Advances in Therapeutics to Alleviate Cognitive Decline and Neuropsychiatric Symptoms of Alzheimer’s Disease
Abstract
:1. Introduction
General Overview of the Pathology of Alzheimer’s Disease
2. Clinical Manifestations and Conventional Therapeutic Approaches for AD to Alleviate Cognitive Decline
Current FDA-Approved Treatments for AD-Associated Cognitive Deficits
| Name of Drug | Class of Drug | Chemical Structure | Action of the Drug | Side Effects |
|---|---|---|---|---|
| Donepezil Approved in 1996 [67,68] | Acetylcholinesterase (AChE) inhibitor used to manage mild to severe symptoms of AD. |  | By inhibiting AChE, donepezil improves the cognitive and behavioural signs and symptoms of AD, which may include apathy, aggression, confusion, and psychosis. | Gastrointestinal side effects including nausea, vomiting, anorexia, and diarrhoea. Nervous side effects including dizziness, confusion, and insomnia. |
| Rivastigmine Approved in 1997 [69,70] | AChE inhibitor used to treat mild to moderate symptoms of AD. | 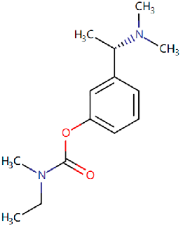 | Rivastigmine inhibits both butyrylcholinesterase and AChE, preventing the hydrolysis of acetylcholine and thus leading to an increased concentration of acetylcholine at cholinergic synapses. | Gastrointestinal side effects including nausea and vomiting, decreased appetite, diarrhoea, and abdominal pain. Nervous side effects including pain, headache, dizziness, syncope, fatigue, and malaise. |
| Galantamine Approved in 2001 [71,72] | AChE inhibitor used to manage mild to moderate AD. |  | Galantamine inhibits AChE in the synaptic cleft, thereby enhancing cholinergic neuron function and signalling. | Gastrointestinal side effects including nausea, vomiting, anorexia, and abdominal pain. Nervous side effects including dizziness, pain, somnolence, and agitation. |
| Memantine Approved in 2013 [73,74] | NMDA receptor antagonist used to treat moderate to severe dementia in AD. |  | Memantine blocks the neurotransmitter glutamate from acting on NMDA receptors that are partly responsible for neuronal excitability, thus preventing hyperexcitability seen in early and late AD. | Gastrointestinal side effects including nausea and vomiting. Nervous side effects including dizziness, headache, insomnia, and confusion. Others including falls and hypertension. |
| Aducanumab Approved in 2021 [75,76] | Monoclonal IgG1 antibody used to treat mild symptoms of AD. | 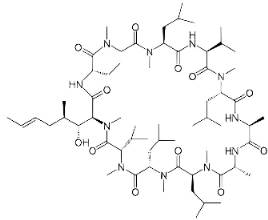 | The drug binds to amyloid-β, reducing amyloid plaques in the brain. The treatment is associated with slowing the rate of progression of AD. | Nervous side effects including headache, dizziness, nausea, and confusion. Others including falls, microhaemorrhages (ARIA-H microhaemorrhage), ARIA-superficial siderosis, and generalized tonic–clonic seizures. |
| Lecanemab Approved in 2023 [46] | Monoclonal IgG1 antibody used to treat mild symptoms of AD. | Not available | The drug reduces Aβ plaques and prevents Aβ deposition in the brain with high selectivity for Aβ protofibrils. | Infusion-related reactions, headache, amyloid-related imaging abnormalities-edema (ARIA-E), ARIA-superficial siderosis, cerebral microhaemorrhages, ARIA cerebral microhaemorrhages, and falls. |
| Donanemab [51,77] | Monoclonal IgG1 antibody for earlier stages of AD reported by Eli Lilly. | Not available | This drug works by inducing microglial-mediated clearance of existing Aβ plaques with the intent of slowing the progressive decline in cognitive function associated with AD. | Gastrointestinal side effects including nausea, diarrhoea, and vomiting. Nervous side effects including cerebral microhaemorrhages and anxiety. Others including urinary tract infection and infusion-related reactions. |
3. Potential Therapy to Target Neuroinflammation in AD
3.1. Repurposing Established Antiviral and Anti-Inflammatory Drugs
3.2. The Endothelin B (ETBR) Receptor as a Potential Therapeutic Target
4. Current Therapies for NPS in AD, Limitations, and Challenges
5. Insights into More Targeted Therapy for NPS in AD
6. Conclusions
Funding
Acknowledgments
Conflicts of Interest
References
- Silva, M.V.F.; Loures, C.d.M.G.; Alves, L.C.V.; de Souza, L.C.; Borges, K.B.G.; Carvalho, M.d.G. Alzheimer’s disease: Risk factors and potentially protective measures. J. Biomed. Sci. 2019, 26, 33. [Google Scholar] [CrossRef] [PubMed]
- Paul, D.; Agrawal, R.; Singh, S. Alzheimer’s disease and clinical trials. J. Basic Clin. Physiol. Pharmacol. 2024, 35, 31–44. [Google Scholar] [CrossRef] [PubMed]
- Aljassabi, A.; Zieneldien, T.; Kim, J.; Regmi, D.; Cao, C. Alzheimer’s Disease Immunotherapy: Current Strategies and Future Prospects. J. Alzheimers Dis. 2024, 98, 755–772. [Google Scholar] [CrossRef] [PubMed]
