Recent Advances in Characterization of Melanin Pigments in Biological Samples
Abstract
1. Introduction
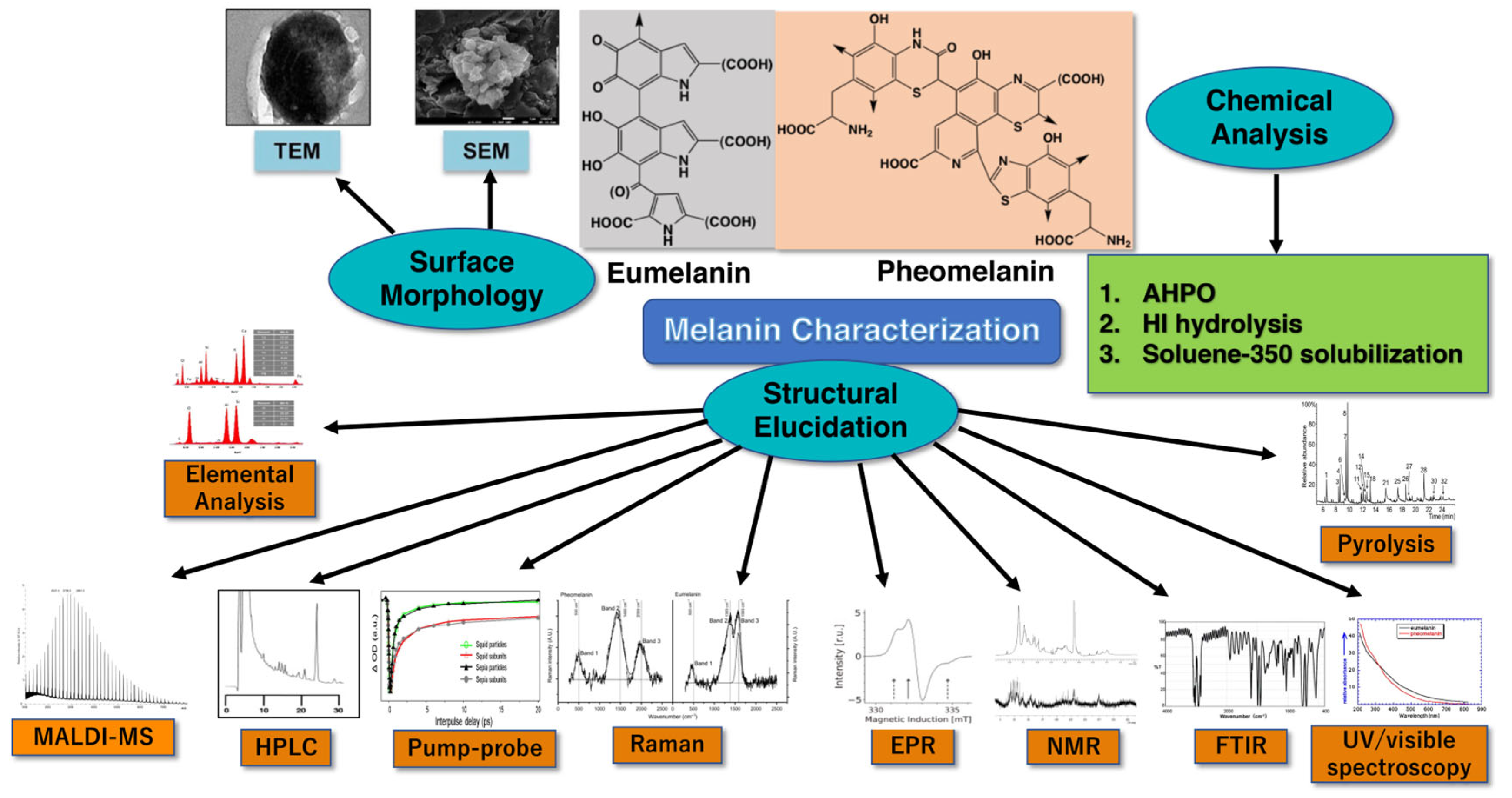
2. Microanalytical Applications of the Chemical Degradation of Melanin
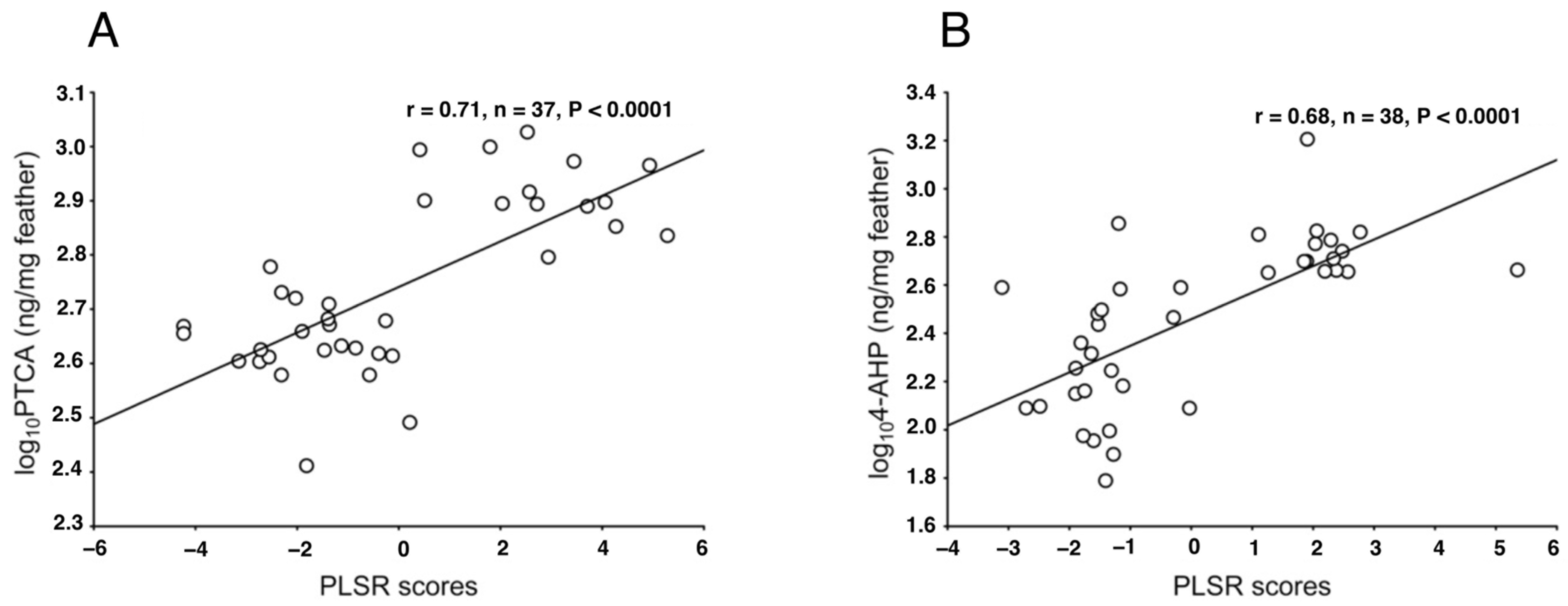
2.1. Analysis of EM and PM Using Chemical Degradation Followed by HPLC
2.2. DA-Derived Melanins
2.3. Usefulness of Melanin Markers in Evaluating Photodegradation of EM and PM
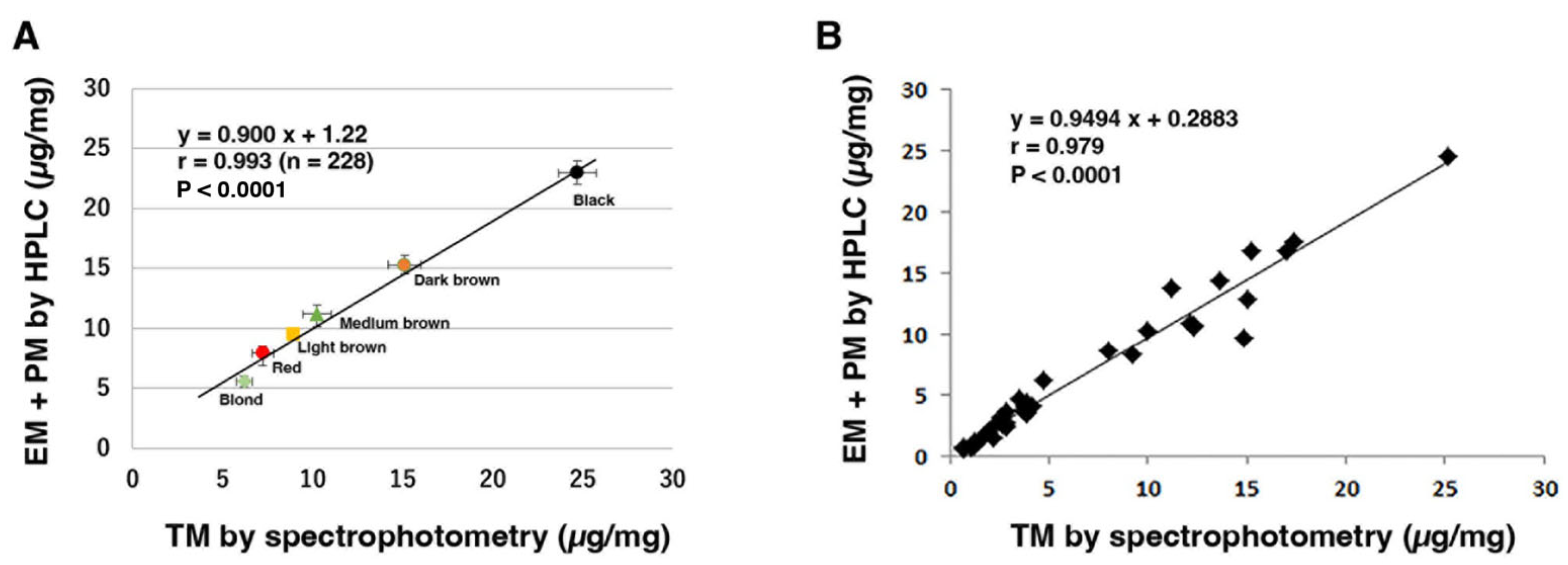
2.4. Spectrophotometric Analysis of Melanins
3. Various Methods Used for Quantification, Imaging, and Structural Characterization of Melanin
3.1. Electron Paramagnetic Resonance (EPR) Spectroscopy
3.2. 1H- and 13C-NMR Spectrometry
3.3. Fourier Transform Infrared Spectroscopy
3.4. Electron Microscopy
3.5. Mass Spectrometry
3.6. Pyrolysis
3.7. Raman Spectroscopy
3.8. Synchrotron Rapid Scanning X-ray Fluorescence
3.9. Near-Infrared Excited Fluorescence
3.10. Fate Tracing of [U-13C]L-Tyrosine Using LC–MS
3.11. Pump–Probe Method
3.12. Hair and Skin Color Parameters—ITA, and Colorimetric Parameters
3.13. Tape Stripping
3.14. Elemental Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- d’Ischia, M.; Wakamatsu, K.; Napolitano, A.; Briganti, S.; Garcia-Borron, J.-C.; Kovacs, D.; Meredith, P.; Pezzella, A.; Picardo, M.; Sarna, T.; et al. Melanins and melanogenesis: Methods, standards, protocols. Pigment Cell Melanoma Res. 2013, 26, 616–633. [Google Scholar] [CrossRef] [PubMed]
- Nicolaus, R.A. Melanins; Hermann: Paris, France, 1969. [Google Scholar]
- Wakamatsu, K.; Ito, S. Melanins in Vertebrates. In Pigments, Pigment Cells and Pigment Patterns; Hashimoto, H., Goda, M., Futahashi, R., Kelsh, R., Akiyama, T., Eds.; Springer: Singapore, 2021; pp. 45–89. [Google Scholar] [CrossRef]
- Wakamatsu, K.; Zippin, J.H.; Ito, S. Chemical and biochemical control of skin pigmentation with special emphasis on mixed melanogenesis. Pigment Cell Melanoma Res. 2021, 34, 730–747. [Google Scholar] [CrossRef] [PubMed]
- Wakamatsu, K.; Ohtara, K.; Ito, S. Chemical analysis of late stages of pheomelanogenesis: Conversion of dihydrobenzothiazine to a benzothiazole structure. Pigment Cell Melanoma Res. 2009, 22, 474–486. [Google Scholar] [CrossRef] [PubMed]
- Zecca, L.; Bellei, C.; Costi, P.; Albertini, A.; Monzani, E.; Casella, L.; Gallorini, M.; Bergamaschi, L.; Moscatelli, A.; Turro, N.J.; et al. New melanic pigments in the human brain that accumulate in aging and block environmental toxic metals. Proc. Natl. Acad. Sci. USA 2008, 105, 17567–17572. [Google Scholar] [CrossRef] [PubMed]
- Wakamatsu, K.; Tabuchi, K.; Ojika, M.; Zucca, F.A.; Zecca, L.; Ito, S. Norephinephrine and its metabolites are involved in the synthesis of neuromelanin derived from the locus coeruleus. J. Neurochem. 2015, 135, 768–776. [Google Scholar] [CrossRef]
- Nagatsu, T.; Nakashima, A.; Watanabe, H.; Ito, S.; Wakamatsu, K. Neuromelanin in Parkinson’s disease: Tyrosine hydroxylase and tyrosinase. Int. J. Mol. Sci. 2022, 23, 4176. [Google Scholar] [CrossRef]
- Lorquin, F.; Piccerelle, P.; Orneto, C.; Robin, M.; Lorquin, J. New insights and advances on pyomelanin production from microbial synthesis to applications. J. Ind. Microbiol. Biotechnol. 2022, 49, kuac013. [Google Scholar] [CrossRef]
- Barek, H.; Sugumaran, M.; Ito, S.; Wakamatsu, K. Insect cuticular melanins are distinctly different from those of mammalian epidermal melanins. Pigment Cell Melanoma Res. 2018, 31, 384–392. [Google Scholar] [CrossRef]
- Simon, J.D.; Peles, D.; Wakamatsu, K.; Ito, S. Current challenges in understanding melanogenesis: Bridging chemistry, biological control, morphology, and function. Pigment Cell Melanoma Res. 2009, 22, 563–579. [Google Scholar] [CrossRef]
- Ito, S.; Wakamatsu, K. Chemistry of mixed melanogenesis—Pivotal roles of dopaquinone. Photochem. Photobiol. 2008, 84, 582–592. [Google Scholar] [CrossRef]
- Ito, S.; Wakamatsu, K. Human hair melanins: What we have learned and have not learned from mouse coat color pigmentation. Pigment Cell Melanoma Res. 2011, 24, 63–74. [Google Scholar] [CrossRef]
- Ito, S.; Sugumaran, M.; Wakamatsu, K. Chemical reactivities of ortho-quinones produced in living organisms: Fate of quinonoid products formed by tyrosinase and phenoloxidase action on phenols and catechols. Int. J. Mol. Sci. 2020, 21, 6080. [Google Scholar] [CrossRef]
- Tse, D.C.S.; McCreery, R.L.; Adams, R.N. Potential oxidative pathways of brain catecholamines. J. Med. Chem. 1976, 19, 37–40. [Google Scholar] [CrossRef]
- Land, E.J.; Ito, S.; Wakamatsu, K.; Riley, P.A. Rate constants for the first two chemical steps of eumelanogenesis. Pigment Cell Res. 2003, 16, 487–493. [Google Scholar] [CrossRef]
- Raper, H.S. The tyrosinase-tyrosine reaction. VI. Production from tyrosine of 5,6-dihydroxyindole and 5,6-dihydroxyindole-2-carboxylic acid—The precursors of melanin. Biochem. J. 1927, 21, 89–96. [Google Scholar] [CrossRef]
- Palumbo, A.; d’Ischia, M.; Misuraca, G.; Prota, G. Effect of metal ions on the rearrangement of dopachrome. Biochim. Biophys. Acta 1987, 925, 203–209. [Google Scholar] [CrossRef]
- Ito, S. Reexamination of the structure of eumelanin. Biochim. Biophys. Acta 1986, 883, 155–161. [Google Scholar] [CrossRef]
- Pawelek, J.M.; Körner, A.M.; Bergstrom, A.; Bolognia, J. New regulators of melanin biosynthesis and the autodestruction of melanoma cells. Nature 1980, 286, 617–619. [Google Scholar] [CrossRef]
- Tsukamoto, K.; Jackson, I.J.; Urabe, K.; Montague, P.M.; Hearing, V.J. A second tyrosinase-related protein, TRP-2, is a melanogenic enzyme termed DOPAchrome tautomerase. EMBO J. 1992, 11, 519–526. [Google Scholar] [CrossRef]
- Kroumpouzos, G.; Urabe, K.; Kobayashi, T.; Sakai, C.; Hearing, V.J. Functional analysis of the slaty gene product (TRP2) as dopachrome tautomerase and the effect of a point mutation on its catalytic function. Biochem. Biophys. Res. Commun. 1994, 202, 1060–1068. [Google Scholar] [CrossRef]
- Ito, S.; Suzuki, N.; Takebayashi, S.; Commo, S.; Wakamatsu, K. Neutral pH and copper ions promote eumelanogenesis after the dopachrome stage. Pigment Cell Melanoma Res. 2013, 26, 817–825. [Google Scholar] [CrossRef] [PubMed]
- Barber, J.I.; Townsend, D.; Olds, D.P.; King, R.A. Dopachrome oxidoreductase: A new enzyme in the pigment pathway. J. Investig. Dermatol. 1984, 83, 145–149. [Google Scholar] [CrossRef] [PubMed]
- Aroca, P.; García-Borrón, J.C.; Solano, F.; Lozano, J.A. Regulation of mammalian melanogenesis I: Partial purification and characterization of a dopachrome converting factor: Dopachrome tautomerase. Biochim. Biophys. Acta—Gen. Subj. 1990, 1035, 266–275. [Google Scholar] [CrossRef] [PubMed]
- Aroca, P.; Solano, F.; García-Borrón, J.; Lozano, J. A new spectrophotometric assay for dopachrome tautomerase. J. Biochem. Biophys. Methods 1990, 21, 35–46. [Google Scholar] [CrossRef]
- Aroca, P.; Solano, F.; Salinas, C.; García-Borrón, J.; Lozano, J. Regulation of the final phase of mammalian melanogenesis. The role of dopachrome tautomerase and the ratio between 5,6-dihydroxyindole-2-carboxylic acid and 5,6-dihydroxyindole. Eur. J. Biochem. 1992, 208, 155–163. [Google Scholar] [CrossRef]
- Orlow, S.J.; Zhou, B.K.; Chakraborty, A.K.; Drucker, M.; Pifko-Hirst, P.; Pawlek, J.M. High-molecular weight forms of tyrosinase and the tyrosinase-related proteins: Evidence for a melanogenic complex. J. Investig. Dermatol. 1994, 103, 196–201. [Google Scholar] [CrossRef]
- Jiménez-Cervantes, C.; Solano, C.F.; Kobayashi, T.; Urabe, K.; Hearing, V.J.; Lozano, J.A.; García-Borrón, J.C. A new enzymatic function in the melanogenic pathway. The 5,6-dihydroxyindole-2-carboxylic acid oxidase activity of tyrosinase-related protein-1 (TRP1). J. Biol. Chem. 1994, 269, 17993–18000. [Google Scholar] [CrossRef]
- Olivares, C.; Jiménez-Cervantes, C.; Lozano, J.A.; Solano, F.; García-Borrón, J.C. The 5,6-dihydroxyindole-2-carboxylic acid (DHICA) oxidase activity of human tyrosinase. Biochem. J. 2001, 354, 131–139. [Google Scholar] [CrossRef]
- Boissy, R.E.; Sakai, C.; Zhao, H.; Kobayashi, T.; Hearing, V.J. Human tyrosinase-related protein-1 (TRP-1) does not function as a DHICA oxidase activity in contrast to murine TRP-1. Exp. Dermatol. 1998, 7, 198–204. [Google Scholar] [CrossRef]
