Piperlongumine Induces Cellular Apoptosis and Autophagy via the ROS/Akt Signaling Pathway in Human Follicular Thyroid Cancer Cells
Abstract
:1. Introduction
2. Results
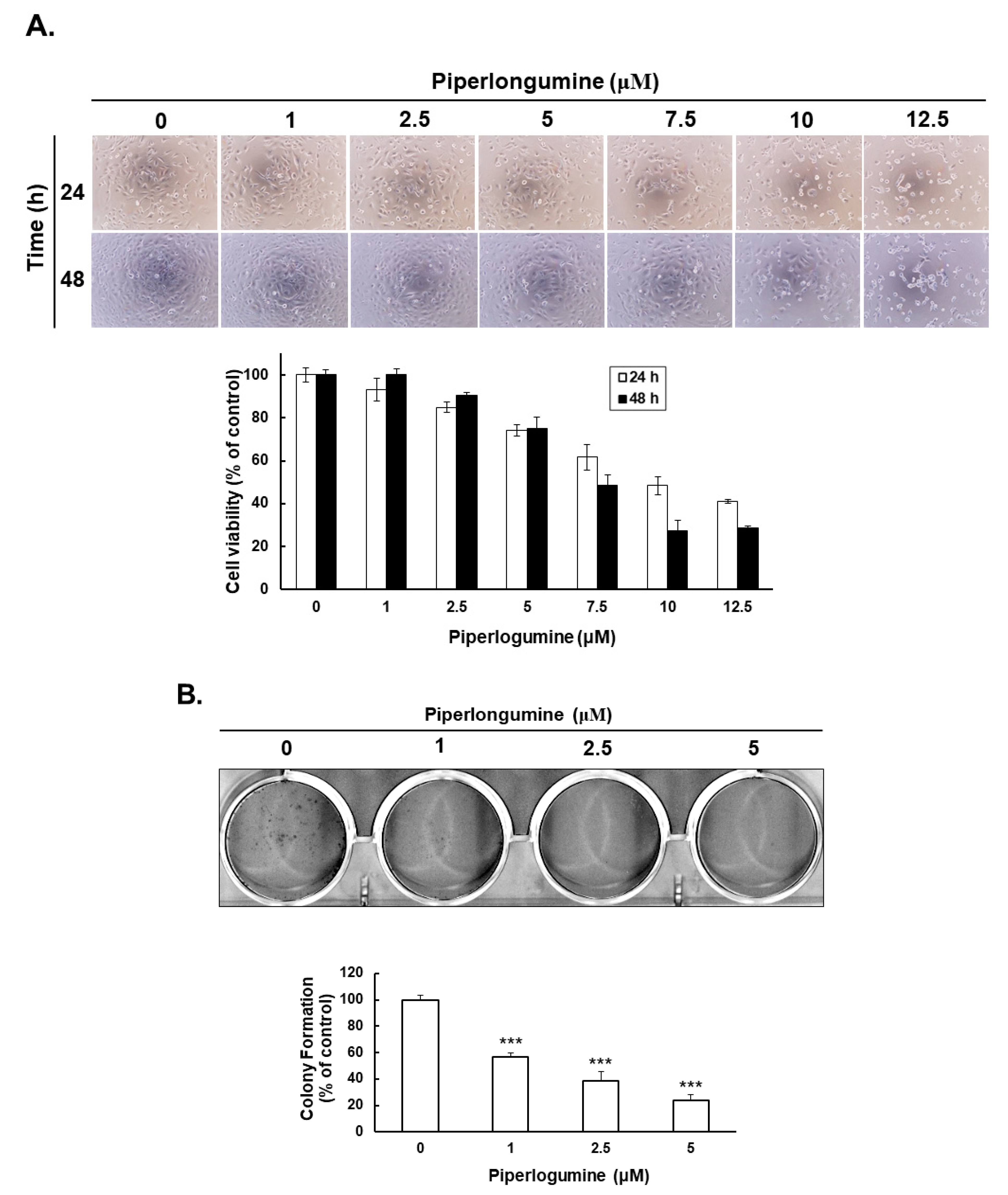
2.1. Piperlongumine Inhibits Cancer Cell Proliferation in Human FTC Cells
2.2. Piperlongumine Induces Cellular Autophagy, Autophagosome Formation and Autophagic Flux in Human FTC Cells
2.3. Piperlongumine Increases ROS, Which Contributes to Anti-Human FTC Activity
2.4. Piperlongumine-Induced ROS Contributes to Autophagy Induction via the Akt Signaling Pathway in Human FTC Cells
3. Discussion
4. Materials and Methods
4.1. Cell Line and Cell Culture
4.2. Cell Viability Examination
4.3. Colony Formation Assay
4.4. Cell Cycle Analysis
4.5. Cellular Apoptosis Determination
4.6. Western Blotting
4.7. Cellular ROS Determination
4.8. Cellular Autophagosome Determination
4.9. Plasmid Transfection
4.10. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hodgson, N.C.; Button, J.; Solorzano, C.C. Thyroid cancer: Is the incidence still increasing? Ann. Surg. Oncol. 2004, 11, 1093–1097. [Google Scholar] [CrossRef] [PubMed]
- Kung, F.P.; Lim, Y.P.; Chao, W.Y.; Zhang, Y.S.; Yu, H.I.; Tai, T.S.; Lu, C.H.; Chen, S.H.; Li, Y.Z.; Zhao, P.W.; et al. Piperlongumine, a Potent Anticancer Phytotherapeutic, Induces Cell Cycle Arrest and Apoptosis In Vitro and In Vivo through the ROS/Akt Pathway in Human Thyroid Cancer Cells. Cancers 2021, 13, 4266. [Google Scholar] [CrossRef] [PubMed]
- Hua, S.C.; Chang, T.C.; Chen, H.R.; Lu, C.H.; Liu, Y.W.; Chen, S.H.; Yu, H.I.; Chang, Y.P.; Lee, Y.R. Reversine, a 2,6-disubstituted purine, as an anti-cancer agent in differentiated and undifferentiated thyroid cancer cells. Pharm. Res. 2012, 29, 1990–2005. [Google Scholar] [CrossRef]
- Haugen, B.R.; Alexander, E.K.; Bible, K.C.; Doherty, G.M.; Mandel, S.J.; Nikiforov, Y.E.; Pacini, F.; Randolph, G.W.; Sawka, A.M.; Schlumberger, M.; et al. 2015 American Thyroid Association Management Guidelines for Adult Patients with Thyroid Nodules and Differentiated Thyroid Cancer: The American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid 2016, 26, 1–133. [Google Scholar] [CrossRef]
- Parameswaran, R.; Shulin Hu, J.; Min En, N.; Tan, W.B.; Yuan, N.K. Patterns of metastasis in follicular thyroid carcinoma and the difference between early and delayed presentation. Ann. R. Coll. Surg. Engl. 2017, 99, 151–154. [Google Scholar] [CrossRef] [PubMed]
- Shaha, A.R.; Shah, J.P.; Loree, T.R. Differentiated thyroid cancer presenting initially with distant metastasis. Am. J. Surg. 1997, 174, 474–476. [Google Scholar] [CrossRef] [PubMed]
- Bezerra, D.P.; Pessoa, C.; de Moraes, M.O.; Saker-Neto, N.; Silveira, E.R.; Costa-Lotufo, L.V. Overview of the therapeutic potential of piplartine (piperlongumine). Eur. J. Pharm. Sci. 2013, 48, 453–463. [Google Scholar] [CrossRef]