- Murphy, M.P.; LeVine, H., 3rd. Alzheimer’s disease and the amyloid-beta peptide. J. Alzheimers Dis. 2010, 19, 311–323. [Google Scholar] [CrossRef] [PubMed]
- Hyman, B.T.; Van Hoesen, G.W.; Damasio, A.R. Alzheimer’s disease: Glutamate depletion in the hippocampal perforant pathway zone. Ann. Neurol. 1987, 22, 37–40. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Hu, J.; Sun, A. The Beneficial Effect of Enriched Environment on Pathogenesis of Alzheimer’s Disease. Yangtze Med. 2018, 2, 225–243. [Google Scholar] [CrossRef]
- Soria Lopez, J.A.; González, H.M.; Léger, G.C. Chapter 13—Alzheimer’s disease. In Handbook of Clinical Neurology; Dekosky, S.T., Asthana, S., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; Volume 167, pp. 231–255. [Google Scholar]
- Breijyeh, Z.; Karaman, R. Comprehensive Review on Alzheimer’s Disease: Causes and Treatment. Molecules 2020, 25, 5789. [Google Scholar] [CrossRef] [PubMed]
- Lanoiselée, H.M.; Nicolas, G.; Wallon, D.; Rovelet-Lecrux, A.; Lacour, M.; Rousseau, S.; Richard, A.-C.; Pasquier, F.; Rollin-Sillaire, A.; Martinaud, O.; et al. APP, PSEN1, and PSEN2 mutations in early-onset Alzheimer disease: A genetic screening study of familial and sporadic cases. PLoS Med. 2017, 14, e1002270. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.X.; Tian, Y.; Wang, Z.T.; Ma, Y.H.; Tan, L.; Yu, J.T. The Epidemiology of Alzheimer’s Disease Modifiable Risk Factors and Prevention. J. Prev. Alzheimers Dis. 2021, 8, 313–321. [Google Scholar] [CrossRef]
- Hasler, G. Pathophysiology of depression: Do we have any solid evidence of interest to clinicians? World Psychiatry 2010, 9, 155–161. [Google Scholar] [CrossRef]
- Kessler, R.C. Epidemiology of women and depression. J. Affect. Disord. 2003, 74, 5–13. [Google Scholar] [CrossRef]
- Dettmann, L.M.; Adams, S.; Taylor, G. Investigating the prevalence of anxiety and depression during the first COVID-19 lockdown in the United Kingdom: Systematic review and meta-analyses. Br. J. Clin. Psychol. 2022, 61, 757–780. [Google Scholar] [CrossRef] [PubMed]
- Penninx, B.W.; Pine, D.S.; Holmes, E.A.; Reif, A. Anxiety disorders. Lancet 2021, 397, 914–927. [Google Scholar] [CrossRef]
- Mendez, M.F. The Relationship Between Anxiety and Alzheimer’s Disease. J. Alzheimers Dis. Rep. 2021, 5, 171–177. [Google Scholar] [CrossRef] [PubMed]
- Botto, R.; Callai, N.; Cermelli, A.; Causarano, L.; Rainero, I. Anxiety and depression in Alzheimer’s disease: A systematic review of pathogenetic mechanisms and relation to cognitive decline. Neurol. Sci. 2022, 43, 4107–4124. [Google Scholar] [CrossRef]
- Vaz, M.; Silvestre, S. Alzheimer’s disease: Recent treatment strategies. Eur. J. Pharmacol. 2020, 887, 173554. [Google Scholar] [CrossRef]
- Kwong, R.Y.; Yucel, E.K. Computed Tomography Scan and Magnetic Resonance Imaging. Circulation 2003, 108, e104–e106. [Google Scholar] [CrossRef] [PubMed]
- Zeisel, J.; Bennett, K.; Fleming, R. World Alzheimer Report 2020: Design, Dignity, Dementia: Dementia-Related Design and the Built Environment; Alzheimer’s Disease International: London, UK, 2020. [Google Scholar]
- Mebane-Sims, I. 2009 Alzheimer’s disease facts and figures. Alzheimer’s Dement. J. Alzheimer’s Assoc. 2009, 5, 234–270. [Google Scholar]
- Apostolova, L.G.; Green, A.E.; Babakchanian, S.; Hwang, K.S.; Chou, Y.-Y.; Toga, A.W.; Thompson, P.M. Hippocampal atrophy and ventricular enlargement in normal aging, mild cognitive impairment and Alzheimer’s disease. Alzheimer Dis. Assoc. Disord. 2012, 26, 17–27. [Google Scholar] [CrossRef]
- Drummond, E.; Wisniewski, T. Alzheimer’s disease: Experimental models and reality. Acta Neuropathol. 2017, 133, 155–175. [Google Scholar] [CrossRef]
- Glenner, G.G.; Wong, C.W. Alzheimer’s disease: Initial report of the purification and characterization of a novel cerebrovascular amyloid protein. Biochem. Biophys. Res. Commun. 1984, 120, 885–890. [Google Scholar] [CrossRef]
- Cooper, M.C.; Kilvert, H.S.; Hodgkins, P.; Roskell, N.S.; Eldar-Lissai, A. Using Matching-Adjusted Indirect Comparisons and Network Meta-analyses to Compare Efficacy of Brexanolone Injection with Selective Serotonin Reuptake Inhibitors for Treating Postpartum Depression. CNS Drugs 2019, 33, 1039–1052. [Google Scholar] [CrossRef] [PubMed]
- Nelson, P.T.; Braak, H.; Markesbery, W.R. Neuropathology and cognitive impairment in Alzheimer disease: A complex but coherent relationship. J. Neuropathol. Exp. Neurol. 2009, 68, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Yiannopoulou, K.G.; Papageorgiou, S.G. Current and future treatments for Alzheimer’s disease. Ther. Adv. Neurol. Disord. 2013, 6, 19–33. [Google Scholar] [CrossRef] [PubMed]
- Cummings, J. New approaches to symptomatic treatments for Alzheimer’s disease. Mol. Neurodegener. 2021, 16, 2. [Google Scholar] [CrossRef] [PubMed]
