Effects of miR-306 Perturbation on Life Parameters in the English Grain Aphid, Sitobion avenae (Homoptera: Aphididae)
Abstract
1. Introduction
2. Results
2.1. Developmental Duration of S. avenae
2.2. Life Table Parameters
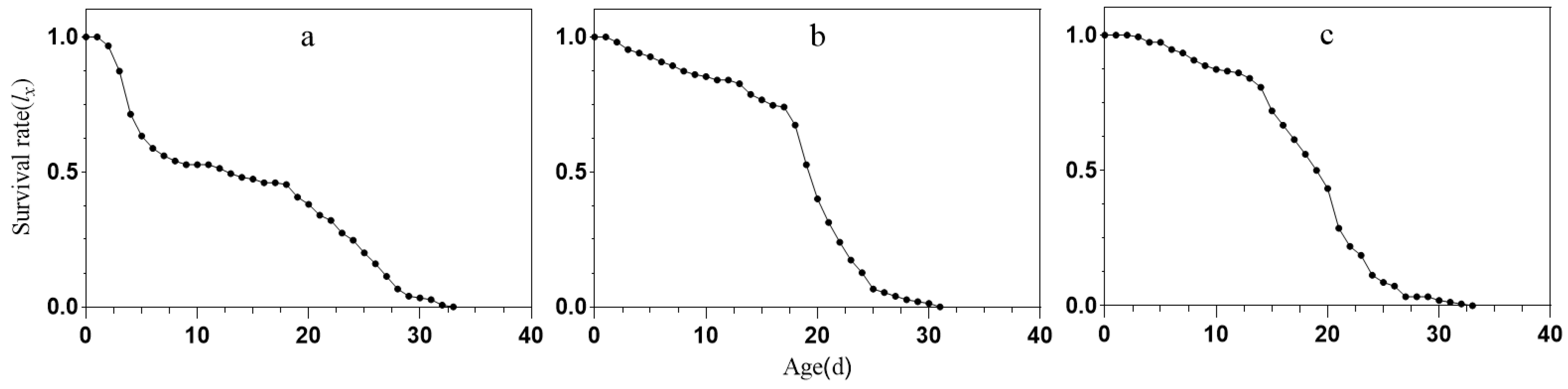
2.3. Survival Curves of S. avenae under Different Treatment Conditions
2.4. Reproductive Rate Curves of S. avenae under Different Treatment Conditions
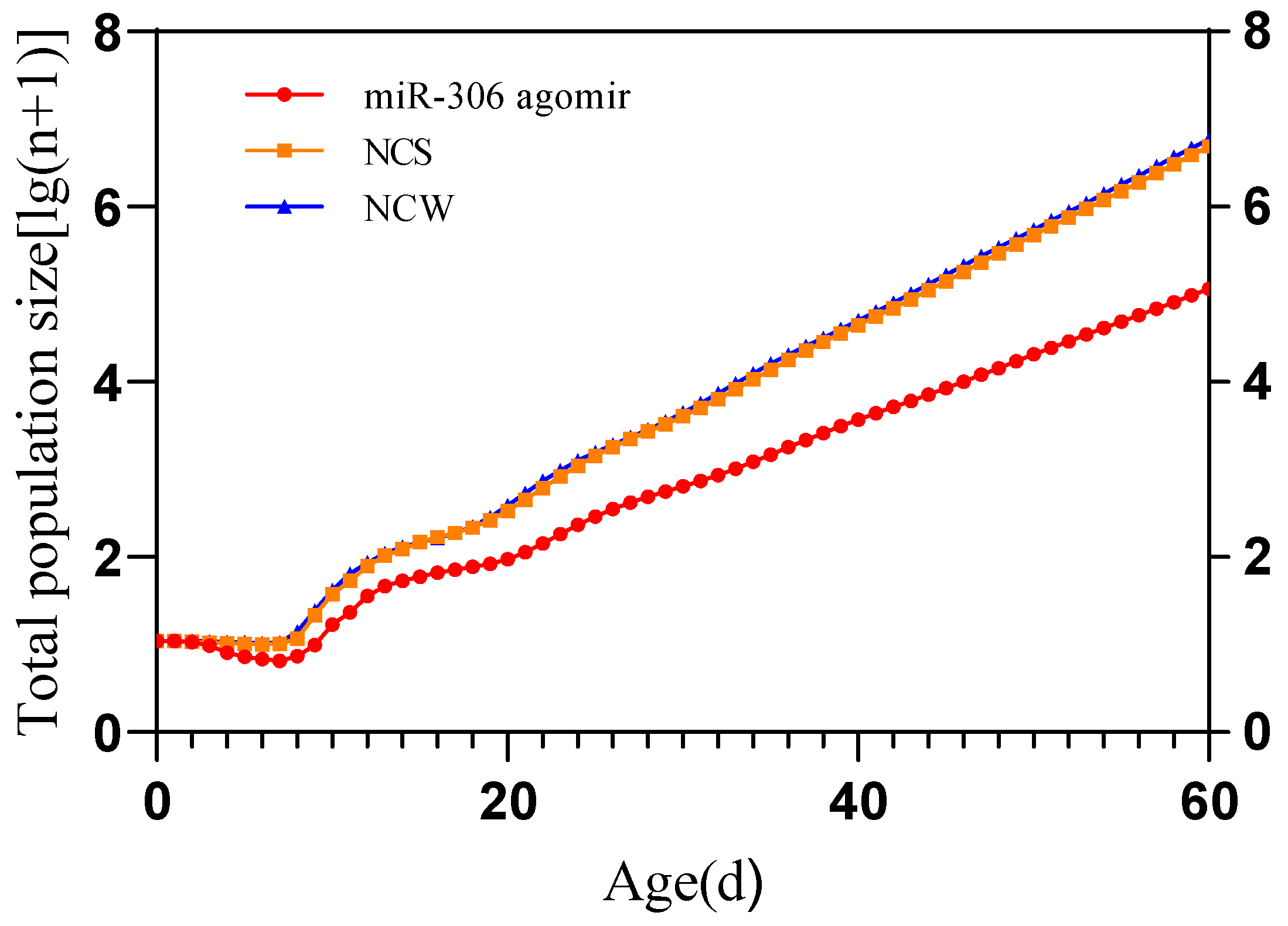
2.5. Simulation of S. avenae Population Projection
3. Discussion
4. Materials and Methods
4.1. Laboratory Aphid Population
4.2. Chemical Agents
4.3. Experimental Design
4.4. Data Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chopa, C.S.; Descamps, L.R. Composition and biological activity of essential oils against Metopolophium dirhodum (Hemiptera: Aphididae) cereal crop pest. Pest. Manag. Sci. 2012, 68, 1492–1500. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Men, X.; Hui, C.; Ge, F.; Ouyang, F. Wheat yield losses from pests and pathogens in China. Agric. Ecosyst. Environ. 2022, 326, 107821. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, Y.; Chen, J. Research progress on mechanism and genetics of aphid resistance in wheat crops. Plant Prot. 2023. [Google Scholar] [CrossRef]
- Morales-Hojas, R.; Sun, J.; Alvira Iraizoz, F.; Tan, X.; Chen, J. Contrasting population structure and demographic history of cereal aphids in different environmental and agricultural landscapes. Ecol. Evol. 2020, 10, 9647–9662. [Google Scholar] [CrossRef] [PubMed]
- Shrestha, R.B.; Parajulee, M.N. Potential cotton aphid, Aphis gossypii, population suppression by arthropod predators in upland cotton. Insect Sci. 2013, 20, 778–788. [Google Scholar] [CrossRef] [PubMed]
- Gong, P.; Li, X.; Gao, H.; Wang, C.; Li, M.; Zhang, Y.; Li, X.; Liu, E.; Zhu, X. Field evolved resistance to pyrethroids, neonicotinoids, organophosphates and macrolides in Rhopalosiphum padi (Linnaeus) and Sitobion avenae (Fabricius) from China. Chemosphere 2021, 269, 128747. [Google Scholar] [CrossRef] [PubMed]
- Fontaine, S.; Caddoux, L.; Barrès, B. First report of the kdr pyrethroid resistance mutation in a French population of the English grain aphid, Sitobion avenae. Crop Prot. 2023, 165, 106153. [Google Scholar] [CrossRef]
