The Role of the Residue at Position 2 in the Catalytic Activity of AA9 Lytic Polysaccharide Monooxygenases
Abstract
1. Introduction
2. Results
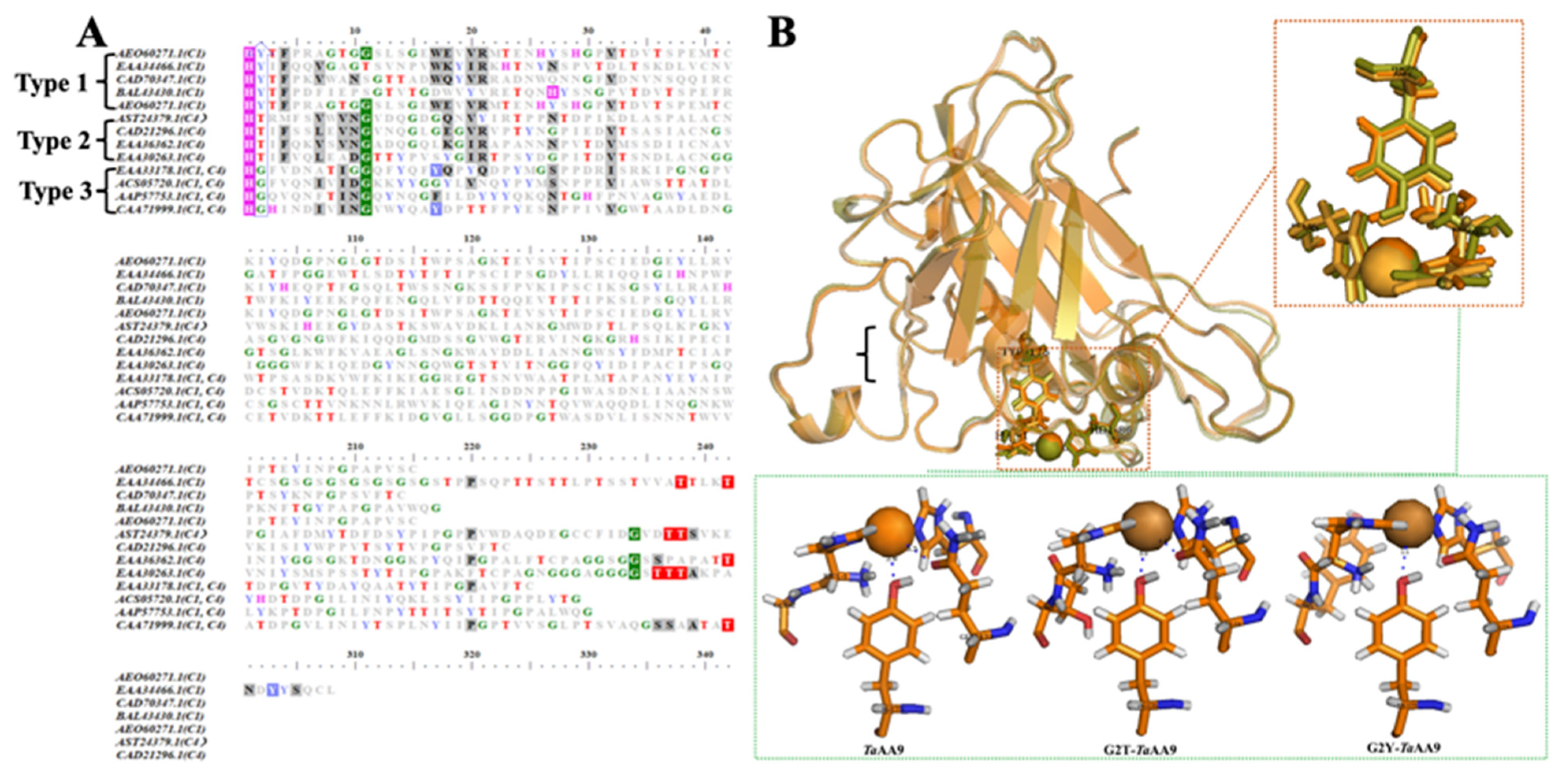
2.1. The Effect of the Residue at Position 2 on the Stabilization of the Copper Ion of TaAA9
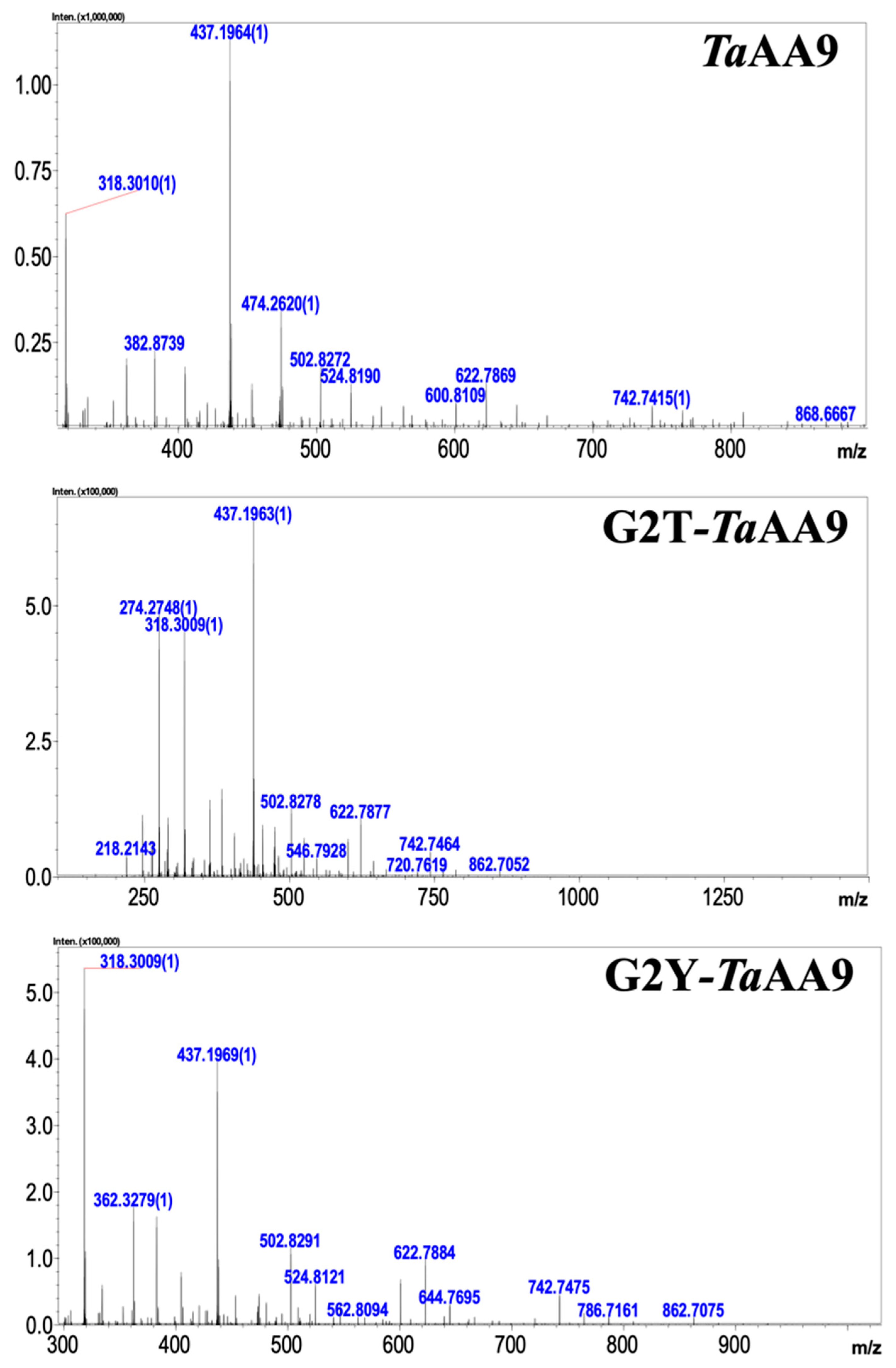
2.2. The Effect of the Residue at Position 2 on the Oxidative Products of TaAA9
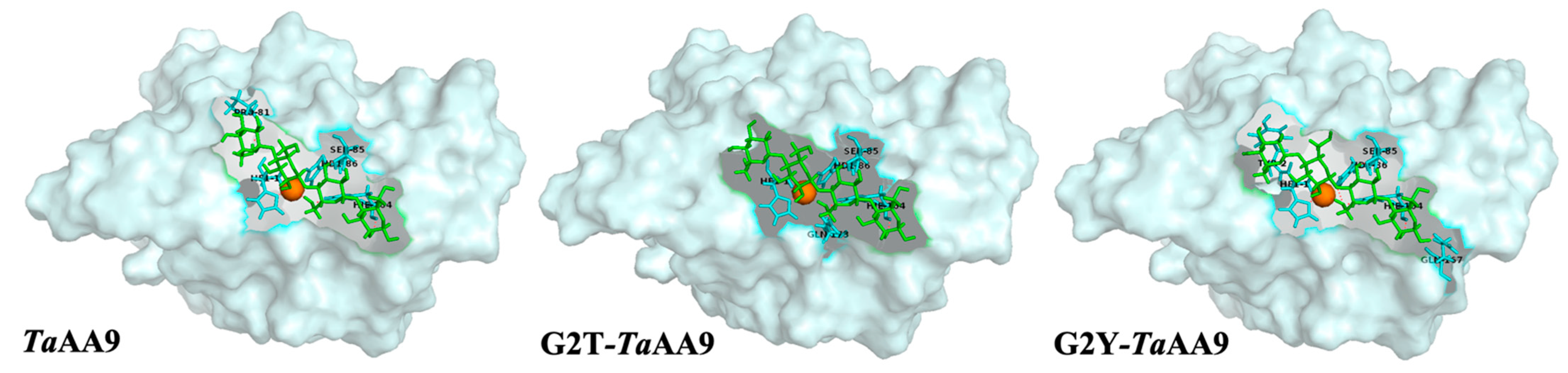
2.3. The Effect of the Residue at Position 2 on Substrate Binding in TaAA9
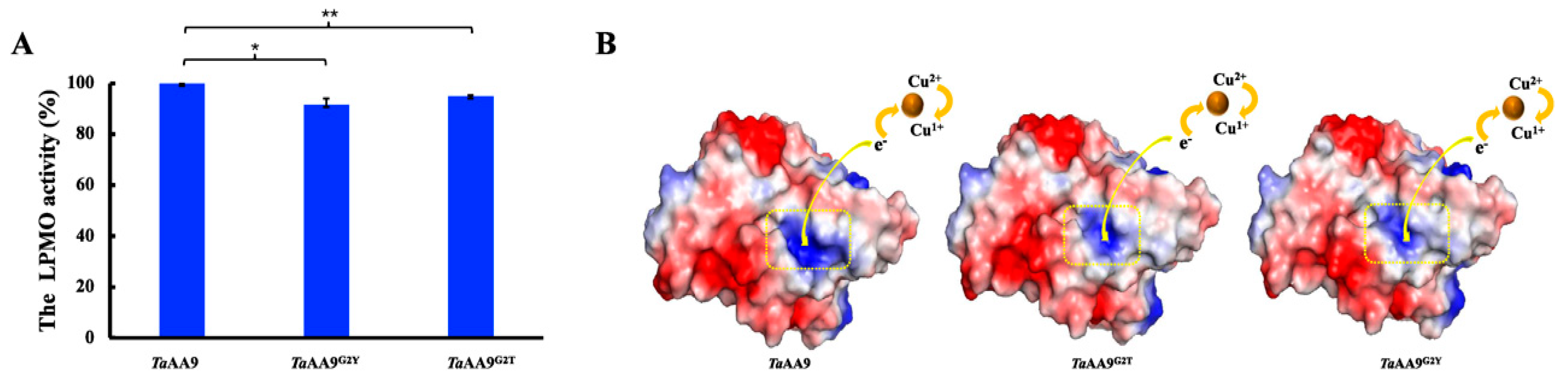
2.4. The Effect of the Residue at Position 2 on Enzymatic Catalysis of TaAA9
2.5. The Effect of the Residue at Position 2 on Product Dissociation from TaAA9
2.6. The Effect of the Residue at Position 2 on the Synergetic Activity of TaAA9
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Strains and Enzymes
4.3. Sequence Alignment Assay and Structural Bioinformation Analysis
4.4. Copper Ion Affinity Assay
4.5. Oxidative Reaction Product Assay
4.6. Cellulose Binding Assay
4.7. Molecular Dynamics (MD) Simulations
4.8. Enzymatic Activity Assay
4.9. Synergy Assay
4.10. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bissaro, B.; Kommedal, E.; Røhr, Å.K.; Eijsink, V.G.H. Controlled depolymerization of cellulose by light-driven lytic polysaccharide oxygenases. Nat. Commun. 2020, 11, 890. [Google Scholar] [CrossRef] [PubMed]
- Himmel, M.E.; Ding, S.Y.; Johnson, D.K.; Adney, W.S.; Nimlos, M.R.; Brady, J.W.; Foust, T.D. Biomass recalcitrance: Engineering plants and enzymes for biofuels production. Science 2007, 315, 804–807. [Google Scholar] [CrossRef] [PubMed]
- Meier, K.K.; Jones, S.M.; Kaper, T.; Hansson, H.; Koetsier, M.J.; Karkehabadi, S.; Solomon, E.I.; Sandgren, M.; Kelemen, B. Oxygen Activation by Cu LPMOs in Recalcitrant Carbohydrate Polysaccharide Conversion to Monomer Sugars. Chem. Rev. 2018, 118, 2593–2635. [Google Scholar] [CrossRef] [PubMed]
