Pramipexole Hyperactivates the External Globus Pallidus and Impairs Decision-Making in a Mouse Model of Parkinson’s Disease
Abstract
:1. Introduction
2. Results
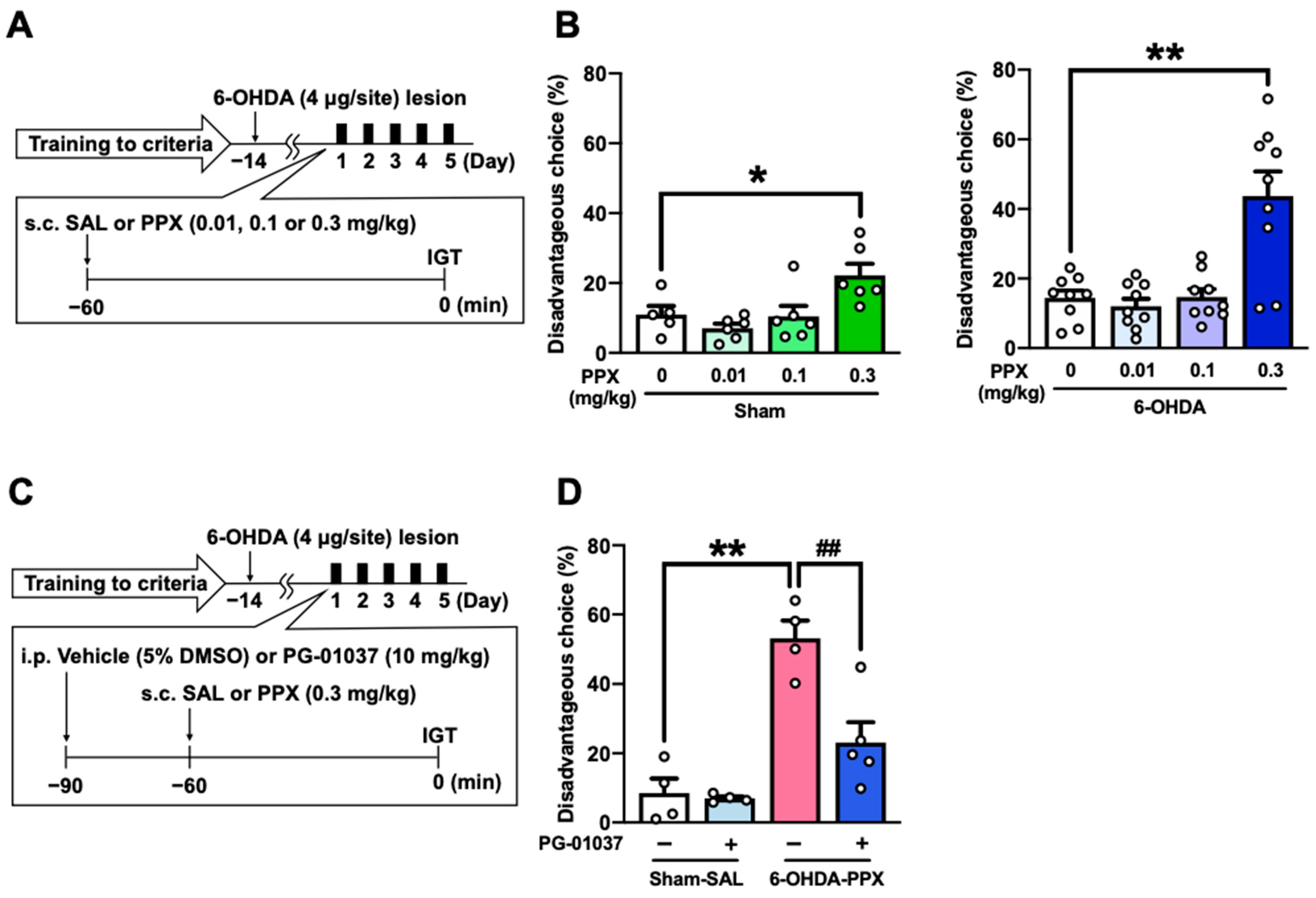
2.1. Effect of PPX Treatment on Decision-Making in Sham- and 6-OHDA-Lesioned Mice
2.2. Effect of D3R Antagonist on PPX-Induced Decision-Making Impairments in 6-OHDA-Lesioned Mice
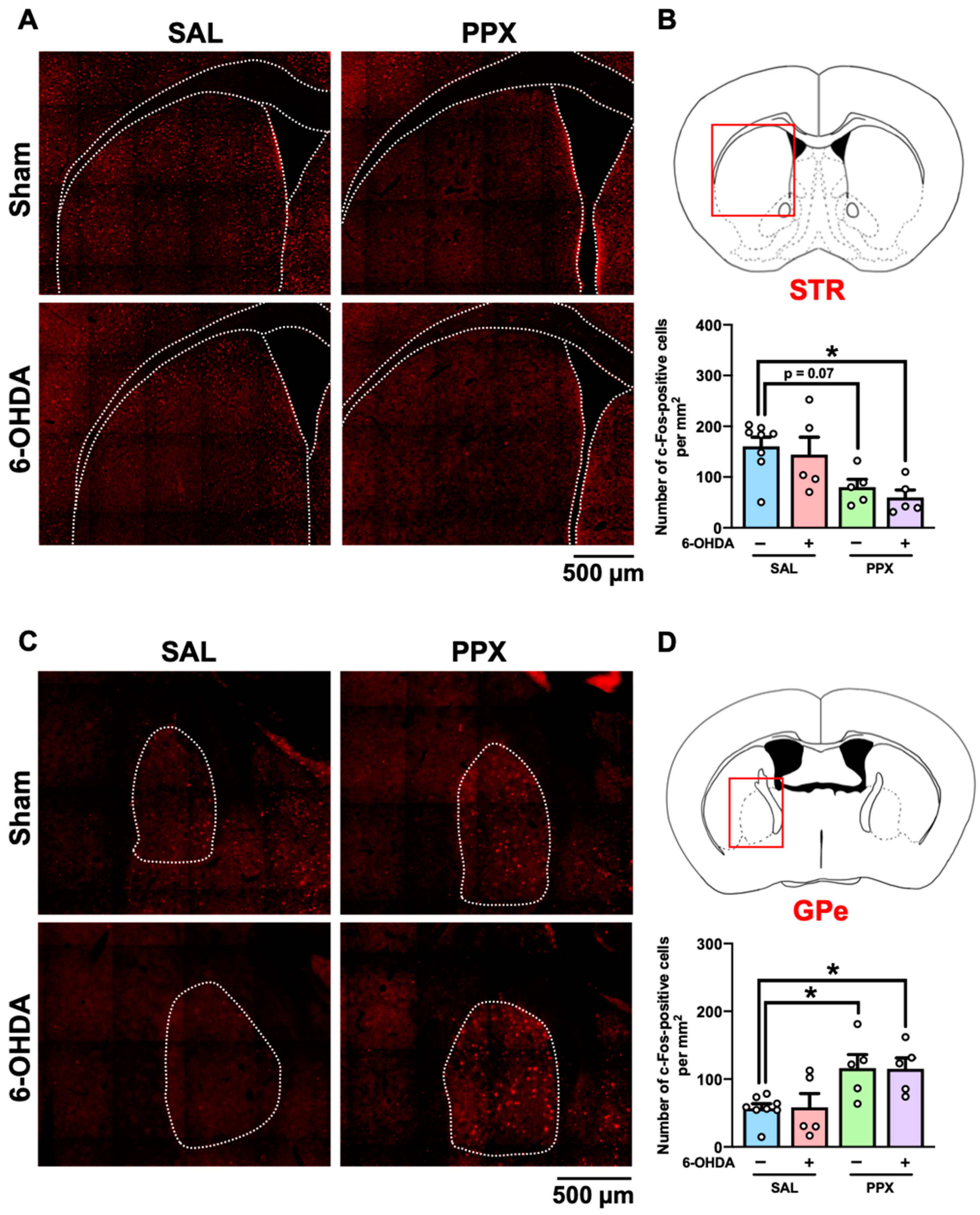
2.3. Expression of D3R in the STR of PPX-Treated Sham- and 6-OHDA-Lesioned Mice
2.4. c-Fos Mapping in the Brain of PPX-Treated Sham- and 6-OHDA-Lesioned Mice
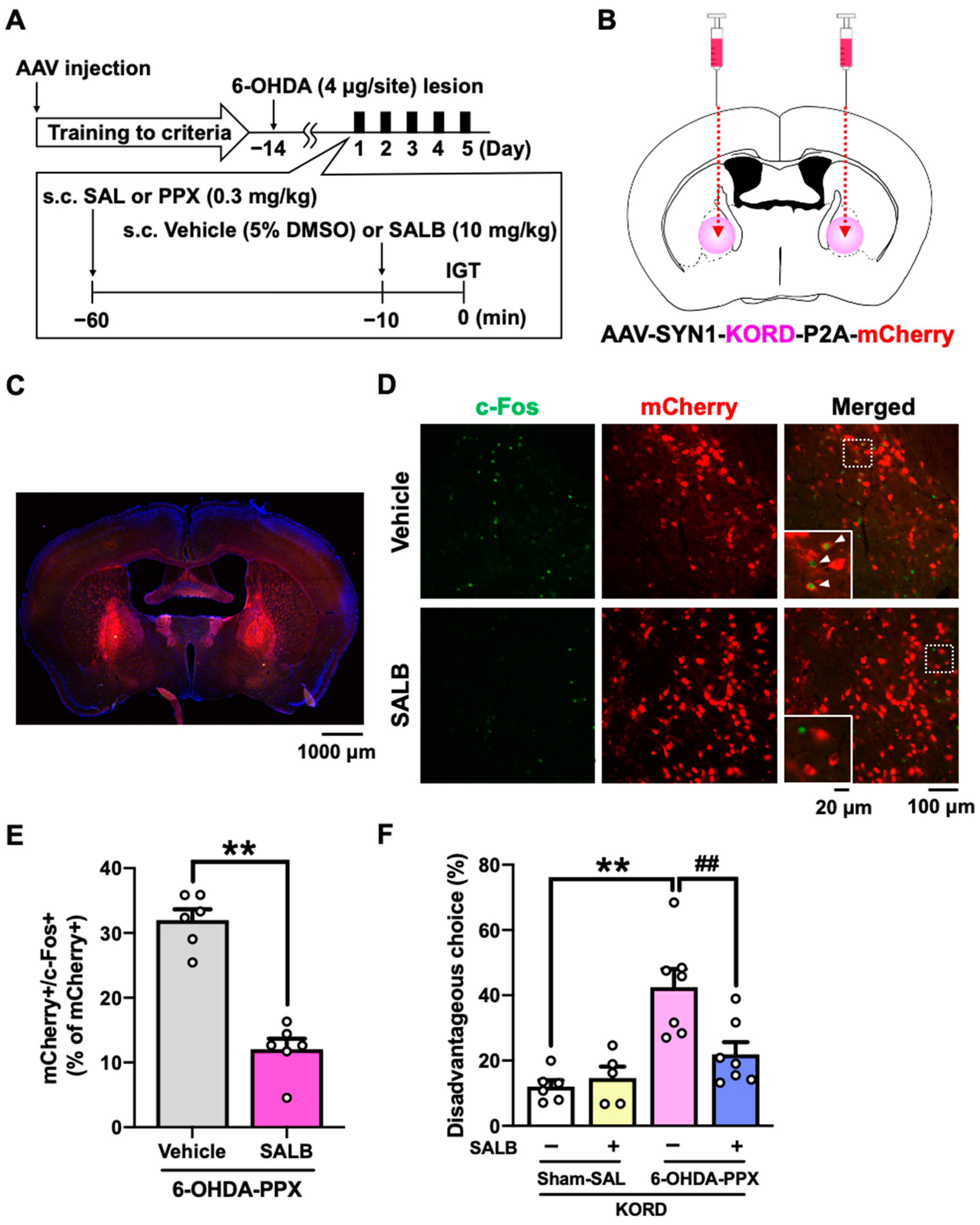
2.5. Effect of Chemogenetic Inhibition of GPe on PPX-Induced Decision-Making Impairments in 6-OHDA-Lesioned Mice
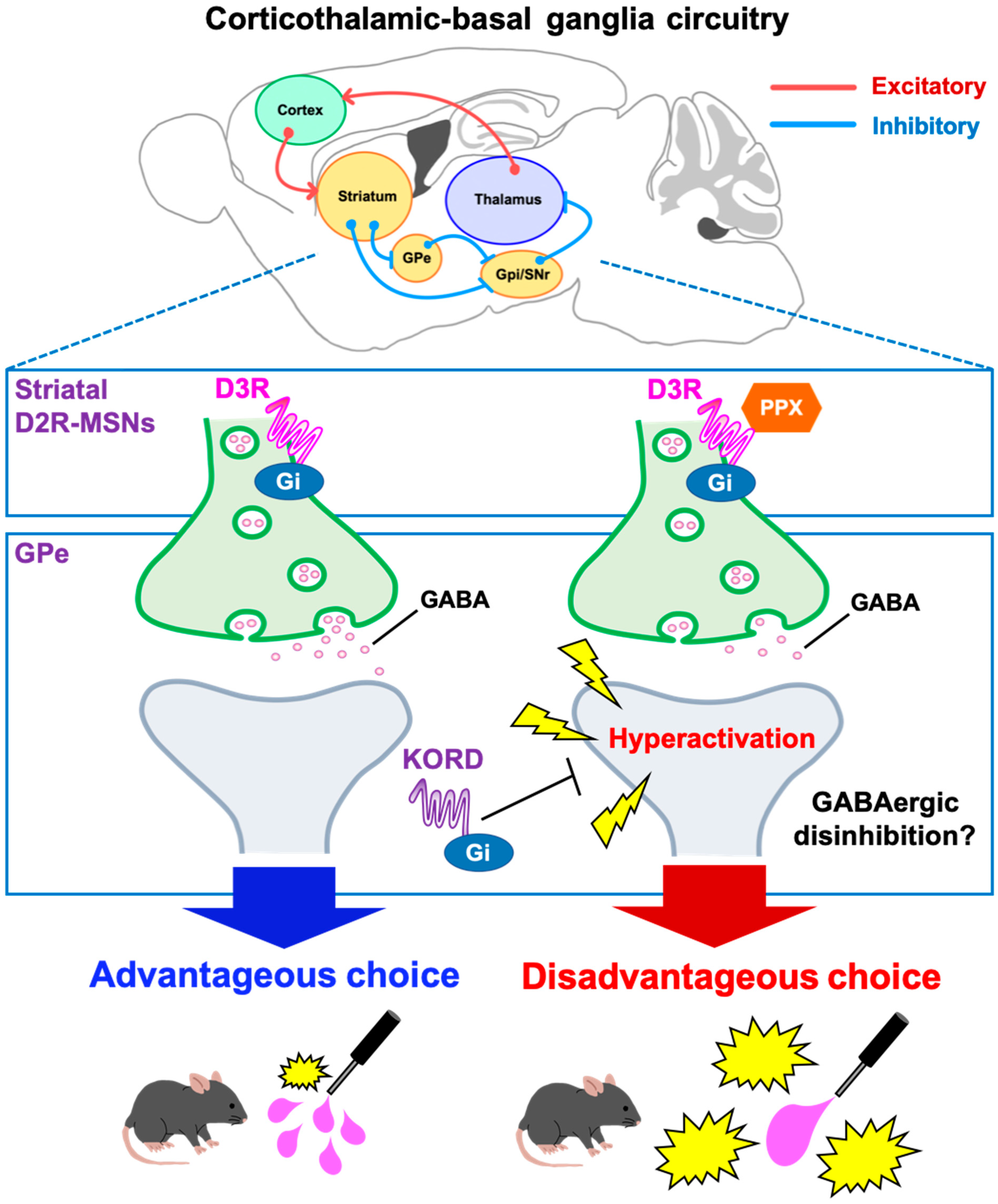
3. Discussion
4. Materials and Methods
4.1. Mice
4.2. Drug Administration
4.3. 6-OHDA Lesion
4.4. Adeno-Associated Virus Preparation
4.5. In Vivo Chemogenetic Manipulation
4.6. Touchscreen-Based IGT
4.6.1. Apparatus
4.6.2. IGT Pre-Training Session
4.6.3. IGT Training Session
4.6.4. IGT Test Session
4.7. Immunohistochemistry
4.8. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jankovic, J. Parkinson’s disease: Clinical features and diagnosis. J. Neurol. Neurosurg. Psychiatry 2008, 79, 368–376. [Google Scholar] [CrossRef] [PubMed]
- Antonini, A.; Barone, P.; Bonuccelli, U.; Annoni, K.; Asgharnejad, M.; Stanzione, P. ICARUS study: Prevalence and clinical features of impulse control disorders in Parkinson’s disease. J. Neurol. Neurosurg. Psychiatry 2017, 88, 317–324. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Ruiz, P.J.; Martinez Castrillo, J.C.; Alonso-Canovas, A.; Herranz Barcenas, A.; Vela, L.; Sanchez Alonso, P.; Mata, M.; Olmedilla Gonzalez, N.; Mahillo Fernandez, I. Impulse control disorder in patients with Parkinson’s disease under dopamine agonist therapy: A multicentre study. J. Neurol. Neurosurg. Psychiatry 2014, 85, 840–844. [Google Scholar] [CrossRef] [PubMed]
- Avanzi, M.; Baratti, M.; Cabrini, S.; Uber, E.; Brighetti, G.; Bonfa, F. Prevalence of pathological gambling in patients with Parkinson’s disease. Mov. Disord. 2006, 21, 2068–2072. [Google Scholar] [CrossRef] [PubMed]
- Santangelo, G.; Barone, P.; Trojano, L.; Vitale, C. Pathological gambling in Parkinson’s disease. A comprehensive review. Park. Relat. Disord. 2013, 19, 645–653. [Google Scholar] [CrossRef] [PubMed]