- Land, E.J.; Riley, P.A. Spontaneous redox reactions of dopaquinone and the balance between the eumelanic and phaeomelanic pathways. Pigment Cell Res. 2000, 13, 273–277. [Google Scholar] [CrossRef]
- Ito, S.; Prota, G.A. A facile one-step synthesis of cysteinyldopa using mushroom tyrosinase. Experientia 1977, 33, 1118–1119. [Google Scholar] [CrossRef]
- Ito, S.; Fujita, K. Microanalysis of eumelanin and pheomelanin in hair and melanomas by chemical degradation and liquid chromatography. Anal. Biochem. 1985, 144, 527–536. [Google Scholar] [CrossRef] [PubMed]
- Wakamatsu, K.; Ito, S. Advanced chemical methods in melanin determination. Pigment Cell Res. 2002, 15, 174–183. [Google Scholar] [CrossRef] [PubMed]
- Ito, S.; Wakamatsu, K. Quantitative analysis of eumelanin and pheomelanin in humans, mice, and other animals: A comparative review. Pigment Cell Res. 2003, 16, 523–531. [Google Scholar] [CrossRef] [PubMed]
- Ito, S.; Wakamatsu, K. Diversity of human hair pigmentation as studied by chemical analysis of eumelanin and pheomelanin. J. Eur. Acad. Dermatol. Venereol. 2011, 25, 1369–1380. [Google Scholar] [CrossRef]
- Ito, S.; Wakamatsu, K.; Sarna, T. Photodegradation of eumelanin and pheomelanin and its pathophysiological implications. Photochem. Photobiol. 2018, 94, 409–420. [Google Scholar] [CrossRef]
- Nicolaus, R.A.; Prota, G.; Santacrose, C.; Scherillo, G.; Sica, D. Struttura e biogenesi delle feomelanine. Nota VII. Sulla structurra delle tricosiderine. Gazz. Chim. Ital. 1967, 99, 323–350. [Google Scholar]
- Prota, G. Melanins and Melanogenesis; Academic Press: New York, NY, USA, 1992; pp. 1–290. [Google Scholar]
- Ito, S.; Wakamatsu, K. An improved modification of permanganate oxidation that gives constant yield of pyrrole-2,3,5-tricarboxylic acid. Pigment Cell Res. 1994, 7, 141–144. [Google Scholar] [CrossRef]
- Ito, S.; Jimbow, K. Quantitative analysis of eumelanin and pheomelanin in hair and melanomas. J. Investig. Dermatol. 1983, 80, 268–272. [Google Scholar] [CrossRef]
- Ito, S.; Nakanishi, Y.; Valenzuela, R.K.; Brilliant, M.H.; Kolbe, L.; Wakamatsu, K. Usefulness of alkaline hydrogen peroxide oxidation to analyze eumelanin and pheomelanin in various tissue samples: Application to chemical analysis of human hair melanins. Pigment Cell Melanoma Res. 2011, 24, 605–613. [Google Scholar] [CrossRef]
- Pralea, I.-E.; Moldovan, R.-C.; Petrache, A.-M.; Ilies, M.; Heghes, S.-C.; Ielciu, I.; Nicoară, R.; Moldovan, M.; Ene, M.; Radu, M.; et al. From Extraction to Advanced Analytical Methods: The Challenges of Melanin Analysis. Int. J. Mol. Sci. 2019, 20, 3943. [Google Scholar] [CrossRef]
- Ito, S. A chemist’s view of melanogenesis. Pigment Cell Res. 2003, 16, 230–236. [Google Scholar] [CrossRef]
- Wakamatsu, K.; Ito, S.; Rees, J.L. The usefulness of 4-amino-3-hydroxyphenylalanine as a specific marker of pheomelanin. Pigment Cell Res. 2002, 15, 225–232. [Google Scholar] [CrossRef]
- Ito, S.; Miyake, S.; Maruyama, S.; Suzuki, I.; Commo, S.; Nakanishi, Y.; Wakamatsu, K. Acid hydrolysis reveals a low but constant level of pheomelanin in human black to brown hair. Pigment Cell Melanoma Res. 2018, 31, 393–403. [Google Scholar] [CrossRef]
- Ito, S.; Del Bino, S.D.; Hirobe, T.; Wakamatsu, K. Improved HPLC conditions to determine eumelanin and pheomelanin contents in biological samples using an ion pair reagent. Int. J. Mol. Sci. 2020, 21, 5134. [Google Scholar] [CrossRef]
- Glass, K.; Ito, S.; Wilby, P.R.; Sota, T.; Nakamura, A.; Bowers, C.R.; Vinther, J.; Dutta, S.; Summons, R.; Briggs, D.E.G.; et al. Direct chemical evidence for eumelanin pigment from the Jurassic period. Proc. Natl. Acad. Sci. USA 2012, 109, 10218–10223. [Google Scholar] [CrossRef]
- Ito, S.; Wakamatsu, K.; Glass, K.; Simon, J.D. High-performance liquid chromatography estimation of cross-linking of dihydroxyindole moiety in eumelanin. Anal. Biochem. 2013, 434, 221–225. [Google Scholar] [CrossRef]
- Hirobe, T.; Ito, S.; Wakamatsu, K. The slaty (slt/Dctslt) allele decreases the content of eumelanin, but not pheomelanin in the mouse hair. Pigment Cell Melanoma Res. 2015, 29, 110–112. [Google Scholar] [CrossRef]
- Del Bino, S.; Ito, S.; Sok, J.; Nakanishi, Y.; Bastien, P.; Wakamatsu, K.; Bernerd, F. Chemical analysis of constitutive pigmentation of human epidermis reveals constant eumelanin to pheomelanin ratio. Pigment Cell Melanoma Res. 2015, 28, 707–717. [Google Scholar] [CrossRef]
- Del Bino, S.; Ito, S.; Sok, J.; Wakamatsu, K. 5,6-Dihydroxyindole eumelanin content in human skin with varying degrees of constitutive pigmentation. Pigment Cell Melanoma Res. 2022, 35, 622–626. [Google Scholar] [CrossRef]
- Lindgren, J.; Sjövall, P.; Thiel, V.; Zheng, W.; Ito, S.; Wakamatsu, K.; Hauff, R.; Kear, B.P.; Engdahl, A.; Alwmark, C.; et al. Soft-tissue evidence for homeothermy and crypsis in a Jurassic ichthyosaur. Nature 2018, 564, 359–365. [Google Scholar] [CrossRef] [PubMed]
- Panzella, L.; Manini, P.; Monfrecola, G.; d’Ischia, M.; Napolitano, A. An easy-to-run method for routine analysis of eumelanin and pheomelanin in pigmented tissues. Pigment Cell Res. 2006, 20, 128–133. [Google Scholar] [CrossRef] [PubMed]
- Petzel-Witt, S.; Meier, S.I.; Schubert-Zsilavecz, M.; Toennes, S.W. PTCA (1H-pyrrole-2,3,5-tricarboxylic acid) as a marker for oxidative hair treatment. Drug Test. Anal. 2018, 10, 768–773. [Google Scholar] [CrossRef] [PubMed]
- Szekely-Klepser, G.; Wade, K.; Woolson, D.; Brown, R.; Fountain, S.; Kindt, E. A validated LC/MS/MS method for the quantification of pyrrole-2,3,5-tricarboxylic acid (PTCA), a eumelanin specific biomarker, in human skin punch biopsies. J. Chromatogr. B 2005, 826, 31–40. [Google Scholar] [CrossRef]
- Eisenbeiss, L.; Binz, T.M.; Baumgartner, M.R.; Steuer, A.E.; Kraemer, T. A possible new oxidation marker for hair adulteration: Detection of PTeCA (1H-pyrrole-2,3,4,5-tetracarboxylic acid) in bleached hair. Drug Test. Anal. 2020, 12, 230–238. [Google Scholar] [CrossRef]
- Lerche, C.M.; Olsen, P.; Nissen, C.V.; Philipsen, P.A.; Wulf, H.C. A novel LC/MS/MS method to quantify eumelanin and pheomelanin and their relation to UVR sensitivity—A study on human skin biopsies. Pigment Cell Melanoma Res. 2019, 32, 809–816. [Google Scholar] [CrossRef]
- Rioux, B.; Rouanet, J.; Akil, H.; Besse, S.; Debiton, E.; Bouchon, B.; Degoul, F.; Quintana, M. Determination of eumelanin and pheomelanin in melanomas using solid-phase extraction and high performance liquid chromatography–diode array detection (HPLC-DAD) analysis. J. Chromatogr. B Biomed. Appl. 2019, 1113, 60–68. [Google Scholar] [CrossRef]
- Affenzeller, S.; Frauendorf, H.; Licha, T.; Jackson, D.J.; Wolkenstein, K. Quantitation of eumelanin and pheomelanin markers in diverse biological samples by HPLC-UV-MS following solid-phase extraction. PLoS ONE 2019, 14, e0223552. [Google Scholar] [CrossRef]
- Affenzeller, S.; Wolkenstein, K.; Frauendorf, H.; Jackson, D.J. Eumelanin and pheomelanin pigmentation in mollusc shells may be less common than expected: Insights from mass spectrometry. Front. Zool. 2019, 16, 44. [Google Scholar] [CrossRef]
- Marsden, C.D. Pigmentation in the nucleus substantiae nigrae of mammals. J. Anat. 1961, 95, 256–261. [Google Scholar]
- Engelen, M.; Vanna, R.; Bellei, C.; Zucca, F.; Wakamatsu, K.; Monzani, E.; Ito, S.; Casella, L.; Zecca, L. Neuromelanins of human brain have soluble and insoluble components with dolichols attached to the melanic structure. PLoS ONE 2012, 7, e48490. [Google Scholar] [CrossRef]
- Wakamatsu, K.; Murase, T.; Zucca, F.A.; Zecca, L.; Ito, S. Biosynthetic pathway to neuromelanin and its aging process. Pigment Cell Melanoma Res. 2012, 25, 792–803. [Google Scholar] [CrossRef]
- Zucca, F.A.; Basso, E.; Cupaioli, F.A.; Ferrari, E.; Sulzer, D.; Casella, L.; Zeccca, L. Neuromelanin of the human substantia nigra: An update. Neurotox. Res. 2014, 25, 13–23. [Google Scholar] [CrossRef]
- Zucca, F.A.; Vanna, R.; Cupaioli, F.A.; Bellei, C.; De Palma, A.; Di Silvestre, D.; Mauri, P.; Grassi, S.; Prinetti, A.; Casella, L.; et al. Neuromelanin organelles are specialized autolysosomes that accumulate undegraded proteins and lipids in aging human brain and are likely involved in Parkinson’s disease. NPJ Park. Dis. 2018, 4, 17. [Google Scholar] [CrossRef]
- Marsden, C.D. Neuromelanin and Parkinson’s disease. J. Neural Transm. 1983, 19, 121–141. [Google Scholar]