- Beeson, W.T.; Vu, V.V.; Span, E.A.; Phillips, C.M.; Marletta, M.A. Cellulose degradation by polysaccharide monooxygenases. Annu. Rev. Biochem. 2015, 84, 923–946. [Google Scholar] [CrossRef]
- Eibinger, M.; Sattelkow, J.; Ganner, T.; Plank, H.; Nidetzky, B. Single-molecule study of oxidative enzymatic deconstruction of cellulose. Nat. Commun. 2017, 8, 894. [Google Scholar] [CrossRef]
- Paradisi, A.; Johnston, E.M.; Tovborg, M.; Nicoll, C.R.; Ciano, L.; Dowle, A.; McMaster, J.; Hancock, Y.; Davies, G.J.; Walton, P.H. Formation of a Copper(II)-Tyrosyl Complex at the Active Site of Lytic Polysaccharide Monooxygenases Following Oxidation by H2O2. J. Am. Chem. Soc. 2019, 141, 18585–18599. [Google Scholar] [CrossRef]
- Sabbadin, F.; Hemsworth, G.R.; Ciano, L.; Henrissat, B.; Dupree, P.; Tryfona, T.; Marques, R.D.S.; Sweeney, S.T.; Besser, K.; Elias, L.; et al. An ancient family of lytic polysaccharide monooxygenases with roles in arthropod development and biomass digestion. Nat. Commun. 2018, 9, 756. [Google Scholar] [CrossRef]
- Vaaje-Kolstad, G.; Westereng, B.; Horn, S.J.; Liu, Z.; Zhai, H.; Sørlie, M.; Eijsink, V.G. An oxidative enzyme boosting the enzymatic conversion of recalcitrant polysaccharides. Science 2010, 330, 219–222. [Google Scholar] [CrossRef]
- Quinlan, R.J.; Sweeney, M.D.; Lo Leggio, L.; Otten, H.; Poulsen, J.C.; Johansen, K.S.; Krogh, K.B.; Jørgensen, C.I.; Tovborg, M.; Anthonsen, A.; et al. Insights into the oxidative degradation of cellulose by a copper metalloenzyme that exploits biomass components. Proc. Natl. Acad. Sci. USA 2011, 108, 15079–15084. [Google Scholar] [CrossRef]
- Ciano, L.; Davies, G.J.; Tolman, W.B.; Walton, P.H. Bracing Copper for the Catalytic Oxidation of C-H bonds. Nat. Catal. 2018, 1, 571–577. [Google Scholar] [CrossRef]
- Kuusk, S.; Kont, R.; Kuusk, P.; Heering, A.; Sørlie, M.; Bissaro, B.; Eijsink, V.G.H.; Väljamäe, P. Kinetic insights into the role of the reductant in H2O2-driven degradation of chitin by a bacterial lytic polysaccharide monooxygenase. J. Biol. Chem. 2019, 294, 1516–1528. [Google Scholar] [CrossRef] [PubMed]
