The State of Research and Weight of Evidence on the Epigenetic Effects of Bisphenol A
Abstract
1. Introduction
2. Human Exposure to BPA
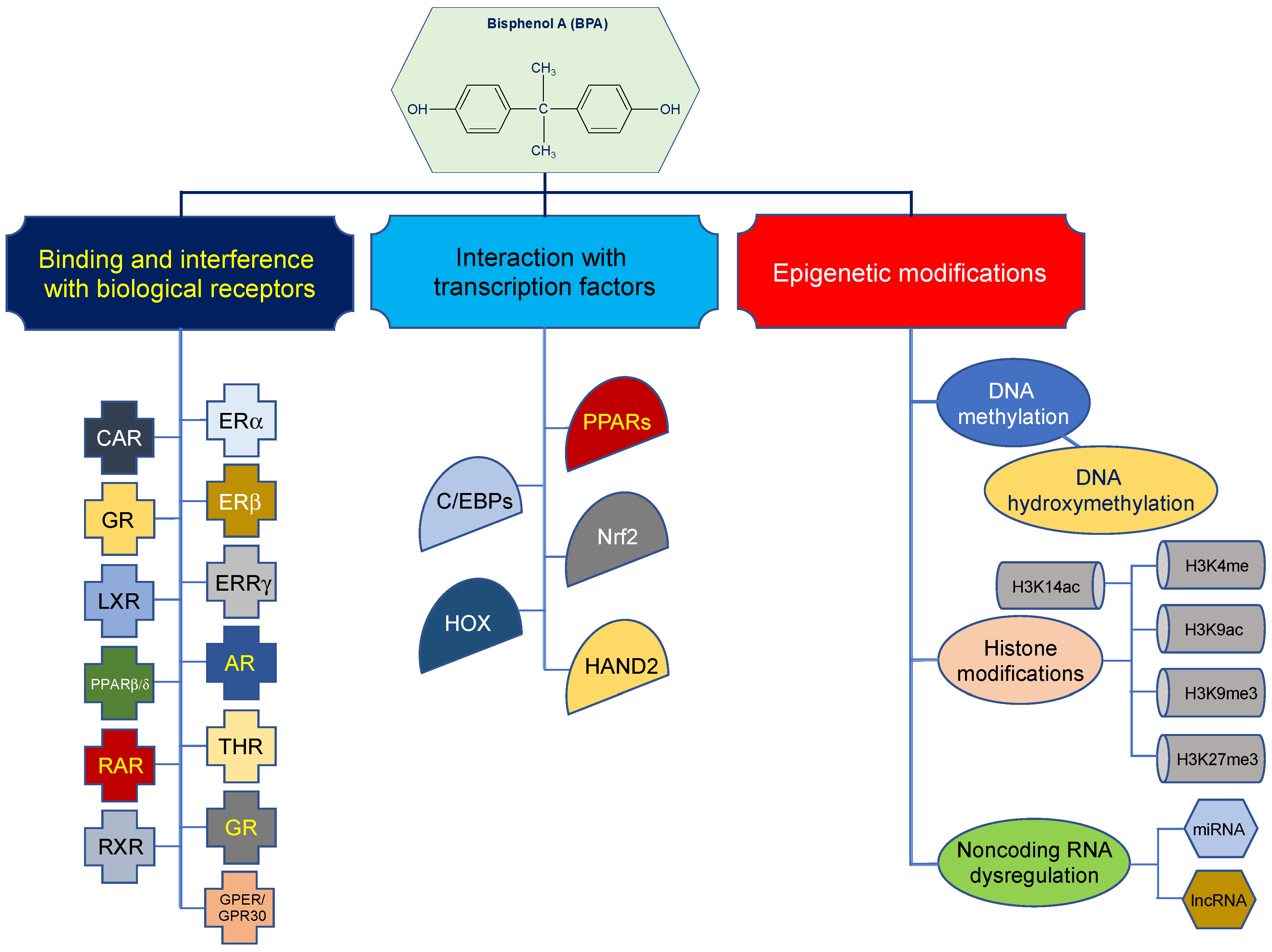
3. Biological Effects of BPA
4. Epigenetic Effects of BPA
5. Limitations of Research on the Epigenetic Effects of BPA in Humans
6. Concluding Remarks
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Dianin, A.P. Condensation of ketones with phenols. Zhurnal Russkogo Fiziko-Khimicheskogo Obshchestva. J. Russ. Phys. Chem. Soc. St. Petersburg. 1891, 23, 601–611. [Google Scholar]
- Goodman, J.E.; Peterson, M.K. Bisphenol A. In Encyclopedia of Toxicology; Elsevier Inc./Academic Press: Amsterdam, The Netherlands, 2014; Volume 1, pp. 514–518. [Google Scholar]
- Delfosse, V.; Grimaldi, M.; Pons, J.L.; Boulahtouf, A.; le Maire, A.; Cavailles, V.; Labesse, G.; Bourguet, W.; Balaguer, P. Structural and mechanistic insights into bisphenols action provide guidelines for risk assessment and discovery of bisphenol A substitutes. Proc. Natl. Acad. Sci. USA 2012, 109, 14930–14935. [Google Scholar] [CrossRef] [PubMed]
- Cheng, V.; Guha, N.; Li, K.; Musa, M.; Ricker, K.; Tsai, F.C.; Elmore, S.; Hsieh, J.C.Y.; Marder, M.E.; O’sborne, G.; et al. Proposition 65: Evidence on the Carcinogenicity of Bisphenol A (BPA), September 2022. In Reproductive and Cancer Hazard Assessment Branch, Office of Environmental Health Hazard Assessmen, California Environmental Protection Agency, Ed. California Environmental Protection Agency. 2022. Available online: https://oehha.ca.gov/media/downloads/crnr/bpahid093022.pdf (accessed on 30 March 2023).
- Michałowicz, J. Bisphenol A—Sources, toxicity and biotransformation. Environ. Toxicol. Pharmacol. 2014, 37, 738–758. [Google Scholar] [CrossRef] [PubMed]
- Kamrin, M.A. Bisphenol A: A scientific evaluation. MedGenMed 2004, 6, 7. [Google Scholar]
- Vogel, S.A. The politics of plastics: The making and unmaking of bisphenol a “safety”. Am. J. Public. Health 2009, 99 (Suppl. 3), S559–S566. [Google Scholar] [CrossRef]
- US EPA. Bisphenol A Action Plan (CASRN 80-05-7) [CA Index Name: Phenol, 4,4′-(1-methylethylidene)bis-] 2010. Available online: https://www.epa.gov/sites/default/files/2015-09/documents/bpa_action_plan.pdf (accessed on 30 March 2023).
- Gao, H.; Yang, B.J.; Li, N.; Feng, L.M.; Shi, X.Y.; Zhao, W.H.; Liu, S.J. Bisphenol A and hormone-associated cancers: Current progress and perspectives. Medicine 2015, 94, e211. [Google Scholar] [CrossRef]
- United Nations Environment Program. An Assessment Report on Issues of Concern: Chemicals and Waste Issues Posing Risks to Human Health and the Environment—September 2020. 2020. Available online: https://wedocs.unep.org/20.500.11822/33807 (accessed on 30 March 2023).
- European Commission Scientific Committee on Consumer Safety. Opinion on the Safety of Presence of Bisphenol A in Clothing Articles. 2021. Available online: https://health.ec.europa.eu/system/files/2021-04/sccs_o_240_0.pdf (accessed on 30 March 2023).
- Vandenberg, L.N.; Chahoud, I.; Heindel, J.J.; Padmanabhan, V.; Paumgartten, F.J.; Schoenfelder, G. Urinary, circulating, and tissue biomonitoring studies indicate widespread exposure to bisphenol A. Environ. Health Perspect. 2010, 118, 1055–1070. [Google Scholar] [CrossRef]
- Corrales, J.; Kristofco, L.A.; Steele, W.B.; Yates, B.S.; Breed, C.S.; Williams, E.S.; Brooks, B.W. Global Assessment of Bisphenol A in the Environment: Review and Analysis of Its Occurrence and Bioaccumulation. Dose Response 2015, 13, 1559325815598308. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention. Fourth Report on Human Exposure to Environmental Chemicals, Updated Tables. 2021. Available online: https://www.cdc.gov/exposurereport/ (accessed on 30 March 2023).
- Haishima, Y.; Hayashi, Y.; Yagami, T.; Nakamura, A. Elution of bisphenol-A from hemodialyzers consisting of polycarbonate and polysulfone resins. J. Biomed. Mater. Res. 2001, 58, 209–215. [Google Scholar] [CrossRef]
- Fleisch, A.F.; Sheffield, P.E.; Chinn, C.; Edelstein, B.L.; Landrigan, P.J. Bisphenol A and related compounds in dental materials. Pediatrics 2010, 126, 760–768. [Google Scholar] [CrossRef]
- Ehrlich, S.; Calafat, A.M.; Humblet, O.; Smith, T.; Hauser, R. Handling of thermal receipts as a source of exposure to bisphenol A. JAMA 2014, 311, 859–860. [Google Scholar] [CrossRef] [PubMed]
- Buckley, J.P.; Kim, H.; Wong, E.; Rebholz, C.M. Ultra-processed food consumption and exposure to phthalates and bisphenols in the US National Health and Nutrition Examination Survey, 2013–2014. Environ. Int. 2019, 131, 105057. [Google Scholar] [CrossRef] [PubMed]
- Iribarne-Durán, L.M.; Artacho-Cordón, F.; Peña-Caballero, M.; Molina-Molina, J.M.; Jiménez-Díaz, I.; Vela-Soria, F.; Serrano, L.; Hurtado, J.A.; Fernández, M.F.; Freire, C.; et al. Presence of Bisphenol A and Parabens in a Neonatal Intensive Care Unit: An Exploratory Study of Potential Sources of Exposure. Environ. Health Perspect. 2019, 127, 117004. [Google Scholar] [CrossRef] [PubMed]
- Research and Markets. Bisphenol-A (BPA) Market—Growth, Trends, COVID-19 Impact, and Forecast (2022–2027). Available online: https://www.researchandmarkets.com/reports/5318392/bisphenol-a-bpa-market-growth-trends-covid#rela1-5438494 (accessed on 30 March 2023).
- US EPA. Toxics Release Inventory (TRI) Program. 2022. Available online: https://www.epa.gov/toxics-release-inventory-tri-program (accessed on 30 March 2023).
- US EPA. Assessing and Managing Chemicals under TSCA: Risk Management for Bisphenol A (BPA). 2022. Available online: https://www.epa.gov/assessing-and-managing-chemicals-under-tsca/risk-management-bisphenol-bpa (accessed on 30 March 2023).
