Peptide Regulation of Chondrogenic Stem Cell Differentiation
Abstract
:1. Introduction
2. Materials and Methods
3. Differentiation of Human Chondrocytes
4. MSC Chondrogenic Factors
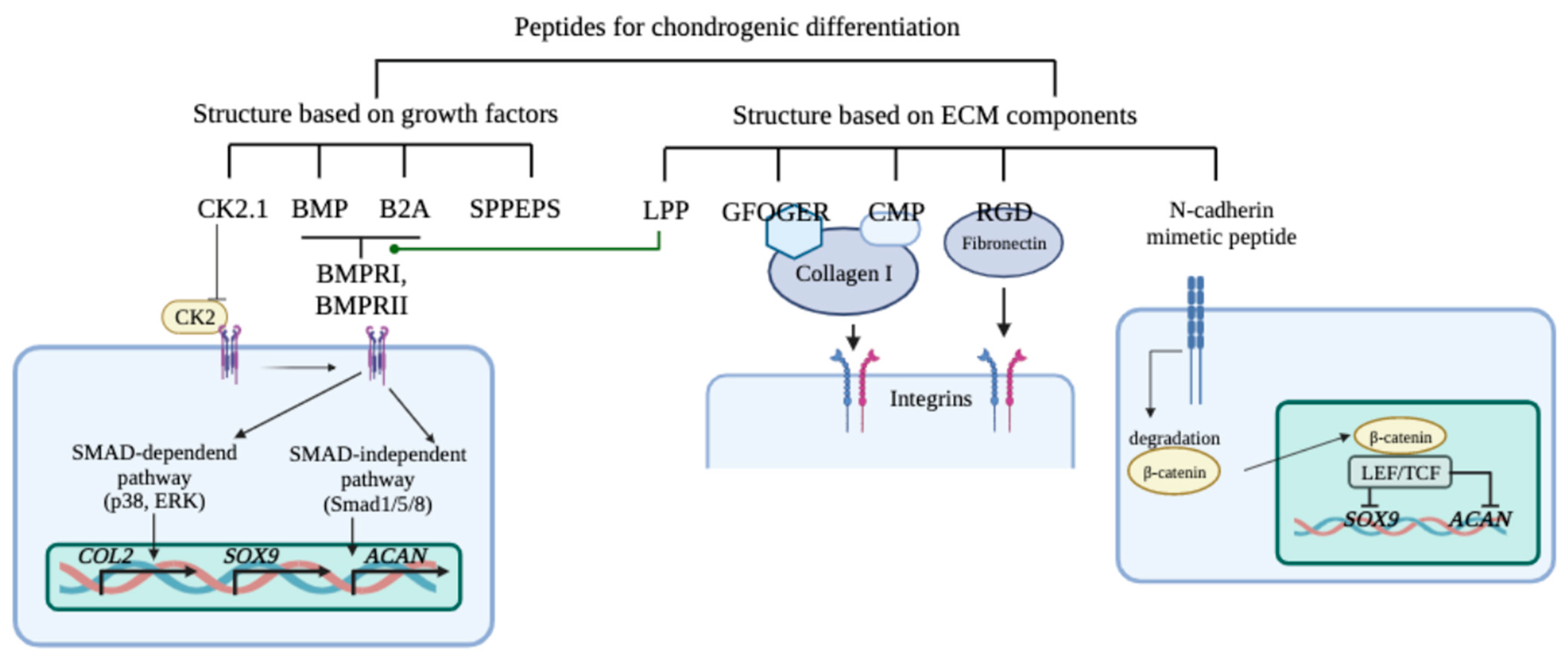
5. Peptides That Stimulate Chondrogenic Differentiation
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sen, R.; Hurley, J.A. Osteoarthritis. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- Hermann, W.; Lambova, S.; Muller-Ladner, U. Current Treatment Options for Osteoarthritis. Curr. Rheumatol. Rev. 2018, 14, 108–116. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Merchan, E.C. Regeneration of Articular Cartilage of the Knee. Rheumatol. Int. 2013, 33, 837–845. [Google Scholar] [CrossRef] [PubMed]
- Rakic, R.; Bourdon, B.; Demoor, M.; Maddens, S.; Saulnier, N.; Galéra, P. Differences in the Intrinsic Chondrogenic Potential of Equine Umbilical Cord Matrix and Cord Blood Mesenchymal Stromal/Stem Cells for Cartilage Regeneration. Sci. Rep. 2018, 8, 13799. [Google Scholar] [CrossRef]
- Mushahary, D.; Spittler, A.; Kasper, C.; Weber, V.; Charwat, V. Isolation, Cultivation, and Characterization of Human Mesenchymal Stem Cells. Cytom. Part A J. Int. Soc. Anal. Cytol. 2018, 93, 19–31. [Google Scholar] [CrossRef] [PubMed]
- Khavinson, V.K.; Popovich, I.G.; Linkova, N.S.; Mironova, E.S.; Ilina, A.R. Peptide Regulation of Gene Expression: A Systematic Review. Molecules 2021, 26, 7053. [Google Scholar] [CrossRef] [PubMed]
- Lin’kova, N.S.; Drobintseva, A.O.; Orlova, O.A.; Kuznetsova, E.P.; Polyakova, V.O.; Kvetnoy, I.M.; Khavinson, V.K. Peptide Regulation of Skin Fibroblast Functions during Their Aging In Vitro. Bull. Exp. Biol. Med. 2016, 161, 175–178. [Google Scholar] [CrossRef]
- Ashapkin, V.V.; Linkova, N.S.; Khavinson, V.K.; Vanyushin, B.F. Epigenetic Mechanisms of Peptidergic Regulation of Gene Expression during Aging of Human Cells. Biochemistry 2015, 80, 310–322. [Google Scholar] [CrossRef]
- Ashapkin, V.; Khavinson, V.; Shilovsky, G.; Linkova, N.; Vanuyshin, B. Gene Expression in Human Mesenchymal Stem Cell Aging Cultures: Modulation by Short Peptides. Mol. Biol. Rep. 2020, 47, 4323–4329. [Google Scholar] [CrossRef]
- Saiki, H.; Okano, Y.; Yasuma, T.; Toda, M.; Takeshita, A.; Abdel-Hamid, A.M.; Fridman D’Alessandro, V.; Tsuruga, T.; D’Alessandro-Gabazza, C.N.; Katayama, K.; et al. A Microbiome-Derived Peptide Induces Apoptosis of Cells from Different Tissues. Cells 2021, 10, 2885. [Google Scholar] [CrossRef]
- Sun, M.; Wang, C.; Lv, M.; Fan, Z.; Du, J. Intracellular Self-Assembly of Peptides to Induce Apoptosis against Drug-Resistant Melanoma. J. Am. Chem. Soc. 2022, 144, 7337–7345. [Google Scholar] [CrossRef]