- Jones, S.M.; Transue, W.J.; Meier, K.K.; Kelemen, B.; Solomon, E.I. Kinetic analysis of amino acid radicals formed in H2O2-driven CuI+ LPMO reoxidation implicates dominant homolytic reactivity. Proc. Natl. Acad. Sci. USA 2020, 117, 11916–11922. [Google Scholar] [CrossRef] [PubMed]
- Harris, P.V.; Welner, D.; McFarland, K.C.; Re, E.; Navarro Poulsen, J.C.; Brown, K.; Salbo, R.; Ding, H.; Vlasenko, E.; Merino, S.; et al. Stimulation of lignocellulosic biomass hydrolysis by proteins of glycoside hydrolase family 61: Structure and function of a large, enigmatic family. Biochemistry 2010, 49, 3305–3316. [Google Scholar] [CrossRef] [PubMed]
- Frandsen, K.E.; Simmons, T.J.; Dupree, P.; Poulsen, J.C.; Hemsworth, G.R.; Ciano, L.; Johnston, E.M.; Tovborg, M.; Johansen, K.S.; von Freiesleben, P.; et al. The molecular basis of polysaccharide cleavage by lytic polysaccharide monooxygenases. Nat. Chem. Biol. 2016, 12, 298–303. [Google Scholar] [CrossRef] [PubMed]
- Johansen, K.S. Discovery and industrial applications of lytic polysaccharide monooxygenases. Biochem. Soc. Trans. 2016, 44, 143–149. [Google Scholar] [CrossRef]
- Kim, S.; Ståhlberg, J.; Sandgren, M.; Paton, R.S.; Beckham, G.T. Quantum mechanical calculations suggest that lytic polysaccharide monooxygenases use a copper-oxyl, oxygen-rebound mechanism. Proc. Natl. Acad. Sci. USA 2014, 111, 149–154. [Google Scholar] [CrossRef]
- Ma, F.; Chung, M.T.; Yao, Y.; Nidetz, R.; Lee, L.M.; Liu, A.P.; Feng, Y.; Kurabayashi, K.; Yang, G.Y. Efficient molecular evolution to generate enantioselective enzymes using a dual-channel microfluidic droplet screening platform. Nat. Commun. 2018, 9, 1030. [Google Scholar] [CrossRef]
- Xu, J.; Cen, Y.; Singh, W.; Fan, J.; Wu, L.; Lin, X.; Zhou, J.; Huang, M.; Reetz, M.T.; Wu, Q. Stereodivergent protein engineering of a lipase to access all possible stereoisomers of chiral esters with two stereocenters. J. Am. Chem. Soc. 2019, 141, 7934–7945. [Google Scholar] [CrossRef]