- Goudriaan, A.E.; Oosterlaan, J.; de Beurs, E.; van den Brink, W. Decision making in pathological gambling: A comparison between pathological gamblers, alcohol dependents, persons with Tourette syndrome, and normal controls. Brain Res. Cogn. Brain Res. 2005, 23, 137–151. [Google Scholar] [CrossRef] [PubMed]
- Kjaer, S.W.; Callesen, M.B.; Larsen, L.; Borghammer, P.; Ostergaard, K.; Damholdt, M.F. Applied strategy in the Iowa Gambling Task: Comparison of individuals with Parkinson’s disease to healthy controls. J. Clin. Exp. Neuropsychol. 2020, 42, 425–435. [Google Scholar] [CrossRef] [PubMed]
- Colautti, L.; Iannello, P.; Silveri, M.C.; Antonietti, A. Decision making in Parkinson’s disease: An analysis of the studies using the Iowa Gambling Task. Eur. J. Neurosci. 2021, 54, 7513–7549. [Google Scholar] [CrossRef] [PubMed]
- Bechara, A.; Damasio, H.; Damasio, A.R.; Lee, G.P. Different contributions of the human amygdala and ventromedial prefrontal cortex to decision-making. J. Neurosci. 1999, 19, 5473–5481. [Google Scholar] [CrossRef]
- Fellows, L.K.; Farah, M.J. Different underlying impairments in decision-making following ventromedial and dorsolateral frontal lobe damage in humans. Cereb. Cortex 2005, 15, 58–63. [Google Scholar] [CrossRef]
- Zeeb, F.D.; Winstanley, C.A. Lesions of the basolateral amygdala and orbitofrontal cortex differentially affect acquisition and performance of a rodent gambling task. J. Neurosci. 2011, 31, 2197–2204. [Google Scholar] [CrossRef]
- Paine, T.A.; Asinof, S.K.; Diehl, G.W.; Frackman, A.; Leffler, J. Medial prefrontal cortex lesions impair decision-making on a rodent gambling task: Reversal by D1 receptor antagonist administration. Behav. Brain Res. 2013, 243, 247–254. [Google Scholar] [CrossRef] [PubMed]
- Cox, J.; Witten, I.B. Striatal circuits for reward learning and decision-making. Nat. Rev. Neurosci. 2019, 20, 482–494. [Google Scholar] [CrossRef] [PubMed]
- Zhao, F.; Zeng, Y.; Xu, B. A brain-inspired decision-making spiking neural network and its application in unmanned aerial vehicle. Front. Neurorobot 2018, 12, 56. [Google Scholar] [CrossRef] [PubMed]