- Mann, D.M.A.; Yates, P.O. Possible role of neuromelanin in the pathogenesis of Parkinson’s disease. Mech. Aging. Dev. 1983, 21, 193–203. [Google Scholar] [CrossRef]
- Zecca, L.; Zucca, F.A.; Albertini, A.; Rizzio, E.; Fariello, R.G. A proposed dual role of neuromelanin in the pathogenesis of Parkinson’s disease. Neurology 2006, 67 (Suppl. 2), S8–S11. [Google Scholar] [CrossRef]
- Holdorff, B. Centenary of Tretiakoff’s thesis on the morphology of Parkinson’s disease, evolved on the grounds of encephalitis lethargica pathology. J. Hist. Neurosci. 2019, 28, 387–398. [Google Scholar] [CrossRef]
- Zecca, L.; Stroppolo, A.; Gatti, A.; Gatti, A.; Tampellini, D.; Toscani, M.; Gallorini, M.; Giaveri, G.; Arosio, P.; Santambrogio, P.; et al. The role of iron and copper molecules in the neuronal vulnerability of locus coeruleus and substantia nigra during aging. Proc. Natl. Acad. Sci. USA 2004, 101, 9843–9848. [Google Scholar] [CrossRef]
- Ikemoto, K.; Nagatsu, I.; Ito, S.; King, R.A.; Nishimura, A.; Nagatsu, T. Does tyrosinase exist in neuromelanin-pigmented neurons in the human substantia nigra? Neurosci. Lett. 1998, 253, 198–200. [Google Scholar] [CrossRef]
- Shen, X.M.; Dryhurst, G. Iron- and manganese-catalyzed autoxidation of dopamine in the presence of L-cysteine: Possible insights into iron- and manganese-mediated dopaminergic neurotoxicity. Chem. Res. Toxicol. 1998, 11, 824–837. [Google Scholar] [CrossRef] [PubMed]
- Tribl, F.; Arzberger, T.; Riederer, P.; Gerlach, M. Tyrosinase is not detected in human catecholaminergic neurons by immunohistochemistry and Western blot analysis. J. Neural Transm. Suppl. 2007, 72, 51–55. [Google Scholar] [CrossRef]
- Plum, S.; Steinbach, S.; Attems, J.; Keers, S.; Riederer, P.; Gerlach, M.; May, C.; Marcus, K. Proteomic characterization of neuromelanin granules isolated from human substantia nigra by laser-microdissection. Sci. Rep. 2016, 6, 37139. [Google Scholar] [CrossRef] [PubMed]
- Nagatsu, T.; Nakashima, A.; Watanabe, H.; Ito, S.; Wakamatsu, K.; Zucca, F.A.; Zecca, L.; Youdim, M.; Wulf, M.; Riederer, P.; et al. The role of tyrosine hydroxylase as a key player in neuromelanin synthesis and the association of neuromelanin with Parkinson’s disease. J. Neural Transm. 2023, 130, 611–625. [Google Scholar] [CrossRef]
- Rosengren, E.; Linder-Eliasson, E.; Carlsson, A. Detection of 5-S-cysteinyldopamine in human brain. J. Neural. Transm. 1985, 63, 247–253. [Google Scholar] [CrossRef]
- Carstam, R.; Brinck, C.; Hindemith-Augustsson, A.; Rorsman, H.; Rosengren, E. The neuromelanin of the human substantia nigra. Biochim. Biophys. Acta 1991, 1097, 152–160. [Google Scholar] [CrossRef]
- Odh, G.; Carstam, R.; Paulson, J.; Wittbjer, A.; Rosengren, E.; Rorsman, H. Neuromelanin of the human substantia nigra: A mixed type melanin. J. Neurochem. 1994, 62, 2030–2036. [Google Scholar] [CrossRef]
- Wakamatsu, K.; Fujikawa, K.; Zucca, F.A.; Zecca, L.; Ito, S. The structure of neuromelanin as studied by chemical degradative methods. J. Neurochem. 2003, 86, 1015–1023. [Google Scholar] [CrossRef]
- Eisenhofer, G.; Kopin, I.J.; Goldstein, D.S. Catecholamine metabolism: A contemporary view with implications for physiology and medicine. Pharmacol. Rev. 2004, 56, 331–349. [Google Scholar] [CrossRef]
- Wakamatsu, K.; Tanaka, H.; Tabuchi, K.; Ojika, M.; Zucca, F.A.; Zecca, L.; Ito, S. Reduction of the nitro group to amine by hydroiodic acid to synthesize o-aminophenol derivatives as putative degradative markers of neuromelanin. Molecules 2014, 19, 8039–8050. [Google Scholar] [CrossRef]
- Krainc, T.; Monje, M.H.G.; Kinsinger, M.; Bustos, B.I.B.; Lubbe, S.J. Melanin and neuromelanin: Linking skin pigmentation and Parkinson’s disease. Mov. Disord. 2023, 38, 185–195. [Google Scholar] [CrossRef]
- Staib, F. Cryptococcus neoformans and Guizotia abyssinica (syn G. Oleifera D.C.) (farbreadktion für C. neoformans). Z. Hyg. Infektionskr. 1962, 148, 466–475. [Google Scholar] [CrossRef]
- Williamson, P.R. Biochemical and molecular characterization of the diphenol oxidase of Cryptococcus neoformans: Identification as a laccase. J. Bacteriol. 1994, 176, 656–664. [Google Scholar] [CrossRef]
- Williamson, P.R.; Wakamatsu, K.; Ito, S. Melanin biosynthesis in Cryptococcus neoformans. J. Bacteriol. 1998, 180, 1570–1572. [Google Scholar] [CrossRef]
- Ito, S.; Kolbe, L.; Weets, G.; Wakamatsu, K. Visible light accelerates the ultraviolet A-induced degradation of eumelanin and pheomelanin. Pigment Cell Melanoma Res. 2019, 32, 441–447. [Google Scholar] [CrossRef]
- Wakamatsu, K.; Nakanishi, Y.; Miyazaki, N.; Kolbe, L.; Ito, S. UVA-induced oxidative degradation of melanins: Fission of indole moiety in eumelanin and conversion to benzothiazole moiety in pheomelanin. Pigment Cell Melanoma Res. 2012, 25, 434–445. [Google Scholar] [CrossRef]
- Ito, S.; Pilat, A.; Gerwat, W.; Skumatz, C.M.B.; Ito, M.; Kiyono, A.; Zadlo, A.; Nakanishi, Y.; Kolbe, L.; Burke, J.M.; et al. Photoaging of human retinal pigment epithelium is accompanied by oxidative modifications of its eumelanin. Pigment Cell Melanoma Res. 2013, 26, 357–366. [Google Scholar] [CrossRef]
- Greco, G.; Wakamatsu, K.; Panzella, L.; Ito, S.; Napolitano, A.; d’Ischia, M. Isomeric cysteinyldopas provide a (photo)degradable bulk component and a robust structural element in red human hair pheomelanin. Pigment Cell Melanoma Res. 2009, 22, 319–327. [Google Scholar] [CrossRef]
- Ito, S.; Kikuta, M.; Koike, S.; Szewczyk, G.; Sarna, M.; Zadlo, A.; Sarna, T.; Wakamatsu, K. Roles of reactive oxygen species in UVA-induced oxidation of 5,6-dihydroxyindole-2-carboxylic acid (DHICA) melanin as studied by differential spectrophotometric method. Pigment Cell Melanoma Res. 2016, 29, 340–351. [Google Scholar] [CrossRef]
- Szewczyk, G.; Zadlo, A.; Sarna, M.; Ito, S.; Wakamatsu, K.; Sarna, T. Aerobic photoreactivity of synthetic eumelanins and pheomelanins: Generation of singlet oxygen and superoxide anion. Pigment Cell Melanoma Res. 2016, 29, 669–678. [Google Scholar] [CrossRef]
- Zadlo, A.; Szewczyk, G.; Sarna, M.; Camenisch, T.G.; Sidabras, J.W.; Ito, S.; Wakamatsu, K.; Sagan, F.; Mitoraj, M.; Sarna, T. Photobleaching of pheomelanin increases its phototoxic potential; physicochemical studies of synthetic pheomelanin subjected to aerobic photolysis. Pigment Cell Melanoma Res. 2019, 32, 359–372. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, H.; Yamashita, Y.; Umezawa, K.; Hirobe, T.; Ito, S.; Wakamatsu, K. The Pro-oxidant activity of pheomelanin is significantly enhanced by UVA irradiation: Benzothiazole moieties are more reactive than benzothiazine moieties. Int. J. Mol. Sci. 2018, 19, 2889. [Google Scholar] [CrossRef] [PubMed]
- Wakamatsu, K.; Munyard, K.; Oddie, C.; Ito, S. Photobleached oxidative degradation of melanins: Chemical characterization of melanins present in alpaca fiber. Photochem. Photobiol. 2021, 97, 1493–1497. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Martínez, S.; Wakamatsu, K.; Galván, I. Increase of the benzothiazole moiety content of pheomelanin pigment after endogenous free radical inducement. Dyes Pigm. 2020, 180, 108516. [Google Scholar] [CrossRef]
- Ozeki, H.; Ito, S.; Wakamatsu, K.; Hirobe, T. Chemical characterization of hair melanins in various coat-color mutants of mice. J. Investig. Dermatol. 1995, 105, 361–366. [Google Scholar] [CrossRef]
- Orlow, S.J.; Osber, M.P.; Pawelek, J.M. Synthesis and characterization of melanins from dihydroxyindole-2-carboxylic acid and dihydroxyindole. Pigment Cell Res. 1992, 5, 113–121. [Google Scholar] [CrossRef]
- Ozeki, H.; Ito, S.; Wakamatsu, K. Chemical characterization of melanins in sheep wool and human hair. Pigment Cell Res. 1996, 9, 51–57. [Google Scholar] [CrossRef]
- Itou, T.; Ito, S.; Wakamatsu, K. Effects of aging on hair color, melanosome morphology, and melanin composition in Japanese females. Int. J. Mol. Sci. 2019, 20, 3739. [Google Scholar] [CrossRef]
- Ito, S.; Napolitano, A.; Sarna, T.; Wakamatsu, K. Iron and copper ions accelerate and modify dopamine oxidation to eumelanin: Implications for neuromelanin genesis. J. Neural Transm. 2023, 130, 29–42. [Google Scholar] [CrossRef]