- Min, K.A.; Maharjan, P.; Ham, S.; Shin, M.C. Pro-Apoptotic Peptides-Based Cancer Therapies: Challenges and Strategies to Enhance Therapeutic Efficacy. Arch. Pharm. Res. 2018, 41, 594–616. [Google Scholar] [CrossRef] [PubMed]
- Zharkova, M.S.; Orlov, D.S.; Golubeva, O.Y.; Chakchir, O.B.; Eliseev, I.E.; Grinchuk, T.M.; Shamova, O.V. Application of Antimicrobial Peptides of the Innate Immune System in Combination With Conventional Antibiotics—A Novel Way to Combat Antibiotic Resistance? Front. Cell. Infect. Microbiol. 2019, 9, 128. [Google Scholar] [CrossRef] [PubMed]
- Hoffknecht, B.C.; Albada, H.B.; Sturm, M.; Prochnow, P.; Bandow, J.E.; Metzler-Nolte, N. Synthesis and Antibacterial Activity of Trivalent Ultrashort Arg-Trp-Based Antimicrobial Peptides (AMPs). MedChemComm 2015, 6, 372–376. [Google Scholar] [CrossRef]
- Liang, W.; Diana, J. The Dual Role of Antimicrobial Peptides in Autoimmunity. Front. Immunol. 2020, 11, 2077. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, T.-H.; Hsu, C.-Y.; Tsai, C.-F.; Chiu, C.-C.; Liang, S.-S.; Wang, T.-N.; Kuo, P.-L.; Long, C.-Y.; Tsai, E.-M. A Novel Cell-Penetrating Peptide Suppresses Breast Tumorigenesis by Inhibiting β-Catenin/LEF-1 Signaling. Sci. Rep. 2016, 6, 19156. [Google Scholar] [CrossRef]
- Pang, Y.; Liu, Z.; Han, H.; Wang, B.; Li, W.; Mao, C.; Liu, S. Peptide SMIM30 Promotes HCC Development by Inducing SRC/YES1 Membrane Anchoring and MAPK Pathway Activation. J. Hepatol. 2020, 73, 1155–1169. [Google Scholar] [CrossRef]
- Sato, K.; Asai, T.T.; Jimi, S. Collagen-Derived Di-Peptide, Prolylhydroxyproline (Pro-Hyp): A New Low Molecular Weight Growth-Initiating Factor for Specific Fibroblasts Associated With Wound Healing. Front. Cell Dev. Biol. 2020, 8, 548975. [Google Scholar] [CrossRef]
- Song, Y.; Wu, C.; Zhang, X.; Bian, W.; Liu, N.; Yin, S.; Yang, M.; Luo, M.; Tang, J.; Yang, X. A Short Peptide Potentially Promotes the Healing of Skin Wound. Biosci. Rep. 2019, 39, BSR20181734. [Google Scholar] [CrossRef]
- Tripathi, J.K.; Pal, S.; Awasthi, B.; Kumar, A.; Tandon, A.; Mitra, K.; Chattopadhyay, N.; Ghosh, J.K. Variants of Self-Assembling Peptide, KLD-12 That Show Both Rapid Fracture Healing and Antimicrobial Properties. Biomaterials 2015, 56, 92–103. [Google Scholar] [CrossRef]
- Zhu, M.; Zhong, W.; Cao, W.; Zhang, Q.; Wu, G. Chondroinductive/Chondroconductive Peptides and Their-Functionalized Biomaterials for Cartilage Tissue Engineering. Bioact. Mater. 2021, 9, 221–238. [Google Scholar] [CrossRef]
- Renner, J.N.; Liu, J.C. Investigating the Effect of Peptide Agonists on the Chondrogenic Differentiation of Human Mesenchymal Stem Cells Using Design of Experiments. Biotechnol. Prog. 2013, 29, 1550–1557. [Google Scholar] [CrossRef] [PubMed]