- Surmeier, D.J.; Song, W.J.; Yan, Z. Coordinated expression of dopamine receptors in neostriatal medium spiny neurons. J. Neurosci. 1996, 16, 6579–6591. [Google Scholar] [CrossRef] [PubMed]
- Pes, R.; Godar, S.C.; Fox, A.T.; Burgeno, L.M.; Strathman, H.J.; Jarmolowicz, D.P.; Devoto, P.; Levant, B.; Phillips, P.E.; Fowler, S.C.; et al. Pramipexole enhances disadvantageous decision-making: Lack of relation to changes in phasic dopamine release. Neuropharmacology 2017, 114, 77–87. [Google Scholar] [CrossRef] [PubMed]
- Mortazavi, L.; Hynes, T.J.; Chernoff, C.S.; Ramaiah, S.; Brodie, H.G.; Russell, B.; Hathaway, B.A.; Kaur, S.; Winstanley, C.A. D2/3 Agonist during learning potentiates cued risky choice. J. Neurosci. 2023, 43, 979–992. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.J.; Swope, D.M.; Dashtipour, K.; Lyons, K.E. Transdermal rotigotine: A clinically innovative dopamine-receptor agonist for the management of Parkinson’s disease. Pharmacotherapy 2009, 29, 1452–1467. [Google Scholar] [CrossRef] [PubMed]
- Barrus, M.M.; Winstanley, C.A. Dopamine D3 receptors modulate the ability of win-paired cues to increase risky choice in a rat gambling task. J. Neurosci. 2016, 36, 785–794. [Google Scholar] [CrossRef]
- Bussey, T.J.; Holmes, A.; Lyon, L.; Mar, A.C.; McAllister, K.A.; Nithianantharajah, J.; Oomen, C.A.; Saksida, L.M. New translational assays for preclinical modelling of cognition in schizophrenia: The touchscreen testing method for mice and rats. Neuropharmacology 2012, 62, 1191–1203. [Google Scholar] [CrossRef]
- Dardou, D.; Chassain, C.; Durif, F. Chronic pramipexole treatment increases tolerance for sucrose in normal and ventral tegmental lesioned rats. Front. Neurosci. 2014, 8, 437. [Google Scholar] [CrossRef] [PubMed]
- Bagetta, V.; Sgobio, C.; Pendolino, V.; Del Papa, G.; Tozzi, A.; Ghiglieri, V.; Giampa, C.; Zianni, E.; Gardoni, F.; Calabresi, P.; et al. Rebalance of striatal NMDA/AMPA receptor ratio underlies the reduced emergence of dyskinesia during D2-like dopamine agonist treatment in experimental Parkinson’s disease. J. Neurosci. 2012, 32, 17921–17931. [Google Scholar] [CrossRef]
- Bogacz, R.; Martin Moraud, E.; Abdi, A.; Magill, P.J.; Baufreton, J. Properties of neurons in external globus pallidus can support optimal action selection. PLoS Comput. Biol. 2016, 12, e1005004. [Google Scholar] [CrossRef]