- Almeida, S.; Raposo, A.; Almeida-González, M.; Carrascosa, C. Bisphenol A: Food Exposure and Impact on Human Health. Compr. Rev. Food Sci. Food Saf. 2018, 17, 1503–1517. [Google Scholar] [CrossRef]
- Cao, X.L.; Corriveau, J.; Popovic, S. Levels of bisphenol A in canned soft drink products in Canadian markets. J. Agric. Food Chem. 2009, 57, 1307–1311. [Google Scholar] [CrossRef] [PubMed]
- Carwile, J.L.; Luu, H.T.; Bassett, L.S.; Driscoll, D.A.; Yuan, C.; Chang, J.Y.; Ye, X.; Calafat, A.M.; Michels, K.B. Polycarbonate bottle use and urinary bisphenol A concentrations. Environ. Health Perspect. 2009, 117, 1368–1372. [Google Scholar] [CrossRef]
- Noonan, G.O.; Ackerman, L.K.; Begley, T.H. Concentration of bisphenol A in highly consumed canned foods on the U. S. market. J. Agric. Food Chem. 2011, 59, 7178–7185. [Google Scholar] [CrossRef]
- Vilarinho, F.; Sendón, R.; van der Kellen, A.; Vaz, M.F.; Sanches Silva, A. Bisphenol A in food as a result of its migration from food packaging. Trends Food Sci. Technol. 2019, 19, 33–65. [Google Scholar] [CrossRef]
- Kubwabo, C.; Kosarac, I.; Stewart, B.; Gauthier, B.R.; Lalonde, K.; Lalonde, P.J. Migration of bisphenol A from plastic baby bottles, baby bottle liners and reusable polycarbonate drinking bottles. Food Addit. Contam. Part. A Chem. Anal. Control. Expo. Risk Assess. 2009, 26, 928–937. [Google Scholar] [CrossRef]
- Cousins, I.T.; Staples, C.A.; Kleĉka, G.M.; Mackay, D. A Multimedia Assessment of the Environmental Fate of Bisphenol A. Hum. Ecol. Risk Assess. Int. J. 2002, 8, 1107–1135. [Google Scholar] [CrossRef]
- Cooper, J.E.; Kendig, E.L.; Belcher, S.M. Assessment of bisphenol A released from reusable plastic, aluminium and stainless steel water bottles. Chemosphere 2011, 85, 943–947. [Google Scholar] [CrossRef] [PubMed]
- Careghini, A.; Mastorgio, A.F.; Saponaro, S.; Sezenna, E. Bisphenol A, nonylphenols, benzophenones, and benzotriazoles in soils, groundwater, surface water, sediments, and food: A review. Environ. Sci. Pollut. Res. Int. 2015, 22, 5711–5741. [Google Scholar] [CrossRef] [PubMed]
- Hanaoka, T.; Kawamura, N.; Hara, K.; Tsugane, S. Urinary bisphenol A and plasma hormone concentrations in male workers exposed to bisphenol A diglycidyl ether and mixed organic solvents. Occup. Environ. Med. 2002, 59, 625–628. [Google Scholar] [CrossRef] [PubMed]
- Loganathan, S.N.; Kannan, K. Occurrence of bisphenol A in indoor dust from two locations in the eastern United States and implications for human exposures. Arch. Environ. Contam. Toxicol. 2011, 61, 68–73. [Google Scholar] [CrossRef] [PubMed]
- Muhamad, M.S.; Salim, M.R.; Lau, W.J.; Yusop, Z. A review on bisphenol A occurrences, health effects and treatment process via membrane technology for drinking water. Environ. Sci. Pollut. Res. Int. 2016, 23, 11549–11567. [Google Scholar] [CrossRef] [PubMed]
- Valentino, R.; D’Esposito, V.; Ariemma, F.; Cimmino, I.; Beguinot, F.; Formisano, P. Bisphenol A environmental exposure and the detrimental effects on human metabolic health: Is it necessary to revise the risk assessment in vulnerable population? J. Endocrinol. Investig. 2016, 39, 259–263. [Google Scholar] [CrossRef] [PubMed]
- US FDA. Bisphenol A (BPA): Use in Food Contact Application. 2018. Available online: https://www.fda.gov/food/food-additives-petitions/bisphenol-bpa-use-food-contact-application (accessed on 30 March 2023).
- Huang, R.P.; Liu, Z.H.; Yin, H.; Dang, Z.; Wu, P.X.; Zhu, N.W.; Lin, Z. Bisphenol A concentrations in human urine, human intakes across six continents, and annual trends of average intakes in adult and child populations worldwide: A thorough literature review. Sci. Total Environ. 2018, 626, 971–981. [Google Scholar] [CrossRef] [PubMed]
- Teeguarden, J.G.; Twaddle, N.C.; Churchwell, M.I.; Yang, X.; Fisher, J.W.; Seryak, L.M.; Doerge, D.R. 24-hour human urine and serum profiles of bisphenol A following ingestion in soup: Individual pharmacokinetic data and emographics. Data Brief. 2015, 4, 83–86. [Google Scholar] [CrossRef] [PubMed]
- EFSA CEF Panel (EFSA Panel on Food Contact Materials, Enzymes, Flavourings and Processing Aids). Scientific Opinion on the risks to public health related to the presence of bisphenol A (BPA) in foodstuffs: Executive summary. EFSA J. 2015, 13, 3978. [Google Scholar] [CrossRef]
- Liu, J.; Martin, J.W. Prolonged Exposure to Bisphenol A from Single Dermal Contact Events. Environ. Sci. Technol. 2017, 51, 9940–9949. [Google Scholar] [CrossRef] [PubMed]
- Sasso, A.F.; Pirow, R.; Andra, S.S.; Church, R.; Nachman, R.M.; Linke, S.; Kapraun, D.F.; Schurman, S.H.; Arora, M.; Thayer, K.A.; et al. Pharmacokinetics of bisphenol A in humans following dermal administration. Environ. Int. 2020, 144, 106031. [Google Scholar] [CrossRef] [PubMed]
- Genuis, S.J.; Beesoon, S.; Birkholz, D.; Lobo, R.A. Human excretion of bisphenol A: Blood, urine, and sweat (BUS) study. J. Environ. Public Health 2012, 2012, 185731. [Google Scholar] [CrossRef] [PubMed]
- Mendonca, K.; Hauser, R.; Calafat, A.M.; Arbuckle, T.E.; Duty, S.M. Bisphenol A concentrations in maternal breast milk and infant urine. Int. Arch. Occup. Environ. Health 2014, 87, 13–20. [Google Scholar] [CrossRef] [PubMed]
- Teeguarden, J.G.; Twaddle, N.C.; Churchwell, M.I.; Doerge, D.R. Urine and serum biomonitoring of exposure to environmental estrogens I: Bisphenol A in pregnant women. Food Chem. Toxicol. 2016, 92, 129–142. [Google Scholar] [CrossRef]
- Pinney, S.E.; Mesaros, C.A.; Snyder, N.W.; Busch, C.M.; Xiao, R.; Aijaz, S.; Ijaz, N.; Blair, I.A.; Manson, J.M. Second trimester amniotic fluid bisphenol A concentration is associated with decreased birth weight in term infants. Reprod. Toxicol. 2017, 67, 1–9. [Google Scholar] [CrossRef]
- Hartle, J.C.; Cohen, R.S.; Sakamoto, P.; Barr, D.B.; Carmichael, S.L. Chemical Contaminants in Raw and Pasteurized Human Milk. J. Hum. Lact. 2018, 34, 340–349. [Google Scholar] [CrossRef]
- Nahar, M.S.; Liao, C.; Kannan, K.; Harris, C.; Dolinoy, D.C. In utero bisphenol A concentration, metabolism, and global DNA methylation across matched placenta, kidney, and liver in the human fetus. Chemosphere 2015, 124, 54–60. [Google Scholar] [CrossRef]
- Vandenberg, L.N.; Maffini, M.V.; Sonnenschein, C.; Rubin, B.S.; Soto, A.M. Bisphenol-A and the great divide: A review of controversies in the field of endocrine disruption. Endocr. Rev. 2009, 30, 75–95. [Google Scholar] [CrossRef]
- Rochester, J.R. Bisphenol A and human health: A review of the literature. Reprod. Toxicol. 2013, 42, 132–155. [Google Scholar] [CrossRef]
- von Goetz, N.; Pirow, R.; Hart, A.; Bradley, E.; Poças, F.; Arcella, D.; Lillegard, I.T.L.; Simoneau, C.; van Engelen, J.; Husoy, T.; et al. Including non-dietary sources into an exposure assessment of the European Food Safety Authority: The challenge of multi-sector chemicals such as Bisphenol A. Regul. Toxicol. Pharmacol. 2017, 85, 70–78. [Google Scholar] [CrossRef]
- Morgan, M.K.; Nash, M.; Barr, D.B.; Starr, J.M.; Scott Clifton, M.; Sobus, J.R. Distribution, variability, and predictors of urinary bisphenol A levels in 50 North Carolina adults over a six-week monitoring period. Environ. Int. 2018, 112, 85–99. [Google Scholar] [CrossRef]
- Mustieles, V.; Zhang, Y.; Yland, J.; Braun, J.M.; Williams, P.L.; Wylie, B.J.; Attaman, J.A.; Ford, J.B.; Azevedo, A.; Calafat, A.M.; et al. Maternal and paternal preconception exposure to phenols and preterm birth. Environ. Int. 2020, 137, 105523. [Google Scholar] [CrossRef] [PubMed]
- Hanioka, N.; Naito, T.; Narimatsu, S. Human UDP-glucuronosyltransferase isoforms involved in bisphenol A glucuronidation. Chemosphere 2008, 74, 33–36. [Google Scholar] [CrossRef]
- Thayer, K.A.; Doerge, D.R.; Hunt, D.; Schurman, S.H.; Twaddle, N.C.; Churchwell, M.I.; Garantziotis, S.; Kissling, G.E.; Easterling, M.R.; Bucher, J.R.; et al. Pharmacokinetics of bisphenol A in humans following a single oral administration. Environ. Int. 2015, 83, 107–115. [Google Scholar] [CrossRef] [PubMed]
- Völkel, W.; Colnot, T.; Csanády, G.A.; Filser, J.G.; Dekant, W. Metabolism and kinetics of bisphenol a in humans at low doses following oral administration. Chem. Res. Toxicol. 2002, 15, 1281–1287. [Google Scholar] [CrossRef] [PubMed]
- Nishiyama, T.; Ogura, K.; Nakano, H.; Kaku, T.; Takahashi, E.; Ohkubo, Y.; Sekine, K.; Hiratsuka, A.; Kadota, S.; Watabe, T. Sulfation of environmental estrogens by cytosolic human sulfotransferases. Drug. Metab. Pharmacokinet. 2002, 17, 221–228. [Google Scholar] [CrossRef] [PubMed]
- Völkel, W.; Bittner, N.; Dekant, W. Quantitation of bisphenol A and bisphenol A glucuronide in biological samples by high performance liquid chromatography-tandem mass spectrometry. Drug. Metab. Dispos. 2005, 33, 1748–1757. [Google Scholar] [CrossRef]
- Trdan Lušin, T.; Roškar, R.; Mrhar, A. Evaluation of bisphenol A glucuronidation according to UGT1A1*28 polymorphism by a new LC-MS/MS assay. Toxicology 2012, 292, 33–41. [Google Scholar] [CrossRef]
- Landolfi, A.; Troisi, J.; Savanelli, M.C.; Vitale, C.; Barone, P.; Amboni, M. Bisphenol A glucuronidation in patients with Parkinson’s disease. Neurotoxicology 2017, 63, 90–96. [Google Scholar] [CrossRef]
- Nakamura, S.; Tezuka, Y.; Ushiyama, A.; Kawashima, C.; Kitagawara, Y.; Takahashi, K.; Ohta, S.; Mashino, T. Ipso substitution of bisphenol A catalyzed by microsomal cytochrome P450 and enhancement of estrogenic activity. Toxicol. Lett. 2011, 203, 92–95. [Google Scholar] [CrossRef]
- Schmidt, J.; Kotnik, P.; Trontelj, J.; Knez, Ž.; Mašič, L.P. Bioactivation of bisphenol A and its analogs (BPF, BPAF, BPZ and DMBPA) in human liver microsomes. Toxicol. Vitro 2013, 27, 1267–1276. [Google Scholar] [CrossRef] [PubMed]