- Berg-Weger, M.; Stewart, D.B. Non-Pharmacologic Interventions for Persons with Dementia. Mo. Med. 2017, 114, 116–119. [Google Scholar] [PubMed]
- Yu, T.W.; Lane, H.Y.; Lin, C.H. Novel Therapeutic Approaches for Alzheimer’s Disease: An Updated Review. Int. J. Mol. Sci. 2021, 22, 8208. [Google Scholar] [CrossRef] [PubMed]
- Jucker, M.; Walker, L.C. Alzheimer’s disease: From immunotherapy to immunoprevention. Cell 2023, 186, 4260–4270. [Google Scholar] [CrossRef] [PubMed]
- Cummings, J.L.; Tong, G.; Ballard, C. Treatment Combinations for Alzheimer’s Disease: Current and Future Pharmacotherapy Options. J. Alzheimers Dis. 2019, 67, 779–794. [Google Scholar] [CrossRef]
- Larson, E.B.; Wang, L.; Bowen, J.D.; McCormick, W.C.; Teri, L.; Crane, P.; Kukull, W. Exercise is associated with reduced risk for incident dementia among persons 65 years of age and older. Ann. Intern. Med. 2006, 144, 73–81. [Google Scholar] [CrossRef]
- Kumar, A.; Singh, A.; Ekavali. A review on Alzheimer’s disease pathophysiology and its management: An update. Pharmacol. Rep. 2015, 67, 195–203. [Google Scholar] [CrossRef] [PubMed]
- McShane, R.; Westby, M.J.; Roberts, E.; Minakaran, N.; Schneider, L.; Farrimond, L.E.; Maayan, N.; Ware, J.; Debarros, J. Memantine for dementia. Cochrane Database Syst. Rev. 2019, 2, CD003154. [Google Scholar] [CrossRef] [PubMed]
- Kuns, B.; Rosani, A.; Patel, P.; Varghese, D. Memantine. In StatPearls; StatPearls Publishing LLC.: Treasure Island, FL, USA, 2024. [Google Scholar]
- Ryu, W.-S. Chapter 4—Diagnosis and Methods. In Molecular Virology of Human Pathogenic Viruses; Ryu, W.-S., Ed.; Academic Press: Boston, MA, USA, 2017; pp. 47–62. [Google Scholar] [CrossRef]
- Reisberg, B.; Doody, R.; Stöffler, A.; Schmitt, F.; Ferris, S.; Möbius, H.J. A 24-week open-label extension study of memantine in moderate to severe Alzheimer disease. Arch. Neurol. 2006, 63, 49–54. [Google Scholar] [CrossRef] [PubMed]
- Dunn, B.; Stein, P.; Cavazzoni, P. Approval of Aducanumab for Alzheimer Disease—The FDA’s Perspective. JAMA Intern. Med. 2021, 181, 1276–1278. [Google Scholar] [CrossRef] [PubMed]
- Tampi, R.R.; Forester, B.P.; Agronin, M. Aducanumab: Evidence from clinical trial data and controversies. Drugs Context 2021, 10. [Google Scholar] [CrossRef]
- Ju, Y.; Tam, K.Y. Pathological mechanisms and therapeutic strategies for Alzheimer’s disease. Neural. Regen. Res. 2022, 17, 543–549. [Google Scholar] [CrossRef] [PubMed]
- Swanson, C.J.; Zhang, Y.; Dhadda, S.; Wang, J.; Kaplow, J.; Lai, R.Y.K.; Lannfelt, L.; Bradley, H.; Rabe, M.; Koyama, A.; et al. A randomized, double-blind, phase 2b proof-of-concept clinical trial in early Alzheimer’s disease with lecanemab, an anti-Aβ protofibril antibody. Alzheimer’s Res. Ther. 2021, 13, 80. [Google Scholar] [CrossRef]
- Landry, I.; Kanekiyo, M.; Aluri, J.; Li, D.J.; Hussein, Z.; Reyderman, L.; Dhadda, S.; Swanson, C.J.; Irizarry, M.C.; Kramer, L.D. Lecanemab (BAN2401) Infusion Reactions and Immunogenicity: Results from Randomized Phase 2 Study and an Open-Label Extension (OLE). Alzheimer’s Dement. 2022, 18, e066289. [Google Scholar] [CrossRef]
- Beshir, S.A.; Aadithsoorya, A.M.; Parveen, A.; Goh, S.S.L.; Hussain, N.; Menon, V.B. Aducanumab Therapy to Treat Alzheimer’s Disease: A Narrative Review. Int. J. Alzheimers Dis. 2022, 2022, 9343514. [Google Scholar] [CrossRef]
- Cummings, J.; Rabinovici, G.D.; Atri, A.; Aisen, P.; Apostolova, L.G.; Hendrix, S.; Sabbagh, M.; Selkoe, D.; Weiner, M.; Salloway, S. Aducanumab: Appropriate Use Recommendations Update. J. Prev. Alzheimers Dis. 2022, 9, 221–230. [Google Scholar] [CrossRef]
- Petch, J.; Bressington, D. Aducanumab for Alzheimer’s disease: The never-ending story that nurses should know. Nurs. Open 2021, 8, 1524–1526. [Google Scholar] [CrossRef]
- van Dyck, C.H.; Swanson, C.J.; Aisen, P.; Bateman, R.J.; Chen, C.; Gee, M.; Kanekiyo, M.; Li, D.; Reyderman, L.; Cohen, S.; et al. Lecanemab in Early Alzheimer’s Disease. N. Engl. J. Med. 2023, 388, 9–21. [Google Scholar] [CrossRef] [PubMed]
- Shi, M.; Chu, F.; Zhu, F.; Zhu, J. Impact of Anti-amyloid-β Monoclonal Antibodies on the Pathology and Clinical Profile of Alzheimer’s Disease: A Focus on Aducanumab and Lecanemab. Front. Aging Neurosci. 2022, 14, 870517. [Google Scholar] [CrossRef]
- Cummings, J.; Lee, G.; Nahed, P.; Kambar, M.; Zhong, K.; Fonseca, J.; Taghva, K. Alzheimer’s disease drug development pipeline: 2022. Alzheimers Dement. 2022, 8, e12295. [Google Scholar] [CrossRef] [PubMed]
- Yi, L.X.; Tan, E.K.; Zhou, Z.D. Passive immunotherapy for Alzheimer's disease: Challenges & future directions. J. Transl. Med. 2024, 22, 430. [Google Scholar] [CrossRef] [PubMed]
