Optimizing Hexose Utilization Pathways of Cupriavidus necator for Improving Growth and L-Alanine Production under Heterotrophic and Autotrophic Conditions
Abstract
:1. Introduction
2. Results
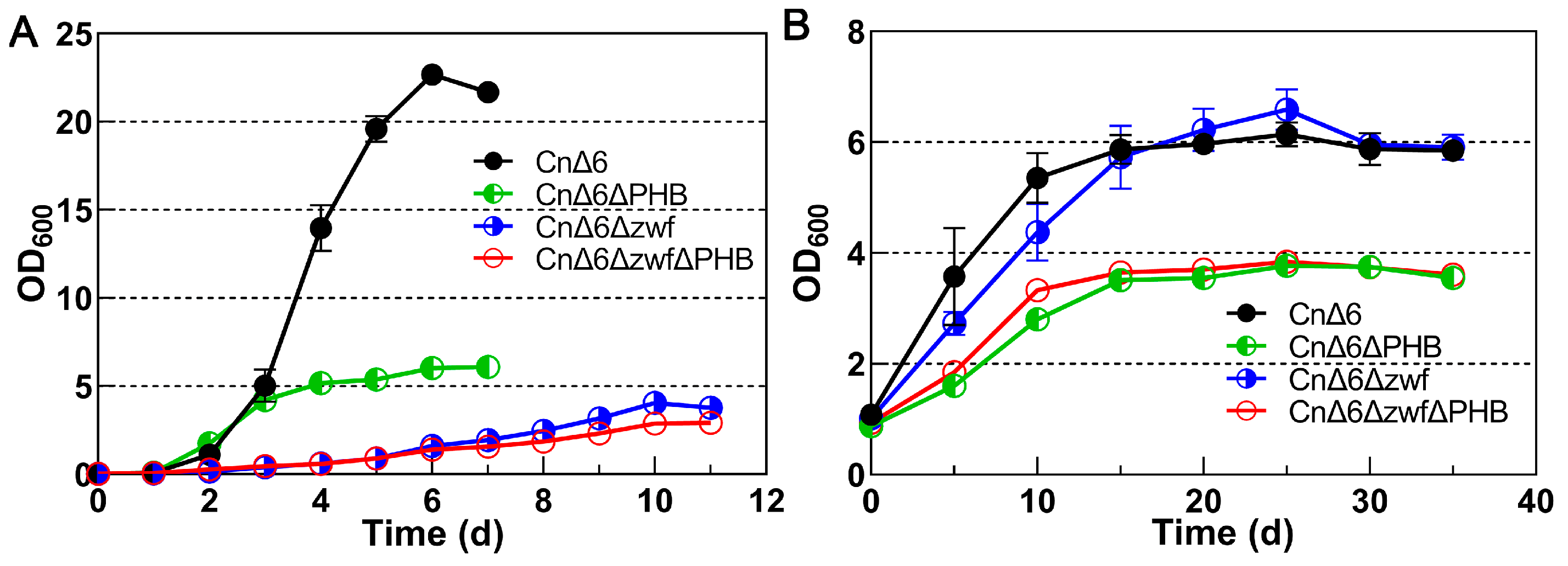
2.1. Effect of Blocking the ED and PHB Synthesis Pathways on Growth
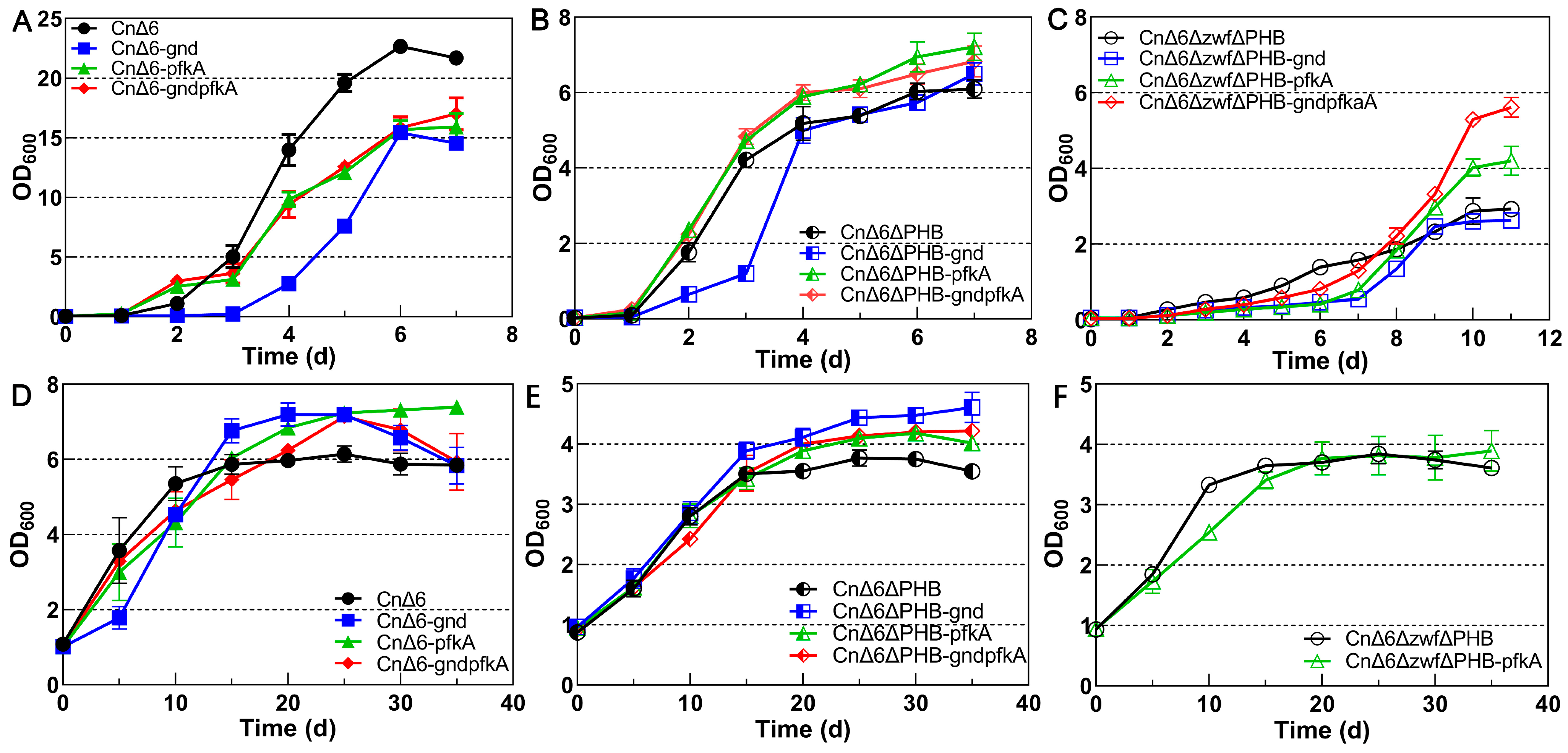
2.2. Effect of Completing the EMP and PP Pathways on Growth
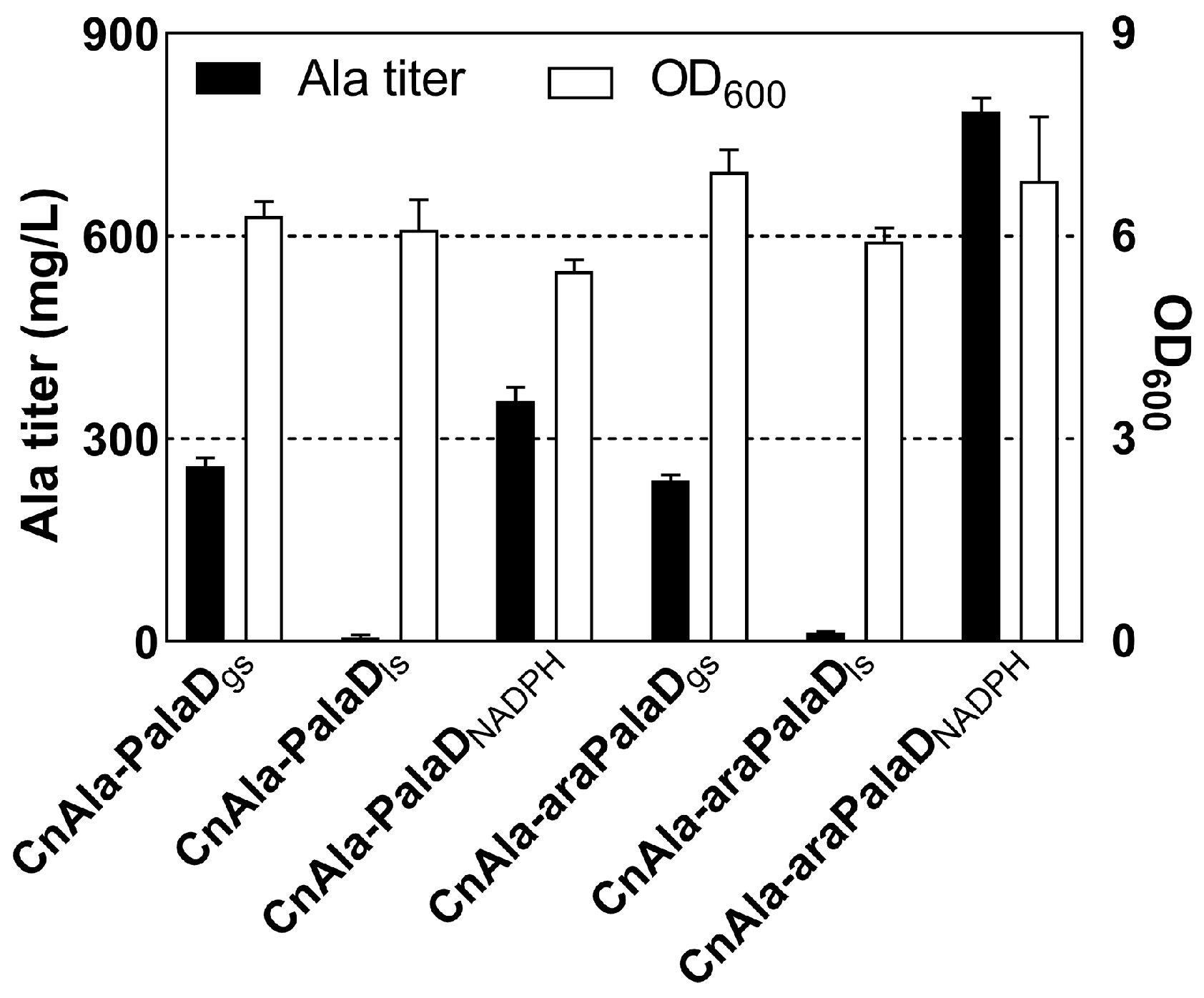
2.3. Construction of the Alanine-Producing Strain
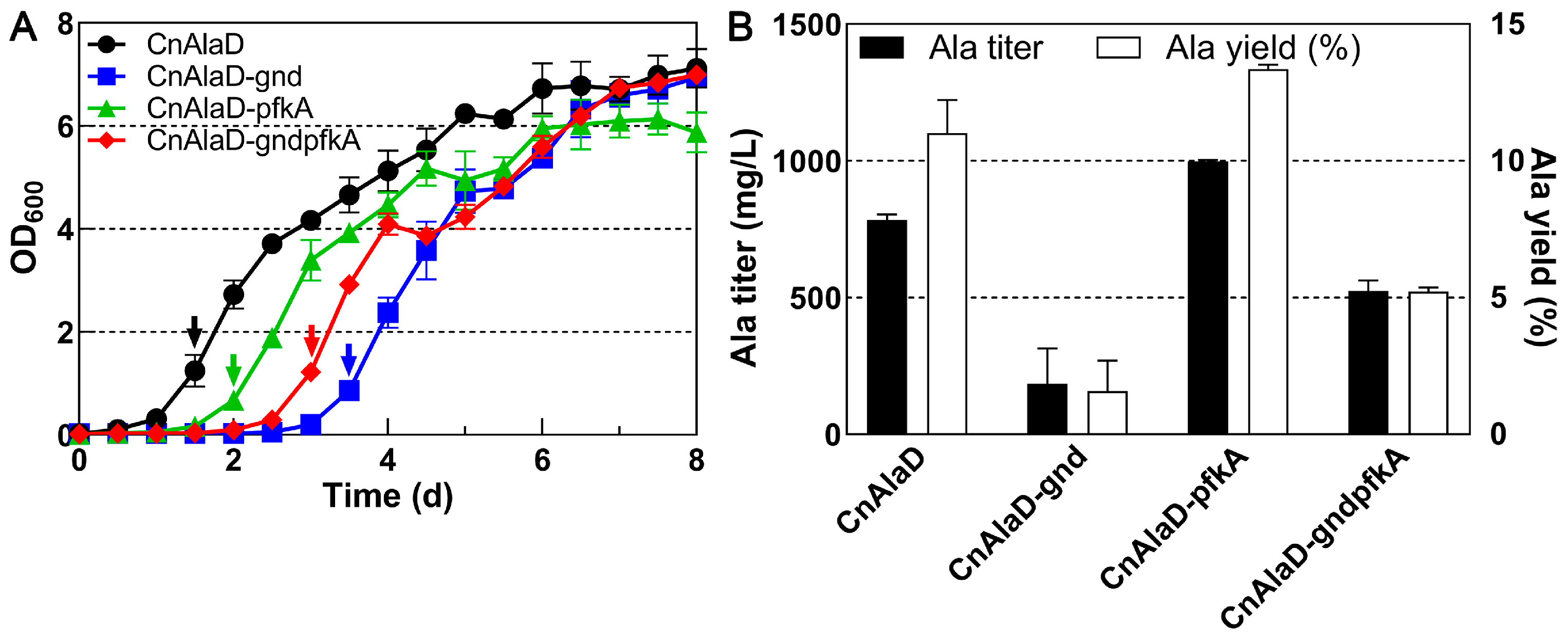
2.4. Effect of Completing the EMP and PP Pathways on Alanine Production
3. Discussion
4. Materials and Methods
4.1. Strains and Culture Medium
4.2. Plasmid Construction
4.3. Strain Modification
4.4. Heterotrophic Fermentation
4.5. Autotrophic Fermentation
4.6. Analytical Methods
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ronďošová, S.; Legerská, B.; Chmelová, D.; Ondrejovič, M.; Miertuš, S. Optimization of growth conditions to enhance PHA production by Cupriavidus necator. Fermentation 2022, 8, 451. [Google Scholar] [CrossRef]
- de Mello, A.F.M.; Vandenberghe, L.P.d.S.; Machado, C.M.B.; Valladares-Diestra, K.K.; de Carvalho, J.C.; Soccol, C.R. Polyhydroxybutyrate production by Cupriavidus necator in a corn biorefinery concept. Bioresour. Technol. 2023, 370, 128537. [Google Scholar] [CrossRef] [PubMed]
- Little, G.T.; Ehsaan, M.; Arenas-Lopez, C.; Jawed, K.; Winzer, K.; Kovacs, K.; Minton, N.P. Complete genome sequence of Cupriavidus necator H16 (DSM 428). Microbiol. Resour. Announc. 2019, 8, e814–e819. [Google Scholar] [CrossRef] [PubMed]
- Sohn, Y.J.; Son, J.; Jo, S.Y.; Park, S.Y.; Yoo, J.I.; Baritugo, K.-A.; Na, J.G.; Choi, J.-i.; Kim, H.T.; Joo, J.C.; et al. Chemoautotroph Cupriavidus necator as a potential game-changer for global warming and plastic waste problem: A review. Bioresour. Technol. 2021, 340, 125693. [Google Scholar] [CrossRef] [PubMed]
- Tu, W.L.; Chu, H.K.; Huang, C.M.; Chen, C.H.; Ou, C.M.; Guo, G.L. Polyhydroxyalkanoate production by Cupriavidus necator with inedible rice. BioResources 2022, 17, 2202–2213. [Google Scholar] [CrossRef]