- Sealy, R.C.; Hyde, J.S.; Felix, C.C.; Menon, I.A.; Prota, G.; Swartz, H.M.; Persad, S.; Haberman, H.F. Novel free radicals in synthetic and natural pheomelanins: Distinction between dopa melanins and cysteinyldopa melanins by ESR spectroscopy. Proc. Natl. Acad. Sci. USA 1982, 79, 2885–2889. [Google Scholar] [CrossRef]
- Sealy, R.C.; Hyde, J.S.; Felix, C.C.; Menon, I.A.; Prota, G. Eumelanins and pheomelanins: Characterization by electron spin resonance spectroscopy. Science 1982, 217, 545–547. [Google Scholar] [CrossRef]
- Vsevolodov, E.B.; Ito, S.; Wakamatsu, K.; Kuchina, I.I.; Latypov, I.F. Comparative analysis of hair melanins by chemical and electron spin resonance methods. Pigment Cell Res. 1991, 3, 30–34. [Google Scholar] [CrossRef]
- Godechal, Q.; Ghanem, G.; Cook, M.G.; Gallez, B. Electron paramagnetic resonance spectrometry and imaging in melanomas: Comparison between pigmented and nonpigmented human malignant melanomas. Mol. Imag. 2013, 12, 218–223. [Google Scholar] [CrossRef]
- Pukalski, J.; Marcol, N.; Wolan, N.; Przemysław Płonka, M.; Ryszka, P.; Kowalski, T.; Latowski, D. Detection of a pheomelanin-like pigment by EPR spectroscopy in the mycelium of Plenodomus biglobosus. Acta Biochim. Pol. 2020, 67, 5405. [Google Scholar] [CrossRef]
- Chikvaidze, E.N.; Partskhaladze, T.M.; Gogoladze, T.V. Electron spin resonance (ESR/EPR) of free radicals observed in human red hair: A new, simple empirical method of determination of pheomelanin/eumelanin ratio in hair. Magn. Reson. Chem. 2014, 52, 377–382. [Google Scholar] [CrossRef]
- Panzella, L.; Benning, K.; Nesbeth, D.N.; Setaro, B.; D’Errico, G.; Napolitano, A.; d’Ischia, M. Identification of black sturgeon caviar pigment as eumelanin. Food Chem. 2022, 373, 131474. [Google Scholar] [CrossRef]
- Ye, M.; Guo, G.Y.; Lu, Y.; Song, S.; Wang, H.Y.; Yang, L. Purification, structure and anti-radiation activity of melanin from Lachnum YM404. Int. J. Biol. Macromol. 2014, 63, 170–176. [Google Scholar] [CrossRef]
- Liu, Q.; Xiao, J.; Liu, B.; Zhuang, Y.; Sun, L. Study on the preparation and chemical structure characterization of melanin from Boletus griseus. Int. J. Mol. Sci. 2018, 19, 3736. [Google Scholar] [CrossRef]
- Xin, C.; Ma, J.H.; Tan, C.J.; Yang, Z.; Ye, F.; Long, C.; Ye, S.; Hou, D.B. Preparation of melanin from Catharsius molossus L. and preliminary study on its chemical structure. J. Biosci. Bioeng. 2015, 119, 446–454. [Google Scholar] [CrossRef]
- Song, S.; Li, S.; Su, N.; Shi, F.; Ye, M. Structural characterization, molecular modification and hepatoprotective effect of melanin from Lachnum YM226 on acute alcohol-induced liver injury in mice. Food Funct. 2016, 7, 3617–3627. [Google Scholar] [CrossRef]
- Ye, Z.; Lu, Y.; Zong, S.; Yang, L.; Shaikh, F.; Li, J.; Ye, M. Structure, molecular modification and anti-tumor activity of melanin from Lachnum singerianum. Process Biochem. 2019, 76, 203–212. [Google Scholar] [CrossRef]
- Camacho, E.; Vij, R.; Chrissian, C.; Prados-Rosales, R.; Gil, D.; O’Meally, R.N.; Cordero, R.J.B.; Cole, R.N.; McCaffery, J.M.; Stark, R.E.; et al. The structural unit of melanin in the cell wall of the fungal pathogen Cryptococcus neoformans. J. Biol. Chem. 2019, 294, 10471–10489. [Google Scholar] [CrossRef] [PubMed]
- Chrissian, C.; Camacho, E.; Kelly, J.E.; Wang, H.; Casadevall, A.; Stark, R.E. Solid-state NMR spectroscopy identifies three classes of lipids in Cryptococcus neoformans melanized cell walls and whole fungal cells. J. Biol. Chem. 2020, 295, 15083–15096. [Google Scholar] [CrossRef] [PubMed]
- Glass, K.; Ito, S.; Wilby, P.R.; Sota, T.; Nakamura, A.; Bowers, C.R.; Miller, K.E.; Dutta, S.; Summons, R.E.; Briggs, D.E.G.; et al. Impact of diagenesis and maturation on the survival of eumelanin in the fossil record. Org. Geochem. 2013, 64, 29–37. [Google Scholar] [CrossRef]
- Sun, S.; Zhang, X.; Sun, S.; Zhang, L.; Shan, S.; Zhu, H. Production of natural melanin by auricularia auricula and study on its molecular structure. Food Chem. 2016, 190, 801–807. [Google Scholar] [CrossRef]
- Correa, N.; Covarrubias, C.; Rodas, P.I.; Hermosilla, G.; Olate, V.R.; Valdés, C.; Meyer, W.; Magne, F.; Tapia, C.V. Differential antifungal activity of human and cryptococcal melanins with structural discrepancies. Front. Microbiol. 2017, 8, 1292. [Google Scholar] [CrossRef]
- El-Naggar, N.E.A.; El-Ewasy, S.M. Bioproduction, characterization, anticancer and antioxidant activities of extracellular melanin pigment produced by newly isolated microbial cell factories Streptomyces glaucescens NEAE-H. Sci. Rep. 2017, 7, 42129. [Google Scholar] [CrossRef]
- Mbonyiryivuze, A.; Nuru, Z.Y.; Ngom, B.D.; Mwakikunga, B.; Dhlamini, S.M.; Park, E.; Maaza, M. Morphological and chemical composition characterization of commercial sepia melanin. Am. J. Nanomater. 2015, 3, 22–27. [Google Scholar] [CrossRef]
- Strube, O.I.; Büngeler, A.; Bremser, W. Site-specific in situ synthesis of eumelanin nanoparticles by an enzymatic autodeposition-like process. Biomacromolecules 2015, 16, 1608–1613. [Google Scholar] [CrossRef]
- McNamara, M.E.; Kaye, J.S.; Benton, M.J.; Orr, P.J.; Rossi, V.; Ito, S.; Wakamatsu, K. Non-integumentary melanosomes can bias reconstructions of the colours of fossil vertebrates. Nat. Commun. 2018, 9, 2878. [Google Scholar] [CrossRef]
- Winey, M.; Meehl, J.B.; O’Toole, E.T.; Giddings, T.H. Conventional transmission electron microscopy. Mol. Biol. Cell 2014, 25, 319–323. [Google Scholar] [CrossRef]
- Zhan, F.; He, Y.; Zu, Y.; Li, T.; Zhao, Z. Characterization of melanin isolated from a dark septate endophyte (DSE), Exophiala pisciphila. World J. Microbiol. Biotechnol. 2011, 27, 2483–2489. [Google Scholar] [CrossRef]
- Ye, M.; Wang, Y.; Guo, G.Y.; He, Y.L.; Lu, Y.; Ye, Y.W.; Yang, Q.H.; Yang, P.Z. Physicochemical characteristics and antioxidant activity of arginine-modified melanin from Lachnum YM-346. Food Chem. 2012, 135, 2490–2497. [Google Scholar] [CrossRef]
- Li, S.; Yang, L.; Li, J.; Chen, T.; Ye, M. Structure, molecular modification, and anti-radiation activity of melanin from Lachnum YM156 on ultraviolet B-induced injury in mice. Appl. Biochem. Biotechnol. 2018, 188, 555–567. [Google Scholar] [CrossRef]
- Yacout, S.M.; McIlwain, K.L.; Mirza, S.P.; Gaillard, E.R. Characterization of retinal pigment epithelial melanin and degraded synthetic melanin using mass spectrometry and in vitro biochemical diagnostics. Photochem. Photobiol. 2019, 95, 183–191. [Google Scholar] [CrossRef]
- Seraglia, R.; Traldi, P.; Elli, G.; Bertazzo, A.; Costa, C.; Allegri, G. Laser desorption ionization mass spectrometry in the study of natural and synthetic melanins. I—Tyrosine melanins. Biol. Mass Spectrom. 1993, 22, 687–697. [Google Scholar] [CrossRef]
- Bertazzo, A.; Biasiolo, M.; Costa, C.; Allegri, G.; Elli, G.; Seraglia, R.; Traldi, P. Laser desorption ionization mass spectrometry in the study of natural and synthetic melanins II—Serotonin melanins. J. Mass Spectrom. 1994, 23, 391–398. [Google Scholar] [CrossRef]
- Bertazzo, A.; Costa, C.; Allegri, G.; Seraglia, R.; Traldi, P. Biosynthesis of melanin from dopamine. An investigation of early oligomerization products. Rapid Commun. Mass Spectrom. 1995, 9, 634–640. [Google Scholar] [CrossRef]
- Napolitano, A.; Pezzella, A.; Prota, G.; Seraglia, R.; Traldi, P. A Reassessment of the Structure of 5,6-Dihydroxyindole-2-carboxylic Acid Melanins by Matrix-assisted Laser Desorption/Ionization Mass Spectrometry. Rapid Commun. Mass Spectrom. 1996, 10, 204–208. [Google Scholar] [CrossRef]
- Sugumaran, M.; Evans, J.; Ito, S.; Wakamatsu, K. Nonenzymatic spontaneous oxidative transformation of 5,6-dihydroxyindole. Int. J. Mol. Sci. 2020, 21, 7321. [Google Scholar] [CrossRef]
- Napolitano, A.; Pezzella, A.; Prota, G.; Seraglia, R.; Traldi, P. Structural analysis of synthetic melanins from 5,6-dihydroxyindole by matrix-assisted laser desorption/ionization mass spectrometry. Rapid Commun. Mass Spectrom. 1996, 10, 468–472. [Google Scholar] [CrossRef]
- Allegri, G.; Bertazzo, A.; Costa, C.; Seraglia, R.; Traldi, P. Investigation on melanin biosynthesis from 5,6-dihydroxy tryptamine by matrix-assisted laser desorption/ionization mass spectrometry. Rapid Commun. Mass Spectrom. 1996, 10, 419–423. [Google Scholar] [CrossRef]
- Bertazzo, A.; Costa, C.V.L.; Allegri, G.; Favretto, D.; Traldi, P. Application of matrix-assisted laser desorption/ionization mass spectrometry to the detection of melanins formed from Dopa and dopamine. J. Mass Spectrom. 1999, 34, 922–929. [Google Scholar] [CrossRef]