- Liu, J.; Liu, W.; Lu, Y.; Tian, H.; Duan, C.; Lu, L.; Gao, G.; Wu, X.; Wang, X.; Yang, H. Piperlongumine restores the balance of autophagy and apoptosis by increasing BCL2 phosphorylation in rotenone-induced Parkinson disease models. Autophagy 2018, 14, 845–861. [Google Scholar] [CrossRef]
- Han, S.S.; Son, D.J.; Yun, H.; Kamberos, N.L.; Janz, S. Piperlongumine inhibits proliferation and survival of Burkitt lymphoma in vitro. Leuk. Res. 2013, 37, 146–154. [Google Scholar] [CrossRef]
- Song, X.; Gao, T.; Lei, Q.; Zhang, L.; Yao, Y.; Xiong, J. Piperlongumine Induces Apoptosis in Human Melanoma Cells Via Reactive Oxygen Species Mediated Mitochondria Disruption. Nutr. Cancer 2018, 70, 502–511. [Google Scholar] [CrossRef]
- Liu, J.M.; Pan, F.; Li, L.; Liu, Q.R.; Chen, Y.; Xiong, X.X.; Cheng, K.; Yu, S.B.; Shi, Z.; Yu, A.C.; et al. Piperlongumine selectively kills glioblastoma multiforme cells via reactive oxygen species accumulation dependent JNK and p38 activation. Biochem. Biophys. Res. Commun. 2013, 437, 87–93. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.Y.; Liu, G.H.; Chao, W.Y.; Shi, C.S.; Lin, C.Y.; Lim, Y.P.; Lu, C.H.; Lai, P.Y.; Chen, H.R.; Lee, Y.R. Piperlongumine Suppresses Proliferation of Human Oral Squamous Cell Carcinoma through Cell Cycle Arrest, Apoptosis and Senescence. Int. J. Mol. Sci. 2016, 17, 616. [Google Scholar] [CrossRef] [PubMed]
- Roh, J.L.; Kim, E.H.; Park, J.Y.; Kim, J.W.; Kwon, M.; Lee, B.H. Piperlongumine selectively kills cancer cells and increases cisplatin antitumor activity in head and neck cancer. Oncotarget 2014, 5, 9227–9238. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; Son, D.J.; Gu, S.M.; Woo, J.R.; Ham, Y.W.; Lee, H.P.; Kim, W.J.; Jung, J.K.; Hong, J.T. Piperlongumine inhibits lung tumor growth via inhibition of nuclear factor kappa B signaling pathway. Sci. Rep. 2016, 6, 26357. [Google Scholar] [CrossRef]
- Lee, H.N.; Jin, H.O.; Park, J.A.; Kim, J.H.; Kim, J.Y.; Kim, B.; Kim, W.; Hong, S.E.; Lee, Y.H.; Chang, Y.H.; et al. Heme oxygenase-1 determines the differential response of breast cancer and normal cells to piperlongumine. Mol. Cells 2015, 38, 327–335. [Google Scholar] [CrossRef]
- Chen, Y.; Liu, J.M.; Xiong, X.X.; Qiu, X.Y.; Pan, F.; Liu, D.; Lan, S.J.; Jin, S.; Yu, S.B.; Chen, X.Q. Piperlongumine selectively kills hepatocellular carcinoma cells and preferentially inhibits their invasion via ROS-ER-MAPKs-CHOP. Oncotarget 2015, 6, 6406–6421. [Google Scholar] [CrossRef]
- Thongsom, S.; Suginta, W.; Lee, K.J.; Choe, H.; Talabnin, C. Piperlongumine induces G2/M phase arrest and apoptosis in cholangiocarcinoma cells through the ROS-JNK-ERK signaling pathway. Apoptosis 2017, 22, 1473–1484. [Google Scholar] [CrossRef]