- Span, E.A.; Suess, D.L.M.; Deller, M.C.; Britt, R.D.; Marletta, M.A. The Role of the Secondary Coordination Sphere in a Fungal Polysaccharide Monooxygenase. ACS Chem. Biol. 2017, 12, 1095–1103. [Google Scholar] [CrossRef]
- Brander, S.; Lausten, S.; Ipsen, J.Ø.; Falkenberg, K.B.; Bertelsen, A.B.; Nørholm, M.H.H.; Østergaard, L.H.; Johansen, K.S. Colorimetric LPMO assay with direct implication for cellulolytic activity. Biotechnol. Biofuels 2021, 14, 51. [Google Scholar] [CrossRef]
- Vu, V.V.; Ngo, S.T. Copper active site in polysaccharide monooxygenases. Coord. Chem. Rev. 2018, 368, 134–157. [Google Scholar] [CrossRef]
- Vu, V.V.; Beeson, W.T.; Phillips, C.M.; Cate, J.H.; Marletta, M.A. Determinants of regioselective hydroxylation in the fungal polysaccharide monooxygenases. J. Am. Chem. Soc. 2014, 136, 562–565. [Google Scholar] [CrossRef] [PubMed]
- Danneels, B.; Tanghe, M.; Desmet, T. Structural Features on the Substrate-Binding Surface of Fungal Lytic Polysaccharide Monooxygenases Determine Their Oxidative Regioselectivity. J. Biotechnol. 2019, 14, e1800211. [Google Scholar] [CrossRef] [PubMed]
- Kruer-Zerhusen, N.; Alahuhta, M.; Lunin, V.V.; Himmel, M.E.; Bomble, Y.J.; Wilson, D.B. Structure of a Thermobifida fusca lytic polysaccharide monooxygenase and mutagenesis of key residues. Biotechnol. Biofuels 2017, 10, 243. [Google Scholar] [CrossRef] [PubMed]
- Danneels, B.; Tanghe, M.; Joosten, H.J.; Gundinger, T.; Spadiut, O.; Stals, I.; Desmet, T. A quantitative indicator diagram for lytic polysaccharide monooxygenases reveals the role of aromatic surface residues in HjLPMO9A regioselectivity. PLoS ONE 2017, 12, e0178446. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Beeson, W.T., 4th; Phillips, C.M.; Marletta, M.A.; Cate, J.H. Structural basis for substrate targeting and catalysis by fungal polysaccharide monooxygenases. Structure 2012, 20, 1051–1061. [Google Scholar] [CrossRef]