- Ousji, O.; Ohlund, L.; Sleno, L. Comprehensive In Vitro Metabolism Study of Bisphenol A Using Liquid Chromatography-High Resolution Tandem Mass Spectrometry. Chem. Res. Toxicol. 2020, 33, 1468–1477. [Google Scholar] [CrossRef] [PubMed]
- Atkinson, A.; Roy, D. In vitro conversion of environmental estrogenic chemical bisphenol A to DNA binding metabolite(s). Biochem. Biophys. Res. Commun. 1995, 210, 424–433. [Google Scholar] [CrossRef] [PubMed]
- Knaak, J.B.; Sullivan, L.J. Metabolism of bisphenol A in the rat. Toxicol. Appl. Pharmacol. 1966, 8, 175–184. [Google Scholar] [CrossRef]
- Zalko, D.; Soto, A.M.; Dolo, L.; Dorio, C.; Rathahao, E.; Debrauwer, L.; Faure, R.; Cravedi, J.P. Biotransformations of bisphenol A in a mammalian model: Answers and new questions raised by low-dose metabolic fate studies in pregnant CD1 mice. Environ. Health Perspect. 2003, 111, 309–319. [Google Scholar] [CrossRef]
- Jaeg, J.P.; Perdu, E.; Dolo, L.; Debrauwer, L.; Cravedi, J.P.; Zalko, D. Characterization of new bisphenol a metabolites produced by CD1 mice liver microsomes and S9 fractions. J. Agric. Food Chem. 2004, 52, 4935–4942. [Google Scholar] [CrossRef]
- Yoshihara, S.; Mizutare, T.; Makishima, M.; Suzuki, N.; Fujimoto, N.; Igarashi, K.; Ohta, S. Potent estrogenic metabolites of bisphenol A and bisphenol B formed by rat liver S9 fraction: Their structures and estrogenic potency. Toxicol. Sci. 2004, 78, 50–59. [Google Scholar] [CrossRef]
- Gramec Skledar, D.; Peterlin Mašič, L. Bisphenol A and its analogs: Do their metabolites have endocrine activity? Environ. Toxicol. Pharmacol. 2016, 47, 182–199. [Google Scholar] [CrossRef]
- Atkinson, A.; Roy, D. In vivo DNA adduct formation by bisphenol A. Environ. Mol. Mutagen. 1995, 26, 60–66. [Google Scholar] [CrossRef]
- Edmonds, J.S.; Nomachi, M.; Terasaki, M.; Morita, M.; Skelton, B.W.; White, A.H. The reaction of bisphenol A 3,4-quinone with DNA. Biochem. Biophys. Res. Commun. 2004, 319, 556–561. [Google Scholar] [CrossRef]
- Izzotti, A.; Kanitz, S.; D’Agostini, F.; Camoirano, A.; De Flora, S. Formation of adducts by bisphenol A, an endocrine disruptor, in DNA in vitro and in liver and mammary tissue of mice. Mutat. Res. 2009, 679, 28–32. [Google Scholar] [CrossRef] [PubMed]
- De Flora, S.; Micale, R.T.; La Maestra, S.; Izzotti, A.; D’Agostini, F.; Camoirano, A.; Davoli, S.A.; Troglio, M.G.; Rizzi, F.; Davalli, P.; et al. Upregulation of clusterin in prostate and DNA damage in spermatozoa from bisphenol A-treated rats and formation of DNA adducts in cultured human prostatic cells. Toxicol. Sci. 2011, 122, 45–51. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Fang, J.; Li, S.; Wei, J.; Yang, Z.; Zhao, H.; Zhao, C.; Cai, Z. Interaction of bisphenol A 3,4-quinone metabolite with glutathione and ribonucleosides/deoxyribonucleosides in vitro. J. Hazard. Mater. 2017, 323, 195–202. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Wei, J.; Xiang, L.; Cai, Z. Mass spectrometry investigation of DNA adduct formation from bisphenol A quinone metabolite and MCF-7 cell DNA. Talanta 2018, 182, 583–589. [Google Scholar] [CrossRef]
- Hu, X.; Wu, J.L.; Miao, W.; Long, F.; Pan, H.; Peng, T.; Yao, X.; Li, N. Covalent Protein Modification: An Unignorable Factor for Bisphenol A-Induced Hepatotoxicity. Environ. Sci. Technol. 2022, 56, 9536–9545. [Google Scholar] [CrossRef] [PubMed]
- Gramec Skledar, D.; Troberg, J.; Lavdas, J.; Peterlin Mašič, L.; Finel, M. Differences in the glucuronidation of bisphenols F and S between two homologous human UGT enzymes, 1A9 and 1A10. Xenobiotica 2015, 45, 511–519. [Google Scholar] [CrossRef] [PubMed]
- Street, C.M.; Zhu, Z.; Finel, M.; Court, M.H. Bisphenol-A glucuronidation in human liver and breast: Identification of UDP-glucuronosyltransferases (UGTs) and influence of genetic polymorphisms. Xenobiotica 2017, 47, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Quesnot, N.; Bucher, S.; Fromenty, B.; Robin, M.A. Modulation of metabolizing enzymes by bisphenol a in human and animal models. Chem. Res. Toxicol. 2014, 27, 1463–1473. [Google Scholar] [CrossRef]
- Calafat, A.M.; Ye, X.; Wong, L.Y.; Reidy, J.A.; Needham, L.L. Exposure of the U.S. population to bisphenol A and 4-tertiary-octylphenol: 2003–2004. Environ. Health Perspect. 2008, 116, 39–44. [Google Scholar] [CrossRef]
- Becker, K.; Göen, T.; Seiwert, M.; Conrad, A.; Pick-Fuss, H.; Müller, J.; Wittassek, M.; Schulz, C.; Kolossa-Gehring, M. GerES IV: Phthalate metabolites and bisphenol A in urine of German children. Int. J. Hyg. Environ. Health 2009, 212, 685–692. [Google Scholar] [CrossRef]
- Gerona, R.R.; Pan, J.; Zota, A.R.; Schwartz, J.M.; Friesen, M.; Taylor, J.A.; Hunt, P.A.; Woodruff, T.J. Direct measurement of Bisphenol A (BPA), BPA glucuronide and BPA sulfate in a diverse and low-income population of pregnant women reveals high exposure, with potential implications for previous exposure estimates: A cross-sectional study. Environ. Health 2016, 15, 50. [Google Scholar] [CrossRef] [PubMed]
- Calafat, A.M.; Longnecker, M.P.; Koch, H.M.; Swan, S.H.; Hauser, R.; Goldman, L.R.; Lanphear, B.P.; Rudel, R.A.; Engel, S.M.; Teitelbaum, S.L.; et al. Optimal Exposure Biomarkers for Nonpersistent Chemicals in Environmental Epidemiology. Environ. Health Perspect. 2015, 123, A166–A168. [Google Scholar] [CrossRef] [PubMed]
- Cox, K.J.; Porucznik, C.A.; Anderson, D.J.; Brozek, E.M.; Szczotka, K.M.; Bailey, N.M.; Wilkins, D.G.; Stanford, J.B. Exposure Classification and Temporal Variability in Urinary Bisphenol A Concentrations among Couples in Utah—The HOPE Study. Environ. Health Perspect. 2016, 124, 498–506. [Google Scholar] [CrossRef] [PubMed]
- Colorado-Yohar, S.M.; Castillo-González, A.C.; Sánchez-Meca, J.; Rubio-Aparicio, M.; Sánchez-Rodríguez, D.; Salamanca-Fernández, E.; Ardanaz, E.; Amiano, P.; Fernández, M.F.; Mendiola, J.; et al. Concentrations of bisphenol-A in adults from the general population: A systematic review and meta-analysis. Sci. Total Environ. 2021, 775, 145755. [Google Scholar] [CrossRef] [PubMed]
- Aylward, L.L.; Hays, S.M.; Zidek, A. Variation in urinary spot sample, 24 h samples, and longer-term average urinary concentrations of short-lived environmental chemicals: Implications for exposure assessment and reverse dosimetry. J. Expo. Sci. Environ. Epidemiol. 2017, 27, 582–590. [Google Scholar] [CrossRef] [PubMed]
- Calafat, A.M. Contemporary Issues in Exposure Assessment Using Biomonitoring. Curr. Epidemiol. Rep. 2016, 3, 145–153. [Google Scholar] [CrossRef]
- Ye, X.; Zhou, X.; Hennings, R.; Kramer, J.; Calafat, A.M. Potential external contamination with bisphenol A and other ubiquitous organic environmental chemicals during biomonitoring analysis: An elusive laboratory challenge. Environ. Health Perspect. 2013, 121, 283–286. [Google Scholar] [CrossRef]
- Longnecker, M.P.; Harbak, K.; Kissling, G.E.; Hoppin, J.A.; Eggesbo, M.; Jusko, T.A.; Eide, J.; Koch, H.M. The concentration of bisphenol A in urine is affected by specimen collection, a preservative, and handling. Environ. Res. 2013, 126, 211–214. [Google Scholar] [CrossRef]
- Ougier, E.; Zeman, F.; Antignac, J.P.; Rousselle, C.; Lange, R.; Kolossa-Gehring, M.; Apel, P. Human biomonitoring initiative (HBM4EU): Human biomonitoring guidance values (HBM-GVs) derived for bisphenol A. Environ. Int. 2021, 154, 106563. [Google Scholar] [CrossRef]
- Dodds, E.C.; Lawson, W. Synthetic strogenic Agents without the Phenanthrene Nucleus. Nature 1936, 137, 996. [Google Scholar] [CrossRef]
- Kuiper, G.G.; Carlsson, B.; Grandien, K.; Enmark, E.; Häggblad, J.; Nilsson, S.; Gustafsson, J.A. Comparison of the ligand binding specificity and transcript tissue distribution of estrogen receptors alpha and beta. Endocrinology 1997, 138, 863–870. [Google Scholar] [CrossRef] [PubMed]
- Acconcia, F.; Pallottini, V.; Marino, M. Molecular Mechanisms of Action of BPA. Dose Response 2015, 13, 1559325815610582. [Google Scholar] [CrossRef] [PubMed]
- Welshons, W.V.; Nagel, S.C.; vom Saal, F.S. Large effects from small exposures. III. Endocrine mechanisms mediating effects of bisphenol A at levels of human exposure. Endocrinology 2006, 147, S56–S69. [Google Scholar] [CrossRef] [PubMed]
- Wetherill, Y.B.; Akingbemi, B.T.; Kanno, J.; McLachlan, J.A.; Nadal, A.; Sonnenschein, C.; Watson, C.S.; Zoeller, R.T.; Belcher, S.M. In vitro molecular mechanisms of bisphenol A action. Reprod. Toxicol. 2007, 24, 178–198. [Google Scholar] [CrossRef]
- Cariati, F.; D’Uonno, N.; Borrillo, F.; Iervolino, S.; Galdiero, G.; Tomaiuolo, R. Bisphenol a: An emerging threat to male fertility. Reprod. Biol. Endocrinol. 2019, 17, 6. [Google Scholar] [CrossRef]
- Pivonello, C.; Muscogiuri, G.; Nardone, A.; Garifalos, F.; Provvisiero, D.P.; Verde, N.; de Angelis, C.; Conforti, A.; Piscopo, M.; Auriemma, R.S.; et al. Bisphenol A: An emerging threat to female fertility. Reprod. Biol. Endocrinol. 2020, 18, 22. [Google Scholar] [CrossRef]
- ECHA. Member State Committee Unanimously Agrees that Bisphenol A Is an Endocrine Disruptor. 2017. Available online: https://echa.europa.eu/documents/10162/769b2777-19cd-9fff-33c4-54fe6d8290d5 (accessed on 30 March 2023).
- NTP. Monograph on the Potential Human Reproductive and Developmental Effects of Bisphenol A. National Toxicology Program. National Toxicology Program-Center for the Evaluation of Risk to Human Reproduction (NTP-CHRHR). 2008. Available online: https://ntp.niehs.nih.gov/ntp/ohat/bisphenol/bisphenol.pdf (accessed on 30 March 2023).