- Baker, M.; Kang, S.; Hong, S.I.; Song, M.; Yang, M.A.; Peyton, L.; Essa, H.; Lee, S.W.; Choi, D.S. External globus pallidus input to the dorsal striatum regulates habitual seeking behavior in male mice. Nat. Commun. 2023, 14, 4085. [Google Scholar] [CrossRef]
- Zhong, C.; Wang, L.; Cao, Y.; Sun, C.; Huang, J.; Wang, X.; Pan, S.; He, S.; Huang, K.; Lu, Z.; et al. A neural circuit from the dorsal CA3 to the dorsomedial hypothalamus mediates balance between risk exploration and defense. Cell Rep. 2022, 41, 111570. [Google Scholar] [CrossRef] [PubMed]
- Vardy, E.; Robinson, J.E.; Li, C.; Olsen, R.H.J.; DiBerto, J.F.; Giguere, P.M.; Sassano, F.M.; Huang, X.P.; Zhu, H.; Urban, D.J.; et al. A new DREADD facilitates the multiplexed chemogenetic interrogation of behavior. Neuron 2015, 86, 936–946. [Google Scholar] [CrossRef]
- Volkmann, J.; Daniels, C.; Witt, K. Neuropsychiatric effects of subthalamic neurostimulation in Parkinson disease. Nat. Rev. Neurol. 2010, 6, 487–498. [Google Scholar] [CrossRef] [PubMed]
- Chandrababu, K.; Radhakrishnan, V.; Anjana, A.S.; Rajan, R.; Sivan, U.; Krishnan, S.; Baby Chakrapani, P.S. Unravelling the Parkinson’s puzzle, from medications and surgery to stem cells and genes: A comprehensive review of current and future management strategies. Exp. Brain Res. 2024, 242, 1–23. [Google Scholar] [CrossRef]
- Saga, Y.; Hoshi, E.; Tremblay, L. Roles of multiple globus pallidus territories of monkeys and humans in motivation, Cognition and action: An anatomical, physiological and pathophysiological review. Front. Neuroanat. 2017, 11, 30. [Google Scholar] [CrossRef]
- Cools, R.; Altamirano, L.; D’Esposito, M. Reversal learning in Parkinson’s disease depends on medication status and outcome valence. Neuropsychologia 2006, 44, 1663–1673. [Google Scholar] [CrossRef]
- Bodi, N.; Keri, S.; Nagy, H.; Moustafa, A.; Myers, C.E.; Daw, N.; Dibo, G.; Takats, A.; Bereczki, D.; Gluck, M.A. Reward-learning and the novelty-seeking personality: A between- and within-subjects study of the effects of dopamine agonists on young Parkinson’s patients. Brain 2009, 132 Pt 9, 2385–2395. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Tsuboi, D.; Funahashi, Y.; Yamahashi, Y.; Kaibuchi, K.; Nagai, T. Phosphorylation signals downstream of dopamine receptors in emotional behaviors: Association with preference and avoidance. Int. J. Mol. Sci. 2022, 23, 11643. [Google Scholar] [CrossRef]