- Bhatia, S.K.; Kim, J.-H.; Kim, M.-S.; Kim, J.; Hong, J.W.; Hong, Y.G.; Kim, H.-J.; Jeon, J.-M.; Kim, S.-H.; Ahn, J.; et al. Production of (3-hydroxybutyrate-co-3-hydroxyhexanoate) copolymer from coffee waste oil using engineered Ralstonia eutropha. Bioprocess Biosyst. Eng. 2017, 41, 229–235. [Google Scholar] [CrossRef] [PubMed]
- Weng, C.; Tang, R.; Peng, X.; Han, Y. Co-conversion of lignocellulose-derived glucose, xylose, and aromatics to polyhydroxybutyrate by metabolically engineered Cupriavidus necator. Bioresour. Technol. 2023, 374, 128762. [Google Scholar] [CrossRef]
- Subagyo, D.C.H.; Shimizu, R.; Orita, I.; Fukui, T. Isopropanol production with reutilization of glucose-derived CO2 by engineered Ralstonia eutropha. J. Biosci. Bioeng. 2021, 132, 479–486. [Google Scholar] [CrossRef]
- Li, Z.; Xiong, B.; Liu, L.; Li, S.; Xin, X.; Li, Z.; Zhang, X.; Bi, C. Development of an autotrophic fermentation technique for the production of fatty acids using an engineered Ralstonia eutropha cell factory. J. Ind. Microbiol. Biotechnol. 2019, 46, 783–790. [Google Scholar] [CrossRef]
- Pan, H.; Wang, J.; Wu, H.; Li, Z.; Lian, J. Synthetic biology toolkit for engineering Cupriviadus necator H16 as a platform for CO2 valorization. Biotechnol. Biofuels 2021, 14, 212. [Google Scholar] [CrossRef]
- Wang, X.; Luo, H.; Wang, Y.; Wang, Y.; Tu, T.; Qin, X.; Su, X.; Huang, H.; Bai, Y.; Yao, B.; et al. Direct conversion of carbon dioxide to glucose using metabolically engineered Cupriavidus necator. Bioresour. Technol. 2022, 362, 127806. [Google Scholar] [CrossRef] [PubMed]
- Windhorst, C.; Gescher, J. Efficient biochemical production of acetoin from carbon dioxide using Cupriavidus necator H16. Biotechnol. Biofuels 2019, 12, 163. [Google Scholar] [CrossRef] [PubMed]
- Przybylski, D.; Rohwerder, T.; Dilssner, C.; Maskow, T.; Harms, H.; Muller, R.H. Exploiting mixtures of H2, CO2, and O2 for improved production of methacrylate precursor 2-hydroxyisobutyric acid by engineered Cupriavidus necator strains. Appl. Microbiol. Biotechnol. 2015, 99, 2131–2145. [Google Scholar] [CrossRef] [PubMed]
- Flamholz, A.; Noor, E.; Bar-Even, A.; Liebermeister, W.; Milo, R. Glycolytic strategy as a tradeoff between energy yield and protein cost. Proc. Natl. Acad. Sci. USA 2013, 110, 10039–10044. [Google Scholar] [CrossRef] [PubMed]