- Vom Saal, F.S.; Vandenberg, L.N. Update on the Health Effects of Bisphenol A: Overwhelming Evidence of Harm. Endocrinology 2021, 162, bqaa171. [Google Scholar] [CrossRef]
- Diamanti-Kandarakis, E.; Bourguignon, J.P.; Giudice, L.C.; Hauser, R.; Prins, G.S.; Soto, A.M.; Zoeller, R.T.; Gore, A.C. Endocrine-disrupting chemicals: An Endocrine Society scientific statement. Endocr. Rev. 2009, 30, 293–342. [Google Scholar] [CrossRef]
- Shankar, A.; Teppala, S.; Sabanayagam, C. Bisphenol A and peripheral arterial disease: Results from the NHANES. Environ. Health Perspect. 2012, 120, 1297–1300. [Google Scholar] [CrossRef]
- Peretz, J.; Vrooman, L.; Ricke, W.A.; Hunt, P.A.; Ehrlich, S.; Hauser, R.; Padmanabhan, V.; Taylor, H.S.; Swan, S.H.; VandeVoort, C.A.; et al. Bisphenol a and reproductive health: Update of experimental and human evidence, 2007–2013. Environ. Health Perspect. 2014, 122, 775–786. [Google Scholar] [CrossRef]
- Mustieles, V.; Pérez-Lobato, R.; Olea, N.; Fernández, M.F. Bisphenol A: Human exposure and neurobehavior. Neurotoxicology 2015, 49, 174–184. [Google Scholar] [CrossRef] [PubMed]
- Bansal, A.; Henao-Mejia, J.; Simmons, R.A. Immune System: An Emerging Player in Mediating Effects of Endocrine Disruptors on Metabolic Health. Endocrinology 2018, 159, 32–45. [Google Scholar] [CrossRef] [PubMed]
- Hafezi, S.A.; Abdel-Rahman, W.M. The Endocrine Disruptor Bisphenol A (BPA) Exerts a Wide Range of Effects in Carcinogenesis and Response to Therapy. Curr. Mol. Pharmacol. 2019, 12, 230–238. [Google Scholar] [CrossRef]
- Lite, C.; Raja, G.L.; Juliet, M.; Sridhar, V.V.; Subhashree, K.D.; Kumar, P.; Chakraborty, P.; Arockiaraj, J. In utero exposure to endocrine-disrupting chemicals, maternal factors and alterations in the epigenetic landscape underlying later-life health effects. Environ. Toxicol. Pharmacol. 2022, 89, 103779. [Google Scholar] [CrossRef] [PubMed]
- Braun, J.M.; Hauser, R. Bisphenol A and children’s health. Curr. Opin. Pediatr. 2011, 23, 233–239. [Google Scholar] [CrossRef]
- Braun, J.M. Early-life exposure to EDCs: Role in childhood obesity and neurodevelopment. Nat. Rev. Endocrinol. 2017, 13, 161–173. [Google Scholar] [CrossRef]
- Lombó, M.; Fernández-Díez, C.; González-Rojo, S.; Herráez, M.P. Genetic and epigenetic alterations induced by bisphenol A exposure during different periods of spermatogenesis: From spermatozoa to the progeny. Sci. Rep. 2019, 9, 18029. [Google Scholar] [CrossRef]
- Rahman, M.S.; Pang, W.K.; Ryu, D.Y.; Park, Y.J.; Pang, M.G. Multigenerational and transgenerational impact of paternal bisphenol A exposure on male fertility in a mouse model. Hum. Reprod. 2020, 35, 1740–1752. [Google Scholar] [CrossRef]
- Ghassabian, A.; Vandenberg, L.; Kannan, K.; Trasande, L. Endocrine-Disrupting Chemicals and Child Health. Annu. Rev. Pharmacol. Toxicol. 2022, 62, 573–594. [Google Scholar] [CrossRef]
- Rubin, B.S. Bisphenol A: An endocrine disruptor with widespread exposure and multiple effects. J. Steroid Biochem. Mol. Biol. 2011, 127, 27–34. [Google Scholar] [CrossRef]
- Shanle, E.K.; Xu, W. Endocrine disrupting chemicals targeting estrogen receptor signaling: Identification and mechanisms of action. Chem. Res. Toxicol. 2011, 24, 6–19. [Google Scholar] [CrossRef]
- Nohynek, G.J.; Borgert, C.J.; Dietrich, D.; Rozman, K.K. Endocrine disruption: Fact or urban legend? Toxicol. Lett. 2013, 223, 295–305. [Google Scholar] [CrossRef] [PubMed]
- Chevalier, N.; Fénichel, P. Bisphenol A: Targeting metabolic tissues. Rev. Endocr. Metab. Disord. 2015, 16, 299–309. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Wang, Q.; Zhang, Y.; Niu, Y.; Yao, X.; Liu, H. The molecular mechanism of bisphenol A (BPA) as an endocrine disruptor by interacting with nuclear receptors: Insights from molecular dynamics (MD) simulations. PLoS ONE 2015, 10, e0120330. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Ding, Z.; Shi, Q.M.; Ge, X.; Wang, H.X.; Li, M.X.; Chen, G.; Wang, Q.; Ju, Q.; Zhang, J.P.; et al. Anti-androgenic mechanisms of Bisphenol A involve androgen receptor signaling pathway. Toxicology 2017, 387, 10–16. [Google Scholar] [CrossRef]
- Grimaldi, M.; Boulahtouf, A.; Toporova, L.; Balaguer, P. Functional profiling of bisphenols for nuclear receptors. Toxicology 2019, 420, 39–45. [Google Scholar] [CrossRef]
- Cimmino, I.; Fiory, F.; Perruolo, G.; Miele, C.; Beguinot, F.; Formisano, P.; Oriente, F. Potential Mechanisms of Bisphenol A (BPA) Contributing to Human Disease. Int. J. Mol. Sci. 2020, 21, 5761. [Google Scholar] [CrossRef]
- Ma, Y.; Liu, H.; Wu, J.; Yuan, L.; Wang, Y.; Du, X.; Wang, R.; Marwa, P.W.; Petlulu, P.; Chen, X.; et al. The adverse health effects of bisphenol A and related toxicity mechanisms. Environ. Res. 2019, 176, 108575. [Google Scholar] [CrossRef]
- Mustieles, V.; D’Cruz, S.C.; Couderq, S.; Rodríguez-Carrillo, A.; Fini, J.B.; Hofer, T.; Steffensen, I.L.; Dirven, H.; Barouki, R.; Olea, N.; et al. Bisphenol A and its analogues: A comprehensive review to identify and prioritize effect biomarkers for human biomonitoring. Environ. Int. 2020, 144, 105811. [Google Scholar] [CrossRef]
- Qin, T.; Zhang, X.; Guo, T.; Yang, T.; Gao, Y.; Hao, W.; Xiao, X. Epigenetic Alteration Shaped by the Environmental Chemical Bisphenol A. Front. Genet. 2020, 11, 618966. [Google Scholar] [CrossRef]
- Cariati, F.; Carbone, L.; Conforti, A.; Bagnulo, F.; Peluso, S.R.; Carotenuto, C.; Buonfantino, C.; Alviggi, E.; Alviggi, C.; Strina, I. Bisphenol A-Induced Epigenetic Changes and Its Effects on the Male Reproductive System. Front. Endocrinol. 2020, 11, 453. [Google Scholar] [CrossRef] [PubMed]
- Ho, S.M.; Tang, W.Y.; Belmonte de Frausto, J.; Prins, G.S. Developmental exposure to estradiol and bisphenol A increases susceptibility to prostate carcinogenesis and epigenetically regulates phosphodiesterase type 4 variant 4. Cancer Res. 2006, 66, 5624–5632. [Google Scholar] [CrossRef] [PubMed]
- Dolinoy, D.C.; Huang, D.; Jirtle, R.L. Maternal nutrient supplementation counteracts bisphenol A-induced DNA hypomethylation in early development. Proc. Natl. Acad. Sci. USA 2007, 104, 13056–13061. [Google Scholar] [CrossRef]
- McGee, M.; Bainbridge, S.; Fontaine-Bisson, B. A crucial role for maternal dietary methyl donor intake in epigenetic programming and fetal growth outcomes. Nutr. Rev. 2018, 76, 469–478. [Google Scholar] [CrossRef]
- Chianese, R.; Troisi, J.; Richards, S.; Scafuro, M.; Fasano, S.; Guida, M.; Pierantoni, R.; Meccariello, R. Bisphenol A in Reproduction: Epigenetic Effects. Curr. Med. Chem. 2018, 25, 748–770. [Google Scholar] [CrossRef] [PubMed]
- Martin, E.M.; Fry, R.C. Environmental Influences on the Epigenome: Exposure- Associated DNA Methylation in Human Populations. Annu. Rev. Public. Health 2018, 39, 309–333. [Google Scholar] [CrossRef]
- Baylin, S.B.; Jones, P.A. Epigenetic Determinants of Cancer. Cold Spring Harb. Perspect. Biol. 2016, 8, a019505. [Google Scholar] [CrossRef]
- Feinberg, A.P.; Koldobskiy, M.A.; Gondor, A. Epigenetic modulators, modifiers and mediators in cancer aetiology and progression. Nat. Rev. Genet. 2016, 17, 284–299. [Google Scholar] [CrossRef]
- Esteller, M.; Pandolfi, P.P. The Epitranscriptome of Noncoding RNAs in Cancer. Cancer Discov. 2017, 7, 359–368. [Google Scholar] [CrossRef]
- Greenberg, M.V.C.; Bourc’his, D. The diverse roles of DNA methylation in mammalian development and disease. Nat. Rev. Mol. Cell. Biol. 2019, 20, 590–607. [Google Scholar] [CrossRef]
- Besaratinia, A.; Tommasi, S. Epigenetics of human melanoma: Promises and challenges. J. Mol. Cell Biol. 2014, 6, 356–367. [Google Scholar] [CrossRef] [PubMed]
- Statello, L.; Guo, C.J.; Chen, L.L.; Huarte, M. Gene regulation by long non-coding RNAs and its biological functions. Nat. Rev. Mol. Cell. Biol. 2021, 22, 96–118. [Google Scholar] [CrossRef] [PubMed]
- Goodall, G.J.; Wickramasinghe, V.O. RNA in cancer. Nat. Rev. Cancer 2021, 21, 22–36. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Maksoud, F.M.; Leasor, K.R.; Butzen, K.; Braden, T.D.; Akingbemi, B.T. Prenatal Exposures of Male Rats to the Environmental Chemicals Bisphenol A and Di(2-Ethylhexyl) Phthalate Impact the Sexual Differentiation Process. Endocrinology 2015, 156, 4672–4683. [Google Scholar] [CrossRef] [PubMed]
- Zheng, H.; Zhou, X.; Li, D.K.; Yang, F.; Pan, H.; Li, T.; Miao, M.; Li, R.; Yuan, W. Genome-wide alteration in DNA hydroxymethylation in the sperm from bisphenol A-exposed men. PLoS ONE 2017, 12, e0178535. [Google Scholar] [CrossRef]
- Kochmanski, J.; Marchlewicz, E.H.; Dolinoy, D.C. Longitudinal effects of developmental bisphenol A, variable diet, and physical activity on age-related methylation in blood. Environ. Epigenet 2018, 4, dvy017. [Google Scholar] [CrossRef]
- Fatma Karaman, E.; Caglayan, M.; Sancar-Bas, S.; Ozal-Coskun, C.; Arda-Pirincci, P.; Ozden, S. Global and region-specific post-transcriptional and post-translational modifications of bisphenol A in human prostate cancer cells. Environ. Pollut. 2019, 255, 113318. [Google Scholar] [CrossRef]
- Song, X.; Miao, M.; Zhou, X.; Li, D.; Tian, Y.; Liang, H.; Li, R.; Yuan, W. Bisphenol A Exposure and Sperm ACHE Hydroxymethylation in Men. Int. J. Environ. Res. Public. Health 2019, 16, 152. [Google Scholar] [CrossRef]
- Li, Z.; Lyu, C.; Ren, Y.; Wang, H. Role of TET Dioxygenases and DNA Hydroxymethylation in Bisphenols-Stimulated Proliferation of Breast Cancer Cells. Environ. Health Perspect. 2020, 128, 27008. [Google Scholar] [CrossRef]
- Besaratinia, A.; Caceres, A.; Tommasi, S. DNA Hydroxymethylation in Smoking-Associated Cancers. Int. J. Mol. Sci. 2022, 23, 2657. [Google Scholar] [CrossRef]