- Tsuboi, D.; Otsuka, T.; Shimomura, T.; Faruk, M.O.; Yamahashi, Y.; Amano, M.; Funahashi, Y.; Kuroda, K.; Nishioka, T.; Kobayashi, K.; et al. Dopamine drives neuronal excitability via KCNQ channel phosphorylation for reward behavior. Cell Rep. 2022, 40, 111309. [Google Scholar] [CrossRef] [PubMed]
- Courtney, C.D.; Pamukcu, A.; Chan, C.S. Cell and circuit complexity of the external globus pallidus. Nat. Neurosci. 2023, 26, 1147–1159. [Google Scholar] [CrossRef]
- Shin, S.; Pribiag, H.; Lilascharoen, V.; Knowland, D.; Wang, X.Y.; Lim, B.K. Drd3 signaling in the lateral septum mediates early life stress-induced social dysfunction. Neuron 2018, 97, 195–208.e6. [Google Scholar] [CrossRef]
- Rizzi-Wise, C.A.; Wang, D.V. Putting together pieces of the lateral septum: Multifaceted functions and its neural pathways. eNeuro 2021, 8, ENEURO.0315-21.2021. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Li, P.; Li, Z.; da Silva, B.S.; Zheng, W.; Xiang, Z.; He, Y.; Xu, T.; Cordeiro, C.; Deng, L.; et al. Lateral septum adenosine A2A receptors control stress-induced depressive-like behaviors via signaling to the hypothalamus and habenula. Nat. Commun. 2023, 14, 1880. [Google Scholar] [CrossRef]
- Schulte-Herbruggen, O.; Vogt, M.A.; Hortnagl, H.; Gass, P.; Hellweg, R. Pramipexole is active in depression tests and modulates monoaminergic transmission, but not brain levels of BDNF in mice. Eur. J. Pharmacol. 2012, 677, 77–86. [Google Scholar] [CrossRef]
- Bonito-Oliva, A.; Masini, D.; Fisone, G. A mouse model of non-motor symptoms in Parkinson’s disease: Focus on pharmacological interventions targeting affective dysfunctions. Front. Behav. Neurosci. 2014, 8, 290. [Google Scholar] [CrossRef]
- Riba, J.; Kramer, U.M.; Heldmann, M.; Richter, S.; Munte, T.F. Dopamine agonist increases risk taking but blunts reward-related brain activity. PLoS ONE 2008, 3, e2479. [Google Scholar] [CrossRef]
- Strejilevich, S.A.; Martino, D.J.; Igoa, A.; Manes, F. Pathological gambling in a bipolar patient treated with pramipexole. J. Neuropsychiatry Clin. Neurosci. 2011, 23, E2–E3. [Google Scholar] [CrossRef] [PubMed]