- Gueorguieva, I.; Willis, B.A.; Chua, L.; Chow, K.; Ernest, C.S.; Shcherbinin, S.; Ardayfio, P.; Mullins, G.R.; Sims, J.R. Donanemab Population Pharmacokinetics, Amyloid Plaque Reduction, and Safety in Participants with Alzheimer's Disease. Clin. Pharmacol. Ther. 2023, 113, 1258–1267. [Google Scholar] [CrossRef]
- Sims, J.R.; Zimmer, J.A.; Evans, C.D.; Lu, M.; Ardayfio, P.; Sparks, J.; Wessels, A.M.; Shcherbinin, S.; Wang, H.; Monkul Nery, E.S.; et al. Donanemab in Early Symptomatic Alzheimer Disease: The TRAILBLAZER-ALZ 2 Randomized Clinical Trial. JAMA 2023, 330, 512–527. [Google Scholar] [CrossRef]
- Lowe, S.L.; Willis, B.A.; Hawdon, A.; Natanegara, F.; Chua, L.; Foster, J.; Shcherbinin, S.; Ardayfio, P.; Sims, J.R. Donanemab (LY3002813) dose-escalation study in Alzheimer’s disease. Alzheimers Dement. 2021, 7, e12112. [Google Scholar] [CrossRef]
- Lu, R.-M.; Hwang, Y.-C.; Liu, I.J.; Lee, C.-C.; Tsai, H.-Z.; Li, H.-J.; Wu, H.-C. Development of therapeutic antibodies for the treatment of diseases. J. Biomed. Sci. 2020, 27, 1. [Google Scholar] [CrossRef]
- Salloway, S.; Chalkias, S.; Barkhof, F.; Burkett, P.; Barakos, J.; Purcell, D.; Suhy, J.; Forrestal, F.; Tian, Y.; Umans, K.; et al. Amyloid-Related Imaging Abnormalities in 2 Phase 3 Studies Evaluating Aducanumab in Patients With Early Alzheimer Disease. JAMA Neurol. 2022, 79, 13–21. [Google Scholar] [CrossRef]
- Agarwal, A.; Gupta, V.; Brahmbhatt, P.; Desai, A.; Vibhute, P.; Joseph-Mathurin, N.; Bathla, G. Amyloid-related Imaging Abnormalities in Alzheimer Disease Treated with Anti–Amyloid-β Therapy. RadioGraphics 2023, 43, e230009. [Google Scholar] [CrossRef] [PubMed]
- Dixit, R.; Ross, J.L.; Goldman, Y.E.; Holzbaur, E.L. Differential regulation of dynein and kinesin motor proteins by tau. Science 2008, 319, 1086–1089. [Google Scholar] [CrossRef] [PubMed]
- Xia, C.; Makaretz, S.J.; Caso, C.; McGinnis, S.; Gomperts, S.N.; Sepulcre, J.; Gomez-Isla, T.; Hyman, B.T.; Schultz, A.; Vasdev, N. Association of in vivo [18F] AV-1451 tau PET imaging results with cortical atrophy and symptoms in typical and atypical Alzheimer disease. JAMA Neurol. 2017, 74, 427–436. [Google Scholar] [CrossRef] [PubMed]
- Hanseeuw, B.J.; Betensky, R.A.; Jacobs, H.I.; Schultz, A.P.; Sepulcre, J.; Becker, J.A.; Cosio, D.M.O.; Farrell, M.; Quiroz, Y.T.; Mormino, E.C. Association of amyloid and tau with cognition in preclinical Alzheimer disease: A longitudinal study. JAMA Neurol. 2019, 76, 915–924. [Google Scholar] [CrossRef] [PubMed]
- Fujio, K.; Sato, M.; Uemura, T.; Sato, T.; Sato-Harada, R.; Harada, A. 14-3-3 proteins and protein phosphatases are not reduced in tau-deficient mice. Neuroreport 2007, 18, 1049–1052. [Google Scholar] [CrossRef] [PubMed]
- Morris, M.; Hamto, P.; Adame, A.; Devidze, N.; Masliah, E.; Mucke, L. Age-appropriate cognition and subtle dopamine-independent motor deficits in aged tau knockout mice. Neurobiol. Aging 2013, 34, 1523–1529. [Google Scholar] [CrossRef] [PubMed]
- Mummery, C.J.; Börjesson-Hanson, A.; Blackburn, D.J.; Vijverberg, E.G.B.; De Deyn, P.P.; Ducharme, S.; Jonsson, M.; Schneider, A.; Rinne, J.O.; Ludolph, A.C.; et al. Tau-targeting antisense oligonucleotide MAPTRx in mild Alzheimer’s disease: A phase 1b, randomized, placebo-controlled trial. Nat. Med. 2023, 29, 1437–1447. [Google Scholar] [CrossRef] [PubMed]
- Biogen. New Data from Biogen’s Investigational Antisense Oligonucleotide (ASO) Targeting Tau Shows Promise for Potential New Generation of Treatments in Early Alzheimer’s Disease. 2023. Available online: https://investors.biogen.com/news-releases/news-release-details/new-data-biogens-investigational-antisense-oligonucleotide-aso (accessed on 25 October 2023).
- Flach, K.; Hilbrich, I.; Schiffmann, A.; Gärtner, U.; Krüger, M.; Leonhardt, M.; Waschipky, H.; Wick, L.; Arendt, T.; Holzer, M. Tau oligomers impair artificial membrane integrity and cellular viability. J. Biol. Chem. 2012, 287, 43223–43233. [Google Scholar] [CrossRef]
- Tai, C.-Y.; Ma, H.-T.; Li, C.-L.; Fang Wu, M.; Wang, S.; Wu, C.-L.; Lai, Y.-T.; Serrano, G.E.; Beach, T.G.; Margolin, R.A.; et al. APNmAb005, an anti-tau antibody targeting synaptic tau oligomers, in Phase 1 for treatment of Alzheimer’s Disease and primary tauopathies. Alzheimer’s Dement. 2023, 19, e076888. [Google Scholar] [CrossRef]
- Chandupatla, R.R.; Flatley, A.; Feederle, R.; Mandelkow, E.M.; Kaniyappan, S. Novel antibody against low-n oligomers of tau protein promotes clearance of tau in cells via lysosomes. Alzheimers Dement. 2020, 6, e12097. [Google Scholar] [CrossRef]
- ClinicalTrials.gov. A Phase 2 Study to Evaluate Efficacy and Safety of AL002 in Participants with Early Alzheimer’s Disease (INVOKE-2). ClinicalTrials.gov. 2024. Available online: https://classic.clinicaltrials.gov/ct2/show/NCT04592874 (accessed on 30 April 2024).