- Camarero-Espinosa, S.; Cooper-White, J.J. Combinatorial Presentation of Cartilage-Inspired Peptides on Nanopatterned Surfaces Enables Directed Differentiation of Human Mesenchymal Stem Cells towards Distinct Articular Chondrogenic Phenotypes. Biomaterials 2019, 210, 105–115. [Google Scholar] [CrossRef]
- Liu, C.-F.; Samsa, W.E.; Zhou, G.; Lefebvre, V. Transcriptional Control of Chondrocyte Specification and Differentiation. Semin. Cell Dev. Biol. 2017, 62, 34–49. [Google Scholar] [CrossRef] [PubMed]
- Yi, S.W.; Kim, H.J.; Oh, H.J.; Shin, H.; Lee, J.S.; Park, J.S.; Park, K.-H. Gene Expression Profiling of Chondrogenic Differentiation by Dexamethasone-Conjugated Polyethyleneimine with SOX Trio Genes in Stem Cells. Stem Cell Res. Ther. 2018, 9, 341. [Google Scholar] [CrossRef] [PubMed]
- Griffin, M.; Hindocha, S.; Khan, W.S. Chondrogenic Differentiation of Adult MSCs. Curr. Stem Cell Res. Ther. 2012, 7, 260–265. [Google Scholar] [CrossRef]
- Zhernasechanka, H.A.; Isaikina, Y.I.; Filipovich, T.V.; Liakh, E.G. Osteogenic and chondrogenic differentiation potential of mesenchymal stem cells obtained from the bone marrow and placenta. Proc. Natl. Acad. Sci. Belarus Med. Ser. 2021, 18, 36–45. [Google Scholar] [CrossRef]
- Bernardo, M.E.; Emons, J.A.M.; Karperien, M.; Nauta, A.J.; Willemze, R.; Roelofs, H.; Romeo, S.; Marchini, A.; Rappold, G.A.; Vukicevic, S.; et al. Human Mesenchymal Stem Cells Derived from Bone Marrow Display a Better Chondrogenic Differentiation Compared with Other Sources. Connect. Tissue Res. 2007, 48, 132–140. [Google Scholar] [CrossRef]
- Zha, K.; Sun, Z.; Yang, Y.; Chen, M.; Gao, C.; Fu, L.; Li, H.; Sui, X.; Guo, Q.; Liu, S. Recent Developed Strategies for Enhancing Chondrogenic Differentiation of MSC: Impact on MSC-Based Therapy for Cartilage Regeneration. Stem Cells Int. 2021, 2021, 8830834. [Google Scholar] [CrossRef]
- Somoza, R.A.; Welter, J.F.; Correa, D.; Caplan, A.I. Chondrogenic Differentiation of Mesenchymal Stem Cells: Challenges and Unfulfilled Expectations. Tissue Eng. Part B Rev. 2014, 20, 596–608. [Google Scholar] [CrossRef]
- Shachpazyan, N.R.; Astrelina, T.A.; Yakovleva, M.V. Mesenchymalstem Cells from Various Human Tissues: Biological Properties, Assessmentof Quality and Safetyfor Clinical Use. Genes Cells 2012, 7, 23–33. [Google Scholar]
- Boeuf, S.; Richter, W. Chondrogenesis of Mesenchymal Stem Cells: Role of Tissue Source and Inducing Factors. Stem Cell Res. Ther. 2010, 1, 31. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Yang, Z.; Ding, J.; Liu, S.; Guo, B.; Yue, Z. Function and Regulation of Transforming Growth Factor β1 Signalling in Antler Chondrocyte Proliferation and Differentiation. Cell Prolif. 2019, 52, e12637. [Google Scholar] [CrossRef] [PubMed]