- Dou, J.; Thangaraj, S.V.; Puttabyatappa, M.; Elangovan, V.R.; Bakulski, K.; Padmanabhan, V. Developmental programming: Adipose depot-specific regulation of non-coding RNAs and their relation to coding RNA expression in prenatal testosterone and prenatal bisphenol-A -treated female sheep. Mol. Cell. Endocrinol. 2023, 564, 111868. [Google Scholar] [CrossRef] [PubMed]
- Puttabyatappa, M.; Saadat, N.; Elangovan, V.R.; Dou, J.; Bakulski, K.; Padmanabhan, V. Developmental programming: Impact of prenatal bisphenol-A exposure on liver and muscle transcriptome of female sheep. Toxicol. Appl. Pharmacol. 2022, 451, 116161. [Google Scholar] [CrossRef] [PubMed]
- Blanc, M.; Rüegg, J.; Scherbak, N.; Keiter, S.H. Environmental chemicals differentially affect epigenetic-related mechanisms in the zebrafish liver (ZF-L) cell line and in zebrafish embryos. Aquat. Toxicol. 2019, 215, 105272. [Google Scholar] [CrossRef] [PubMed]
- Weinhouse, C.; Bergin, I.L.; Harris, C.; Dolinoy, D.C. Stat3 is a candidate epigenetic biomarker of perinatal Bisphenol A exposure associated with murine hepatic tumors with implications for human health. Epigenetics 2015, 10, 1099–1110. [Google Scholar] [CrossRef] [PubMed]
- Bouwmeester, M.C.; Ruiter, S.; Lommelaars, T.; Sippel, J.; Hodemaekers, H.M.; van den Brandhof, E.J.; Pennings, J.L.; Kamstra, J.H.; Jelinek, J.; Issa, J.P.; et al. Zebrafish embryos as a screen for DNA methylation modifications after compound exposure. Toxicol. Appl. Pharmacol. 2016, 291, 84–96. [Google Scholar] [CrossRef]
- Laing, L.V.; Viana, J.; Dempster, E.L.; Trznadel, M.; Trunkfield, L.A.; Uren Webster, T.M.; van Aerle, R.; Paull, G.C.; Wilson, R.J.; Mill, J.; et al. Bisphenol A causes reproductive toxicity, decreases dnmt1 transcription, and reduces global DNA methylation in breeding zebrafish (Danio rerio). Epigenetics 2016, 11, 526–538. [Google Scholar] [CrossRef]
- Santangeli, S.; Maradonna, F.; Gioacchini, G.; Cobellis, G.; Piccinetti, C.C.; Dalla Valle, L.; Carnevali, O. BPA-Induced Deregulation Of Epigenetic Patterns: Effects On Female Zebrafish Reproduction. Sci. Rep. 2016, 6, 21982. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, Y.; Tao, S.; Guan, Y.; Zhang, T.; Wang, Z. Global DNA methylation in gonads of adult zebrafish Danio rerio under bisphenol A exposure. Ecotoxicol. Environ. Saf. 2016, 130, 124–132. [Google Scholar] [CrossRef]
- Zhao, F.; Wei, P.; Wang, J.; Yu, M.; Zhang, X.; Tian, H.; Wang, W.; Ru, S. Estrogenic effects associated with bisphenol a exposure in male zebrafish (Danio rerio) is associated with changes of endogenous 17β-estradiol and gene specific DNA methylation levels. Gen. Comp. Endocrinol. 2017, 252, 27–35. [Google Scholar] [CrossRef]
- Zhang, T.; Guan, Y.; Wang, S.; Wang, L.; Cheng, M.; Yuan, C.; Liu, Y.; Wang, Z. Bisphenol A induced abnormal DNA methylation of ovarian steroidogenic genes in rare minnow Gobiocypris rarus. Gen. Comp. Endocrinol. 2018, 269, 156–165. [Google Scholar] [CrossRef]
- Lombó, M.; González-Rojo, S.; Fernández-Díez, C.; Herráez, M.P. Cardiogenesis impairment promoted by bisphenol A exposure is successfully counteracted by epigallocatechin gallate. Environ. Pollut. 2019, 246, 1008–1019. [Google Scholar] [CrossRef] [PubMed]
- González-Rojo, S.; Lombó, M.; Fernández-Díez, C.; Herráez, M.P. Male exposure to bisphenol a impairs spermatogenesis and triggers histone hyperacetylation in zebrafish testes. Environ. Pollut. 2019, 248, 368–379. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Yuan, C.; Wang, M.; Liu, Y.; Wang, Z.; Seif, M.M. Bisphenol A-associated alterations in DNA and histone methylation affects semen quality in rare minnow Gobiocypris rarus. Aquat. Toxicol. 2020, 226, 105580. [Google Scholar] [CrossRef] [PubMed]
- Gyimah, E.; Dong, X.; Xu, H.; Zhang, Z.; Mensah, J.K. Embryonic Exposure to Low Concentrations of Bisphenol A and S Altered Genes Related to Pancreatic β-Cell Development and DNA Methyltransferase in Zebrafish. Arch. Environ. Contam. Toxicol. 2021, 80, 450–460. [Google Scholar] [CrossRef]
- Ruiz, T.F.R.; Colleta, S.J.; Zuccari, D.; Vilamaior, P.S.L.; Leonel, E.C.R.; Taboga, S.R. Hormone receptor expression in aging mammary tissue and carcinoma from a rodent model after xenoestrogen disruption. Life Sci. 2021, 285, 120010. [Google Scholar] [CrossRef]
- Qin, X.Y.; Fukuda, T.; Yang, L.; Zaha, H.; Akanuma, H.; Zeng, Q.; Yoshinaga, J.; Sone, H. Effects of bisphenol A exposure on the proliferation and senescence of normal human mammary epithelial cells. Cancer Biol. Ther. 2012, 13, 296–306. [Google Scholar] [CrossRef]
- Fernandez, S.V.; Huang, Y.; Snider, K.E.; Zhou, Y.; Pogash, T.J.; Russo, J. Expression and DNA methylation changes in human breast epithelial cells after bisphenol A exposure. Int. J. Oncol. 2012, 41, 369–377. [Google Scholar] [CrossRef]
- Bastos Sales, L.; Kamstra, J.H.; Cenijn, P.H.; van Rijt, L.S.; Hamers, T.; Legler, J. Effects of endocrine disrupting chemicals on in vitro global DNA methylation and adipocyte differentiation. Toxicol. Vitro 2013, 27, 1634–1643. [Google Scholar] [CrossRef]
- Ribeiro-Varandas, E.; Pereira, H.S.; Monteiro, S.; Neves, E.; Brito, L.; Ferreira, R.B.; Viegas, W.; Delgado, M. Bisphenol A disrupts transcription and decreases viability in aging vascular endothelial cells. Int. J. Mol. Sci. 2014, 15, 15791–15805. [Google Scholar] [CrossRef]
- Yin, L.; Dai, Y.; Jiang, X.; Liu, Y.; Chen, H.; Han, F.; Cao, J.; Liu, J. Role of DNA methylation in bisphenol A exposed mouse spermatocyte. Environ. Toxicol. Pharmacol. 2016, 48, 265–271. [Google Scholar] [CrossRef]
- Huang, B.; Ning, S.; Zhang, Q.; Chen, A.; Jiang, C.; Cui, Y.; Hu, J.; Li, H.; Fan, G.; Qin, L.; et al. Bisphenol A Represses Dopaminergic Neuron Differentiation from Human Embryonic Stem Cells through Downregulating the Expression of Insulin-like Growth Factor 1. Mol. Neurobiol. 2017, 54, 3798–3812. [Google Scholar] [CrossRef] [PubMed]
- Senyildiz, M.; Karaman, E.F.; Bas, S.S.; Pirincci, P.A.; Ozden, S. Effects of BPA on global DNA methylation and global histone 3 lysine modifications in SH-SY5Y cells: An epigenetic mechanism linking the regulation of chromatin modifiying genes. Toxicol. Vitro 2017, 44, 313–321. [Google Scholar] [CrossRef] [PubMed]
- Sidorkiewicz, I.; Czerniecki, J.; Jarząbek, K.; Zbucka-Krętowska, M.; Wołczyński, S. Cellular, transcriptomic and methylome effects of individual and combined exposure to BPA, BPF, BPS on mouse spermatocyte GC-2 cell line. Toxicol. Appl. Pharmacol. 2018, 359, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Wei, Y.; Guo, T.; Yuan, J.; Zhao, R. Epigenetic effect of long-term bisphenol A exposure on human breast adenocarcinoma cells. Toxicol. Environ. Chem. 2018, 100, 258–266. [Google Scholar] [CrossRef]
- Li, Q.; Lawrence, C.R.; Nowak, R.A.; Flaws, J.A.; Bagchi, M.K.; Bagchi, I.C. Bisphenol A and Phthalates Modulate Peritoneal Macrophage Function in Female Mice Involving SYMD2-H3K36 Dimethylation. Endocrinology 2018, 159, 2216–2228. [Google Scholar] [CrossRef]
- Awada, Z.; Nasr, R.; Akika, R.; Cahais, V.; Cuenin, C.; Zhivagui, M.; Herceg, Z.; Ghantous, A.; Zgheib, N.K. DNA methylome-wide alterations associated with estrogen receptor-dependent effects of bisphenols in breast cancer. Clin. Epigenetics 2019, 11, 138. [Google Scholar] [CrossRef]
- Longo, M.; Zatterale, F.; Naderi, J.; Nigro, C.; Oriente, F.; Formisano, P.; Miele, C.; Beguinot, F. Low-dose Bisphenol-A Promotes Epigenetic Changes at Pparγ Promoter in Adipose Precursor Cells. Nutrients 2020, 12, 3498. [Google Scholar] [CrossRef]
- Nair, V.A.; Valo, S.; Peltomäki, P.; Bajbouj, K.; Abdel-Rahman, W.M. Oncogenic Potential of Bisphenol A and Common Environmental Contaminants in Human Mammary Epithelial Cells. Int. J. Mol. Sci. 2020, 21, 3735. [Google Scholar] [CrossRef]
- Oldenburg, J.; Fürhacker, M.; Hartmann, C.; Steinbichl, P.; Banaderakhshan, R.; Haslberger, A. Different bisphenols induce non-monotonous changes in miRNA expression and LINE-1 methylation in two cell lines. Environ. Epigenet 2021, 7, dvab011. [Google Scholar] [CrossRef]
- Cao, Y.; Chen, Z.; Zhang, M.; Shi, L.; Qin, S.; Lv, D.; Li, D.; Ma, L.; Zhang, Y. Maternal exposure to bisphenol A induces fetal growth restriction via upregulating the expression of estrogen receptors. Chemosphere 2022, 287, 132244. [Google Scholar] [CrossRef]
- O’Brien, E.; Dolinoy, D.C.; Mancuso, P. Perinatal bisphenol A exposures increase production of pro-inflammatory mediators in bone marrow-derived mast cells of adult mice. J. Immunotoxicol. 2014, 11, 205–212. [Google Scholar] [CrossRef] [PubMed]
- Patkin, E.L.; Grudinina, N.A.; Sasina, L.K.; Noniashvili, E.M.; Pavlinova, L.I.; Suchkova, I.O.; Kustova, M.E.; Kolmakov, N.N.; Van Truong, T.; Sofronov, G.A. Asymmetric DNA methylation between sister chromatids of metaphase chromosomes in mouse embryos upon bisphenol A action. Reprod. Toxicol. 2017, 74, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Faulk, C.; Kim, J.H.; Jones, T.R.; McEachin, R.C.; Nahar, M.S.; Dolinoy, D.C.; Sartor, M.A. Bisphenol A-associated alterations in genome-wide DNA methylation and gene expression patterns reveal sequence-dependent and non-monotonic effects in human fetal liver. Environ. Epigenet 2015, 1, dvv006. [Google Scholar] [CrossRef] [PubMed]
- Du, L.; Sun, W.; Li, X.M.; Li, X.Y.; Liu, W.; Chen, D. DNA methylation and copy number variation analyses of human embryonic stem cell-derived neuroprogenitors after low-dose decabromodiphenyl ether and/or bisphenol A exposure. Hum. Exp. Toxicol. 2018, 37, 475–485. [Google Scholar] [CrossRef] [PubMed]
- Suchkova, I.O.; Sasina, L.K.; Dergacheva, N.I.; Sofronov, G.A.; Patkin, E.L. The influence of low dose Bisphenol A on whole genome DNA methylation and chromatin compaction in different human cell lines. Toxicol. Vitro 2019, 58, 26–34. [Google Scholar] [CrossRef]