- Burns, A.; Rossor, M.; Hecker, J.; Gauthier, S.; Petit, H.; Möller, H.J.; Rogers, S.L.; Friedhoff, L.T. The effects of donepezil in Alzheimer’s disease—Results from a multinational trial. Dement. Geriatr. Cogn. Disord. 1999, 10, 237–244. [Google Scholar] [CrossRef]
- Mohs, R.C.; Doody, R.S.; Morris, J.C.; Ieni, J.R.; Rogers, S.L.; Perdomo, C.A.; Pratt, R.D. A 1-year, placebo-controlled preservation of function survival study of donepezil in AD patients. Neurology 2001, 57, 481–488. [Google Scholar] [CrossRef] [PubMed]
- Rösler, M.; Anand, R.; Cicin-Sain, A.; Gauthier, S.; Agid, Y.; Dal-Bianco, P.; Stähelin, H.B.; Hartman, R.; Gharabawi, M. Efficacy and safety of rivastigmine in patients with Alzheimer’s disease: International randomised controlled trial. BMJ 1999, 318, 633–638. [Google Scholar] [CrossRef] [PubMed]
- Birks, J.; Grimley Evans, J.; Iakovidou, V.; Tsolaki, M.; Holt, F.E. Rivastigmine for Alzheimer’s disease. Cochrane Database Syst. Rev. 2009, 2, Cd001191. [Google Scholar] [CrossRef]
- Rockwood, K.; Mintzer, J.; Truyen, L.; Wessel, T.; Wilkinson, D. Effects of a flexible galantamine dose in Alzheimer’s disease: A randomised, controlled trial. J. Neurol. Neurosurg. Psychiatry 2001, 71, 589–595. [Google Scholar] [CrossRef]
- Raskind, M.A.; Peskind, E.R.; Wessel, T.; Yuan, W. Galantamine in AD: A 6-month randomized, placebo-controlled trial with a 6-month extension. The Galantamine USA-1 Study Group. Neurology 2000, 54, 2261–2268. [Google Scholar] [CrossRef] [PubMed]
- del Río-Sancho, S. Chapter 32—Memantine and Alzheimer's disease: A comprehensive review. In Diagnosis and Management in Dementia; Martin, C.R., Preedy, V.R., Eds.; Academic Press: Cambridge, MA, USA, 2020; pp. 511–527. [Google Scholar] [CrossRef]
- Battle, C.E.; Abdul-Rahim, A.H.; Shenkin, S.D.; Hewitt, J.; Quinn, T.J. Cholinesterase inhibitors for vascular dementia and other vascular cognitive impairments: A network meta-analysis. Cochrane Database Syst. Rev. 2021, 2, Cd013306. [Google Scholar] [CrossRef]
- Rahman, A.; Hossen, M.A.; Chowdhury, M.F.I.; Bari, S.; Tamanna, N.; Sultana, S.S.; Haque, S.N.; Al Masud, A.; Saif-Ur-Rahman, K.M. Aducanumab for the treatment of Alzheimer’s disease: A systematic review. Psychogeriatrics 2023, 23, 512–522. [Google Scholar] [CrossRef] [PubMed]
- Budd Haeberlein, S.; Aisen, P.S.; Barkhof, F.; Chalkias, S.; Chen, T.; Cohen, S.; Dent, G.; Hansson, O.; Harrison, K.; von Hehn, C.; et al. Two Randomized Phase 3 Studies of Aducanumab in Early Alzheimer’s Disease. J. Prev. Alzheimers Dis. 2022, 9, 197–210. [Google Scholar] [CrossRef]
- Ebell, M.H.; Barry, H.C.; Baduni, K.; Grasso, G. Clinically Important Benefits and Harms of Monoclonal Antibodies Targeting Amyloid for the Treatment of Alzheimer Disease: A Systematic Review and Meta-Analysis. Ann. Fam. Med. 2024, 22, 50–62. [Google Scholar] [CrossRef]
- Baier, P.C.; May, U.; Scheller, J.; Rose-John, S.; Schiffelholz, T. Impaired hippocampus-dependent and-independent learning in IL-6 deficient mice. Behav. Brain Res. 2009, 200, 192–196. [Google Scholar] [CrossRef] [PubMed]
- Semmler, A.; Hermann, S.; Mormann, F.; Weberpals, M.; Paxian, S.A.; Okulla, T.; Schäfers, M.; Kummer, M.P.; Klockgether, T.; Heneka, M.T. Sepsis causes neuroinflammation and concomitant decrease of cerebral metabolism. J. Neuroinflamm. 2008, 5, 38. [Google Scholar] [CrossRef] [PubMed]
- Bartsch, T. The Clinical Neurobiology of the Hippocampus: An Integrative View; Oxford University Press: Oxford, UK, 2012. [Google Scholar] [CrossRef]
- Lee, Y.J.; Han, S.B.; Nam, S.Y.; Oh, K.W.; Hong, J.T. Inflammation and Alzheimer’s disease. Arch. Pharmacal Res. 2010, 33, 1539–1556. [Google Scholar] [CrossRef] [PubMed]
- Wyss-Coray, T.; Rogers, J. Inflammation in Alzheimer disease—A brief review of the basic science and clinical literature. Cold Spring Harb. Perspect. Med. 2012, 2, a006346. [Google Scholar] [CrossRef] [PubMed]
- Kale, M.A.; Shamkuwar, P.B.; Mourya, V.K.; Deshpande, A.B.; Shelke, P.A. Drug Repositioning: A Unique Approach to Refurbish Drug Discovery. Curr. Drug Discov. Technol. 2022, 19, e140122192307. [Google Scholar] [CrossRef] [PubMed]
- Roy, S.; Dhaneshwar, S.; Bhasin, B. Drug Repurposing: An Emerging Tool for Drug Reuse, Recycling and Discovery. Curr. Drug Res. Rev. 2021, 13, 101–119. [Google Scholar] [CrossRef] [PubMed]