- Futrega, K.; Robey, P.G.; Klein, T.J.; Crawford, R.W.; Doran, M.R. A Single Day of TGF-β1 Exposure Activates Chondrogenic and Hypertrophic Differentiation Pathways in Bone Marrow-Derived Stromal Cells. Commun. Biol. 2021, 4, 29. [Google Scholar] [CrossRef] [PubMed]
- Lefebvre, V.; Angelozzi, M.; Haseeb, A. SOX9 in Cartilage Development and Disease. Curr. Opin. Cell Biol. 2019, 61, 39–47. [Google Scholar] [CrossRef] [PubMed]
- Sock, E.; Schmidt, K.; Hermanns-Borgmeyer, I.; Bösl, M.R.; Wegner, M. Idiopathic Weight Reduction in Mice Deficient in the High-Mobility-Group Transcription Factor Sox8. Mol. Cell. Biol. 2001, 21, 6951–6959. [Google Scholar] [CrossRef] [PubMed]
- Bhattaram, P.; Penzo-Méndez, A.; Kato, K.; Bandyopadhyay, K.; Gadi, A.; Taketo, M.M.; Lefebvre, V. SOXC Proteins Amplify Canonical WNT Signaling to Secure Nonchondrocytic Fates in Skeletogenesis. J. Cell Biol. 2014, 207, 657–671. [Google Scholar] [CrossRef]
- Liu, J.; Xiao, Q.; Xiao, J.; Niu, C.; Li, Y.; Zhang, X.; Zhou, Z.; Shu, G.; Yin, G. Wnt/β-Catenin Signalling: Function, Biological Mechanisms, and Therapeutic Opportunities. Signal Transduct. Target. Ther. 2022, 7, 3. [Google Scholar] [CrossRef]
- Mikasa, M.; Rokutanda, S.; Komori, H.; Ito, K.; Tsang, Y.S.; Date, Y.; Yoshida, C.A.; Komori, T. Regulation of Tcf7 by Runx2 in Chondrocyte Maturation and Proliferation. J. Bone Miner. Metab. 2011, 29, 291–299. [Google Scholar] [CrossRef]
- Ma, B.; Zhong, L.; van Blitterswijk, C.A.; Post, J.N.; Karperien, M. T Cell Factor 4 Is a Pro-Catabolic and Apoptotic Factor in Human Articular Chondrocytes by Potentiating Nuclear Factor κB Signaling. J. Biol. Chem. 2013, 288, 17552–17558. [Google Scholar] [CrossRef]
- Wu, H.; Whitfield, T.W.; Gordon, J.A.R.; Dobson, J.R.; Tai, P.W.L.; van Wijnen, A.J.; Stein, J.L.; Stein, G.S.; Lian, J.B. Genomic Occupancy of Runx2 with Global Expression Profiling Identifies a Novel Dimension to Control of Osteoblastogenesis. Genome Biol. 2014, 15, R52. [Google Scholar] [CrossRef]
- LeBlanc, K.T.; Walcott, M.E.; Gaur, T.; O’Connell, S.L.; Basil, K.; Tadiri, C.P.; Mason-Savas, A.; Silva, J.A.; van Wijnen, A.J.; Stein, J.L.; et al. Runx1 Activities in Superficial Zone Chondrocytes, Osteoarthritic Chondrocyte Clones and Response to Mechanical Loading. J. Cell. Physiol. 2015, 230, 440–448. [Google Scholar] [CrossRef] [PubMed]
- Potthoff, M.J.; Olson, E.N. MEF2: A Central Regulator of Diverse Developmental Programs. Dev. Camb. Engl. 2007, 134, 4131–4140. [Google Scholar] [CrossRef] [PubMed]