- Novellino, L. Isolation and characterization of mammalian eumelanins from hair and irides. Biochim. Biophys. Acta (BBA) Gen. Subj. 2000, 1475, 295–306. [Google Scholar] [CrossRef]
- Rosen, E.P.; Thompson, C.G.; Bokhart, M.T.; Prince, H.M.A.; Sykes, C.; Muddiman, D.C.; Kashuba, A.D.M. Analysis of antiretrovirals in single hair strands for evaluation of drug adherence with infrared-matrix-assisted laser desorption electrospray ionization mass spectrometry imaging. Anal. Chem. 2016, 88, 1336–1344. [Google Scholar] [CrossRef]
- Tanaka, G.; Parker, A.R.; Hasegawa, Y.; Siveter, D.J.; Yamamoto, R.; Miyashita, K.; Takahashi, Y.; Ito, S.; Wakamatsu, K.; Mukuda, T.; et al. Mineralized rods and cones suggest colour vision in a 300 Myr-old fossil fish. Nat. Commun. 2014, 5, 5920. [Google Scholar] [CrossRef]
- Pinheiro, F.; Prado, G.; Ito, S.; Simon, J.; Wakamatsu, K.; Anelli, L.; Andrade, J.; Glass, K. Chemical characterization of pterosaur melanin challenges color inferences for extinct animals. Sci. Rep. 2019, 9, 15947. [Google Scholar] [CrossRef]
- Briggs, D.E.G.; Evershed, R.P.; Lockheart, M.J. The biomolecular paleontology of continental fossils. Paleobiology 2000, 26, 169–193. [Google Scholar] [CrossRef]
- Vinther, J.; Briggs, D.E.G.; Prum, R.O.; Saranathan, V. The color of fossil feathers. Biol. Lett. 2008, 4, 522–525. [Google Scholar] [CrossRef]
- Vinther, J.; Briggs, D.E.G.; Clarke, J.; Mayr, G.; Prum, R.O. Structural coloration in a fossil feather. Biol. Lett. 2009, 6, 128–131. [Google Scholar] [CrossRef]
- Li, Q.; Gao, K.-Q.; Vinther, J.; Shawkey, M.D.; Clarke, J.; D’Alba, L.; Meng, Q.; Briggs, D.E.G.; Prum, R.O. Plumage color patterns of an extinct dinosaur. Science 2010, 327, 1369–1372. [Google Scholar] [CrossRef]
- Zhang, F.; Kearns, S.L.; Orr, P.J.; Benton, M.J.; Zhou, Z.; Johnson, D.; Xu, X.; Wang, X. Fossilized melanosomes and the color of Cretaceous dinosaurs and birds. Nature 2000, 463, 1075–1078. [Google Scholar] [CrossRef]
- Barden, H.E.; Wogelius, R.A.; Li, D.; Manning, P.L.; Edwards, N.P.; van Dongen, B.E. Morphological and Geochemical Evidence of Eumelanin Preservation in the Feathers of the Early Cretaceous Bird, Gansus yumenensis. PLoS ONE 2011, 6, e25494. [Google Scholar] [CrossRef]
- Wogelius, R.A.; Manning, P.L.; Barden, H.E.; Edwards, N.P.; Webb, S.M.; Sellers, W.I.; Taylor, K.G.; Larson, L.; Dodson, P.; You, H.; et al. Trace metals as biomarkers for eumelanin pigment in the fossil record. Science 2011, 333, 1622–1626. [Google Scholar] [CrossRef]
- Lindgren, J.; Uvdal, P.; Sjövall, P.; Nilsson, D.E.; Engdahl, A.; Schultz, B.P.; Thiel, V. Molecular preservation of the pigment melanin in fossil melanosomes. Nat. Commun. 2012, 3, 824. [Google Scholar] [CrossRef]
- Sanyova, J.; Cersoy, S.; Richardin, P.; Laprévote, O.; Walter, P.; Brunelle, A. Unexpected materials in a Rembrandt painting characterized by high spatial resolution cluster-TOF-SIMS imaging. Anal. Chem. 2011, 83, 753–760. [Google Scholar] [CrossRef]
- Jarenmark, M.; Sjövall, P.; Ito, S.; Wakamatsu, K.; Lindgren, J. Chemical evaluation of eumelanin maturation by ToF-SIMS and alkaline peroxide oxidation HPLC analysis. Int. J. Mol. Sci. 2021, 22, 161. [Google Scholar] [CrossRef]
- Castaing, R.; Slodzian, G. Analytical microscopy by secondary ion imaging techniques. J. Phys. E Sci. Instrum. 1981, 14, 1119. [Google Scholar] [CrossRef]
- Shimomura, Y.; Aoki, N.; Rogers, M.A.; Langbein, L.; Schweizer, J.; Ito, M. hKAP1.6 and hKAP1.7, two novel human high sulfur keratin-associated proteins are expressed in the hair follicle cortex. J. Investig. Dermatol. 2002, 118, 226–231. [Google Scholar] [CrossRef]
- Dworzanski, J.P. Pyrolysis—Gas chromatography of natural and synthetic melanins. J. Anal. Appl. Pyrolysis 1983, 5, 69–79. [Google Scholar] [CrossRef]
- Stępień, K.B.; Dworzanski, J.P.; Imielski, S.; Wilczok, T.; Stępień, K.B. Study of chloroquine binding to melanins by pyrolysis-gas chromatography and electron spin resonance spectroscopy. J. Anal. Appl. Pyrolysis 1986, 9, 297–307. [Google Scholar] [CrossRef]
- Dzierżęga-Lęcznar, A.; Chodurek, E.; Stępień, K.; Wilczok, T. Pyrolysis-gas chromatography-mass spectrometry of synthetic neuromelanins. J. Anal. Appl. Pyrolysis 2002, 62, 239–248. [Google Scholar] [CrossRef]
- Stępień, K.; Dzierżęga-Lęcznar, A.; Kurkiewicz, S.; Tam, I. Melanin from epidermal human melanocytes: Study by pyrolytic GC/MS. J. Am. Soc. Mass Spectrom. 2009, 20, 464–468. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kurkiewicz, S.; Marek, Ł.; Kurkiewicz, M.; Adam Kurkiewicz, A.; Dzierżęga-Lęcznar, A. Are plants capable of pheomelanin synthesis? Gas chromatography/tandem mass spectrometry characterization of thermally degraded melanin isolated from Echinaces purpures. Processes 2022, 10, 2465. [Google Scholar] [CrossRef]
- Dzierżęga-Lęcznar, A.; Kurkiewicz, S.; Stępień, K. Detection and quantitation of a pheomelanin component in melanin pigments using pyrolysis–gas chromatography/tandem mass spectrometry system with multiple reaction monitoring mode. J. Mass Spectrom. 2012, 47, 242–245. [Google Scholar] [CrossRef]
- Galván, I.; Jorge, A.; Solano, F.; Wakamatsu, K. Vibrational characterization of pheomelanin and trichochromes F by Raman spectroscopy. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2013, 110, 55–59. [Google Scholar] [CrossRef]
- Galván, I.; Jorge, A.; Ito, K.; Tabuchi, K.; Solano, F.; Wakamatsu, K. Raman spectroscopy as a non-invasive technique for the quantification of melanins in feathers and hairs. Pigment Cell Melanoma Res. 2013, 26, 917–923. [Google Scholar] [CrossRef]
- Galván, I.; Jorge, A. Dispersive Raman spectroscopy allows the identification and quantification of melanin types. Ecol. Evol. 2015, 5, 1425–1431. [Google Scholar] [CrossRef]
- Galván, I.; Araujo-Andrade, C.; Marro, M.; Loza-Alvarez, P.; Wakamatsu, K. Raman spectroscopy quantification of eumelanin subunits in natural unaltered pigments. Pigment Cell Melanoma Res. 2018, 31, 673–682. [Google Scholar] [CrossRef]
- Yakimov, B.P.; Shirshin, E.A.; Schleusener, J.; Allenova, A.S.; Fadeev, V.V.; Darvin, M.E. Melanin distribution from the dermal–epidermal junction to the stratum corneum: Non-invasive in vivo assessment by fluorescence and Raman microspectroscopy. Sci. Rep. 2020, 10, 14374. [Google Scholar] [CrossRef]
- Saha, A.; Arora, R.; Yakovlev, V.V.; Burke, J.M. Raman microspectroscopy of melanosomes: The effect of long term light irradiation. J. Biophotonics 2011, 4, 805–813. [Google Scholar] [CrossRef]
- Kim, E.; Panzella, L.; Micillo, R.; Bentley, W.E.; Napolitano, A.; Payne, G.F. Reverse engineering applied to red human hair pheomelanin reveals redox-buffering as a pro-oxidant mechanism. Sci. Rep. 2015, 5, 18447. [Google Scholar] [CrossRef]
- Huang, Z.; Lui, H.; Cehn, X.K.; Alajlan, A.; McLean, D.I.; Zeng, H. Raman spectroscopy of in vivo cutaneous melanin. J. Biomed. Opt. 2004, 9, 1198–1206. [Google Scholar] [CrossRef]
- Galván, I.; Erritzøe, J.; Wakamatsu, K.; Møller, A.P. High prevalence of cataracts in birds with pheomelanin-based colouration. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2012, 162, 259–264. [Google Scholar] [CrossRef]
- Neves, A.; Zougagh, M.; Ríos, Á.; Tauler, R.; Wakamatsu, K.; Galván, I. Pheomelanin subunit non-destructive quantification by Raman spectroscopy and multivariate curve resolution-alternating least squares (MCR-ALS). Chemom. Intell. Lab. Syst. 2021, 217, 104406. [Google Scholar] [CrossRef]
- Ruiz, J.J.; Marro, M.; Galván, I.; Bernabeu-Wittel, J.; Conejo-Mir, J.; Zulueta-Dorado, T.; Guisado-Gil, A.B.; Loza-Álvarez, P. Novel Non-Invasive Quantification and Imaging of Eumelanin and DHICA Subunit in Skin Lesions by Raman Spectroscopy and MCR Algorithm: Improving Dysplastic Nevi Diagnosis. Cancers 2022, 14, 1056. [Google Scholar] [CrossRef]
- Rossi, V.; McNamara, M.E.; Webb, S.M.; Ito, S.; Wakamatsu, K. Tissue-specific geometry and chemistry of modern and fossilized melanosomes reveal internal anatomy of extinct vertebrates. Proc. Natl. Acad. Sci. USA 2019, 116, 17880–17889. [Google Scholar] [CrossRef]