- Rokosik, S.L.; Napier, T.C. Pramipexole-induced increased probabilistic discounting: Comparison between a rodent model of Parkinson’s disease and controls. Neuropsychopharmacology 2012, 37, 1397–1408. [Google Scholar] [CrossRef]
- Wang, S.; Zhang, Q.J.; Liu, J.; Wu, Z.H.; Ali, U.; Wang, Y.; Chen, L.; Gui, Z.H. The firing activity of pyramidal neurons in medial prefrontal cortex and their response to 5-hydroxytryptamine-1A receptor stimulation in a rat model of Parkinson’s disease. Neuroscience 2009, 162, 1091–1100. [Google Scholar] [CrossRef]
- Li, M.; Xu, H.; Chen, G.; Sun, S.; Wang, Q.; Liu, B.; Wu, X.; Zhou, L.; Chai, Z.; Sun, X.; et al. Impaired D2 receptor-dependent dopaminergic transmission in prefrontal cortex of awake mouse model of Parkinson’s disease. Brain 2019, 142, 3099–3115. [Google Scholar] [CrossRef] [PubMed]
- Palmas, M.F.; Etzi, M.; Pisanu, A.; Camoglio, C.; Sagheddu, C.; Santoni, M.; Manchinu, M.F.; Pala, M.; Fusco, G.; De Simone, A.; et al. The intranigral infusion of human-alpha synuclein oligomers induces a cognitive impairment in rats associated with changes in neuronal firing and neuroinflammation in the anterior cingulate cortex. Cells 2022, 11, 2628. [Google Scholar] [CrossRef]
- Cho, E.Y.; Cho, D.I.; Park, J.H.; Kurose, H.; Caron, M.G.; Kim, K.M. Roles of protein kinase C and actin-binding protein 280 in the regulation of intracellular trafficking of dopamine D3 receptor. Mol. Endocrinol. 2007, 21, 2242–2254. [Google Scholar] [CrossRef] [PubMed]
- Fiorentini, C.; Busi, C.; Gorruso, E.; Gotti, C.; Spano, P.; Missale, C. Reciprocal regulation of dopamine D1 and D3 receptor function and trafficking by heterodimerization. Mol. Pharmacol. 2008, 74, 59–69. [Google Scholar] [CrossRef]
- Xu, W.; Reith, M.E.A.; Liu-Chen, L.Y.; Kortagere, S. Biased signaling agonist of dopamine D3 receptor induces receptor internalization independent of beta-arrestin recruitment. Pharmacol. Res. 2019, 143, 48–57. [Google Scholar] [CrossRef] [PubMed]
- Prieto, G.A. Abnormalities of Dopamine D3 Receptor Signaling in the Diseased Brain. J. Cent. Nerv. Syst. Dis. 2017, 9, 1179573517726335. [Google Scholar] [CrossRef]
- Prieto, G.A.; Perez-Burgos, A.; Palomero-Rivero, M.; Galarraga, E.; Drucker-Colin, R.; Bargas, J. Upregulation of D2-class signaling in dopamine-denervated striatum is in part mediated by D3 receptors acting on Ca V 2.1 channels via PIP2 depletion. J. Neurophysiol. 2011, 105, 2260–2274. [Google Scholar] [CrossRef]