- Golovine, K.; Makhov, P.; Naito, S.; Raiyani, H.; Tomaszewski, J.; Mehrazin, R.; Tulin, A.; Kutikov, A.; Uzzo, R.G.; Kolenko, V.M. Piperlongumine and its analogs down-regulate expression of c-Met in renal cell carcinoma. Cancer Biol. Ther. 2015, 16, 743–749. [Google Scholar] [CrossRef]
- Mohammad, J.; Singh, R.R.; Riggle, C.; Haugrud, B.; Abdalla, M.Y.; Reindl, K.M. JNK inhibition blocks piperlongumine-induced cell death and transcriptional activation of heme oxygenase-1 in pancreatic cancer cells. Apoptosis 2019, 24, 730–744. [Google Scholar] [CrossRef]
- Duan, C.; Zhang, B.; Deng, C.; Cao, Y.; Zhou, F.; Wu, L.; Chen, M.; Shen, S.; Xu, G.; Zhang, S.; et al. Piperlongumine induces gastric cancer cell apoptosis and G2/M cell cycle arrest both in vitro and in vivo. Tumor Biol. 2016, 37, 10793–10804. [Google Scholar] [CrossRef]
- Kumar, S.; Agnihotri, N. Piperlongumine, a piper alkaloid targets Ras/PI3K/Akt/mTOR signaling axis to inhibit tumor cell growth and proliferation in DMH/DSS induced experimental colon cancer. Biomed. Pharmacother. 2019, 109, 1462–1477. [Google Scholar] [CrossRef]
- Liu, D.; Qiu, X.Y.; Wu, X.; Hu, D.X.; Li, C.Y.; Yu, S.B.; Pan, F.; Chen, X.Q. Piperlongumine suppresses bladder cancer invasion via inhibiting epithelial mesenchymal transition and F-actin reorganization. Biochem. Biophys. Res. Commun. 2017, 494, 165–172. [Google Scholar] [CrossRef]
- Oblad, R.; Doughty, H.; Lawson, J.; Christensen, M.; Kenealey, J. Application of Mixture Design Response Surface Methodology for Combination Chemotherapy in PC-3 Human Prostate Cancer Cells. Mol. Pharmacol. 2018, 94, 907–916. [Google Scholar] [CrossRef] [PubMed]
- Nan, X.W.; Gong, L.H.; Chen, X.; Zhou, H.H.; Ye, P.P.; Yang, Y.; Xing, Z.H.; Wei, M.N.; Li, Y.; Wang, S.T.; et al. Survivin Promotes Piperlongumine Resistance in Ovarian Cancer. Front. Oncol. 2019, 9, 1345. [Google Scholar] [CrossRef] [PubMed]
- Seber, S.; Sirin, D.Y.; Yetisyigit, T.; Bilgen, T. Piperlongumine increases the apoptotic effect of doxorubicin and paclitaxel in a cervical cancer cell line. Niger. J. Clin. Pract. 2020, 23, 386–391. [Google Scholar] [PubMed]
- Chen, S.Y.; Huang, H.Y.; Lin, H.P.; Fang, C.Y. Piperlongumine induces autophagy in biliary cancer cells via reactive oxygen species-activated Erk signaling pathway. Int. J. Mol. Med. 2019, 44, 1687–1696. [Google Scholar] [CrossRef]
- Zhu, P.; Qian, J.; Xu, Z.; Meng, C.; Liu, J.; Shan, W.; Zhu, W.; Wang, Y.; Yang, Y.; Zhang, W.; et al. Piperlonguminine and Piperine Analogues as TrxR Inhibitors that Promote ROS and Autophagy and Regulate p38 and Akt/mTOR Signaling. J. Nat. Prod. 2020, 83, 3041–3049. [Google Scholar] [CrossRef]
- Kma, L.; Baruah, T.J. The interplay of ROS and the PI3K/Akt pathway in autophagy regulation. Biotechnol. Appl. Biochem. 2022, 69, 248–264. [Google Scholar] [CrossRef]