- Tan, T.C.; Kracher, D.; Gandini, R.; Sygmund, C.; Kittl, R.; Haltrich, D.; Hällberg, B.M.; Ludwig, R.; Divne, C. Structural basis for cellobiose dehydrogenase action during oxidative cellulose degradation. Nat. Commun. 2015, 6, 7542. [Google Scholar] [CrossRef]
- Jing, Y.; Song, X.F. Progress in Properties of Amino Acids on Surface of Protein Molecules. Progr. Mod. Biomed. 2011, 11, 10. [Google Scholar] [CrossRef]
- Monclaro, A.V.; Filho, E.X.F. Fungal lytic polysaccharide monooxygenases from family AA9: Recent developments and application in lignocelullose breakdown. Intl. J. Biol. Macromol. 2017, 102, 771–778. [Google Scholar] [CrossRef]
- Wang, J.; Bie, M.; Zhou, W.; Xie, B.; Sun, Z. Interaction between carboxymethyl pachyman and lotus seedpod oligomeric procyanidins with superior synergistic antibacterial activity. Carbohydr. Polym. 2019, 212, 11–20. [Google Scholar] [CrossRef]
- Vaaje-Kolstad, G.; Forsberg, Z.; Loose, J.S.; Bissaro, B.; Eijsink, V.G. Structural diversity of lytic polysaccharide monooxygenases. Curr. Opin. Strucl. Biol. 2017, 44, 67–76. [Google Scholar] [CrossRef] [PubMed]
- Frommhagen, M.; Westphal, A.H.; van Berkel, W.J.H.; Kabel, M.A. Distinct substrate specificities and electron-donating systems of fungal lytic polysaccharide monooxygenases. Front. Microbiol. 2018, 9, 1080. [Google Scholar] [CrossRef] [PubMed]
- Kracher, D.; Scheiblbrandner, S.; Felice, A.K.; Breslmayr, E.; Preims, M.; Ludwicka, K.; Haltrich, D.; Eijsink, V.G.; Ludwig, R. Extracellular electron transfer systems fuel cellulose oxidative degradation. Science 2016, 352, 1098–1101. [Google Scholar] [CrossRef] [PubMed]
- Cannella, D.; Möllers, K.B.; Frigaard, N.U.; Jensen, P.E.; Bjerrum, M.J.; Johansen, K.S.; Felby, C. Light-driven oxidation of polysaccharides by photosynthetic pigments and a metalloenzyme. Nat. Commun. 2016, 4, 11134. [Google Scholar] [CrossRef] [PubMed]