- Fire, A.; Xu, S.; Montgomery, M.K.; Kostas, S.A.; Driver, S.E.; Mello, C.C. Potent and specific genetic interference by double-stranded RNA in Caenorhabditis elegans. Nature 1998, 391, 806–811. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; De Schutter, K. Biosafety aspects of RNAi-based pests control. Pest. Manag. Sci. 2024. [Google Scholar] [CrossRef] [PubMed]
- Bolognesi, R.; Ramaseshadri, P.; Anderson, J.; Bachman, P.; Clinton, W.; Flannagan, R.; Ilagan, O.; Lawrence, C.; Levine, S.; Moar, W.; et al. Characterizing the mechanism of action of double-stranded RNA activity against western corn rootworm (Diabrotica virgifera virgifera LeConte). PLoS ONE 2012, 7, e47534. [Google Scholar] [CrossRef]
- Price, D.R.; Gatehouse, J.A. RNAi-mediated crop protection against insects. Trends Biotechnol. 2008, 26, 393–400. [Google Scholar] [CrossRef]
- Whangbo, J.S.; Hunter, C.P. Environmental RNA interference. Trends Genet. 2008, 24, 297–305. [Google Scholar] [CrossRef] [PubMed]
- Zotti, M.J.; Smagghe, G. RNAi Technology for Insect Management and Protection of Beneficial Insects from Diseases: Lessons, Challenges and Risk Assessments. Neotrop. Entomol. 2015, 44, 197–213. [Google Scholar] [CrossRef]
- Guan, M.; Chao, Z.; Yan, S.; Shen, J. Research status and development prospect of RNA pesticide. Mod. Agrochem. 2023, 22, 11–18. [Google Scholar]
- Hafeez, M.; Li, X.; Chen, L.; Ullah, F.; Huang, J.; Zhang, Z.; Zhang, J.; Siddiqui, J.A.; Zhou, S.X.; Ren, X.Y.; et al. Molecular characterization and functional analysis of cytochrome P450-mediated detoxification CYP302A1 gene involved in host plant adaptation in Spodoptera frugieprda. Front. Plant Sci. 2022, 13, 1079442. [Google Scholar] [CrossRef] [PubMed]
- Ullah, F.; Gul, H.; Tariq, K.; Hafeez, M.; Desneux, N.; Song, D. Silencing of Cytochrome P450 genes CYP6CY14 and CYP6DC1 in Aphis gossypii by RNA interference enhances susceptibility to clothianidin. Entomol. Gen. 2023, 43, 669–678. [Google Scholar] [CrossRef]
- Li, J.; Qian, J.; Xu, Y.; Yan, S.; Shen, J.; Yin, M. A Facile-Synthesized Star Polycation Constructed as a Highly Efficient Gene Vector in Pest Management. ACS Sustain. Chem. Eng. 2019, 7, 6316–6322. [Google Scholar] [CrossRef]
- Liz, J.; Portela, A.; Soler, M.; Gomez, A.; Ling, H.; Michlewski, G.; Calin, G.A.; Guil, S.; Esteller, M. Regulation of pri-miRNA processing by a long noncoding RNA transcribed from an ultraconserved region. Mol. Cell 2014, 55, 138–147. [Google Scholar] [CrossRef]