- Rogers, C.S.; Astrop, T.I.; Webb, S.M.; Ito, S.; Wakamatsu, K.; McNamara, M.E. Synchrotron X-ray absorption spectroscopy of melanosomes in vertebrates and cephalopods: Implications for the affinity of Tullimonstrum. Proc. R. Soc. B Biol. Sci. 2019, 286, 20191649. [Google Scholar] [CrossRef]
- Manning, P.L.; Edwards, N.P.; Bergmann, U.; Anné, J.; Sellers, W.I.; van Veelen, A.; Sokaras, D.; Egerton, V.M.; Alonso-Mori, R.; Ignatyev, K.; et al. Pheomelanin pigment remnants mapped in fossils of an extinct mammal. Nat. Commun. 2019, 10, 2250. [Google Scholar] [CrossRef]
- Kawasaki, T.; Zen, H.; Ozaki, K.; Yamada, H.; Wakamatsu, K.; Ito, S. Application of mid-infrared free-electron laser for structural analysis of biological materials. J. Synchrotron Radiat. 2021, 28, 28–35. [Google Scholar] [CrossRef]
- d’Ischia, M.; Napolitano, A.; Michalczyk-Wetula, D.; Płonka, P.M. Melanin ‘dust’ or ‘ghost’? Exp. Dermatol. 2016, 25, 505–506. [Google Scholar] [CrossRef] [PubMed]
- Joly-Tonetti, N.; Wibawa, J.I.; Bell, M.; Tobin, D. Melanin fate in the human epidermis: A reassessment of how best to detect and analyse histologically. Exp. Dermatol. 2016, 25, 501–504. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Lui, H.; McLean, D.I.; Zeng, H. Near-infrared autofluorescence imaging of cutaneous melanins and human skin in vivo. J. Biomed. Opt. 2009, 14, 024017. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Zhao, J.; Lui, H.; He, Q.; Zeng, H. In vivo near-infrared autofluorescence imaging of pigmented skin lesions: Methods, technical improvements and preliminary clinical results. Skin Res. Technol. 2013, 19, 20–26. [Google Scholar] [CrossRef]
- Kalia, S.; Zhao, J.; Zeng, H.; McLean, D.; Kollias, N.; Lui, H. Melanin quantification by in vitro and in vivo analysis of near-infrared fluorescence. Pigment Cell Melanoma Res. 2018, 31, 31–38. [Google Scholar] [CrossRef]
- Ashtikar, M.; Matthäus, C.; Schmitt, M.; Krafft, C.; Fahr, A.; Popp, J. Non-invasive depth profile imaging of the stratum corneum using confocal Raman microscopy: First insights into the method. Eur. J. Pharm. Sci. 2013, 50, 601–608. [Google Scholar] [CrossRef]
- Bratchenko, I.A.; Artemyev, D.N.; Myakinin, O.O.; Khristoforova, Y.A.; Moryatov, A.A.; Kozlov, S.V.; Zakharov, V.P. Combined Raman and autofluorescence ex vivo diagnostics of skin cancer in near-infrared and visible regions. J. Biomed. Opt. 2017, 22, 027005. [Google Scholar] [CrossRef]
- Hani, A.F.M.; Baba, R.; Shamsuddin, N.; Nugroho, H. Determination of melanin types and relative concentrations: An observational study using a non-invasive inverse skin reflectance analysis. Int. J. Cosmet. Sci. 2014, 36, 451–458. [Google Scholar] [CrossRef]
- Teuchner, K.; Ehlert, J.; Freyer, W.; Leupold, D.; Altmeyer, P.; Stücker, M.; Hoffmann, K. Fluorescence studies of melanin by stepwise two-photon femtosecond laser excitation. J. Fluoresc. 2000, 10, 275. [Google Scholar] [CrossRef]
- Dimitrow, E.; Riemann, I.; Ehlers, A.; Koehler, M.J.; Norgauer, J.; Elsner, P.; König, K.; Kaatz, M. Spectral fluorescence lifetime detection and selective melanin imaging by multiphoton laser tomography for melanoma diagnosis. Exp. Dermatol. 2009, 18, 509–515. [Google Scholar] [CrossRef]
- Lai, Z.; Kerimo, J.; Mega, Y.J.; DiMarzio, C.A. Stepwise multiphoton activation fluorescence reveals a new method of melanin detection. J. Biomed. Opt. 2013, 18, 061225. [Google Scholar] [CrossRef]
- Dancik, Y.; Favre, A.; Loy, C.J.; Zvyagin, A.V.; Roberts, M.S. Use of multiphoton tomography and fluorescence lifetime imaging to investigate skin pigmentation in vivo. J. Biomed. Opt. 2013, 18, 026022. [Google Scholar] [CrossRef]
- Shirshin, E.A.; Gurfinkel, Y.I.; Proezzhev, A.V.; Fadeev, V.V.; Lademann, J.; Darvin, M.E. Two-photon autofluorescence lifetime imaging of human skin papillary dermis in vivo: Assessment of blood capillaries and structural proteins localization. Sci. Rep. 2017, 7, 1171. [Google Scholar] [CrossRef]
- Pena, A.-M.; Ito, S.; Bornschlögl, T.; Brizion, S.; Wakamatsu, K.; Del Bino, S. Multiphoton FLIM analyses of native and UVA modified synthetic melanins. Int. J. Mol. Sci. 2023, 24, 4517. [Google Scholar] [CrossRef]
- Rajadhyaksha, M.; Grossman, M.; Esterowitz, D.; Webb, R.H.; Anderson, R.R. In vivo confocal scanning laser microscopy of human skin: Melanin provides strong contrast. J. Investig. Dermatol. 1995, 104, 946–952. [Google Scholar] [CrossRef]
- Charles, C.A.; Marghoob, A.A.; Busam, K.J.; Clark-Loeser, L.; Halpern, A.C. Melanoma or pigmented basal cell carcinoma: A clinical-pathologic correlation with dermoscopy, in vivo confocal scanning laser microscopy, and routine histology. Skin Res. Technol. 2002, 8, 282–287. [Google Scholar] [CrossRef]
- Agero, A.L.C.; Busam, K.J.; Benvenuto-Andrade, C.; Scope, A.; Gill, M.; Marghoob, A.A.; González, S.; Halpern, A.C. Reflectance confocal microscopy of pigmented basal cell carcinoma. J. Am. Acad. Dermatol. 2006, 54, 638–643. [Google Scholar] [CrossRef]
- Gambichler, T.; Regeniter, P.; Bechara, F.; Orlikov, A.; Vasa, R.; Moussa, G.; Stücker, M.; Altmeyer, P.; Hoffmann, K. Characterization of benign and malignant melanocytic skin lesions using optical coherence tomography in vivo. J. Am. Acad. Dermatol. 2007, 57, 629–637. [Google Scholar] [CrossRef]
- Baumann, B.; Baumann, S.O.; Konegger, T.; Pircher, M.; Götzinger, E.; Schlanitz, F.; Schütze, C.; Sattmann, H.; Litschauer, M.; Schmidt-Erfurth, U.; et al. Polarization sensitive optical coherence tomography of melanin provides intrinsic contrast based on depolarization. Biomed. Opt. Express 2012, 3, 1670–1683. [Google Scholar] [CrossRef]
- Viator, J.A.; Komadina, J.; Svaasand, L.O.; Aguilar, G.; Choi, B.; Nelson, J.S. A comparative study of photoacoustic and reflectance methods for determination of epidermal melanin content. J. Investig. Dermatol. 2004, 122, 1432–1439. [Google Scholar] [CrossRef]
- Swearingen, J.A.; Holan, S.; Feldman, M.M.; Viator, J.A. Photoacoustic discrimination of vascular and pigmented lesions using classical and Bayesian methods. J. Biomed. Opt. 2010, 15, 016019. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Dinish, U.S.; Aguirre, J.; Bi, R.; Dev, K.; Attia, A.B.E.; Nitkunanatharajah, S.; Lim, Q.H.; Schwarz, M.; Yew, Y.W.; et al. Optoacoustic mesoscopy analysis and quantitative estimation of specific imaging metrics in Fitzpatrick skin phototypes II to V. J. Biophotonics 2019, 12, e201800442. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Zhou, D.; Abdel-Malek, Z.; Zhang, F.; Goff, P.S.; Sviderskaya, E.V.; Wakamatsu, K.; Ito, S.; Gross, S.S.; Zippin, J.H. Measurement of melanin metabolism in live cells by [U-13C]-tyrosine fate tracing using LC-MS. J. Investig. Dermatol. 2021, 141, 1810–1818.e6. [Google Scholar] [CrossRef] [PubMed]
- Kadekaro, A.L.; Leachman, S.; Kavanagh, R.J.; Swope, V.; Cassidy, P.; Supp, D.; Sartor, M.; Schwemberger, S.; Babcock, G.; Wakamatsu, K.; et al. Melanocortin 1 receptor genotype: An important determinant of the damage response of melanocytes to ultraviolet radiation. FASEB J. 2010, 24, 3850–3960. [Google Scholar] [CrossRef]
- Matthews, T.E.; Wilson, J.W.; Degan, S.; Simpson, M.J.; Jin, J.Y.; Zhang, J.Y.; Warren, S.; Warren, W.S. In vivo and ex vivo epi-mode pump-probe imaging of melanin and microvasculature. Biomed. Opt. Express 2011, 2, 1576–1583. [Google Scholar] [CrossRef]
- Simpson, M.J.; Glass, K.E.; Wilson, J.W.; Wilby, P.R.; Simon, J.D.; Warren, W.S. Pump-Probe Microscopic Imaging of Jurassic-Aged Eumelanin. J. Phys. Chem. Lett. 2013, 4, 1924–1927. [Google Scholar] [CrossRef]
- Simpson, M.J.; Wilson, J.W.; Phipps, M.A.; Robles, F.E.; Selim, M.A.; Warren, W.S. Nonlinear microscopy of eumelanin and pheomelanin with sub-cellular resolution. J. Investig. Dermatol. 2013, 133, 1822–1826. [Google Scholar] [CrossRef]
- Wilson, J.W.; Degan, S.; Gainey, C.S.; Mitropoulos, T.; Simpson, M.J.; Zhang, J.Y.; Warren, W.S. Comparing in vivo pump–probe and multiphoton fluorescence microscopy of melanoma and pigmented lesions. J. Biomed. Opt. 2014, 20, 051012. [Google Scholar] [CrossRef]
- Robles, F.E.; Wilson, J.W.; Warren, W.S. Quantifying melanin spatial distribution using pump-probe microscopy and a 2-D morphological autocorrelation transformation for melanoma diagnosis. J. Biomed. Opt. 2013, 18, 120502. [Google Scholar] [CrossRef]