- Basak, D.; Punganuru, S.R.; Srivenugopal, K.S. Piperlongumine exerts cytotoxic effects against cancer cells with mutant p53 proteins at least in part by restoring the biological functions of the tumor suppressor. Int. J. Oncol. 2016, 48, 1426–1436. [Google Scholar] [CrossRef]
- Reeb, A.N.; Ziegler, A.; Lin, R.Y. Characterization of human follicular thyroid cancer cell lines in preclinical mouse models. Endocr. Connect. 2016, 5, 47–54. [Google Scholar] [CrossRef]
- Landa, I.; Pozdeyev, N.; Korch, C.; Marlow, L.A.; Smallridge, R.C.; Copland, J.A.; Henderson, Y.C.; Lai, S.Y.; Clayman, G.L.; Onoda, N.; et al. Comprehensive Genetic Characterization of Human Thyroid Cancer Cell Lines: A Validated Panel for Preclinical Studies. Clin. Cancer Res. 2019, 25, 3141–3151. [Google Scholar] [CrossRef] [PubMed]
- Pan, X.; Chen, G.; Hu, W. Piperlongumine increases the sensitivity of bladder cancer to cisplatin by mitochondrial ROS. J. Clin. Lab. Anal. 2022, 36, e24452. [Google Scholar] [CrossRef] [PubMed]
- Mohler, H.; Pfirrmann, R.W.; Frei, K. Redox-directed cancer therapeutics: Taurolidine and Piperlongumine as broadly effective antineoplastic agents (review). Int. J. Oncol. 2014, 45, 1329–1336. [Google Scholar] [CrossRef] [PubMed]
- Gorrini, C.; Harris, I.S.; Mak, T.W. Modulation of oxidative stress as an anticancer strategy. Nat. Rev. Drug Discov. 2013, 12, 931–947. [Google Scholar] [CrossRef]
- Wondrak, G.T. Redox-directed cancer therapeutics: Molecular mechanisms and opportunities. Antioxid. Redox Signal. 2009, 11, 3013–3069. [Google Scholar] [CrossRef]
- Wang, H.; Jiang, H.; Corbet, C.; de Mey, S.; Law, K.; Gevaert, T.; Feron, O.; De Ridder, M. Piperlongumine increases sensitivity of colorectal cancer cells to radiation: Involvement of ROS production via dual inhibition of glutathione and thioredoxin systems. Cancer Lett. 2019, 450, 42–52. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, X.; Vikash, V.; Ye, Q.; Wu, D.; Liu, Y.; Dong, W. ROS and ROS-Mediated Cellular Signaling. Oxid. Med. Cell. Longev. 2016, 2016, 4350965. [Google Scholar] [CrossRef]
- Liu, P.F.; Farooqi, A.A.; Peng, S.Y.; Yu, T.J.; Dahms, H.U.; Lee, C.H.; Tang, J.Y.; Wang, S.C.; Shu, C.W.; Chang, H.W. Regulatory effects of noncoding RNAs on the interplay of oxidative stress and autophagy in cancer malignancy and therapy. Semin. Cancer Biol. 2022, 83, 269–282. [Google Scholar] [CrossRef]
- Panigrahi, D.P.; Praharaj, P.P.; Bhol, C.S.; Mahapatra, K.K.; Patra, S.; Behera, B.P.; Mishra, S.R.; Bhutia, S.K. The emerging, multifaceted role of mitophagy in cancer and cancer therapeutics. Semin. Cancer Biol. 2020, 66, 45–58. [Google Scholar] [CrossRef]