- Kim, V.N. MicroRNA biogenesis: Coordinated cropping and dicing. Nat. Rev. Mol. Cell Biol. 2005, 6, 376–385. [Google Scholar] [CrossRef]
- Kuehbacher, A.; Urbich, C.; Zeiher, A.M.; Dimmeler, S. Role of Dicer and Drosha for endothelial microRNA expression and angiogenesis. Circ. Res. 2007, 101, 59–68. [Google Scholar] [CrossRef]
- Tang, G. siRNA and miRNA: An insight into RISCs. Trends Biochem. Sci. 2005, 30, 106–114. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.Z.; Zhang, M.Y.; Li, Y.S.; Hu, G.L.; Fan, X.Z.; Guo, T.X.; Zhou, F.; Zhang, P.; Wu, Y.B.; Gao, Y.F.; et al. MicroRNA-263b confers imidacloprid resistance in Sitobion miscanthi (Takahashi) by regulating the expression of the nAChRbeta1 subunit. Pestic. Biochem. Physiol. 2022, 187, 105218. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Dou, W.; Taning, C.N.T.; Smagghe, G.; Wang, J.J. Regulatory roles of microRNAs in insect pests: Prospective targets for insect pest control. Curr. Opin. Biotechnol. 2021, 70, 158–166. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Wang, G. RNA Interference: From Gene Function to Biopesticides; China Science Publishing & Media Ltd.: Beijing, China, 2021. [Google Scholar]
- Zheng, X.; Weng, Z.; Li, H.; Kong, Z.; Zhou, Z.; Li, F.; Ma, W.; Lin, Y.; Chen, H. Transgenic rice overexpressing insect endogenous microRNA csu-novel-260 is resistant to striped stem borer under field conditions. Plant Biotechnol. J. 2021, 19, 421–423. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Li, T.; Pang, R. miR-2703 regulates the chitin biosynthesis pathway by targeting chitin synthase 1a in Nilaparvata lugens. Insect Mol. Biol. 2020, 29, 38–47. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.L.; Wu, L.X.; Li, H.Y.; Wen, X.Q.; Ma, E.B.; Zhu, K.Y.; Zhang, J.Z. The microRNA miR-184 regulates the CYP303A1 transcript level to control molting of Locusta migratoria. Insect Sci. 2021, 28, 941–951. [Google Scholar] [CrossRef] [PubMed]
- Chang, M.; Cheng, H.; Cai, Z.; Qian, Y.; Zhang, K.; Yang, L.; Ma, N.; Li, D. miR-92a-1-p5 Modulated Expression of the flightin Gene Regulates Flight Muscle Formation and Wing Extension in the Pea Aphid, Acyrthosiphon pisum (Hemiptera: Aphidoidea). J. Insect Sci. 2022, 22. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Li, X.; Mohammed, A.; Liu, Y.; Gao, X. miR-147b-modulated expression of vestigial regulates wing development in the bird cherry-oat aphid Rhopalosiphum padi. BMC Genom. 2020, 21, 71. [Google Scholar] [CrossRef]