- Pohlmann, A.; Fricke, W.F.; Reinecke, F.; Kusian, B.; Liesegang, H.; Cramm, R.; Eitinger, T.; Ewering, C.; Potter, M.; Schwartz, E.; et al. Genome sequence of the bioplastic-producing “Knallgas” bacterium Ralstonia eutropha H16. Nat. Biotechnol. 2006, 24, 1257–1262. [Google Scholar] [CrossRef] [PubMed]
- Hanko, E.K.R.; Sherlock, G.; Minton, N.P.; Malys, N. Biosensor-informed engineering of Cupriavidus necator H16 for autotrophic D-mannitol production. Metab. Eng. 2022, 72, 24–34. [Google Scholar] [CrossRef] [PubMed]
- Peplinski, K.; Ehrenreich, A.; Doring, C.; Bomeke, M.; Reinecke, F.; Hutmacher, C.; Steinbuchel, A. Genome-wide transcriptome analyses of the ‘Knallgas’ bacterium Ralstonia eutropha H16 with regard to polyhydroxyalkanoate metabolism. Microbiology 2010, 156 Pt 7, 2136–2152. [Google Scholar] [CrossRef]
- Müller, J.; MacEachran, D.; Burd, H.; Sathitsuksanoh, N.; Bi, C.; Yeh, Y.-C.; Lee, T.S.; Hillson, N.J.; Chhabra, S.R.; Singer, S.W.; et al. Engineering of Ralstonia eutropha H16 for autotrophic and heterotrophic production of methyl ketones. Appl. Environ. Microbiol. 2013, 79, 4433–4439. [Google Scholar] [CrossRef]
- Han, X.; Liu, J.; Wu, Y.; Yang, Y.; Tao, F.; Xu, P. Activating a dormant metabolic pathway for high-temperature L-alanine production in Bacillus licheniformis. iScience 2023, 26, 106397. [Google Scholar] [CrossRef]
- Liu, P.; Xu, H.; Zhang, X. Metabolic engineering of microorganisms for L-alanine production. J. Ind. Microbiol. Biotechnol. 2022, 49, kuab057. [Google Scholar] [CrossRef]
- Khattab, S.N.; Massoud, M.I.; Jad Yel, S.; Bekhit, A.A.; El-Faham, A. Production and physicochemical assessment of new stevia amino acid sweeteners from the natural stevioside. Food Chem. 2015, 173, 979–985. [Google Scholar] [CrossRef] [PubMed]
- Porcellati, F.; Pampanelli, S.; Rossetti, P.; Busciantella Ricci, N.; Marzotti, S.; Lucidi, P.; Santeusanio, F.; Bolli, G.B.; Fanelli, C.G. Effect of the amino acid alanine on glucagon secretion in non-diabetic and type 1 diabetic subjects during hyperinsulinaemic euglycaemia, hypoglycaemia and post-hypoglycaemic hyperglycaemia. Diabetologia 2007, 50, 422–430. [Google Scholar] [CrossRef] [PubMed]