- Dy, P.; Wang, W.; Bhattaram, P.; Wang, Q.; Wang, L.; Ballock, R.T.; Lefebvre, V. Sox9 Directs Hypertrophic Maturation and Blocks Osteoblast Differentiation of Growth Plate Chondrocytes. Dev. Cell 2012, 22, 597–609. [Google Scholar] [CrossRef] [PubMed]
- Arnold, M.A.; Kim, Y.; Czubryt, M.P.; Phan, D.; McAnally, J.; Qi, X.; Shelton, J.M.; Richardson, J.A.; Bassel-Duby, R.; Olson, E.N. MEF2C Transcription Factor Controls Chondrocyte Hypertrophy and Bone Development. Dev. Cell 2007, 12, 377–389. [Google Scholar] [CrossRef]
- Wu, S.; Morrison, A.; Sun, H.; De Luca, F. Nuclear Factor-κB (NF-κB) p65 Interacts with Stat5b in Growth Plate Chondrocytes and Mediates the Effects of Growth Hormone on Chondrogenesis and on the Expression of Insulin-like Growth Factor-1 and Bone Morphogenetic Protein-2. J. Biol. Chem. 2011, 286, 24726–24734. [Google Scholar] [CrossRef]
- Legeai-Mallet, L.; Benoist-Lasselin, C.; Munnich, A.; Bonaventure, J. Overexpression of FGFR3, Stat1, Stat5 and p21Cip1 Correlates with Phenotypic Severity and Defective Chondrocyte Differentiation in FGFR3-Related Chondrodysplasias. Bone 2004, 34, 26–36. [Google Scholar] [CrossRef]
- Agar, G.; Blumenstein, S.; Bar-Ziv, Y.; Kardosh, R.; Schrift-Tzadok, M.; Gal-Levy, R.; Fischler, T.; Goldschmid, R.; Yayon, A. The Chondrogenic Potential of Mesenchymal Cells and Chondrocytes from Osteoarthritic Subjects: A Comparative Analysis. Cartilage. 2011, 2, 40–49. [Google Scholar] [CrossRef]
- Cai, H.; Guo, H. Mesenchymal Stem Cells and Their Exocytotic Vesicles. Int. J. Mol. Sci. 2023, 24, 2085. [Google Scholar] [CrossRef]
- Zhang, J.; Rong, Y.; Luo, C.; Cui, W. Bone Marrow Mesenchymal Stem Cell-Derived Exosomes Prevent Osteoarthritis by Regulating Synovial Macrophage Polarization. Aging 2020, 12, 25138–25152. [Google Scholar] [CrossRef]
- Liang, W.; Han, B.; Hai, Y.; Sun, D.; Yin, P. Mechanism of Action of Mesenchymal Stem Cell-Derived Exosomes in the Intervertebral Disc Degeneration Treatment and Bone Repair and Regeneration. Front. Cell Dev. Biol. 2021, 9, 833840. [Google Scholar] [CrossRef]
- Sun, Y.; Liu, G.; Zhang, K.; Cao, Q.; Liu, T.; Li, J. Mesenchymal Stem Cells-Derived Exosomes for Drug Delivery. Stem Cell Res. Ther. 2021, 12, 561. [Google Scholar] [CrossRef] [PubMed]
- Rao, D.; Huang, D.; Sang, C.; Zhong, T.; Zhang, Z.; Tang, Z. Advances in Mesenchymal Stem Cell-Derived Exosomes as Drug Delivery Vehicles. Front. Bioeng. Biotechnol. 2021, 9, 797359. [Google Scholar] [CrossRef] [PubMed]
- Joshi, B.S.; de Beer, M.A.; Giepmans, B.N.G.; Zuhorn, I.S. Endocytosis of Extracellular Vesicles and Release of Their Cargo from Endosomes. ACS Nano 2020, 14, 4444–4455. [Google Scholar] [CrossRef] [PubMed]