- Laurent, C.V.F.P.; Breslmayr, E.; Tunega, D.; Ludwig, R.; Oostenbrink, C. Interaction between Cellobiose Dehydrogenase and Lytic Polysaccharide Monooxygenase. Biochemistry 2019, 58, 1226–1235. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Feng, S.; Rovira, C.; Wang, B. How Oxygen Binding Enhances Long-Range Electron Transfer: Lessons from Reduction of Lytic Polysaccharide Monooxygenases by Cellobiose Dehydrogenase. Angew. Chem. Int. Ed. 2021, 60, 2385–2392. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Li, T.; Yu, Z.; Ju, J.; Zhang, H.; Tan, H.; Li, K.; Yin, H. A lytic polysaccharide monooxygenase from Myceliophthora thermophila and its synergism with cellobiohydrolases in cellulose hydrolysis. Intl. J. Biol. Macromol. 2019, 139, 570–576. [Google Scholar] [CrossRef]
- Wang, F.; Jiang, Y.; Guo, W.; Niu, K.; Zhang, R.; Hou, S.; Wang, M.; Yi, Y.; Zhu, C.; Jia, C.; et al. An environmentally friendly and productive process for bioethanol production from potato waste. Biotechnol. Biofuel 2016, 9, 50–59. [Google Scholar] [CrossRef]
- Wang, M.; Han, L.; Liu, S.; Zhao, X.; Yang, J.; Loh, S.K.; Sun, X.; Zhang, C.; Fang, X. A Weibull statistics-based lignocellulose saccharification model and a built-in parameter accurately predict lignocellulose hydrolysis performance. J. Biotechnol. 2015, 10, 1424–1433. [Google Scholar] [CrossRef]
- Borisova, A.S.; Isaksen, T.; Dimarogona, M.; Kognole, A.A.; Mathiesen, G.; Várnai, A.; Røhr, Å.K.; Payne, C.M.; Sørlie, M.; Sandgren, M.; et al. Structural and Functional Characterization of a Lytic Polysaccharide Monooxygenase with Broad Substrate Specificity. J. Biol. Chem. 2015, 290, 22955–22969. [Google Scholar] [CrossRef]
- Zhang, R.; Liu, Y.; Zhang, Y.; Feng, D.; Hou, S.; Guo, W.; Niu, K.; Jiang, Y.; Han, L.; Sindhu, L.; et al. Identification of a thermostable fungal lytic polysaccharide monooxygenase and evaluation of its effect on lignocellulosic degradation. Appl. Microbiol. Biot. 2019, 103, 5739–5750. [Google Scholar] [CrossRef] [PubMed]