- Bromer, J.G.; Zhou, Y.; Taylor, M.B.; Doherty, L.; Taylor, H.S. Bisphenol-A exposure in utero leads to epigenetic alterations in the developmental programming of uterine estrogen response. Faseb J. 2010, 24, 2273–2280. [Google Scholar] [CrossRef]
- Doshi, T.; Mehta, S.S.; Dighe, V.; Balasinor, N.; Vanage, G. Hypermethylation of estrogen receptor promoter region in adult testis of rats exposed neonatally to bisphenol A. Toxicology 2011, 289, 74–82. [Google Scholar] [CrossRef]
- Tang, W.Y.; Morey, L.M.; Cheung, Y.Y.; Birch, L.; Prins, G.S.; Ho, S.M. Neonatal exposure to estradiol/bisphenol A alters promoter methylation and expression of Nsbp1 and Hpcal1 genes and transcriptional programs of Dnmt3a/b and Mbd2/4 in the rat prostate gland throughout life. Endocrinology 2012, 153, 42–55. [Google Scholar] [CrossRef]
- Anderson, O.S.; Nahar, M.S.; Faulk, C.; Jones, T.R.; Liao, C.; Kannan, K.; Weinhouse, C.; Rozek, L.S.; Dolinoy, D.C. Epigenetic responses following maternal dietary exposure to physiologically relevant levels of bisphenol A. Environ. Mol. Mutagen. 2012, 53, 334–342. [Google Scholar] [CrossRef]
- Kundakovic, M.; Gudsnuk, K.; Franks, B.; Madrid, J.; Miller, R.L.; Perera, F.P.; Champagne, F.A. Sex-specific epigenetic disruption and behavioral changes following low-dose in utero bisphenol A exposure. Proc. Natl. Acad. Sci. USA 2013, 110, 9956–9961. [Google Scholar] [CrossRef]
- Ma, Y.; Xia, W.; Wang, D.Q.; Wan, Y.J.; Xu, B.; Chen, X.; Li, Y.Y.; Xu, S.Q. Hepatic DNA methylation modifications in early development of rats resulting from perinatal BPA exposure contribute to insulin resistance in adulthood. Diabetologia 2013, 56, 2059–2067. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Sartor, M.A.; Rozek, L.S.; Faulk, C.; Anderson, O.S.; Jones, T.R.; Nahar, M.S.; Dolinoy, D.C. Perinatal bisphenol A exposure promotes dose-dependent alterations of the mouse methylome. BMC Genom. 2014, 15, 30. [Google Scholar] [CrossRef] [PubMed]
- Patel, B.B.; Raad, M.; Sebag, I.A.; Chalifour, L.E. Lifelong exposure to bisphenol a alters cardiac structure/function, protein expression, and DNA methylation in adult mice. Toxicol. Sci. 2013, 133, 174–185. [Google Scholar] [CrossRef] [PubMed]
- Wong, R.L.; Wang, Q.; Treviño, L.S.; Bosland, M.C.; Chen, J.; Medvedovic, M.; Prins, G.S.; Kannan, K.; Ho, S.M.; Walker, C.L. Identification of secretaglobin Scgb2a1 as a target for developmental reprogramming by BPA in the rat prostate. Epigenetics 2015, 10, 127–134. [Google Scholar] [CrossRef]
- Chang, H.; Wang, M.; Xia, W.; Chen, T.; Huo, W.; Mao, Z.; Zhu, Y.; Li, Y.; Xu, S. Perinatal exposure to low-dose bisphenol A disrupts learning/memory and DNA methylation of estrogen receptor alpha in the hippocampus. Toxicol. Res. 2016, 5, 828–835. [Google Scholar] [CrossRef]
- Jorgensen, E.M.; Alderman, M.H., 3rd; Taylor, H.S. Preferential epigenetic programming of estrogen response after in utero xenoestrogen (bisphenol-A) exposure. FASEB J. 2016, 30, 3194–3201. [Google Scholar] [CrossRef]
- Jadhav, R.R.; Santucci-Pereira, J.; Wang, Y.V.; Liu, J.; Nguyen, T.D.; Wang, J.; Jenkins, S.; Russo, J.; Huang, T.H.; Jin, V.X.; et al. DNA Methylation Targets Influenced by Bisphenol A and/or Genistein Are Associated with Survival Outcomes in Breast Cancer Patients. Genes 2017, 8, 144. [Google Scholar] [CrossRef]
- Kochmanski, J.; Marchlewicz, E.H.; Savidge, M.; Montrose, L.; Faulk, C.; Dolinoy, D.C. Longitudinal effects of developmental bisphenol A and variable diet exposures on epigenetic drift in mice. Reprod. Toxicol. 2017, 68, 154–163. [Google Scholar] [CrossRef]
- Anderson, O.S.; Kim, J.H.; Peterson, K.E.; Sanchez, B.N.; Sant, K.E.; Sartor, M.A.; Weinhouse, C.; Dolinoy, D.C. Novel Epigenetic Biomarkers Mediating Bisphenol A Exposure and Metabolic Phenotypes in Female Mice. Endocrinology 2017, 158, 31–40. [Google Scholar] [CrossRef]
- Prins, G.S.; Ye, S.H.; Birch, L.; Zhang, X.; Cheong, A.; Lin, H.; Calderon-Gierszal, E.; Groen, J.; Hu, W.Y.; Ho, S.M.; et al. Prostate Cancer Risk and DNA Methylation Signatures in Aging Rats following Developmental BPA Exposure: A Dose-Response Analysis. Environ. Health Perspect. 2017, 125, 077007. [Google Scholar] [CrossRef]
- Junge, K.M.; Leppert, B.; Jahreis, S.; Wissenbach, D.K.; Feltens, R.; Grützmann, K.; Thürmann, L.; Bauer, T.; Ishaque, N.; Schick, M.; et al. MEST mediates the impact of prenatal bisphenol A exposure on long-term body weight development. Clin. Epigenetics 2018, 10, 58. [Google Scholar] [CrossRef] [PubMed]
- Bhandari, R.K.; Taylor, J.A.; Sommerfeld-Sager, J.; Tillitt, D.E.; Ricke, W.A.; Vom Saal, F.S. Estrogen receptor 1 expression and methylation of Esr1 promoter in mouse fetal prostate mesenchymal cells induced by gestational exposure to bisphenol A or ethinylestradiol. Environ. Epigenet 2019, 5, dvz012. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Han, C.; Li, S.; Cui, Y.; Bao, Y.; Shi, W. Cuscuta chinensis flavonoids down-regulate the DNA methylation of the H19/Igf2 imprinted control region and estrogen receptor alpha promoter of the testis in bisphenol A exposed mouse offspring. Food Funct. 2020, 11, 787–798. [Google Scholar] [CrossRef] [PubMed]
- Strakovsky, R.S.; Wang, H.; Engeseth, N.J.; Flaws, J.A.; Helferich, W.G.; Pan, Y.X.; Lezmi, S. Developmental bisphenol A (BPA) exposure leads to sex-specific modification of hepatic gene expression and epigenome at birth that may exacerbate high-fat diet-induced hepatic steatosis. Toxicol. Appl. Pharmacol. 2015, 284, 101–112. [Google Scholar] [CrossRef] [PubMed]
- Bird, A. DNA methylation patterns and epigenetic memory. Genes. Dev. 2002, 16, 6–21. [Google Scholar] [CrossRef]
- Tommasi, S.; Zheng, A.; Yoon, J.I.; Besaratinia, A. Epigenetic targeting of the Nanog pathway and signaling networks during chemical carcinogenesis. Carcinogenesis 2014, 35, 1726–1736. [Google Scholar] [CrossRef]
- Warita, K.; Mitsuhashi, T.; Ohta, K.; Suzuki, S.; Hoshi, N.; Miki, T.; Takeuchi, Y. Gene expression of epigenetic regulatory factors related to primary silencing mechanism is less susceptible to lower doses of bisphenol A in embryonic hypothalamic cells. J. Toxicol. Sci. 2013, 38, 285–289. [Google Scholar] [CrossRef]
- El Henafy, H.M.A.; Ibrahim, M.A.; Abd El Aziz, S.A.; Gouda, E.M. Oxidative Stress and DNA methylation in male rat pups provoked by the transplacental and translactational exposure to bisphenol A. Environ. Sci. Pollut. Res. Int. 2020, 27, 4513–4519. [Google Scholar] [CrossRef]
- Escarda-Castro, E.; Herráez, M.P.; Lombó, M. Effects of bisphenol A exposure during cardiac cell differentiation. Environ. Pollut. 2021, 286, 117567. [Google Scholar] [CrossRef]
- Tommasi, S.; Zheng, A.; Weninger, A.; Bates, S.E.; Li, X.A.; Wu, X.; Hollstein, M.; Besaratinia, A. Mammalian cells acquire epigenetic hallmarks of human cancer during immortalization. Nucleic Acids Res. 2013, 41, 182–195. [Google Scholar] [CrossRef]
- Song, X.; Wang, Z.; Zhang, Z.; Miao, M.; Liu, J.; Luan, M.; Du, J.; Liang, H.; Yuan, W. Differential methylation of genes in the human placenta associated with bisphenol A exposure. Environ. Res. 2021, 200, 111389. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Rozek, L.S.; Soliman, A.S.; Sartor, M.A.; Hablas, A.; Seifeldin, I.A.; Colacino, J.A.; Weinhouse, C.; Nahar, M.S.; Dolinoy, D.C. Bisphenol A-associated epigenomic changes in prepubescent girls: A cross-sectional study in Gharbiah, Egypt. Environ. Health 2013, 12, 33. [Google Scholar] [CrossRef] [PubMed]
- Miao, M.; Zhou, X.; Li, Y.; Zhang, O.; Zhou, Z.; Li, T.; Yuan, W.; Li, R.; Li, D.K. LINE-1 hypomethylation in spermatozoa is associated with Bisphenol A exposure. Andrology 2014, 2, 138–144. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.F.; Karmaus, W.J.J.; Yang, C.C.; Chen, M.L.; Wang, I.J. Bisphenol a Exposure, DNA Methylation, and Asthma in Children. Int. J. Environ. Res. Public. Health 2020, 17, 298. [Google Scholar] [CrossRef]
- Almstrup, K.; Frederiksen, H.; Andersson, A.M.; Juul, A. Levels of endocrine-disrupting chemicals are associated with changes in the peri-pubertal epigenome. Endocr. Connect. 2020, 9, 845–857. [Google Scholar] [CrossRef]
- Awada, Z.; Sleiman, F.; Mailhac, A.; Mouneimne, Y.; Tamim, H.; Zgheib, N.K. BPA exposure is associated with non-monotonic alteration in ESR1 promoter methylation in peripheral blood of men and shorter relative telomere length in peripheral blood of women. J. Expo. Sci. Environ. Epidemiol. 2019, 29, 118–128. [Google Scholar] [CrossRef]
- Mustieles, V.; Rodríguez-Carrillo, A.; Vela-Soria, F.; D’Cruz, S.C.; David, A.; Smagulova, F.; Mundo-López, A.; Olivas-Martínez, A.; Reina-Pérez, I.; Olea, N.; et al. BDNF as a potential mediator between childhood BPA exposure and behavioral function in adolescent boys from the INMA-Granada cohort. Sci. Total Environ. 2022, 803, 150014. [Google Scholar] [CrossRef]
- Miura, R.; Araki, A.; Minatoya, M.; Miyake, K.; Chen, M.-L.; Kobayashi, S.; Miyashita, C.; Yamamoto, J.; Matsumura, T.; Ishizuka, M.; et al. An epigenome-wide analysis of cord blood DNA methylation reveals sex-specific effect of exposure to bisphenol A. Sci. Rep. 2019, 9, 12369. [Google Scholar] [CrossRef]
- Oluwayiose, O.A.; Wu, H.; Saddiki, H.; Whitcomb, B.W.; Balzer, L.B.; Brandon, N.; Suvorov, A.; Tayyab, R.; Sites, C.K.; Hill, L.; et al. Sperm DNA methylation mediates the association of male age on reproductive outcomes among couples undergoing infertility treatment. Sci. Rep. 2021, 11, 3216. [Google Scholar] [CrossRef]