- Robles, F.E.; Deb, S.; Wilson, J.W.; Gainey, C.S.; Selim, M.A.; Mosca, P.J.; Tyler, D.S.; Fischer, M.C.; Warren, W.S. Pump-probe imaging of pigmented cutaneous melanoma primary lesions gives insight into metastatic potential. Biomed. Opt. Express 2015, 6, 3631–3645. [Google Scholar] [CrossRef]
- Fischer, M.C.; Wilson, J.W.; Robles, F.E.; Warren, W.S. Invited review article: Pump-probe microscopy. Rev. Sci. Instrum. 2016, 87, 031101. [Google Scholar] [CrossRef]
- Robles, F.E.; Deb, S.; Fischer, M.C.; Warren, W.S.; Selim, M.A. Label-free imaging of female genital tract melanocytic lesions with pump-probe microscopy: A promising diagnostic tool. J. Low. Genit. Tract. Dis. 2017, 21, 137. [Google Scholar] [CrossRef]
- Ju, K.-Y.; Degan, S.; Zhou, K.C.; Jia, X.; Yu, J.; Warren, W.S. Unraveling the molecular nature of melanin changes in metastatic cancer. J. Biomed. Opt. 2019, 24, 051414. [Google Scholar] [CrossRef]
- Grass, D.; Beasley, G.M.; Fischer, M.C.; Selim, M.A.; Zhou, Y.; Warren, W.S. Contrast mechanisms in pump-probe microscopy of melanin. Optics Express 2022, 30, 31852. [Google Scholar] [CrossRef]
- Thompson, A.; Robles, F.E.; Wilson, J.W.; Deb, S.; Calderbank, R.; Warren, W.S. Dual-wavelength pump-probe microscopy analysis of melanin composition. Sci. Rep. 2016, 6, 36871. [Google Scholar] [CrossRef]
- Del Bino, S.; Duval, C.; Bernerd, F. Clinical and biological characterization of skin pigmentation diversity and its consequences on UV impact. Int. J. Mol. Sci. 2018, 19, 2668. [Google Scholar] [CrossRef]
- Yamaguchi, Y.; Brenner, M.; Hearing, V.J. The regulation of skin pigmentation. J. Biol. Chem. 2007, 282, 27557–27561. [Google Scholar] [CrossRef]
- Del Bino, S.; Bernerd, F. Variations in skin colour and the biological consequences of ultraviolet radiation exposure. Br. J. Dermatol. 2013, 169 (Suppl. 3), 33–40. [Google Scholar] [CrossRef]
- Chardon, A.; Cretois, I.; Hourseau, C. Skin colour typology and suntanning pathways. Int. J. Cosmet. Sci. 1991, 13, 191–208. [Google Scholar] [CrossRef]
- Del Bino, S.; Sok, J.; Bessac, E.; Bernerd, F. Relationship between skin response to ultraviolet exposure and skin color type. Pigment Cell Res. 2006, 19, 606–614. [Google Scholar] [CrossRef]
- Itou, T.; Ito, S.; Wakamatsu, K. Effects of aging on hair color, melanosomes, and melanin composition in Japanese males and their sex differences. Int. J. Mol. Sci. 2022, 23, 14459. [Google Scholar] [CrossRef] [PubMed]
- Matias, A.R.; Ferreira, M.; Costa, P.; Neto, P. Skin colour, skin redness and melanin biometric measurements: Comparison study between Antera(®) 3D, Mexameter(®) and colorimeter(®). Skin Res. Technol. 2015, 21, 346–362. [Google Scholar] [CrossRef] [PubMed]
- Xiao, K.; Zhu, Y.; Li, C.; Connah, D.; Yates, J.M.; Wuerger, S. Improved method for skin reflectance reconstruction from camera images. Opt. Express 2016, 24, 14934–14950. [Google Scholar] [CrossRef] [PubMed]
- Masuda, Y.; Yamashita, T.; Hirao, T.; Takahashi, M. An innovative method to measure skin pigmentation. Skin Res. Technol. 2009, 15, 224–229. [Google Scholar] [CrossRef]
- Alaluf, S.; Atkins, D.; Barrett, K.; Blount, M.; Carter, N.; Heath, A. Ethnic variation in melanin content and composition in photoexposed and photoprotected human skin. Pigment Cell Res. 2002, 15, 112–118. [Google Scholar] [CrossRef]
- Alaluf, S.; Atkins, D.; Barrett, K.; Blount, M.; Carter, N.; Heath, A. The impact of epidermal melanin on objective measurements of human skin colour. Pigment Cell Res. 2002, 15, 119–126. [Google Scholar] [CrossRef]
- Matsunaka, H.; Yamamoto, Y.; Furukawa, F. Non-invasive quantification of melanin in the stratum corneum: A novel indicator of skin lesions in pigmentation diseases. Skin Res. Technol. 2017, 23, 104–111. [Google Scholar] [CrossRef]
- Hughes, A.J.; Tawfik, S.S.; Baruah, K.P.; O’Toole, E.A.; O’shaughnessy, R.F.L. Tape strips in dermatology research. Br. J. Dermatol. 2021, 185, 26–35. [Google Scholar] [CrossRef]
- Keurentjes, A.J.; Jakasa, I.; Kezic, S. Research techniques made simple: Stratum corneum tape stripping. J. Investig. Dermatol. 2021, 141, 1129–1133.e1. [Google Scholar] [CrossRef]
- Ito, S.; Imai, Y.; Jimbow, K.; Fujita, K. Incorporation of sulfhydryl compounds into melanins in vitro. Biochim. Biophys. Acta 1988, 964, 1–7. [Google Scholar] [CrossRef]
- Ito, S. Optimization of conditions for preparing synthetic pheomelanin. Pigment Cell Res. 1989, 2, 53–56. [Google Scholar] [CrossRef]
- Ma, X.X.; Sun, X.X. Melanin: Biosynthesis, Functions and Health Effects; Nova Science Publishers: Hauppauge, NY, USA, 2012; ISBN 978-1-62100-991-7. [Google Scholar]
- Pombeiro-Sponchiado, S.R.; Sousa, G.S.; Andrade, J.C.R.; Lisboa, H.F.; Gonçalves, R.C.R. Production of Melanin Pigment by Fungi and Its Biotechnological Applications. In Melanin; IntechOpen: London, UK, 2017. [Google Scholar]
- Gonçalves, R.C.R.; Lisboa, H.C.F.; Pombeiro-Sponchiado, S.R. Characterization of melanin pigment produced by Aspergillus nidulans. World J. Microbiol. Biotechnol. 2012, 28, 1467–1474. [Google Scholar] [CrossRef]
- Jiang, S.; Liu, X.-M.; Dai, X.; Zhou, Q.; Lei, T.-C.; Beermann, F.; Wakamatsu, K.; Xu, S.-Z. Regulation of DHICA-mediated antioxidation by dopachrome tautomerase: Implication for skin photoprotection against UVA radiation. Free Radic. Biol. Med. 2010, 48, 1144–1151. [Google Scholar] [CrossRef]
- Cao, W.; Mao, H.; McCallum, N.C.; Zhou, X.; Sun, H.; Sharpe, C.; Korpanty, J.; Hu, Z.; Ni, Q.Z.; Burkart, M.D.; et al. Biomimetic pheomelanin to unravel the electronic, molecular and supramolecular structure of the natural product. Chem. Sci. 2023, 14, 4183. [Google Scholar] [CrossRef]
- Singh, S.; Nimse, S.B.; Mathew, D.E.; Dhimmar, A.; Sahastrabudhe, H.; Gajjar, A.; Ghadge, V.A.; Kumar, P.; Shinde, P.B. Microbial melanin: Recent advances in biosynthesis, extraction, characterization, and applications. Biotechnol. Adv. 2021, 53, 107773. [Google Scholar] [CrossRef]

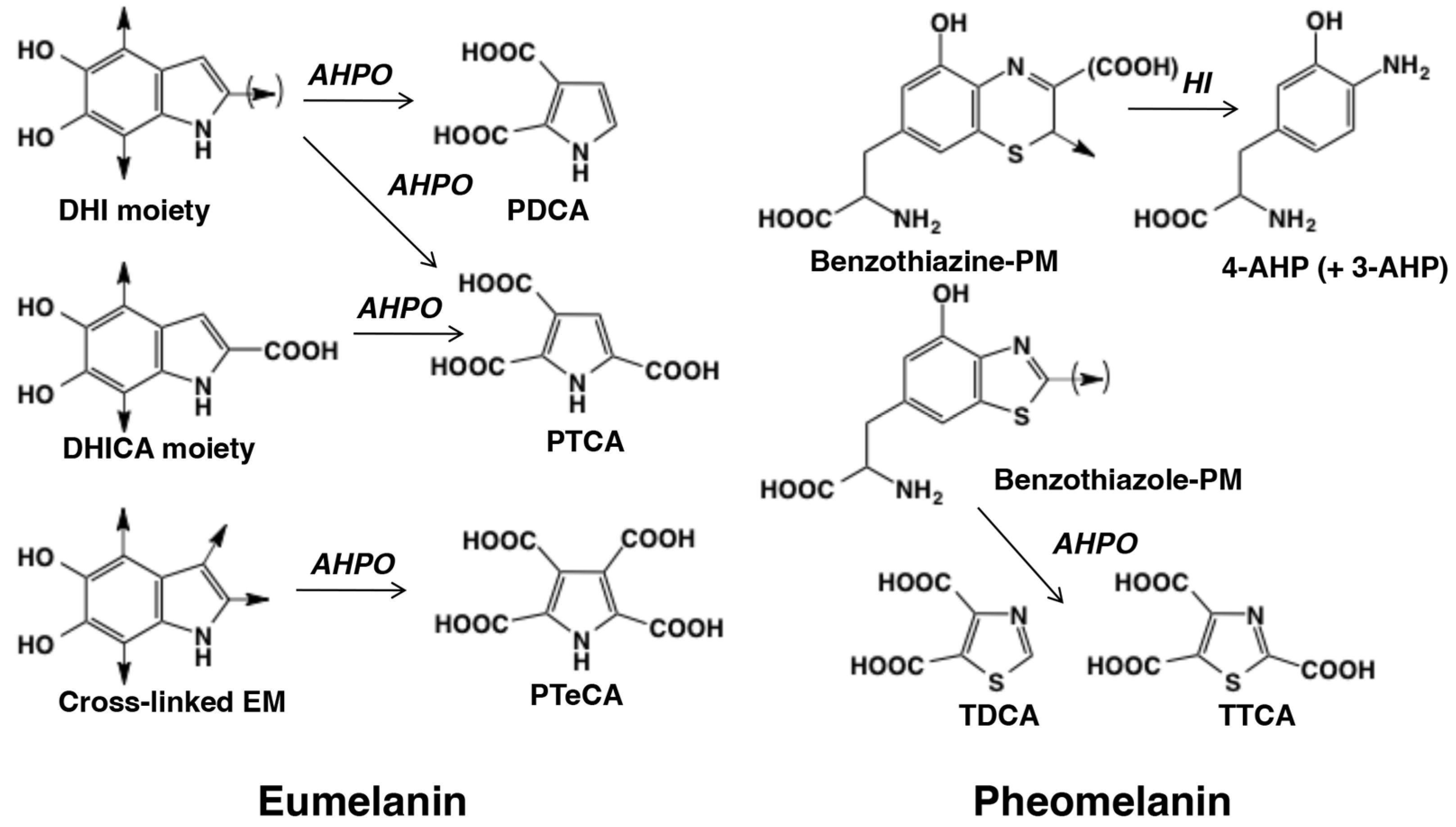
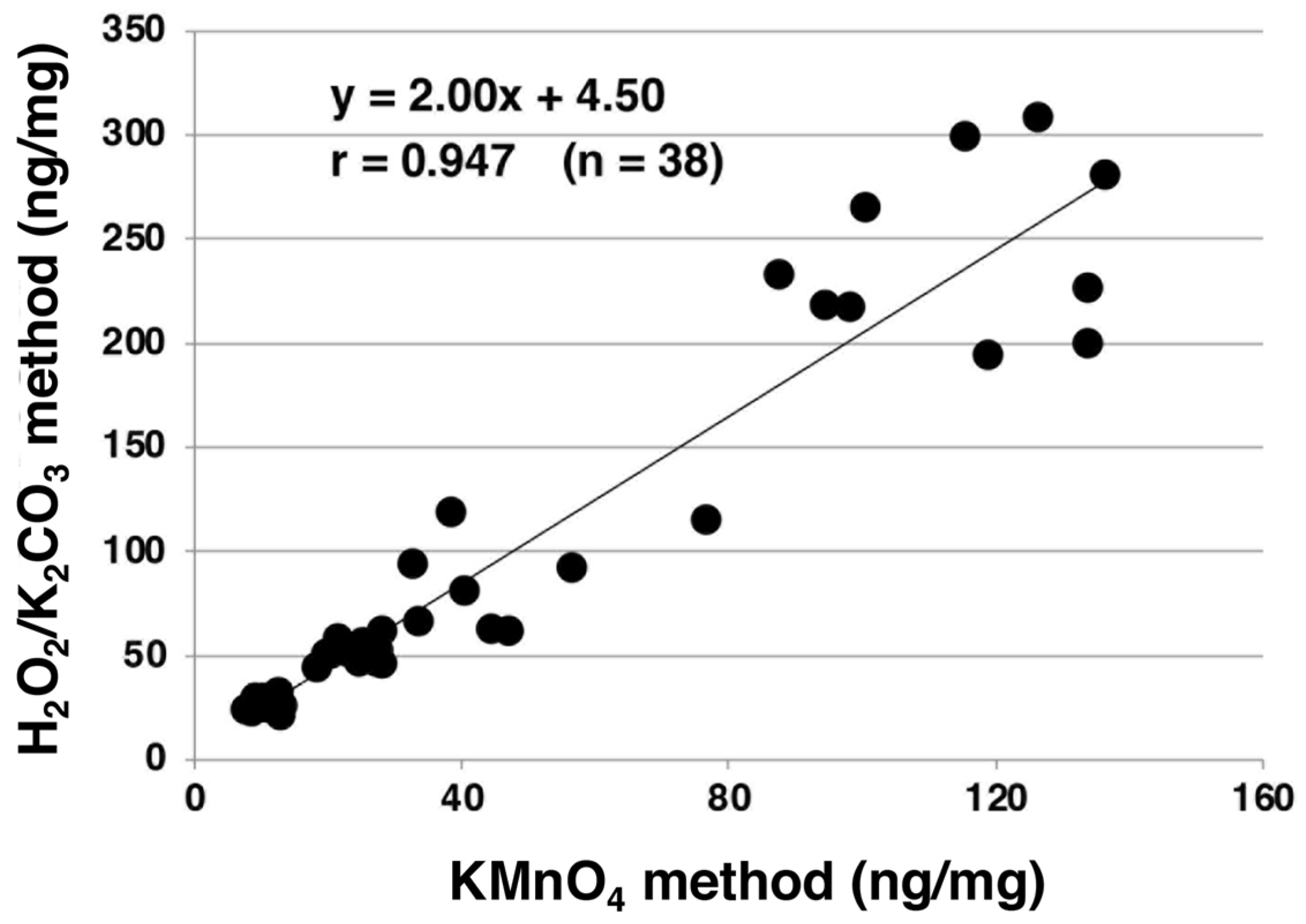



| Eumelanin (EM) | Methods | Advantages | Limitations |
| Acidic KMnO4 oxidation [34,35,36,41] | PTCA is highly specific | (1) Difficult to perform (ether extraction) (2) PDCA cannot be determined | |
| Alkaline H2O2 oxidation (AHPO) [43,47,48] | (1) Easy to perform by direct injection (2) PDCA can also be determined | Artificial production of PTCA from PM | |
| Pheomelanin (PM) | Methods | Advantages | Limitations |
| HI hydrolysis [34,46] | (1) 4-AHP is highly specific (2) Electrochemical detection (ECD) is highly sensitive | Difficult to perform (HI evaporation) | |
| Alkaline H2O2 oxidation (AHPO) [43,47,48] | (1) Easy to perform (2) TTCA and TDCA can be used as markers | Lower sensitivity for TTCA and TDCA |
| Hair | PTCA | PDCA | PTeCA | TTCA | TDCA |
|---|---|---|---|---|---|
| Black | 205 ± 4.4 (2.1%) | 19.2 ± 0.60 (3.2%) | 82.0 ± 3.6 (4.4%) | 43.4 ± 0.94 (2.2%) | 19.4 ± 1.9 (9.7%) |
| Red | 23.4 ± 1.2 (5.2%) | 10.7 ± 0.52 (4.8%) | 12.9 ± 1.6 (12.4%) | 169 ± 6.7 (4.0%) | 26.7 ± 0.86 (3.2%) |
| Analytical Method | Principles of Methods | Advantages | Limitations |
|---|---|---|---|
| Spectrophotometric analysis | (1) Soluene-350 plus water (2) NaOH | (1) Conventional and inexpensive (2) Solubilizes both EM and PM (Soluene-350) (3) Good correlation between TM using spectrometry and melanin contents using HPLC | (1) Some background of absorbance from tissue constituents such as proteins and the viscosity of the solvent (Soluene-350) (2) Low sensitivity and selectivity |
| Electron paramagnetic resonance (EPR) spectroscopy | Method for studying materials that have unpaired electrons | (1) Non-invasive and non-destructive (2) Method allows to distinguish between EM and PM (3) Possibility to study physicochemical properties of melanins after binding chemicals and metals | (1) Short lifespan of some radicals (2) Instrument is expensive (3) Sub-units of EM and PM cannot be quantified (4) Unable to distinguish between 1,8-dihydroxynaphthalene (DHN)-, pyo-, and eumelanins |
| 1H and 13C-NMR spectrometry | The method is a physicochemical technique and based on the physical phenomenon of magnetic resonance | (1) High selectivity (2) Solid samples can be analyzed (3) Useful for characterizing structural features such as the ratios of aliphatic and aromatic 1H and 13C | (1) Not laboratory-based (2) Instrument is expensive and large (3) Not suited for the quantitative analysis of melanins in tissue samples |