- Krishnamurthy, N.; Grimshaw, A.A.; Axson, S.A.; Choe, S.H.; Miller, J.E. Drug repurposing: A systematic review on root causes, barriers and facilitators. BMC Health Serv. Res. 2022, 22, 970. [Google Scholar] [CrossRef] [PubMed]
- Sahoo, B.M.; Ravi Kumar, B.V.V.; Sruti, J.; Mahapatra, M.K.; Banik, B.K.; Borah, P. Drug Repurposing Strategy (DRS): Emerging Approach to Identify Potential Therapeutics for Treatment of Novel Coronavirus Infection. Front. Mol. Biosci. 2021, 8, 628144. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, U.H.; Zeng, E.; Pasinetti, G.M. The Use of Antimicrobial and Antiviral Drugs in Alzheimer’s Disease. Int. J. Mol. Sci. 2020, 21, 4920. [Google Scholar] [CrossRef]
- Hui, Z.; Zhijun, Y.; Yushan, Y.; Liping, C.; Yiying, Z.; Difan, Z.; Chunglit, C.T.; Wei, C. The combination of acyclovir and dexamethasone protects against Alzheimer’s disease-related cognitive impairments in mice. Psychopharmacology 2020, 237, 1851–1860. [Google Scholar] [CrossRef]
- Wozniak, M.A.; Frost, A.L.; Preston, C.M.; Itzhaki, R.F. Antivirals reduce the formation of key Alzheimer’s disease molecules in cell cultures acutely infected with herpes simplex virus type 1. PLoS ONE 2011, 6, e25152. [Google Scholar] [CrossRef] [PubMed]
- Van Eldik, L.J.; Carrillo, M.C.; Cole, P.E.; Feuerbach, D.; Greenberg, B.D.; Hendrix, J.A.; Kennedy, M.; Kozauer, N.; Margolin, R.A.; Molinuevo, J.L. The roles of inflammation and immune mechanisms in Alzheimer’s disease. Alzheimer’s Dement. Transl. Res. Clin. Interv. 2016, 2, 99–109. [Google Scholar] [CrossRef]
- Rivers-Auty, J.; Mather, A.E.; Peters, R.; Lawrence, C.B.; Brough, D. Anti-inflammatories in Alzheimer’s disease—Potential therapy or spurious correlate? Brain Commun. 2020, 2, fcaa109. [Google Scholar] [CrossRef]
- Gupta, P.P.; Pandey, R.D.; Jha, D.; Shrivastav, V.; Kumar, S. Role of traditional nonsteroidal anti-inflammatory drugs in Alzheimer’s disease: A meta-analysis of randomized clinical trials. Am. J. Alzheimer’s Dis. Other Dement. 2015, 30, 178–182. [Google Scholar] [CrossRef] [PubMed]
- Ali, M.M.; Ghouri, R.G.; Ans, A.H.; Akbar, A.; Toheed, A. Recommendations for Anti-inflammatory Treatments in Alzheimer’s Disease: A Comprehensive Review of the Literature. Cureus 2019, 11, e4620. [Google Scholar] [CrossRef]
- Tran, V.T.A.; Lee, L.P.; Cho, H. Neuroinflammation in neurodegeneration via microbial infections. Front. Immunol. 2022, 13, 907804. [Google Scholar] [CrossRef]
- DiSabato, D.J.; Quan, N.; Godbout, J.P. Neuroinflammation: The devil is in the details. J. Neurochem. 2016, 139 (Suppl. S2), 136–153. [Google Scholar] [CrossRef]
- Yang, I.; Han, S.J.; Kaur, G.; Crane, C.; Parsa, A.T. The role of microglia in central nervous system immunity and glioma immunology. J. Clin. Neurosci. 2010, 17, 6–10. [Google Scholar] [CrossRef] [PubMed]
- Colonna, M.; Butovsky, O. Microglia Function in the Central Nervous System During Health and Neurodegeneration. Annu. Rev. Immunol. 2017, 35, 441–468. [Google Scholar] [CrossRef]
- Bachiller, S.; Jiménez-Ferrer, I.; Paulus, A.; Yang, Y.; Swanberg, M.; Deierborg, T.; Boza-Serrano, A. Microglia in Neurological Diseases: A Road Map to Brain-Disease Dependent-Inflammatory Response. Front. Cell. Neurosci. 2018, 12, 488. [Google Scholar] [CrossRef]
- Jäkel, S.; Dimou, L. Glial Cells and Their Function in the Adult Brain: A Journey through the History of Their Ablation. Front. Cell. Neurosci. 2017, 11, 24. [Google Scholar] [CrossRef] [PubMed]
- Sofroniew, M.V.; Vinters, H.V. Astrocytes: Biology and pathology. Acta Neuropathol. 2010, 119, 7–35. [Google Scholar] [CrossRef]
- Davenport, A.P.; Hyndman, K.A.; Dhaun, N.; Southan, C.; Kohan, D.E.; Pollock, J.S.; Pollock, D.M.; Webb, D.J.; Maguire, J.J. Endothelin. Pharmacol. Rev. 2016, 68, 357–418. [Google Scholar] [CrossRef] [PubMed]
- Michinaga, S.; Inoue, A.; Yamamoto, H.; Ryu, R.; Inoue, A.; Mizuguchi, H.; Koyama, Y. Endothelin receptor antagonists alleviate blood-brain barrier disruption and cerebral edema in a mouse model of traumatic brain injury: A comparison between bosentan and ambrisentan. Neuropharmacology 2020, 175, 108182. [Google Scholar] [CrossRef]
- Smit, T.; Deshayes, N.A.C.; Borchelt, D.R.; Kamphuis, W.; Middeldorp, J.; Hol, E.M. Reactive astrocytes as treatment targets in Alzheimer’s disease—Systematic review of studies using the APPswePS1dE9 mouse model. Glia 2021, 69, 1852–1881. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Li, J.; Zheng, J.; Qin, S. Reactive Astrocytes in Neurodegenerative Diseases. Aging Dis. 2019, 10, 664–675. [Google Scholar] [CrossRef]