- Chourasia, A.H.; Tracy, K.; Frankenberger, C.; Boland, M.L.; Sharifi, M.N.; Drake, L.E.; Sachleben, J.R.; Asara, J.M.; Locasale, J.W.; Karczmar, G.S.; et al. Mitophagy defects arising from BNip3 loss promote mammary tumor progression to metastasis. EMBO Rep. 2015, 16, 1145–1163. [Google Scholar] [CrossRef]
- Klionsky, D.J.; Abdel-Aziz, A.K.; Abdelfatah, S.; Abdellatif, M.; Abdoli, A.; Abel, S.; Abeliovich, H.; Abildgaard, M.H.; Abudu, Y.P.; Acevedo-Arozena, A.; et al. Guidelines for the use and interpretation of assays for monitoring autophagy (4th edition). Autophagy 2021, 17, 1–382. [Google Scholar] [PubMed]
- Yun, C.W.; Jeon, J.; Go, G.; Lee, J.H.; Lee, S.H. The Dual Role of Autophagy in Cancer Development and a Therapeutic Strategy for Cancer by Targeting Autophagy. Int. J. Mol. Sci. 2020, 22, 179. [Google Scholar] [CrossRef] [PubMed]
- Zheng, L.; Fang, S.; Chen, A.; Chen, W.; Qiao, E.; Chen, M.; Shu, G.; Zhang, D.; Kong, C.; Weng, Q.; et al. Piperlongumine synergistically enhances the antitumour activity of sorafenib by mediating ROS-AMPK activation and targeting CPSF7 in liver cancer. Pharmacol. Res. 2022, 177, 106140. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Shi, L.; Zhang, T.; Hong, L.; He, W.; Cao, P.; Shen, X.; Zheng, P.; Xia, Y.; Zou, P. Piperlongumine potentiates the antitumor efficacy of oxaliplatin through ROS induction in gastric cancer cells. Cell. Oncol. 2019, 42, 847–860. [Google Scholar] [CrossRef]
- Zhang, C.; He, L.J.; Zhu, Y.B.; Fan, Q.Z.; Miao, D.D.; Zhang, S.P.; Zhao, W.Y.; Liu, X.P. Piperlongumine Inhibits Akt Phosphorylation to Reverse Resistance to Cisplatin in Human Non-Small Cell Lung Cancer Cells via ROS Regulation. Front. Pharmacol. 2019, 10, 1178. [Google Scholar] [CrossRef]
- Xiong, X.; Lu, B.; Tian, Q.; Zhang, H.; Wu, M.; Guo, H.; Zhang, Q.; Li, X.; Zhou, T.; Wang, Y. Inhibition of autophagy enhances cinobufagininduced apoptosis in gastric cancer. Oncol. Rep. 2019, 41, 492–500. [Google Scholar]
- Chen, K.; Shi, W. Autophagy regulates resistance of non-small cell lung cancer cells to paclitaxel. Tumor Biol. 2016, 37, 10539–10544. [Google Scholar] [CrossRef]
- Sadani, G.R.; Nadkarni, G.D. Changes in lipid peroxide levels and the activity of reactive oxygen scavenging systems in thyroid tissue after exposure to radioactive iodine in rats. Thyroid 1997, 7, 937–941. [Google Scholar] [CrossRef]
- Vrndic, O.B.; Radivojevic, S.D.; Jovanovic, M.D.; Djukic, S.M.; Teodorovic, L.C.; Simonovic, S.T. Oxidative stress in patients with differentiated thyroid cancer: Early effects of radioiodine therapy. Indian J. Biochem. Biophys. 2014, 51, 223–229. [Google Scholar]
- Stepniak, J.; Krawczyk-Lipiec, J.; Lewinski, A.; Karbownik-Lewinska, M. Sorafenib versus Lenvatinib Causes Stronger Oxidative Damage to Membrane Lipids in Noncancerous Tissues of the Thyroid, Liver, and Kidney: Effective Protection by Melatonin and Indole-3-Propionic Acid. Biomedicines 2022, 10, 2890. [Google Scholar] [CrossRef]