| Fourier transform infrared spectroscopy (FTIR) | (1) The technique is used to obtain an infrared spectrum of absorption or emission from a solid, liquid, or gas (2) Method based on absorption of infrared radiation and excitation of oscillatory levels, to study the presence of chemical functional groups | (1) Highly sensitive and quick method to achieve a high-quality spectrum (2) Ability to analyze solid, liquid, or gas phase samples (3) Good signal-to-noise ratio (4) Non-destructive (5) Scan within 1–2 s (6) Capability to create chemical distribution images | (1) Only single beam (2) Instrument is expensive (3) The Rayleigh criterion reduces the spatial resolution of chemical images compared to resonance Raman spectroscopy images |
| Electron microscopy | The method uses a beam of electrons and their wave-like characteristics to magnify an object’s image | (1) Powerful method for investigating the morphology and particle size distribution of various types of melanin | (1) Instrument is expensive and large |
| Mass Spectrometry (MALDI–MS, ToF–SIMS, GC/MS) | The method is to generate ions from either organic or inorganic compounds, using any suitable method to separate these ions by their mass-to-charge ratio (m/z) and to detect them qualitatively and quantitatively by their respective m/z and abundance | (1) Highly sensitive and selective (2) Able to identify/quantify components of mixtures (3) Possibility of combination with other techniques such as HPLC (LC–MS) and gas chromatography (GC–MS) (4) Very precise, rapid, and sensitive method (5) Measurability with very small amounts (ppm levels) of samples (6) Able to be used for both qualitative and quantitative analysis of chemicals | (1) Costly, the system needs a skilled technician and is not a portable system (2) Large instrument (3) Not laboratory-based |
| Pyrolysis | The method is a process by the thermal decomposition occurring in the absence of oxygen or any other oxidants, and one of the most common methods in thermal conversion technology of biomass | (1) Simple and fast process (2) More suitable technology for bio-oil production (3) Scale-up is economically feasible (4) Efficient energy conversion | (1) Possibility of requiring additional energy (2) Increased biochar production (3) Biomass collection is its main problem of industrialization (4) Limited commercial experience (5) Low thermal stability (6) Production of pyrolytic water |
| Raman spectroscopy | Raman is a scattering technique which is based on Raman effect, i.e., the frequency of a small fraction of scattered radiation is different from the frequency of monochromatic incident radiation. It is based on the inelastic scattering of incident radiation through its interaction with vibrating molecules. | (1) Non-invasive and non-destructive (2) Good correlation between the results obtained from the chemical degradations and the Raman spectrum (3) Laboratory-based and portable instruments (4) Ability to analyze materials in sealed transparent containers (5) Capability to create chemical distribution images | (1) Difficulty to build calibration curves (2) Trouble with fluorescent or strongly absorbing materials (black materials) (3) Chemical species with a high Raman scattering cross-section, even in small concentrations, may give intensive bands covering other, important analytical bands in the spectrum (4) Problematical to obtain an accurate spectrum of amorphous materials (5) Potential for ignition of explosives (6) Possibly long collection times (7) Low sensitivity (8) Instrument is expensive (9) Specific power requirements |
| Synchrotron rapid scanning X-ray fluorescence | The method provides spatial distribution and quantification of ions in samples ranging in length from mm to submicron | (1) Able to visualize the distribution of ions. (2) Useful to study ionic processes at very small scales. (3) Useful for in vivo analysis that requires room temperatures and pressures or high detection limits of about 1-100 mg/kg or nanoscale resolutions of about 50 nm. | (1) Necessitates very large and sophisticated facilities that are not readily available to the public. (2) Sample preparation necessitates extensive technical knowledge and is delicate. (3) The weight of elements influences the distribution of elements in samples. |
| Near-infrared excited fluorescence | Light of 800 to 2500 nm, which is said to be in the near-infrared region, irradiates the sample, and other components are simultaneously measured by making full use of statistical methods for the absorbed wavelengths. | (1) High sensitivity, non-invasiveness, and lack of radiation hazard (2) Low background interference (3) High penetration (1–10 mm) (4) Low tissue damage (5) Fast, real-time display, relatively low cost, portability (6) Sample preparation is almost unnecessary | (1) Broad peak shape (2) Quantitative analysis is difficult. (3) Moisture and granularity are influential factors |
| Pump–probe method | Using two beams of light (or particle beams), one beam (the pump beam) illuminates a material to induce changes in the material, and the other beam (the probe beam) measures those changes | (1) Non-destructive to cells and tissues (2) Imaging of endogenous pigments with three-dimensional spatial resolution (3) Efficient discrimination between hemoglobin and melanin (4) Possible discrimination of melanoma based on EM/PM ratio | (1) Difficulty of accurate detection in samples with weak signals, such as highly diluted solutions (2) Difficulty in suppressing scattered light, especially when the pump and probe spectra overlap (3) Contamination of the signal from the probe beam by a strong pump, when the pump and probe pulses overlap in both space and time at the sample surface (4) Instrument is expensive |
| ITA and colorimetric parameters | (1) A colorimeter is a light-sensitive tool used to measure the absorption and transmission of light passing through a sample matrix. (2) ITA measures constitutive pigmentation. (3) ITA and colorimetric parameters are facultative. | (1) Inexpensive, fast, and simple operation of the colorimetric method (2) A fast and convenient method compared to the volumetric or gravimetric process (3) Does not require an experienced person to handle the colorimetric method (4) Applied to the quantitative analysis of colored compounds with the colorimetric method (5) Portable system to easily carry for the colorimetric method (6) Low cost | (1) Unable to analyze colorless compounds (2) Needs a high number of samples for analysis (3) Low sensitivity (4) Generation of errors in the results by interference from material of the same color (5) Reflection of light on some surfaces makes measurements difficult |
| Tape stripping | A method to collect and remove the stratum corneum by attaching adhesive tape such as cellophane tape to the skin | (1) Minimally invasive (2) Simple and easy to perform (3) Easy collection of stratum corneum | (1) Unknown kinetics of biomarker translocation from the living epidermis to stratum corneum |
| Elemental analysis of C, H, N, and S | Method to determine the elemental composition of the sample | (1) Ability to distinguish EM, PM, DHN-, and pyomelanins (2) Fast (3) Low cost | (1) High purity of the sample required (2) It may be difficult to distinguish between DHN- and pyomelanins. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wakamatsu, K.; Ito, S. Recent Advances in Characterization of Melanin Pigments in Biological Samples. Int. J. Mol. Sci. 2023, 24, 8305. https://doi.org/10.3390/ijms24098305
Wakamatsu K, Ito S. Recent Advances in Characterization of Melanin Pigments in Biological Samples. International Journal of Molecular Sciences. 2023; 24(9):8305. https://doi.org/10.3390/ijms24098305
Chicago/Turabian StyleWakamatsu, Kazumasa, and Shosuke Ito. 2023. "Recent Advances in Characterization of Melanin Pigments in Biological Samples" International Journal of Molecular Sciences 24, no. 9: 8305. https://doi.org/10.3390/ijms24098305
APA StyleWakamatsu, K., & Ito, S. (2023). Recent Advances in Characterization of Melanin Pigments in Biological Samples. International Journal of Molecular Sciences, 24(9), 8305. https://doi.org/10.3390/ijms24098305