- Eun, S.H.; Stoiber, P.M.; Wright, H.J.; McMurdie, K.E.; Choi, C.H.; Gan, Q.; Lim, C.; Chen, X. MicroRNAs downregulate Bag of marbles to ensure proper terminal differentiation in the Drosophila male germline. Development 2013, 140, 23–30. [Google Scholar] [CrossRef] [PubMed]
- Simoes da Silva, C.J.; Sospedra, I.; Aparicio, R.; Busturia, A. The microRNA-306/abrupt regulatory axis controls wing and haltere growth in Drosophila. Mech. Dev. 2019, 158, 103555. [Google Scholar] [CrossRef]
- Wang, Z.; Xia, X.; Li, J.; Igaki, T. Tumor elimination by clustered microRNAs miR-306 and miR-79 via noncanonical activation of JNK signaling. eLife 2022, 11, e77340. [Google Scholar] [CrossRef]
- Li, X.; Zhang, F.; Coates, B.; Wei, C.; Zhu, X.; Zhang, Y.; Zhou, X. Temporal analysis of microRNAs associated with wing development in the English grain aphid, Sitobion avenae (F.) (Homoptera: Aphidiae). Insect Biochem. Mol. Biol. 2022, 142, 103579. [Google Scholar] [CrossRef]
- Huvenne, H.; Smagghe, G. Mechanisms of dsRNA uptake in insects and potential of RNAi for pest control: A review. J. Insect Physiol. 2010, 56, 227–235. [Google Scholar] [CrossRef] [PubMed]
- Baum, J.A.; Bogaert, T.; Clinton, W.; Heck, G.R.; Feldmann, P.; Ilagan, O.; Johnson, S.; Plaetinck, G.; Munyikwa, T.; Pleau, M.; et al. Control of coleopteran insect pests through RNA interference. Nat. Biotechnol. 2007, 25, 1322–1326. [Google Scholar] [CrossRef] [PubMed]
- Mao, Y.B.; Cai, W.J.; Wang, J.W.; Hong, G.J.; Tao, X.Y.; Wang, L.J.; Huang, Y.P.; Chen, X.Y. Silencing a cotton bollworm P450 monooxygenase gene by plant-mediated RNAi impairs larval tolerance of gossypol. Nat. Biotechnol. 2007, 25, 1307–1313. [Google Scholar] [CrossRef] [PubMed]
- Howard, J.D.; Beghyn, M.; Dewulf, N.; De Vos, Y.; Philips, A.; Portwood, D.; Kilby, P.M.; Oliver, D.; Maddelein, W.; Brown, S.; et al. Chemically modified dsRNA induces RNAi effects in insects in vitro and in vivo: A potential new tool for improving RNA-based plant protection. J. Biol. Chem. 2022, 298, 102311. [Google Scholar] [CrossRef]