- Sichwart, S.; Hetzler, S.; Broker, D.; Steinbuchel, A. Extension of the substrate utilization range of Ralstonia eutropha strain H16 by metabolic engineering to include mannose and glucose. Appl. Environ. Microbiol. 2011, 77, 1325–1334. [Google Scholar] [CrossRef] [PubMed]
- Orita, I.; Iwazawa, R.; Nakamura, S.; Fukui, T. Identification of mutation points in Cupriavidus necator NCIMB 11599 and genetic reconstitution of glucose-utilization ability in wild strain H16 for polyhydroxyalkanoate production. J. Biosci. Bioeng. 2012, 113, 63–69. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Deng, C.; Cui, W.J.; Liu, Z.M.; Zhou, Z.M. Efficient L-alanine production by a thermo-regulated switch in Escherichia coli. Appl. Biochem. Biotechnol. 2016, 178, 324–337. [Google Scholar] [CrossRef]
- Jojima, T.; Fujii, M.; Mori, E.; Inui, M.; Yukawa, H. Engineering of sugar metabolism of Corynebacterium glutamicum for production of amino acid L-alanine under oxygen deprivation. Appl. Microbiol. Biotechnol. 2010, 87, 159–165. [Google Scholar] [CrossRef]
- Lerchner, A.; Jarasch, A.; Skerra, A. Engineering of alanine dehydrogenase from Bacillus subtilis for novel cofactor specificity. Biotechnol. Appl. Biochem. 2016, 63, 616–624. [Google Scholar] [CrossRef]
- Lu, J.; Brigham, C.J.; Gai, C.S.; Sinskey, A.J. Studies on the production of branched-chain alcohols in engineered Ralstonia eutropha. Appl. Microbiol. Biotechnol. 2012, 96, 283–297. [Google Scholar] [CrossRef]
- Wang, X.; Wang, K.; Wang, L.; Luo, H.; Wang, Y.; Wang, Y.; Tu, T.; Qin, X.; Su, X.; Bai, Y.; et al. Engineering Cupriavidus necator H16 for heterotrophic and autotrophic production of myo-inositol. Bioresour. Technol. 2023, 368, 128321. [Google Scholar] [CrossRef]
- Volodina, E.; Raberg, M.; Steinbüchel, A. Engineering the heterotrophic carbon sources utilization range of Ralstonia eutropha H16 for applications in biotechnology. Crit. Rev. Biotechnol. 2016, 36, 978–991. [Google Scholar] [CrossRef]
- Yu, T.; Liu, Q.; Wang, X.; Liu, X.; Chen, Y.; Nielsen, J. Metabolic reconfiguration enables synthetic reductive metabolism in yeast. Nat. Metab. 2022, 4, 1551–1559. [Google Scholar] [CrossRef] [PubMed]