- Yu, D.-A.; Han, J.; Kim, B.-S. Stimulation of Chondrogenic Differentiation of Mesenchymal Stem Cells. Int. J. Stem Cells 2012, 5, 16–22. [Google Scholar] [CrossRef]
- Yu, C.-Y.; Huang, W.; Li, Z.-P.; Lei, X.-Y.; He, D.-X.; Sun, L. Progress in Self-Assembling Peptide-Based Nanomaterials for Biomedical Applications. Curr. Top. Med. Chem. 2016, 16, 281–290. [Google Scholar] [CrossRef]
- Verrecchio, A.; Germann, M.W.; Schick, B.P.; Kung, B.; Twardowski, T.; San Antonio, J.D. Design of Peptides with High Affinities for Heparin and Endothelial Cell Proteoglycans. J. Biol. Chem. 2000, 275, 7701–7707. [Google Scholar] [CrossRef]
- Lin, X.; Shanmugasundaram, S.; Liu, Y.; Derrien, A.; Nurminskaya, M.; Zamora, P.O. B2A Peptide Induces Chondrogenic Differentiation In Vitro and Enhances Cartilage Repair in Rats. J. Orthop. Res. 2012, 30, 1221–1228. [Google Scholar] [CrossRef]
- Akkiraju, H.; Bonor, J.; Nohe, A. CK2.1, a Novel Peptide, Induces Articular Cartilage Formation In Vivo. J. Orthop. Res. Off. Publ. Orthop. Res. Soc. 2017, 35, 876–885. [Google Scholar] [CrossRef]
- Mahzoon, S.; Townsend, J.M.; Lam, T.N.; Sjoelund, V.; Detamore, M.S. Effects of a Bioactive SPPEPS Peptide on Chondrogenic Differentiation of Mesenchymal Stem Cells. Ann. Biomed. Eng. 2019, 47, 2308–2321. [Google Scholar] [CrossRef]
- Sun, J.; Zheng, Q. Experimental Study on Self-Assembly of KLD-12 Peptide Hydrogel and 3-D Culture of MSC Encapsulated Within Hydrogel in Vitro. J. Huazhong Univ. Sci. Technol. 2009, 29, 512–516. [Google Scholar] [CrossRef]
- Li, R.; Xu, J.; Wong, D.S.H.; Li, J.; Zhao, P.; Bian, L. Self-Assembled N-Cadherin Mimetic Peptide Hydrogels Promote the Chondrogenesis of Mesenchymal Stem Cells through Inhibition of Canonical Wnt/β-Catenin Signaling. Biomaterials 2017, 145, 33–43. [Google Scholar] [CrossRef] [PubMed]
- Re’em, T.; Tsur-Gang, O.; Cohen, S. The Effect of Immobilized RGD Peptide in Macroporous Alginate Scaffolds on TGFbeta1-Induced Chondrogenesis of Human Mesenchymal Stem Cells. Biomaterials 2010, 31, 6746–6755. [Google Scholar] [CrossRef] [PubMed]
- Andersen, T.; Auk-Emblem, P.; Dornish, M. 3D Cell Culture in Alginate Hydrogels. Microarrays 2015, 4, 133–161. [Google Scholar] [CrossRef] [PubMed]
- Mhanna, R.; Öztürk, E.; Vallmajo-Martin, Q.; Millan, C.; Müller, M.; Zenobi-Wong, M. GFOGER-Modified MMP-Sensitive Polyethylene Glycol Hydrogels Induce Chondrogenic Differentiation of Human Mesenchymal Stem Cells. Tissue Eng. Part A 2014, 20, 1165. [Google Scholar] [CrossRef]