- Liddelow, S.A.; Barres, B.A. Reactive Astrocytes: Production, Function, and Therapeutic Potential. Immunity 2017, 46, 957–967. [Google Scholar] [CrossRef]
- Palmer, J.C.; Barker, R.; Kehoe, P.G.; Love, S. Endothelin-1 is elevated in Alzheimer’s disease and upregulated by amyloid-β. J. Alzheimers Dis. 2012, 29, 853–861. [Google Scholar] [CrossRef]
- Michinaga, S.; Kimura, A.; Hatanaka, S.; Minami, S.; Asano, A.; Ikushima, Y.; Matsui, S.; Toriyama, Y.; Fujii, M.; Koyama, Y. Delayed Administration of BQ788, an ET(B) Antagonist, after Experimental Traumatic Brain Injury Promotes Recovery of Blood-Brain Barrier Function and a Reduction of Cerebral Edema in Mice. J. Neurotrauma 2018, 35, 1481–1494. [Google Scholar] [CrossRef]
- Oliva, A.A., Jr.; Kang, Y.; Sanchez-Molano, J.; Furones, C.; Atkins, C.M. STAT3 signaling after traumatic brain injury. J. Neurochem. 2012, 120, 710–720. [Google Scholar] [CrossRef]
- Zhou, Y.; Shao, A.; Yao, Y.; Tu, S.; Deng, Y.; Zhang, J. Dual roles of astrocytes in plasticity and reconstruction after traumatic brain injury. Cell Commun. Signal. 2020, 18, 62. [Google Scholar] [CrossRef] [PubMed]
- Yu, G.; Zhang, Y.; Ning, B. Reactive Astrocytes in Central Nervous System Injury: Subgroup and Potential Therapy. Front. Cell. Neurosci. 2021, 15, 792764. [Google Scholar] [CrossRef] [PubMed]
- Koyama, Y. Endothelin ET(B) Receptor-Mediated Astrocytic Activation: Pathological Roles in Brain Disorders. Int. J. Mol. Sci. 2021, 22, 4333. [Google Scholar] [CrossRef] [PubMed]
- Migliorelli, R.; Petracca, G.; Teson, A.; Sabe, L.; Leiguarda, R.; Starkstein, S. Neuropsychiatric and neuropsychological correlates of delusions in Alzheimer’s disease. Psychol. Med. 1995, 25, 505–513. [Google Scholar] [CrossRef] [PubMed]
- Pentkowski, N.S.; Rogge-Obando, K.K.; Donaldson, T.N.; Bouquin, S.J.; Clark, B.J. Anxiety and Alzheimer’s disease: Behavioral analysis and neural basis in rodent models of Alzheimer’s-related neuropathology. Neurosci. Biobehav. Rev. 2021, 127, 647–658. [Google Scholar] [CrossRef] [PubMed]
- Teri, L.; Ferretti, L.E.; Gibbons, L.E.; Logsdon, R.G.; McCurry, S.M.; Kukull, W.A.; McCormick, W.C.; Bowen, J.D.; Larson, E.B. Anxiety in Alzheimer’s Disease: Prevalence and Comorbidity. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 1999, 54, M348–M352. [Google Scholar] [CrossRef] [PubMed]
- Trivedi, M.H.; Fava, M.; Wisniewski, S.R.; Thase, M.E.; Quitkin, F.; Warden, D.; Ritz, L.; Nierenberg, A.A.; Lebowitz, B.D.; Biggs, M.M.; et al. Medication Augmentation after the Failure of SSRIs for Depression. N. Engl. J. Med. 2006, 354, 1243–1252. [Google Scholar] [CrossRef] [PubMed]
- Hirschfeld, R.M. History and evolution of the monoamine hypothesis of depression. J. Clin. Psychiatry 2000, 61 (Suppl. S6), 4–6. [Google Scholar]
- Zhu, S.; Noviello, C.M.; Teng, J.; Walsh, R.M.; Kim, J.J.; Hibbs, R.E. Structure of a human synaptic GABAA receptor. Nature 2018, 559, 67–72. [Google Scholar] [CrossRef]
- Dixon, C.; Sah, P.; Lynch, J.W.; Keramidas, A. GABAA receptor α and γ subunits shape synaptic currents via different mechanisms. J. Biol. Chem. 2014, 289, 5399–5411. [Google Scholar] [CrossRef]
- Castellano, D.; Shepard, R.D.; Lu, W. Looking for Novelty in an “Old” Receptor: Recent Advances Toward Our Understanding of GABAARs and Their Implications in Receptor Pharmacology. Front. Neurosci. 2021, 14, 616298. [Google Scholar] [CrossRef] [PubMed]
- Engin, E.; Liu, J.; Rudolph, U. α2-containing GABA(A) receptors: A target for the development of novel treatment strategies for CNS disorders. Pharmacol. Ther. 2012, 136, 142–152. [Google Scholar] [CrossRef] [PubMed]
- Perry, E.K.; Gibson, P.H.; Blessed, G.; Perry, R.H.; Tomlinson, B.E. Neurotransmitter enzyme abnormalities in senile dementia: Choline acetyltransferase and glutamic acid decarboxylase activities in necropsy brain tissue. J. Neurol. Sci. 1977, 34, 247–265. [Google Scholar] [CrossRef] [PubMed]
- Rossor, M.; Garrett, N.; Johnson, A.; Mountjoy, C.; Roth, M.; Iversen, L. A post-mortem study of the cholinergic and GABA systems in senile dementia. Brain J. Neurol. 1982, 105, 313–330. [Google Scholar] [CrossRef] [PubMed]
- Millar, A. The Drugs Fighting Memory Loss. 2019. Available online: https://www.pharmaceutical-technology.com/features/memory-loss-drugs-reverse/ (accessed on 13 December 2021).