- Hansson, H.; Karkehabadi, S.; Mikkelsen, N.; Douglas, N.R.; Kim, S.; Lam, A.; Kaper, T.; Kelemen, B.; Meier, K.K.; Jones, S.M.; et al. High-resolution structure of a lytic polysaccharide monooxygenase from Hypocrea jecorina reveals a predicted linker as an integral part of the catalytic domain. J. Biol. Chem. 2017, 292, 19099–19109. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Lin, X.; Yue, J.; Li, X.; Fang, X.; Zhu, M.; Lin, J.; Qu, Y.; Xiao, L. High concentration ethanol production from corncob residues by fed-batch strategy. Bioresour. Technol. 2010, 101, 4952–4958. [Google Scholar] [CrossRef] [PubMed]


| Product Profiles | TaAA9 | G2T-TaAA9 | G2Y-TaAA9 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| DP2 | DP3 | DP4 | DP5 | DP2 | DP3 | DP4 | DP5 | DP2 | DP3 | DP4 | DP5 | |
| C1 (m/z + 16) | − | − | − | + | − | − | − | − | − | − | − | − |
| C4/C6 (m/z − 2) | + | − | + | − | + | + | + | − | + | + | + | − |
| C1 + C4 (m/z + 14; m/z + 32) | + | + | + | + | + | + | − | − | + | + | − | − |
| C1 + C6 (m/z + 30) | + | + | − | − | − | − | − | − | − | − | − | − |
| C4 + C6 (m/z − 4) | − | − | − | − | − | − | − | − | − | − | − | − |
| C1 + C4 + C6 (m/z + 28) | + | + | + | + | + | + | − | − | + | + | − | − |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Y.; Ma, W.; Fang, X. The Role of the Residue at Position 2 in the Catalytic Activity of AA9 Lytic Polysaccharide Monooxygenases. Int. J. Mol. Sci. 2023, 24, 8300. https://doi.org/10.3390/ijms24098300
Liu Y, Ma W, Fang X. The Role of the Residue at Position 2 in the Catalytic Activity of AA9 Lytic Polysaccharide Monooxygenases. International Journal of Molecular Sciences. 2023; 24(9):8300. https://doi.org/10.3390/ijms24098300
Chicago/Turabian StyleLiu, Yucui, Wei Ma, and Xu Fang. 2023. "The Role of the Residue at Position 2 in the Catalytic Activity of AA9 Lytic Polysaccharide Monooxygenases" International Journal of Molecular Sciences 24, no. 9: 8300. https://doi.org/10.3390/ijms24098300
APA StyleLiu, Y., Ma, W., & Fang, X. (2023). The Role of the Residue at Position 2 in the Catalytic Activity of AA9 Lytic Polysaccharide Monooxygenases. International Journal of Molecular Sciences, 24(9), 8300. https://doi.org/10.3390/ijms24098300


_Kim.png)