- Wu, S.Y.; Chen, Y.L.; Lee, Y.R.; Lin, C.F.; Lan, S.H.; Lan, K.Y.; Chu, M.L.; Lin, P.W.; Yang, Z.L.; Chen, Y.H.; et al. The Autophagosomes Containing Dengue Virus Proteins and Full-Length Genomic RNA Are Infectious. Viruses 2021, 13, 2034. [Google Scholar] [CrossRef]
- Wan, S.W.; Lee, Y.R.; Ho, T.S.; Chang, C.P. Regulation of innate immune signaling pathways by autophagy in dengue virus infection. IUBMB Life 2022, 74, 170–179. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.R.; Wu, S.Y.; Chen, R.Y.; Lin, Y.S.; Yeh, T.M.; Liu, H.S. Regulation of autophagy, glucose uptake, and glycolysis under dengue virus infection. Kaohsiung J. Med. Sci. 2020, 36, 911–919. [Google Scholar] [CrossRef] [PubMed]
- Solvik, T.A.; Nguyen, T.A.; Lin, Y.H.T.; Marsh, T.; Huang, E.J.; Wiita, A.P.; Debnath, J.; Leidal, A.M. Secretory autophagy maintains proteostasis upon lysosome inhibition. J. Cell Biol. 2022, 221, e202110151. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.Y.; Wu, H.T.; Wang, Y.C.; Chang, C.J.; Shan, Y.S.; Wu, S.R.; Chiu, Y.C.; Hsu, C.L.; Juan, H.F.; Lan, K.Y.; et al. Secretory autophagy promotes RAB37-mediated insulin secretion under glucose stimulation both in vitro and in vivo. Autophagy 2022, 19, 1239–1257. [Google Scholar] [CrossRef]
- New, J.; Arnold, L.; Ananth, M.; Alvi, S.; Thornton, M.; Werner, L.; Tawfik, O.; Dai, H.; Shnayder, Y.; Kakarala, K.; et al. Secretory Autophagy in Cancer-Associated Fibroblasts Promotes Head and Neck Cancer Progression and Offers a Novel Therapeutic Target. Cancer Res. 2017, 77, 6679–6691. [Google Scholar] [CrossRef]
- Mahapatra, K.K.; Patra, S.; Mishra, S.R.; Behera, B.P.; Patil, S.; Bhutia, S.K. Autophagy for secretory protein: Therapeutic targets in cancer. Adv. Protein Chem. Struct. Biol. 2023, 133, 159–180. [Google Scholar]
- Gonzalez, C.D.; Alvarez, S.; Ropolo, A.; Rosenzvit, C.; Bagnes, M.F.; Vaccaro, M.I. Autophagy, Warburg, and Warburg reverse effects in human cancer. Biomed Res. Int. 2014, 2014, 926729. [Google Scholar] [CrossRef]
- Lu, C.H.; Liu, Y.W.; Hua, S.C.; Yu, H.I.; Chang, Y.P.; Lee, Y.R. Autophagy induction of reversine on human follicular thyroid cancer cells. Biomed. Pharmacother. 2012, 66, 642–647. [Google Scholar] [CrossRef]
- Chang, J.M.; Wu, J.Y.; Chen, S.H.; Chao, W.Y.; Chuang, H.H.; Kam, K.H.; Zhao, P.W.; Li, Y.Z.; Yen, Y.P.; Lee, Y.R. 9-O-Terpenyl-Substituted Berberrubine Derivatives Suppress Tumor Migration and Increase Anti-Human Non-Small-Cell Lung Cancer Activity. Int. J. Mol. Sci. 2021, 22, 9864. [Google Scholar] [CrossRef]