- Kanehisa, M.; Furumichi, M.; Sato, Y.; Ishiguro-Watanabe, M.; Tanabe, M. KEGG: Integrating viruses and cellular organisms. Nucleic Acids Res. 2021, 49, D545–D551. [Google Scholar] [CrossRef] [PubMed]
- Tang, R.; Yuan, X.; Yang, J. Problems and corresponding strategies for converting CO2 into value-added products in Cupriavidus necator H16 cell factories. Biotechnol. Adv. 2023, 67, 108183. [Google Scholar] [CrossRef] [PubMed]
- Bowien, B.; Kusian, B. Genetics and control of CO2 assimilation in the chemoautotroph Ralstonia eutropha. Arch. Microbiol. 2002, 178, 85–93. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.N.; Shin, H.D.; Lee, Y.H. Metabolic engineering of pentose phosphate pathway in Ralstonia eutropha for enhanced biosynthesis of poly-β-hydroxybutyrate. Biotechnol. Prog. 2003, 19, 1444–1449. [Google Scholar] [CrossRef] [PubMed]
- Steinbüchel, A. Expression of the Escherichia coli pfkA gene in Alcaligenes eutrophus and in other Gram-negative bacteria. J. Bacteriol. 1986, 166, 319–327. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Yao, L.; Xu, G.; Zhu, J.; Zhang, X.; Shi, J.; Xu, Z. Enhancement of fructose utilization from sucrose in the cell for improved L-serine production in engineered Corynebacterium glutamicum. Biochem. Eng. J. 2017, 118, 113–122. [Google Scholar] [CrossRef]
- Chavarría, M.; Nikel, P.I.; Pérez-Pantoja, D.; de Lorenzo, V. The Entner-Doudoroff pathway empowers Pseudomonas putida KT2440 with a high tolerance to oxidative stress. Environ. Microbiol. 2013, 15, 1772–1785. [Google Scholar] [CrossRef]
- Ventura, J.-R.S.; Hu, H.; Jahng, D. Enhanced butanol production in Clostridium acetobutylicum ATCC 824 by double overexpression of 6-phosphofructokinase and pyruvate kinase genes. Appl. Microbiol. Biotechnol. 2013, 97, 7505–7516. [Google Scholar] [CrossRef]
- Tsuge, Y.; Uematsu, K.; Yamamoto, S.; Suda, M.; Yukawa, H.; Inui, M. Glucose consumption rate critically depends on redox state in Corynebacterium glutamicum under oxygen deprivation. Appl. Microbiol. Biotechnol. 2015, 99, 5573–5582. [Google Scholar] [CrossRef]
- Gu, P.; Ma, Q.; Zhao, S.; Li, Q.; Gao, J. Alanine dehydrogenases from four different microorganisms: Characterization and their application in L-alanine production. Biotechnol. Biofuels Bioprod. 2023, 16, 123. [Google Scholar] [CrossRef] [PubMed]