- List, F.; Tarone, A.M.; Zhu-Salzman, K.; Vargo, E.L. RNA meets toxicology: Efficacy indicators from the experimental design of RNAi studies for insect pest management. Pest. Manag. Sci. 2022, 78, 3215–3225. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.S.; Abdellah, Y.A.Y.; Hafeez, M.; Yang, X.; Hou, W.T.; Kong, X.H.; Wang, R.L. Herbivore-induced tomato plant volatiles lead to the reduction of insecticides susceptibility in Spodoptera litura. Pestic. Biochem. Physiol. 2022, 187, 105215. [Google Scholar] [CrossRef] [PubMed]
- Ullah, F.; Gul, H.; Tariq, K.; Desneux, N.; Gao, X.; Song, D. Acetamiprid resistance and fitness costs of melon aphid, Aphis gossypii: An age-stage, two-sex life table study. Pestic. Biochem. Physiol. 2021, 171, 104729. [Google Scholar] [CrossRef]
- Zeng, X.; He, Y.; Wu, J.; Tang, Y.; Gu, J.; Ding, W.; Zhang, Y. Sublethal Effects of Cyantraniliprole and Imidacloprid on Feeding Behavior and Life Table Parameters of Myzus persicae (Hemiptera: Aphididae). J. Econ. Entomol. 2016, 109, 1595–1602. [Google Scholar] [CrossRef]
- Zhang, Q.-Q.; Li, W.-Q.; Lu, Z.-B.; Li, L.-L.; Yu, Y.; Li, C.; Men, X.-Y. Sublethal effects of beta-cypermethrin on the bird cherry-oat aphid Rhopalosiphum padi (Hemiptera: Aphididae). J. Asia-Pac. Entomol. 2019, 22, 693–698. [Google Scholar] [CrossRef]
- Qu, Y.; Xiao, D.; Liu, J.; Chen, Z.; Song, L.; Desneux, N.; Benelli, G.; Gao, X.; Song, D. Sublethal and hormesis effects of beta-cypermethrin on the biology, life table parameters and reproductive potential of soybean aphid Aphis glycines. Ecotoxicology 2017, 26, 1002–1009. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Ma, K.; Li, F.; Liang, P.; Liu, Y.; Guo, T.; Song, D.; Desneux, N.; Gao, X. Sublethal and transgenerational effects of sulfoxaflor on the biological traits of the cotton aphid, Aphis gossypii Glover (Hemiptera: Aphididae). Ecotoxicology 2016, 25, 1841–1848. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; Desneux, N.; Sonoda, S.; Liang, P.; Han, P.; Gao, X.-W. Sublethal and transgenerational effects of chlorantraniliprole on biological traits of the diamondback moth, Plutella xylostella L. Crop Prot. 2013, 48, 29–34. [Google Scholar] [CrossRef]
- Farhadi, R.; Allahyari, H.; Chi, H. Life table and predation capacity of Hippodamia variegata (Coleoptera: Coccinellidae) feeding on Aphis fabae (Hemiptera: Aphididae). Biol. Control. 2011, 59, 83–89. [Google Scholar] [CrossRef]
- Zhang, R.; Liu, W.; Zhang, Z. miR-306-5p is involved in chitin metabolism in Aedes albopictus pupae via linc8338-miR-306-5p-XM_019678125.2 axis. Pestic. Biochem. Physiol. 2024, 200, 105811. [Google Scholar] [CrossRef]
