hauschildt, S. The Impact of Rubella Virus Infection on a Secondary Inflammatory Response in Polarized Human Macrophages. Front. Immunol. 2021, 12, 772595. [Google Scholar] [CrossRef] [PubMed]
- Sakuragi, S.; Liao, H.; Yajima, K.; Fujiwara, S.; Nakamura, H. Rubella Virus Triggers Type I Interferon Antiviral Response in Cultured Human Neural Cells: Involvement in the Control of Viral Gene Expression and Infectious Progeny Production. Int. J. Mol. Sci. 2022, 23, 9799. [Google Scholar] [CrossRef]
- Takeuchi, O.; Akira, S. Pattern recognition receptors and inflammation. Cell 2010, 140, 805–820. [Google Scholar] [CrossRef]
- Gierman, L.M.; Stodle, G.S.; Tangeras, L.H.; Austdal, M.; Olsen, G.D.; Follestad, T.; Skei, B.; Rian, K.; Gundersen, A.S.; Austgulen, R.; et al. Toll-like receptor profiling of seven trophoblast cell lines warrants caution for translation to primary trophoblasts. Placenta 2015, 36, 1246–1253. [Google Scholar] [CrossRef]
- Weisblum, Y.; Oiknine-Djian, E.; Vorontsov, O.M.; Haimov-Kochman, R.; Zakay-Rones, Z.; Meir, K.; Shveiky, D.; Elgavish, S.; Nevo, Y.; Roseman, M.; et al. Zika Virus Infects Early-and Midgestation Human Maternal Decidual Tissues, Inducing Distinct Innate Tissue Responses in the Maternal-Fetal Interface. J. Virol. 2017, 91, e01905-16. [Google Scholar] [CrossRef]
- Gwon, Y.D.; Nematollahi Mahani, S.A.; Nagaev, I.; Mincheva-Nilsson, L.; Evander, M. Rift Valley Fever Virus Propagates in Human Villous Trophoblast Cell Lines and Induces Cytokine mRNA Responses Known to Provoke Miscarriage. Viruses 2021, 13, 2265. [Google Scholar] [CrossRef] [PubMed]
- Yockey, L.J.; Jurado, K.A.; Arora, N.; Millet, A.; Rakib, T.; Milano, K.M.; Hastings, A.K.; Fikrig, E.; Kong, Y.; Horvath, T.L.; et al. Type I interferons instigate fetal demise after Zika virus infection. Sci. Immunol. 2018, 3, eaao1680. [Google Scholar] [CrossRef] [PubMed]
- Schilling, E.; Wald, M.E.; Schulz, J.; Werner, L.E.; Claus, C. Interferon Signaling-Dependent Contribution of Glycolysis to Rubella Virus Infection. Pathogens 2022, 11, 537. [Google Scholar] [CrossRef]
- Chen, Q.; Gouilly, J.; Ferrat, Y.J.; Espino, A.; Glaziou, Q.; Cartron, G.; El Costa, H.; Al-Daccak, R.; Jabrane-Ferrat, N. Metabolic reprogramming by Zika virus provokes inflammation in human placenta. Nat. Commun. 2020, 11, 2967. [Google Scholar] [CrossRef]
- Easton, Z.J.W.; Delhaes, F.; Mathers, K.; Zhao, L.; Vanderboor, C.M.G.; Regnault, T.R.H. Syncytialization and prolonged exposure to palmitate impacts BeWo respiration. Reproduction 2021, 161, 73–88. [Google Scholar] [CrossRef] [PubMed]
- Stanifer, M.L.; Rippert, A.; Kazakov, A.; Willemsen, J.; Bucher, D.; Bender, S.; Bartenschlager, R.; Binder, M.; Boulant, S. Reovirus intermediate subviral particles constitute a strategy to infect intestinal epithelial cells by exploiting TGF-beta dependent pro-survival signaling. Cell Microbiol. 2016, 18, 1831–1845. [Google Scholar] [CrossRef]
- Bender, S.; Reuter, A.; Eberle, F.; Einhorn, E.; Binder, M.; Bartenschlager, R. Activation of Type I and III Interferon Response by Mitochondrial and Peroxisomal MAVS and Inhibition by Hepatitis C Virus. PLoS Pathog. 2015, 11, e1005264. [Google Scholar] [CrossRef]
- Wu, X.; Dao Thi, V.L.; Huang, Y.; Billerbeck, E.; Saha, D.; Hoffmann, H.H.; Wang, Y.; Silva, L.A.V.; Sarbanes, S.; Sun, T.; et al. Intrinsic Immunity Shapes Viral Resistance of Stem Cells. Cell 2018, 172, 423–438.e25. [Google Scholar] [CrossRef]
- Chiang, C.; Beljanski, V.; Yin, K.; Olagnier, D.; Ben Yebdri, F.; Steel, C.; Goulet, M.L.; DeFilippis, V.R.; Streblow, D.N.; Haddad, E.K.; et al. Sequence-Specific Modifications Enhance the Broad-Spectrum Antiviral Response Activated by RIG-I Agonists. J. Virol. 2015, 89, 8011–8025. [Google Scholar] [CrossRef]
- Claus, C.; Bergs, S.; Emmrich, N.C.; Hubschen, J.M.; Mankertz, A.; Liebert, U.G. A sensitive one-step TaqMan amplification approach for detection of rubella virus clade I and II genotypes in clinical samples. Arch Virol. 2017, 162, 477–486. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schulz, J.; Schilling, E.; Fabian, C.; Zenclussen, A.C.; Stojanovska, V.; Claus, C. Dissecting Rubella Placental Infection in an In Vitro Trophoblast Model. Int. J. Mol. Sci. 2023, 24, 7894. https://doi.org/10.3390/ijms24097894
Schulz J, Schilling E, Fabian C, Zenclussen AC, Stojanovska V, Claus C. Dissecting Rubella Placental Infection in an In Vitro Trophoblast Model. International Journal of Molecular Sciences. 2023; 24(9):7894. https://doi.org/10.3390/ijms24097894
Chicago/Turabian StyleSchulz, Juliane, Erik Schilling, Claire Fabian, Ana Claudia Zenclussen, Violeta Stojanovska, and Claudia Claus. 2023. "Dissecting Rubella Placental Infection in an In Vitro Trophoblast Model" International Journal of Molecular Sciences 24, no. 9: 7894. https://doi.org/10.3390/ijms24097894
APA StyleSchulz, J., Schilling, E., Fabian, C., Zenclussen, A. C., Stojanovska, V., & Claus, C. (2023). Dissecting Rubella Placental Infection in an In Vitro Trophoblast Model. International Journal of Molecular Sciences, 24(9), 7894. https://doi.org/10.3390/ijms24097894