- Nangle, S.N.; Ziesack, M.; Buckley, S.; Trivedi, D.; Loh, D.M.; Nocera, D.G.; Silver, P.A. Valorization of CO2 through lithoautotrophic production of sustainable chemicals in Cupriavidus necator. Metab. Eng. 2020, 62, 207–220. [Google Scholar] [CrossRef] [PubMed]
- Aragao, G.M.F.; Lindley, N.D.; Uribelarrea, J.L.; Pareilleux, A. Maintaining a controlled residual growth capacity increases the production of polyhydroxyalkanoate copolymers by Alcaligenes eutrophus. Biotechnol. Lett. 1996, 18, 937–942. [Google Scholar] [CrossRef]
- Shang, L.; Jiang, M.; Ryu, C.H.; Chang, H.N.; Cho, S.H.; Lee, J.W. Inhibitory effect of carbon dioxide on the fed-batch culture of Ralstonia eutropha: Evaluation by CO2 pulse injection and autogenous CO2 methods. Biotechnol. Bioeng. 2003, 83, 312–320. [Google Scholar] [CrossRef]
- Gascoyne, J.L.; Bommareddy, R.R.; Heeb, S.; Malys, N. Engineering Cupriavidus necator H16 for the autotrophic production of (R)-1,3-butanediol. Metab. Eng. 2021, 67, 262–276. [Google Scholar] [CrossRef]

| Plasmids | Characteristics | Sources |
|---|---|---|
| pK18mobsacB | Plasmid for conjugation and genomic editing, Kmr | Laboratory |
| pK18-phaC1AB1 | pK18mobsacB-derived, for phaC1AB1 operon deletion | This study |
| pK18-nagR | pK18mobsacB-derived, for nagR deletion | This study |
| pK18-nagEG793C | pK18mobsacB-derived, for mutation of nagE(G793C) | This study |
| pK18-alaE-ldhA1A2 | pK18mobsacB-derived, for the replacement of ldhA1A2 with alaE from E. coli fused with PphaC1 | This study |
| pBBR1-MCS2 | Wide host plasmid for gene expression, with Cpa fdx terminator at the end of MCS, Kmr | Laboratory |
| gnd-pBBR1 | gnd gene from E. coli fused with PphaC1 and assembled into pBBR1-MCS2 | This study |
| pfkA-pBBR1 | pfkA gene from E. coli fused with PphaC1 and assembled into pBBR1-MCS2 | This study |
| gnd-pfkA-pBBR1 | pfkA gene from E. coli fused with PphaC1 and assembled after the gnd gene of gnd-pBBR1 | This study |
| alaDgs-pBBR1 | Codon-optimized alaD gene from Geobacillus stearothermophilus fused with PphaC1 and assembled into pBBR1-MCS2 | This study |