- Liu, Z.F.; Feng, Y.T.; Gao, Y.; Guo, X.J.; Zang, P.J.; Fan, R.J. Effects of sublethal dose of imidacloprid on life table of experimental populations of lacewing Chrysoperla nipponensis (Okamoto)(Neuroptera: Chrysopidae). J. Plant Prot. 2016, 43, 1014–1019. [Google Scholar] [PubMed]
- Goodman, D. Optiaml life histpries, optiaml notation, and the value of reproductive value. Am. Nat. 1982, 119, 803–823. [Google Scholar] [CrossRef]
- Chi, H. TWOSEX-MSChart-exe.rar. 2024. Available online: http://140.120.197.173/ecology/prod102.htm (accessed on 10 May 2023).
- Huang, Y.B.; Chi, H. Life tables of Bactrocera cucurbitae (Diptera Tephritidae) with an invalidation of the jackknife technique. J. Appl. Èntomol. 2012, 137, 327–339. [Google Scholar] [CrossRef]
- Efron, B.; Tibshirani, R.J.; Hall, C. An Introduction to the Bootstrap; Chapman and Hall: New York, NY, USA, 1993; pp. 1–456. [Google Scholar]
- Akca, I.; Ayvaz, T.; Yazici, E.; Smith, C.L.; Chi, H. Demography and Population Projection of Aphis fabae (Hemiptera: Aphididae): With Additional Comments on Life Table Research Criteria. J. Econ. Entomol. 2015, 108, 1466–1478. [Google Scholar] [CrossRef]
- Akkopru, E.P.; Atlihan, R.; Okut, H.; Chi, H. Demographic Assessment of Plant Cultivar Resistance to Insect Pests: A Case Study of the Dusky-Veined Walnut Aphid (Hemiptera: Callaphididae) on Five Walnut Cultivars. J. Econ. Entomol. 2015, 108, 378–387. [Google Scholar] [CrossRef] [PubMed]
- Smucker, M.D.; Allan, J.; Carterette, B. A comparison of statistical significance tests for information retrieval evaluation. In Proceedings of the Sixteenth ACM Conference on Conference on Information and Knowledge Management 2007, Lisbon, Portugal, 6–10 November 2007; Association for Computing Machinery: New York, NY, USA, 2007; pp. 623–632. [Google Scholar]
- Wei, M.; Chi, H.; Guo, Y.; Li, X.; Zhao, L.; Ma, R. Demography of Cacopsylla chinensis (Hemiptera: Psyllidae) Reared on Four Cultivars of Pyrus bretschneideri (Rosales: Rosaceae) and P. communis Pears With Estimations of Confidence Intervals of Specific Life Table Statistics. J. Econ. Entomol. 2020, 113, 2343–2353. [Google Scholar] [CrossRef] [PubMed]


| Life Parameters | NCW | NCS | miR-306 |
|---|---|---|---|
| Mean ± SE | Mean ± SE | Mean ± SE | |
| 1st instar nymph (N1) (d) | 2.07 ± 0.06 a | 2.06 ± 0.07 a | 2.16 ± 0.07 a |