- Chang, J.M.; Kam, K.H.; Chao, W.Y.; Zhao, P.W.; Chen, S.H.; Chung, H.C.; Li, Y.Z.; Wu, J.Y.; Lee, Y.R. Berberine Derivatives Suppress Cellular Proliferation and Tumorigenesis In Vitro in Human Non-Small-Cell Lung Cancer Cells. Int. J. Mol. Sci. 2020, 21, 4218. [Google Scholar] [CrossRef] [PubMed]
- Shi, C.S.; Li, J.M.; Chin, C.C.; Kuo, Y.H.; Lee, Y.R.; Huang, Y.C. Evodiamine Induces Cell Growth Arrest, Apoptosis and Suppresses Tumorigenesis in Human Urothelial Cell Carcinoma Cells. Anticancer Res. 2017, 37, 1149–1159. [Google Scholar]
- Lee, Y.R.; Chen, S.H.; Lin, C.Y.; Chao, W.Y.; Lim, Y.P.; Yu, H.I.; Lu, C.H. In Vitro Antitumor Activity of Aloperine on Human Thyroid Cancer Cells through Caspase-Dependent Apoptosis. Int. J. Mol. Sci. 2018, 19, 312. [Google Scholar] [CrossRef]
- Ullah, A.; Leong, S.W.; Wang, J.; Wu, Q.; Ghauri, M.A.; Sarwar, A.; Su, Q.; Zhang, Y. Cephalomannine inhibits hypoxia-induced cellular function via the suppression of APEX1/HIF-1alpha interaction in lung cancer. Cell Death Dis. 2021, 12, 490. [Google Scholar] [CrossRef]
- Ullah, A.; Ullah, N.; Nawaz, T.; Aziz, T. Molecular Mechanisms of Sanguinarine in Cancer Prevention and Treatment. Anti-Cancer Agents Med. Chem. 2023, 23, 765–778. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.I.; Shen, H.C.; Chen, S.H.; Lim, Y.P.; Chuang, H.H.; Tai, T.S.; Kung, F.P.; Lu, C.H.; Hou, C.Y.; Lee, Y.R. Autophagy Modulation in Human Thyroid Cancer Cells following Aloperine Treatment. Int. J. Mol. Sci. 2019, 20, 5315. [Google Scholar] [CrossRef] [PubMed]








Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, T.-H.; Kuo, C.-H.; Zhang, Y.-S.; Chen, P.-T.; Chen, S.-H.; Li, Y.-Z.; Lee, Y.-R. Piperlongumine Induces Cellular Apoptosis and Autophagy via the ROS/Akt Signaling Pathway in Human Follicular Thyroid Cancer Cells. Int. J. Mol. Sci. 2023, 24, 8048. https://doi.org/10.3390/ijms24098048
Lin T-H, Kuo C-H, Zhang Y-S, Chen P-T, Chen S-H, Li Y-Z, Lee Y-R. Piperlongumine Induces Cellular Apoptosis and Autophagy via the ROS/Akt Signaling Pathway in Human Follicular Thyroid Cancer Cells. International Journal of Molecular Sciences. 2023; 24(9):8048. https://doi.org/10.3390/ijms24098048
Chicago/Turabian StyleLin, Tsung-Hsing, Chin-Ho Kuo, Yi-Sheng Zhang, Pin-Tzu Chen, Shu-Hsin Chen, Yi-Zhen Li, and Ying-Ray Lee. 2023. "Piperlongumine Induces Cellular Apoptosis and Autophagy via the ROS/Akt Signaling Pathway in Human Follicular Thyroid Cancer Cells" International Journal of Molecular Sciences 24, no. 9: 8048. https://doi.org/10.3390/ijms24098048
APA StyleLin, T. -H., Kuo, C. -H., Zhang, Y. -S., Chen, P. -T., Chen, S. -H., Li, Y. -Z., & Lee, Y. -R. (2023). Piperlongumine Induces Cellular Apoptosis and Autophagy via the ROS/Akt Signaling Pathway in Human Follicular Thyroid Cancer Cells. International Journal of Molecular Sciences, 24(9), 8048. https://doi.org/10.3390/ijms24098048