- McCabe, C.F.; Goodrich, J.M.; Bakulski, K.M.; Domino, S.E.; Jones, T.R.; Colacino, J.; Dolinoy, D.C.; Padmanabhan, V. Probing prenatal bisphenol exposures and tissue-specific DNA methylation responses in cord blood, cord tissue, and placenta. Reprod. Toxicol. 2023, 115, 74–84. [Google Scholar] [CrossRef]
- Faulk, C.; Kim, J.H.; Anderson, O.S.; Nahar, M.S.; Jones, T.R.; Sartor, M.A.; Dolinoy, D.C. Detection of differential DNA methylation in repetitive DNA of mice and humans perinatally exposed to bisphenol A. Epigenetics 2016, 11, 489–500. [Google Scholar] [CrossRef] [PubMed]
- Navarro-Lafuente, F.; Adoamnei, E.; Arense-Gonzalo, J.J.; Prieto-Sánchez, M.T.; Sánchez-Ferrer, M.L.; Parrado, A.; Fernández, M.F.; Suarez, B.; López-Acosta, A.; Sánchez-Guillamón, A.; et al. Maternal urinary concentrations of bisphenol A during pregnancy are associated with global DNA methylation in cord blood of newborns in the “NELA” birth cohort. Sci. Total Environ. 2022, 838, 156540. [Google Scholar] [CrossRef] [PubMed]
- Hanna, C.W.; Bloom, M.S.; Robinson, W.P.; Kim, D.; Parsons, P.J.; vom Saal, F.S.; Taylor, J.A.; Steuerwald, A.J.; Fujimoto, V.Y. DNA methylation changes in whole blood is associated with exposure to the environmental contaminants, mercury, lead, cadmium and bisphenol A, in women undergoing ovarian stimulation for IVF. Hum. Reprod. 2012, 27, 1401–1410. [Google Scholar] [CrossRef] [PubMed]
- Lander, E.S.; Linton, L.M.; Birren, B.; Nusbaum, C.; Zody, M.C.; Baldwin, J.; Devon, K.; Dewar, K.; Doyle, M.; FitzHugh, W.; et al. Initial sequencing and analysis of the human genome. Nature 2001, 409, 860–921. [Google Scholar] [CrossRef]
- Venter, J.C.; Adams, M.D.; Myers, E.W.; Li, P.W.; Mural, R.J.; Sutton, G.G.; Smith, H.O.; Yandell, M.; Evans, C.A.; Holt, R.A.; et al. The sequence of the human genome. Science 2001, 291, 1304–1351. [Google Scholar] [CrossRef]
- Sabry, R.; Yamate, J.; Favetta, L.; LaMarre, J. MicroRNAs: Potential targets and agents of endocrine disruption in female reproduction. J. Toxicol. Pathol. 2019, 32, 213–221. [Google Scholar] [CrossRef]
- Farahani, M.; Rezaei-Tavirani, M.; Arjmand, B. A systematic review of microRNA expression studies with exposure to bisphenol A. J. Appl. Toxicol. 2021, 41, 4–19. [Google Scholar] [CrossRef]
- Doherty, L.F.; Bromer, J.G.; Zhou, Y.; Aldad, T.S.; Taylor, H.S. In utero exposure to diethylstilbestrol (DES) or bisphenol-A (BPA) increases EZH2 expression in the mammary gland: An epigenetic mechanism linking endocrine disruptors to breast cancer. Horm. Cancer 2010, 1, 146–155. [Google Scholar] [CrossRef]
- Trapphoff, T.; Heiligentag, M.; El Hajj, N.; Haaf, T.; Eichenlaub-Ritter, U. Chronic exposure to a low concentration of bisphenol A during follicle culture affects the epigenetic status of germinal vesicles and metaphase II oocytes. Fertil. Steril. 2013, 100, 1758–1767.e1751. [Google Scholar] [CrossRef]
- Bhan, A.; Hussain, I.; Ansari, K.I.; Bobzean, S.A.; Perrotti, L.I.; Mandal, S.S. Histone methyltransferase EZH2 is transcriptionally induced by estradiol as well as estrogenic endocrine disruptors bisphenol-A and diethylstilbestrol. J. Mol. Biol. 2014, 426, 3426–3441. [Google Scholar] [CrossRef]
- Hussain, I.; Bhan, A.; Ansari, K.I.; Deb, P.; Bobzean, S.A.; Perrotti, L.I.; Mandal, S.S. Bisphenol-A induces expression of HOXC6, an estrogen-regulated homeobox-containing gene associated with breast cancer. Biochim. Biophys. Acta 2015, 1849, 697–708. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Han, J.; Duan, X.; Xiong, B.; Cui, X.S.; Kim, N.H.; Liu, H.L.; Sun, S.C. The toxic effects and possible mechanisms of Bisphenol A on oocyte maturation of porcine in vitro. Oncotarget 2016, 7, 32554–32565. [Google Scholar] [CrossRef] [PubMed]
- Hong, J.; Chen, F.; Wang, X.; Bai, Y.; Zhou, R.; Li, Y.; Chen, L. Exposure of preimplantation embryos to low-dose bisphenol A impairs testes development and suppresses histone acetylation of StAR promoter to reduce production of testosterone in mice. Mol. Cell. Endocrinol. 2016, 427, 101–111. [Google Scholar] [CrossRef] [PubMed]
- Kurian, J.R.; Louis, S.; Keen, K.L.; Wolfe, A.; Terasawa, E.; Levine, J.E. The Methylcytosine Dioxygenase Ten-Eleven Translocase-2 (tet2) Enables Elevated GnRH Gene Expression and Maintenance of Male Reproductive Function. Endocrinology 2016, 157, 3588–3603. [Google Scholar] [CrossRef] [PubMed]
- Deb, P.; Bhan, A.; Hussain, I.; Ansari, K.I.; Bobzean, S.A.; Pandita, T.K.; Perrotti, L.I.; Mandal, S.S. Endocrine disrupting chemical, bisphenol-A, induces breast cancer associated gene HOXB9 expression in vitro and in vivo. Gene 2016, 590, 234–243. [Google Scholar] [CrossRef]
- Altamirano, G.A.; Ramos, J.G.; Gomez, A.L.; Luque, E.H.; Muñoz-de-Toro, M.; Kass, L. Perinatal exposure to bisphenol A modifies the transcriptional regulation of the β-Casein gene during secretory activation of the rat mammary gland. Mol. Cell. Endocrinol. 2017, 439, 407–418. [Google Scholar] [CrossRef]
- Li, Y.; Duan, F.; Zhou, X.; Pan, H.; Li, R. Differential responses of GC-1 spermatogonia cells to high and low doses of bisphenol A. Mol. Med. Rep. 2018, 18, 3034–3040. [Google Scholar] [CrossRef]
- Shi, M.; Sekulovski, N.; MacLean, J.A., 2nd; Hayashi, K. Prenatal Exposure to Bisphenol A Analogues on Male Reproductive Functions in Mice. Toxicol. Sci. 2018, 163, 620–631. [Google Scholar] [CrossRef]
- Shi, M.; Whorton, A.E.; Sekulovski, N.; MacLean, J.A.; Hayashi, K. Prenatal Exposure to Bisphenol A, E, and S Induces Transgenerational Effects on Male Reproductive Functions in Mice. Toxicol. Sci. 2019, 172, 303–315. [Google Scholar] [CrossRef]
- Xiong, Y.; Wen, X.; Liu, H.; Zhang, M.; Zhang, Y. Bisphenol a affects endometrial stromal cells decidualization, involvement of epigenetic regulation. J. Steroid Biochem. Mol. Biol. 2020, 200, 105640. [Google Scholar] [CrossRef]
- Sowers, M.L.; Tang, H.; Tian, B.; Goldblum, R.; Midoro-Horiuti, T.; Zhang, K. Bisphenol A Activates an Innate Viral Immune Response Pathway. J. Proteome Res. 2020, 19, 644–654. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Liu, Y.; Xue, X.; Yuan, C.; Wang, Z. BPA’s transgenerational disturbance to transcription of ovarian steroidogenic genes in rare minnow Gobiocypris rarus via DNA and histone methylation. Sci. Total Environ. 2021, 762, 143055. [Google Scholar] [CrossRef] [PubMed]
- Almeida, T.F.A.; Oliveira, S.R.; Mayra da Silva, J.; Fernandes de Oliveira, A.L.; de Lourdes Cardeal, Z.; Menezes, H.C.; Gomes, J.M.; Campolina-Silva, G.H.; Oliveira, C.A.; Macari, S.; et al. Effects of high-dose bisphenol A on the mouse oral mucosa: A possible link with oral cancers. Environ. Pollut. 2021, 286, 117296. [Google Scholar] [CrossRef] [PubMed]
- Bi, N.; Gu, X.; Fan, A.; Li, D.; Wang, M.; Zhou, R.; Sun, Q.C.; Wang, H.L. Bisphenol-A exposure leads to neurotoxicity through upregulating the expression of histone deacetylase 2 in vivo and in vitro. Toxicology 2022, 465, 153052. [Google Scholar] [CrossRef] [PubMed]
- Chang, H.; Wang, D.; Xia, W.; Pan, X.; Huo, W.; Xu, S.; Li, Y. Epigenetic disruption and glucose homeostasis changes following low-dose maternal bisphenol A exposure. Toxicol. Res. 2016, 5, 1400–1409. [Google Scholar] [CrossRef]
- Dhimolea, E.; Wadia, P.R.; Murray, T.J.; Settles, M.L.; Treitman, J.D.; Sonnenschein, C.; Shioda, T.; Soto, A.M. Prenatal exposure to BPA alters the epigenome of the rat mammary gland and increases the propensity to neoplastic development. PLoS ONE 2014, 9, e99800. [Google Scholar] [CrossRef]
- Chen, Z.; Zuo, X.; He, D.; Ding, S.; Xu, F.; Yang, H.; Jin, X.; Fan, Y.; Ying, L.; Tian, C.; et al. Long-term exposure to a ‘safe’ dose of bisphenol A reduced protein acetylation in adult rat testes. Sci. Rep. 2017, 7, 40337. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, L.; Zhu, L.; Ran, B.; Wang, Z. Bisphenol A disturbs transcription of steroidogenic genes in ovary of rare minnow Gobiocypris rarus via the abnormal DNA and histone methylation. Chemosphere 2020, 240, 124935. [Google Scholar] [CrossRef]
- Torres, T.; Ruivo, R.; Santos, M.M. Epigenetic biomarkers as tools for chemical hazard assessment: Gene expression profiling using the model Danio rerio. Sci. Total Environ. 2021, 773, 144830. [Google Scholar] [CrossRef]
- Cho, H.; Kim, S.J.; Park, H.-W.; Oh, M.-J.; Yu, S.Y.; Lee, S.Y.; Park, C.; Han, J.; Oh, J.-H.; Hwang, S.Y.; et al. A relationship between miRNA and gene expression in the mouse Sertoli cell line after exposure to bisphenol A. BioChip J. 2010, 4, 75–81. [Google Scholar] [CrossRef]
- Tilghman, S.L.; Bratton, M.R.; Segar, H.C.; Martin, E.C.; Rhodes, L.V.; Li, M.; McLachlan, J.A.; Wiese, T.E.; Nephew, K.P.; Burow, M.E. Endocrine disruptor regulation of microRNA expression in breast carcinoma cells. PLoS ONE 2012, 7, e32754. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Xu, B.; Han, X.; Mao, Z.; Talbot, P.; Chen, M.; Du, G.; Chen, A.; Liu, J.; Wang, X.; et al. Effect of bisphenol A on pluripotency of mouse embryonic stem cells and differentiation capacity in mouse embryoid bodies. Toxicol. Vitro 2013, 27, 2249–2255. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Kappil, M.A.; Li, A.; Dassanayake, P.S.; Darrah, T.H.; Friedman, A.E.; Friedman, M.; Lambertini, L.; Landrigan, P.; Stodgell, C.J.; et al. Exploring the associations between microRNA expression profiles and environmental pollutants in human placenta from the National Children’s Study (NCS). Epigenetics 2015, 10, 793–802. [Google Scholar] [CrossRef] [PubMed]
- De Felice, B.; Manfellotto, F.; Palumbo, A.; Troisi, J.; Zullo, F.; Di Carlo, C.; Di Spiezio Sardo, A.; De Stefano, N.; Ferbo, U.; Guida, M.; et al. Genome-wide microRNA expression profiling in placentas from pregnant women exposed to BPA. BMC Med. Genom. 2015, 8, 56. [Google Scholar] [CrossRef]