| alaDls-pBBR1 | Codon-optimized alaD gene from Lysinibacillus sphaericus fused with PphaC1 and assembled into pBBR1-MCS2 | This study |
| alaDNADPH-pBBR1 | Codon-optimized alaD gene from Bacillus subtilis, mutated to NADPH preference, fused with PphaC1, and assembled into pBBR1-MCS2 | This study |
| araPalaDgs-pBBR1 | Codon-optimized alaD gene from G. stearothermophilus fused with araCPBAD and assembled into pBBR1-MCS2 | This study |
| araPalaDls-pBBR1 | Codon-optimized alaD gene from L. sphaericus fused with araCPBAD and assembled into pBBR1-MCS2 | This study |
| araPalaDNADPH-pBBR1 | Codon-optimized alaD gene from B. subtilis, mutated to NADPH preference, fused with araCPBAD, and assembled into pBBR1-MCS2 | This study |
| alaD-gnd-pBBR1 | gnd gene from E. coli fused with PphaC1 and assembled after the alaD gene of araPalaDNADPH-pBBR1 | This study |
| alaD-pfkA-pBBR1 | pfkA gene from E. coli fused with PphaC1 and assembled after the alaD gene of araPalaDNADPH-pBBR1 | This study |
| alaD-gnd-pfkA-pBBR1 | pfkA gene from E. coli fused with PphaC1 and assembled after the gnd gene of alaD-gnd-pBBR1 | This study |
| Strain | Characteristics | Sources |
|---|---|---|
| E. coli | ||
| Trans1-T1 | F−φ80(lacZ)ΔM15ΔlacX74hsdR(rk−,mk+)ΔrecA1398endA1tonA | Transgen, Beijing, China |
| S17-1 | thi pro hsdR recA; chromosomal RP4; Tra+; Tmpr Str/Spcr | ATCC47055 |
| C. necator | ||
| H16 | Wild-type, Genr | DSM 428 |
| CnΔ6 | H16ΔH16_A0006 | Laboratory |
| CnΔ6-gnd | CnΔ6 harboring plasmid gnd-pBBR1 | This study |
| CnΔ6-pfkA | CnΔ6 harboring plasmid pfkA-pBBR1 | This study |
| CnΔ6-gndpfkA | CnΔ6 harboring plasmid gnd-pfkA-pBBR1 | This study |
| CnΔ6Δzwf | CnΔ6 derived, Δzwf1, Δzwf2, Δzwf3 | Laboratory |
| CnΔ6ΔPHB | CnΔ6 derived, ΔphaC1AB1 | This study |
| CnΔ6ΔPHB-gnd | CnΔ6ΔPHB harboring plasmid gnd-pBBR1 | This study |
| CnΔ6ΔPHB-pfkA | CnΔ6ΔPHB harboring plasmid pfkA-pBBR1 | This study |
| CnΔ6ΔPHB-gndpfkA | CnΔ6ΔPHB harboring plasmid gnd-pfkA-pBBR1 | This study |
| CnΔ6ΔzwfΔPHB | CnΔ6Δzwf-derived, ΔphaC1AB1 | This study |
| CnΔ6ΔzwfΔPHB-gnd | CnΔ6ΔzwfΔPHB harboring plasmid gnd-pBBR1 | This study |
| CnΔ6ΔzwfΔPHB-pfkA | CnΔ6ΔzwfΔPHB harboring plasmid pfkA-pBBR1 | This study |