- Aldabbagh, Y.; Islam, A.; Zhang, W.; Whiting, P.; Ali, A.B. Alzheimer’s Disease Enhanced Tonic Inhibition is Correlated With Upregulated Astrocyte GABA Transporter-3/4 in a Knock-In APP Mouse Model. Front. Pharmacol. 2022, 13, 822499. [Google Scholar] [CrossRef] [PubMed]
- Petrache, A.L.; Rajulawalla, A.; Shi, A.; Wetzel, A.; Saito, T.; Saido, T.C.; Harvey, K.; Ali, A.B. Aberrant Excitatory-Inhibitory Synaptic Mechanisms in Entorhinal Cortex Microcircuits During the Pathogenesis of Alzheimer’s Disease. Cereb. Cortex 2019, 29, 1834–1850. [Google Scholar] [CrossRef] [PubMed]
- Petrache, A.L.; Khan, A.A.; Nicholson, M.W.; Monaco, A.; Kuta-Siejkowska, M.; Haider, S.; Hilton, S.; Jovanovic, J.N.; Ali, A.B. Selective Modulation of alpha5 GABA(A) Receptors Exacerbates Aberrant Inhibition at Key Hippocampal Neuronal Circuits in APP Mouse Model of Alzheimer’s Disease. Front. Cell Neurosci. 2020, 14, 568194. [Google Scholar] [CrossRef]
- Shi, A.; Petrache, A.L.; Shi, J.; Ali, A.B. Preserved Calretinin Interneurons in an App Model of Alzheimer’s Disease Disrupt Hippocampal Inhibition via Upregulated P2Y1 Purinoreceptors. Cereb. Cortex 2019, 30, 1272–1290. [Google Scholar] [CrossRef]
- Ali, A.B.; Islam, A.; Constanti, A. The fate of interneurons, GABA(A) receptor sub-types and perineuronal nets in Alzheimer’s disease. Brain Pathol. 2023, 33, e13129. [Google Scholar] [CrossRef]
- Belelli, D.; Harrison, N.L.; Maguire, J.; Macdonald, R.L.; Walker, M.C.; Cope, D.W. Extrasynaptic GABAA receptors: Form, pharmacology, and function. J. Neurosci. 2009, 29, 12757–12763. [Google Scholar] [CrossRef]
- Brickley, S.G.; Mody, I. Extrasynaptic GABA(A) receptors: Their function in the CNS and implications for disease. Neuron 2012, 73, 23–34. [Google Scholar] [CrossRef] [PubMed]
- Nusser, Z.; Mody, I. Selective modulation of tonic and phasic inhibitions in dentate gyrus granule cells. J. Neurophysiol. 2002, 87, 2624–2628. [Google Scholar] [CrossRef] [PubMed]
- Althaus, A.L.; Ackley, M.A.; Belfort, G.M.; Gee, S.M.; Dai, J.; Nguyen, D.P.; Kazdoba, T.M.; Modgil, A.; Davies, P.A.; Moss, S.J.; et al. Preclinical characterization of zuranolone (SAGE-217), a selective neuroactive steroid GABA(A) receptor positive allosteric modulator. Neuropharmacology 2020, 181, 108333. [Google Scholar] [CrossRef] [PubMed]
- Zanettini, C.; Pressly, J.D.; Ibarra, M.H.; Smith, K.R.; Gerak, L.R. Comparing the discriminative stimulus effects of modulators of GABAA receptors containing α4-δ subunits with those of gaboxadol in rats. Psychopharmacology 2016, 233, 2005–2013. [Google Scholar] [CrossRef] [PubMed]
- Whissell, P.D.; Lecker, I.; Wang, D.S.; Yu, J.; Orser, B.A. Altered expression of δGABAA receptors in health and disease. Neuropharmacology 2015, 88, 24–35. [Google Scholar] [CrossRef]
- Hoehn-Saric, R. Effects of THIP on chronic anxiety. Psychopharmacology 1983, 80, 338–341. [Google Scholar] [CrossRef]
- Cogram, P.; Deacon, R.M.J.; Warner-Schmidt, J.L.; von Schimmelmann, M.J.; Abrahams, B.S.; During, M.J. Gaboxadol Normalizes Behavioral Abnormalities in a Mouse Model of Fragile X Syndrome. Front. Behav. Neurosci. 2019, 13, 141. [Google Scholar] [CrossRef]


| Treatment of Drugs | Phase | Ages for Study | AD Stages | Treatment Purpose |
|---|---|---|---|---|
| AL002 and placebo | Phase 2 | 50–85 | Early | Efficacy and safety |
| SHR-1707 and SHR-1707 placebo | Phase 2 | 50–85 | Mild | Safety and pharmacodynamics |
| ALZ-801 | Phase 3 | 50–85 | Early | Long-term safety and efficacy |
| ADEL-Y01 and placebo | Phase 1 | 18–80 | Mild | Safety, tolerability, PK, and PD |
| ACU193 | Phase 2 and Phase 3 | 50–90 | Early | Efficacy and safety |
| Benfotiamine and placebo | Phase 2 | 50–89 | Early | Safety, effectiveness, and tolerability |
| Masitinib and placebo | Phase 3 | ≥50 | Mild to moderate | As an adjunct to a cholinesterase inhibitor |
| DDN-A-0101 and placebo | Phase 1 | 19–75 | Early | Safety, tolerability, and pharmacokinetics |
| BMS-986446 and placebo | Phase 2 | 50–80 | Early | Efficacy, safety, and tolerability |
| Daridorexant and placebo | Phase 4 | 60–85 | Mild to moderate | Efficacy and safety |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, J.; Haj Ebrahimi, A.; Ali, A.B. Advances in Therapeutics to Alleviate Cognitive Decline and Neuropsychiatric Symptoms of Alzheimer’s Disease. Int. J. Mol. Sci. 2024, 25, 5169. https://doi.org/10.3390/ijms25105169
Li J, Haj Ebrahimi A, Ali AB. Advances in Therapeutics to Alleviate Cognitive Decline and Neuropsychiatric Symptoms of Alzheimer’s Disease. International Journal of Molecular Sciences. 2024; 25(10):5169. https://doi.org/10.3390/ijms25105169
Chicago/Turabian StyleLi, Jialin, Anita Haj Ebrahimi, and Afia B. Ali. 2024. "Advances in Therapeutics to Alleviate Cognitive Decline and Neuropsychiatric Symptoms of Alzheimer’s Disease" International Journal of Molecular Sciences 25, no. 10: 5169. https://doi.org/10.3390/ijms25105169
APA StyleLi, J., Haj Ebrahimi, A., & Ali, A. B. (2024). Advances in Therapeutics to Alleviate Cognitive Decline and Neuropsychiatric Symptoms of Alzheimer’s Disease. International Journal of Molecular Sciences, 25(10), 5169. https://doi.org/10.3390/ijms25105169