- Chou, W.C.; Lee, P.H.; Tan, Y.Y.; Lin, H.C.; Yang, C.W.; Chen, K.H.; Chuang, C.Y. An integrative transcriptomic analysis reveals bisphenol A exposure-induced dysregulation of microRNA expression in human endometrial cells. Toxicol. Vitro 2017, 41, 133–142. [Google Scholar] [CrossRef]
- Verbanck, M.; Canouil, M.; Leloire, A.; Dhennin, V.; Coumoul, X.; Yengo, L.; Froguel, P.; Poulain-Godefroy, O. Low-dose exposure to bisphenols A, F and S of human primary adipocyte impacts coding and non-coding RNA profiles. PLoS ONE 2017, 12, e0179583. [Google Scholar] [CrossRef]
- Gao, G.Z.; Zhao, Y.; Li, H.X.; Li, W. Bisphenol A-elicited miR-146a-5p impairs murine testicular steroidogenesis through negative regulation of Mta3 signaling. Biochem. Biophys. Res. Commun. 2018, 501, 478–485. [Google Scholar] [CrossRef]
- Reed, B.G.; Babayev, S.N.; Chen, L.X.; Carr, B.R.; Word, R.A.; Jimenez, P.T. Estrogen-regulated miRNA-27b is altered by bisphenol A in human endometrial stromal cells. Reproduction 2018, 156, 559–567. [Google Scholar] [CrossRef]
- Martínez-Ibarra, A.; Martínez-Razo, L.D.; Vázquez-Martínez, E.R.; Martínez-Cruz, N.; Flores-Ramírez, R.; García-Gómez, E.; López-López, M.; Ortega-González, C.; Camacho-Arroyo, I.; Cerbón, M. Unhealthy Levels of Phthalates and Bisphenol A in Mexican Pregnant Women with Gestational Diabetes and Its Association to Altered Expression of miRNAs Involved with Metabolic Disease. Int. J. Mol. Sci. 2019, 20, 3343. [Google Scholar] [CrossRef]
- Kim, J.H.; Cho, Y.H.; Hong, Y.C. MicroRNA expression in response to bisphenol A is associated with high blood pressure. Environ. Int. 2020, 141, 105791. [Google Scholar] [CrossRef]
- Deng, P.; Tan, M.; Zhou, W.; Chen, C.; Xi, Y.; Gao, P.; Ma, Q.; Liang, Y.; Chen, M.; Tian, L.; et al. Bisphenol A promotes breast cancer cell proliferation by driving miR-381-3p-PTTG1-dependent cell cycle progression. Chemosphere 2021, 268, 129221. [Google Scholar] [CrossRef] [PubMed]
- Kaur, S.; Kinkade, J.A.; Green, M.T.; Martin, R.E.; Willemse, T.E.; Bivens, N.J.; Schenk, A.K.; Helferich, W.G.; Trainor, B.C.; Fass, J.; et al. Disruption of global hypothalamic microRNA (miR) profiles and associated behavioral changes in California mice (Peromyscus californicus) developmentally exposed to endocrine disrupting chemicals. Horm. Behav. 2021, 128, 104890. [Google Scholar] [CrossRef] [PubMed]
- Palak, E.; Lebiedzińska, W.; Anisimowicz, S.; Sztachelska, M.; Pierzyński, P.; Wiczkowski, W.; Żelazowska-Rutkowska, B.; Niklińska, G.N.; Ponikwicka-Tyszko, D.; Wołczyński, S. The Association between Bisphenol A, Steroid Hormones, and Selected MicroRNAs Levels in Seminal Plasma of Men with Infertility. J. Clin. Med. 2021, 10, 5945. [Google Scholar] [CrossRef] [PubMed]
- Luo, M.; Li, L.; Ding, M.; Niu, Y.; Xu, X.; Shi, X.; Shan, N.; Qiu, Z.; Piao, F.; Zhang, C. Long-term potentiation and depression regulatory microRNAs were highlighted in Bisphenol A induced learning and memory impairment by microRNA sequencing and bioinformatics analysis. PLoS ONE 2023, 18, e0279029. [Google Scholar] [CrossRef]
- Pang, W.; Lian, F.Z.; Leng, X.; Wang, S.M.; Li, Y.B.; Wang, Z.Y.; Li, K.R.; Gao, Z.X.; Jiang, Y.G. Microarray expression profiling and co-expression network analysis of circulating LncRNAs and mRNAs associated with neurotoxicity induced by BPA. Environ. Sci. Pollut. Res. Int. 2018, 25, 15006–15018. [Google Scholar] [CrossRef]
- Santoro, A.; Scafuro, M.; Troisi, J.; Piegari, G.; Di Pietro, P.; Mele, E.; Cappetta, D.; Marino, M.; De Angelis, A.; Vecchione, C.; et al. Multi-Systemic Alterations by Chronic Exposure to a Low Dose of Bisphenol A in Drinking Water: Effects on Inflammation and NAD(+)-Dependent Deacetylase Sirtuin1 in Lactating and Weaned Rats. Int. J. Mol. Sci. 2021, 22, 9666. [Google Scholar] [CrossRef]
- Flavahan, W.A.; Gaskell, E.; Bernstein, B.E. Epigenetic plasticity and the hallmarks of cancer. Science 2017, 357, eaal2380. [Google Scholar] [CrossRef]
- Caliri, A.W.; Caceres, A.; Tommasi, S.; Besaratinia, A. Hypomethylation of LINE-1 repeat elements and global loss of DNA hydroxymethylation in vapers and smokers. Epigenetics 2020, 15, 816–829. [Google Scholar] [CrossRef]
- Tommasi, S.; Yoon, J.I.; Besaratinia, A. Secondhand Smoke Induces Liver Steatosis through Deregulation of Genes Involved in Hepatic Lipid Metabolism. Int. J. Mol. Sci. 2020, 21, 1296. [Google Scholar] [CrossRef]
- Tommasi, S.; Pabustan, N.; Li, M.; Chen, Y.; Siegmund, K.D.; Besaratinia, A. A novel role for vaping in mitochondrial gene dysregulation and inflammation fundamental to disease development. Sci. Rep. 2021, 11, 22773. [Google Scholar] [CrossRef]
- Goodman, S.; Chappell, G.; Guyton, K.Z.; Pogribny, I.P.; Rusyn, I. Epigenetic alterations induced by genotoxic occupational and environmental human chemical carcinogens: An update of a systematic literature review. Mutat. Res. Rev. Mutat. Res. 2022, 789, 108408. [Google Scholar] [CrossRef] [PubMed]
- Goodrich, J.M.; Dolinoy, D.C.; Sánchez, B.N.; Zhang, Z.; Meeker, J.D.; Mercado-Garcia, A.; Solano-González, M.; Hu, H.; Téllez-Rojo, M.M.; Peterson, K.E. Adolescent epigenetic profiles and environmental exposures from early life through peri-adolescence. Environ. Epigenet 2016, 2, dvw018. [Google Scholar] [CrossRef] [PubMed]
- Alavian-Ghavanini, A.; Lin, P.I.; Lind, P.M.; Risén Rimfors, S.; Halin Lejonklou, M.; Dunder, L.; Tang, M.; Lindh, C.; Bornehag, C.G.; Rüegg, J. Prenatal Bisphenol A Exposure is Linked to Epigenetic Changes in Glutamate Receptor Subunit Gene Grin2b in Female Rats and Humans. Sci. Rep. 2018, 8, 11315. [Google Scholar] [CrossRef] [PubMed]
- Montrose, L.; Padmanabhan, V.; Goodrich, J.M.; Domino, S.E.; Treadwell, M.C.; Meeker, J.D.; Watkins, D.J.; Dolinoy, D.C. Maternal levels of endocrine disrupting chemicals in the first trimester of pregnancy are associated with infant cord blood DNA methylation. Epigenetics 2018, 13, 301–309. [Google Scholar] [CrossRef]
- Song, X.; Zhou, X.; Yang, F.; Liang, H.; Wang, Z.; Li, R.; Miao, M.; Yuan, W. Association between prenatal bisphenol a exposure and promoter hypermethylation of CAPS2, TNFRSF25, and HKR1 genes in cord blood. Environ. Res. 2020, 190, 109996. [Google Scholar] [CrossRef]
- Choi, Y.J.; Lee, Y.A.; Hong, Y.C.; Cho, J.; Lee, K.S.; Shin, C.H.; Kim, B.N.; Kim, J.I.; Park, S.J.; Bisgaard, H.; et al. Effect of prenatal bisphenol A exposure on early childhood body mass index through epigenetic influence on the insulin-like growth factor 2 receptor (IGF2R) gene. Environ. Int. 2020, 143, 105929. [Google Scholar] [CrossRef]
- Besaratinia, A.; Bates, S.E.; Pfeifer, G.P. Mutational signature of the proximate bladder carcinogen N-hydroxy-4-acetylaminobiphenyl: Inconsistency with the p53 mutational spectrum in bladder cancer. Cancer Res. 2002, 62, 4331–4338. [Google Scholar]
- Besaratinia, A.; Pfeifer, G.P. Enhancement of the mutagenicity of benzo(a)pyrene diol epoxide by a nonmutagenic dose of ultraviolet A radiation. Cancer Res. 2003, 63, 8708–8716. [Google Scholar]
- Lambert, I.B.; Singer, T.M.; Boucher, S.E.; Douglas, G.R. Detailed review of transgenic rodent mutation assays. Mutat. Res. 2005, 590, 1–280. [Google Scholar] [CrossRef]
- Besaratinia, A.; Pfeifer, G.P. Applications of the human p53 knock-in (Hupki) mouse model for human carcinogen testing. FASEB J. 2010, 24, 2612–2619. [Google Scholar] [CrossRef]
- Besaratinia, A.; Li, H.; Yoon, J.I.; Zheng, A.; Gao, H.; Tommasi, S. A high-throughput next-generation sequencing-based method for detecting the mutational fingerprint of carcinogens. Nucleic Acids Res. 2012, 40, e116. [Google Scholar] [CrossRef] [PubMed]
- Besaratinia, A.; Tommasi, S. An opportune and unique research to evaluate the public health impact of electronic cigarettes. Cancer Causes Control. 2017, 28, 1167–1171. [Google Scholar] [CrossRef] [PubMed]
- Caliri, A.W.; Tommasi, S.; Bates, S.E.; Besaratinia, A. Spontaneous and photosensitization-induced mutations in primary mouse cells transitioning through senescence and immortalization. J. Biol. Chem. 2020, 295, 9974–9985. [Google Scholar] [CrossRef] [PubMed]
- Besaratinia, A.; Caliri, A.W.; Tommasi, S. Hydroxychloroquine induces oxidative DNA damage and mutation in mammalian cells. DNA Repair. 2021, 106, 103180. [Google Scholar] [CrossRef] [PubMed]
- Vandenberg, L.N.; Hunt, P.A.; Gore, A.C. Endocrine disruptors and the future of toxicology testing—Lessons from CLARITY-BPA. Nat. Rev. Endocrinol. 2019, 15, 366–374. [Google Scholar] [CrossRef]
- NIEHS. Toxicant Exposures and Responses by Genomic and Epigenomic Regulators of Transcription (TaRGET) Program. Available online: https://www.niehs.nih.gov/research/supported/health/envepi/target/index.cfm (accessed on 30 March 2023).

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Besaratinia, A. The State of Research and Weight of Evidence on the Epigenetic Effects of Bisphenol A. Int. J. Mol. Sci. 2023, 24, 7951. https://doi.org/10.3390/ijms24097951
Besaratinia A. The State of Research and Weight of Evidence on the Epigenetic Effects of Bisphenol A. International Journal of Molecular Sciences. 2023; 24(9):7951. https://doi.org/10.3390/ijms24097951
Chicago/Turabian StyleBesaratinia, Ahmad. 2023. "The State of Research and Weight of Evidence on the Epigenetic Effects of Bisphenol A" International Journal of Molecular Sciences 24, no. 9: 7951. https://doi.org/10.3390/ijms24097951
APA StyleBesaratinia, A. (2023). The State of Research and Weight of Evidence on the Epigenetic Effects of Bisphenol A. International Journal of Molecular Sciences, 24(9), 7951. https://doi.org/10.3390/ijms24097951