| 2nd instar nymph (N2) (d) | 1.98 ± 0.04 a | 2.07 ± 0.06 a | 2.13 ± 0.08 a |
| 3rd instar nymph (N3) (d) | 1.99 ± 0.05 b | 1.97 ± 0.06 b | 2.20 ± 0.07 a |
| 4th instar nymph (N4) (d) | 2.13 ± 0.05 b | 2.17 ± 0.06 b | 2.41 ± 0.06 a |
| Pre-adult (d) | 8.15 ± 0.10 b | 8.28 ± 0.09 b | 9.02 ± 0.14 a |
| Adult longevity (d) | 11.64 ± 0.41 b | 12.25 ± 0.33 b | 14.26 ± 0.61 a |
| Mean longevity (d) | 18.46 ± 0.49 a | 18.41 ± 0.53 a | 14.40 ± 0.83 b |
| Pre-adult survival (%) | 89.99 ± 2.45 a | 86.01 ± 2.84 a | 52.69 ± 4.09 b |
| TPOP (d) | 8.64 ± 0.12 b | 8.91 ± 0.12 b | 9.60 ± 0.16 a |
| APOP (d) | 0.50 ± 0.05 a | 0.63 ± 0.06 a | 0.57 ± 0.07 a |
| Mean fecundity (individuals) | 25.19 ± 1.14 ab | 27.29 ± 1.10 a | 23.73 ± 1.39 b |
| Parameters | NCW | NCS | miR-306 |
|---|---|---|---|
| Mean ± SE | Mean ± SE | Mean ± SE | |
| Intrinsic rate of increase (r) (d−1) | 0.2375 ± 0.0041 a | 0.2351 ± 0.0047 a | 0.1728 ± 0.0077 b |
| Finite rate of increase (λ) (d−1) | 1.2681 ± 0.0052 a | 1.2650 ± 0.0059 a | 1.1886 ± 0.0091 b |
| Net reproductive rate (R0) | 22.67 ± 1.45 a | 23.48 ± 1.22 a | 12.50 ± 1.22 b |
| Mean generation time (T) (d) | 13.14 ± 0.15 b | 13.42 ± 0.14 b | 14.60 ± 0.21 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, L.; Wei, G.; Yan, Y.; Zhou, X.; Zhu, X.; Zhang, Y.; Li, X. Effects of miR-306 Perturbation on Life Parameters in the English Grain Aphid, Sitobion avenae (Homoptera: Aphididae). Int. J. Mol. Sci. 2024, 25, 5680. https://doi.org/10.3390/ijms25115680
Wu L, Wei G, Yan Y, Zhou X, Zhu X, Zhang Y, Li X. Effects of miR-306 Perturbation on Life Parameters in the English Grain Aphid, Sitobion avenae (Homoptera: Aphididae). International Journal of Molecular Sciences. 2024; 25(11):5680. https://doi.org/10.3390/ijms25115680
Chicago/Turabian StyleWu, Linyuan, Guohua Wei, Yi Yan, Xuguo Zhou, Xun Zhu, Yunhui Zhang, and Xiangrui Li. 2024. "Effects of miR-306 Perturbation on Life Parameters in the English Grain Aphid, Sitobion avenae (Homoptera: Aphididae)" International Journal of Molecular Sciences 25, no. 11: 5680. https://doi.org/10.3390/ijms25115680
APA StyleWu, L., Wei, G., Yan, Y., Zhou, X., Zhu, X., Zhang, Y., & Li, X. (2024). Effects of miR-306 Perturbation on Life Parameters in the English Grain Aphid, Sitobion avenae (Homoptera: Aphididae). International Journal of Molecular Sciences, 25(11), 5680. https://doi.org/10.3390/ijms25115680