- Hultman, C.; Tjernstrom, N.; Vadlin, S.; Rehn, M.; Nilsson, K.W.; Roman, E.; Aslund, C. Exploring decision-making strategies in the Iowa gambling task and rat gambling task. Front. Behav. Neurosci. 2022, 16, 964348. [Google Scholar] [CrossRef] [PubMed]
- Rentsch, P.; Stayte, S.; Morris, G.P.; Vissel, B. Time dependent degeneration of the nigrostriatal tract in mice with 6-OHDA lesioned medial forebrain bundle and the effect of activin A on L-Dopa induced dyskinesia. BMC Neurosci. 2019, 20, 5. [Google Scholar] [CrossRef] [PubMed]
- Sano, H.; Nambu, A. The effects of zonisamide on L-DOPA-induced dyskinesia in Parkinson’s disease model mice. Neurochem. Int. 2019, 124, 171–180. [Google Scholar] [CrossRef] [PubMed]
- Masini, D.; Plewnia, C.; Bertho, M.; Scalbert, N.; Caggiano, V.; Fisone, G. A guide to the generation of a 6-hydroxydopamine mouse model of Parkinson’s disease for the study of non-motor symptoms. Biomedicines 2021, 9, 598. [Google Scholar] [CrossRef] [PubMed]
- Djamshidian, A.; Cardoso, F.; Grosset, D.; Bowden-Jones, H.; Lees, A.J. Pathological gambling in Parkinson’s disease—A review of the literature. Mov. Disord. 2011, 26, 1976–1984. [Google Scholar] [CrossRef] [PubMed]
- Meziane, H.; Ouagazzal, A.M.; Aubert, L.; Wietrzych, M.; Krezel, W. Estrous cycle effects on behavior of C57BL/6J and BALB/cByJ female mice: Implications for phenotyping strategies. Genes. Brain Behav. 2007, 6, 192–200. [Google Scholar] [CrossRef] [PubMed]
- Botz-Zapp, C.A.; Foster, S.L.; Pulley, D.M.; Hempel, B.; Bi, G.H.; Xi, Z.X.; Newman, A.H.; Weinshenker, D.; Manvich, D.F. Effects of the selective dopamine D3 receptor antagonist PG01037 on morphine-induced hyperactivity and antinociception in mice. Behav. Brain Res. 2021, 415, 113506. [Google Scholar] [CrossRef]
- van Enkhuizen, J.; Geyer, M.A.; Young, J.W. Differential effects of dopamine transporter inhibitors in the rodent Iowa gambling task: Relevance to mania. Psychopharmacology 2013, 225, 661–674. [Google Scholar] [CrossRef]
- Kim, W.Y.; Cho, B.R.; Kwak, M.J.; Kim, J.H. Interaction between trait and housing condition produces differential decision-making toward risk choice in a rat gambling task. Sci. Rep. 2017, 7, 5718. [Google Scholar] [CrossRef]