| CnΔ6ΔzwfΔPHB-gndpfkA | CnΔ6ΔzwfΔPHB harboring plasmid gnd-pfkA-pBBR1 | This study |
| CnΔ6RE | CnΔ6 derived, ΔnagR nagEG793C, able to use glucose | This study |
| CnΔ6REalaEΔldhA12 | CnΔ6RE-derived, ldhA1A2::PphaC1-alaE | This study |
| CnAla | CnΔ6REalaEΔldhA12-derived, ΔphaC1AB1 | This study |
| CnAla-PalaDgs | CnAla harboring plasmid alaDgs-pBBR1 | This study |
| CnAla-PalaDls | CnAla harboring plasmid alaDls-pBBR1 | This study |
| CnAla-PalaDNADPH | CnAla harboring plasmid alaDNADPH-pBBR1 | This study |
| CnAla-araPalaDgs | CnAla harboring plasmid araPalaDgs-pBBR1 | This study |
| CnAla-araPalaDls | CnAla harboring plasmid araPalaDls-pBBR1 | This study |
| CnAla-araPalaDNADPH (CnAlaD) | CnAla harboring plasmid araPalaDNADPH-pBBR1 | This study |
| CnAlaD-gnd | CnAla harboring plasmid alaD-gnd-pBBR1 | This study |
| CnAlaD-pfkA | CnAla harboring plasmid alaD-pfkA-pBBR1 | This study |
| CnAlaD-gndpfkA | CnAla harboring plasmid alaD-gnd-pfkA-pBBR1 | This study |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, L.; Luo, H.; Yao, B.; Yao, J.; Zhang, J. Optimizing Hexose Utilization Pathways of Cupriavidus necator for Improving Growth and L-Alanine Production under Heterotrophic and Autotrophic Conditions. Int. J. Mol. Sci. 2024, 25, 548. https://doi.org/10.3390/ijms25010548
Wang L, Luo H, Yao B, Yao J, Zhang J. Optimizing Hexose Utilization Pathways of Cupriavidus necator for Improving Growth and L-Alanine Production under Heterotrophic and Autotrophic Conditions. International Journal of Molecular Sciences. 2024; 25(1):548. https://doi.org/10.3390/ijms25010548
Chicago/Turabian StyleWang, Lei, Huiying Luo, Bin Yao, Junhu Yao, and Jie Zhang. 2024. "Optimizing Hexose Utilization Pathways of Cupriavidus necator for Improving Growth and L-Alanine Production under Heterotrophic and Autotrophic Conditions" International Journal of Molecular Sciences 25, no. 1: 548. https://doi.org/10.3390/ijms25010548
APA StyleWang, L., Luo, H., Yao, B., Yao, J., & Zhang, J. (2024). Optimizing Hexose Utilization Pathways of Cupriavidus necator for Improving Growth and L-Alanine Production under Heterotrophic and Autotrophic Conditions. International Journal of Molecular Sciences, 25(1), 548. https://doi.org/10.3390/ijms25010548