- Wang, Z.; Weitzmann, M.N.; Sangadala, S.; Hutton, W.C.; Yoon, S.T. Link Protein N-Terminal Peptide Binds to Bone Morphogenetic Protein (BMP) Type II Receptor and Drives Matrix Protein Expression in Rabbit Intervertebral Disc Cells. J. Biol. Chem. 2013, 288, 28243–28253. [Google Scholar] [CrossRef]
- Lee, H.J.; Yu, C.; Chansakul, T.; Hwang, N.S.; Varghese, S.; Yu, S.M.; Elisseeff, J.H. Enhanced Chondrogenesis of Mesenchymal Stem Cells in Collagen Mimetic Peptide-Mediated Microenvironment. Tissue Eng. Part A 2008, 14, 1843–1851. [Google Scholar] [CrossRef]
- Ryzhak, G.A.; Popovich, I.G.; Khavinson, V.K. Prospects for using peptide bioregulators for prevention and treatment of age-associated diseases of the musculoskeletal system (review of experimental data). Pathogenesis 2019, 17, 13–24. [Google Scholar] [CrossRef]
- Zhurkovich, I.K.; Kovrova, N.G.; Ryzhak, G.A.; Mironova, E.S.; Khavinson, V.K. Identification of short peptides as part of polypeptide complexes isolated from animal organs. Biol. Bull. Rev. 2020, 140, 140–148. [Google Scholar] [CrossRef]
- Khavinson, V.K.; Grigoriev, E.I.; Malinin, V.V.; Ryzhak, G.A. Peptide Normalizing Osseous and Cartilaginous Tissue Methabolism, Pharmacological Substance Based Thereon and Method of Its Application. Eurasia Patent EA 010574, 30 October 2008. [Google Scholar]

| Peptide | Structure | Function |
|---|---|---|
| B2A | - | |
| CK2.1 CK2.2 CK2.3 | - |
|
| SPPEPS | Ser-Pro-Pro-Glu-Pro-Ser | |
| KLD-12 | AcN- Lys-Leu-Asp-Leu-Lys-Leu-Asp-Leu-Lys-Leu-Asp-Leu-CNH(2) |
|
| RGD | Arg-Gly-Asp |
|
| GFOGER | (Gly-Pro-Cys-(Gly-Pro-Pro)5-Gly-Phe-Gly-Glu-Arg-(Gly-Pro-Pro)5-Gly-Pro-Cys-NH2), |
|
| LPP | Asp-His-Leu-Ser-Asp-Asn-Tyr-Thr-Leu-Asp-His-Asp-Arg-Ala-Ile-His |
|
| CMPs |
| |
| AED | Ala-Glu-Asp |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Linkova, N.; Khavinson, V.; Diatlova, A.; Myakisheva, S.; Ryzhak, G. Peptide Regulation of Chondrogenic Stem Cell Differentiation. Int. J. Mol. Sci. 2023, 24, 8415. https://doi.org/10.3390/ijms24098415
Linkova N, Khavinson V, Diatlova A, Myakisheva S, Ryzhak G. Peptide Regulation of Chondrogenic Stem Cell Differentiation. International Journal of Molecular Sciences. 2023; 24(9):8415. https://doi.org/10.3390/ijms24098415
Chicago/Turabian StyleLinkova, Natalia, Vladimir Khavinson, Anastasiia Diatlova, Svetlana Myakisheva, and Galina Ryzhak. 2023. "Peptide Regulation of Chondrogenic Stem Cell Differentiation" International Journal of Molecular Sciences 24, no. 9: 8415. https://doi.org/10.3390/ijms24098415
APA StyleLinkova, N., Khavinson, V., Diatlova, A., Myakisheva, S., & Ryzhak, G. (2023). Peptide Regulation of Chondrogenic Stem Cell Differentiation. International Journal of Molecular Sciences, 24(9), 8415. https://doi.org/10.3390/ijms24098415