- Wulaer, B.; Nagai, T.; Sobue, A.; Itoh, N.; Kuroda, K.; Kaibuchi, K.; Nabeshima, T.; Yamada, K. Repetitive and compulsive-like behaviors lead to cognitive dysfunction in Disc1Δ2-3/Δ2-3 mice. Genes. Brain Behav. 2018, 17, e12478. [Google Scholar] [CrossRef]


| Measure & Description | Outcome |
|---|---|
| Total trials initiation: Number of trials completed per session (Max = 100) | Degree of session completion |
| Response (%): Response to P1, P2, P3 or P4 as a percentage of the total cue windows touched | Preference for 1 cue compared to others |
| Disadvantageous choice (%): Disadvantageous response options (P3 + P4)/total (P1 + P2 + P3 + P4) × 100 | Risk preference |
| Premature response (%): Responding in any cue window during the inter-trial interval of 5 s | Motoric impulsivity |
| Omission (%): Failure to respond in any cue window during the light stimulus duration of 10 s | Inattention/amotivation |
| Collect latency: The latency to collect rewards after responding in a cue window | Motivation |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kubota, H.; Zhou, X.; Zhang, X.; Watanabe, H.; Nagai, T. Pramipexole Hyperactivates the External Globus Pallidus and Impairs Decision-Making in a Mouse Model of Parkinson’s Disease. Int. J. Mol. Sci. 2024, 25, 8849. https://doi.org/10.3390/ijms25168849
Kubota H, Zhou X, Zhang X, Watanabe H, Nagai T. Pramipexole Hyperactivates the External Globus Pallidus and Impairs Decision-Making in a Mouse Model of Parkinson’s Disease. International Journal of Molecular Sciences. 2024; 25(16):8849. https://doi.org/10.3390/ijms25168849
Chicago/Turabian StyleKubota, Hisayoshi, Xinzhu Zhou, Xinjian Zhang, Hirohisa Watanabe, and Taku Nagai. 2024. "Pramipexole Hyperactivates the External Globus Pallidus and Impairs Decision-Making in a Mouse Model of Parkinson’s Disease" International Journal of Molecular Sciences 25, no. 16: 8849. https://doi.org/10.3390/ijms25168849
APA StyleKubota, H., Zhou, X., Zhang, X., Watanabe, H., & Nagai, T. (2024). Pramipexole Hyperactivates the External Globus Pallidus and Impairs Decision-Making in a Mouse Model of Parkinson’s Disease. International Journal of Molecular Sciences, 25(16), 8849. https://doi.org/10.3390/ijms25168849






