Enhancing Heart Transplantation: Utilizing Gas-Loaded Nanocarriers to Mitigate Cold/Hypoxia Stress
Abstract
:1. Introduction
2. Results
2.1. Characteristics of Cardioplegic Solutions Containing CNN or CNN-NS
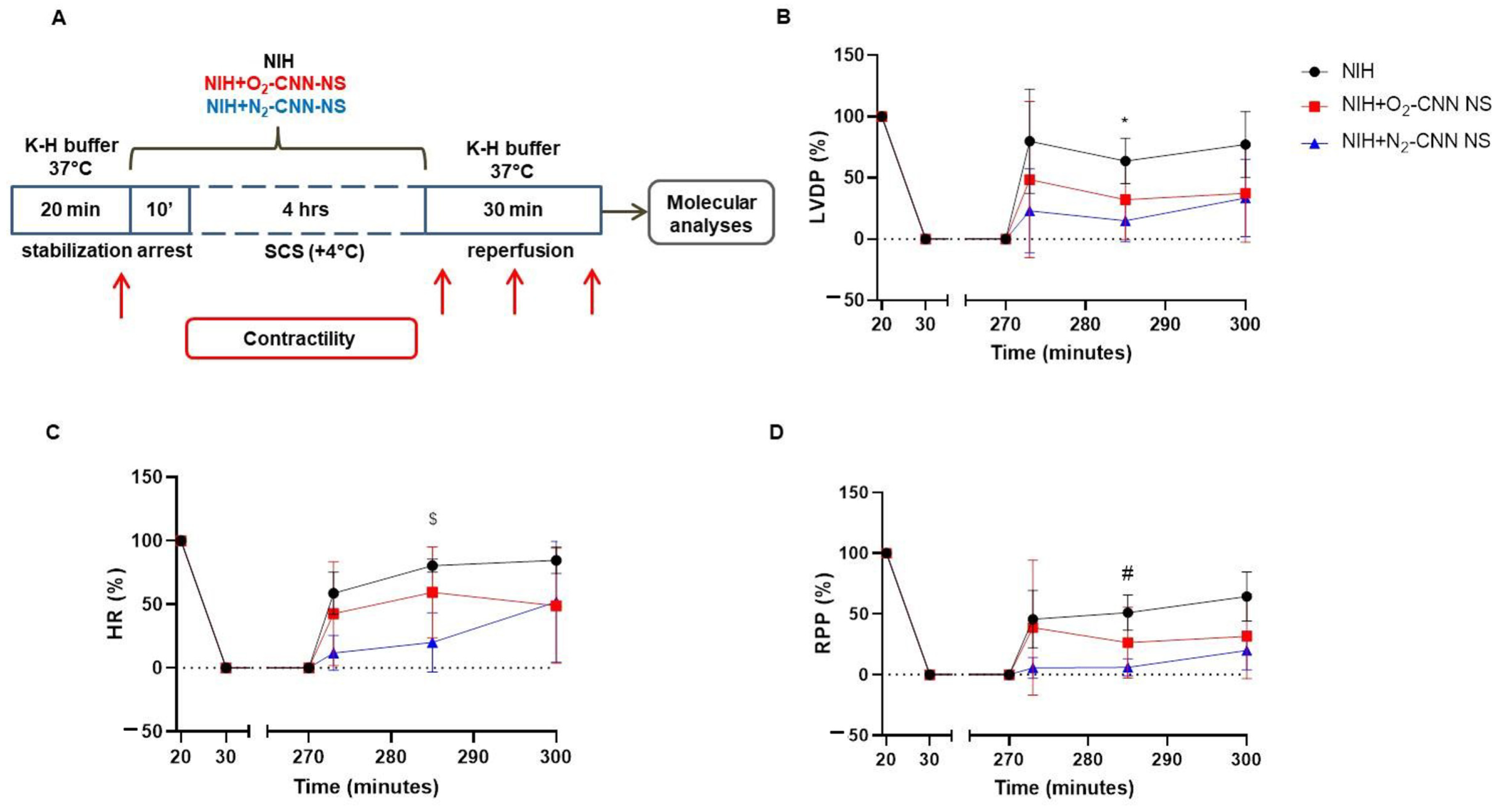
2.2. Contractile Recovery of Hearts Arrested and Preserved with CNN
2.3. Contracture Index in Hearts Arrested and Preserved with CNN
2.4. Contractile Recovery of Hearts Arrested and Preserved with CNN-NS
2.5. Contracture Index in Hearts Arrested and Preserved with CNN-NS
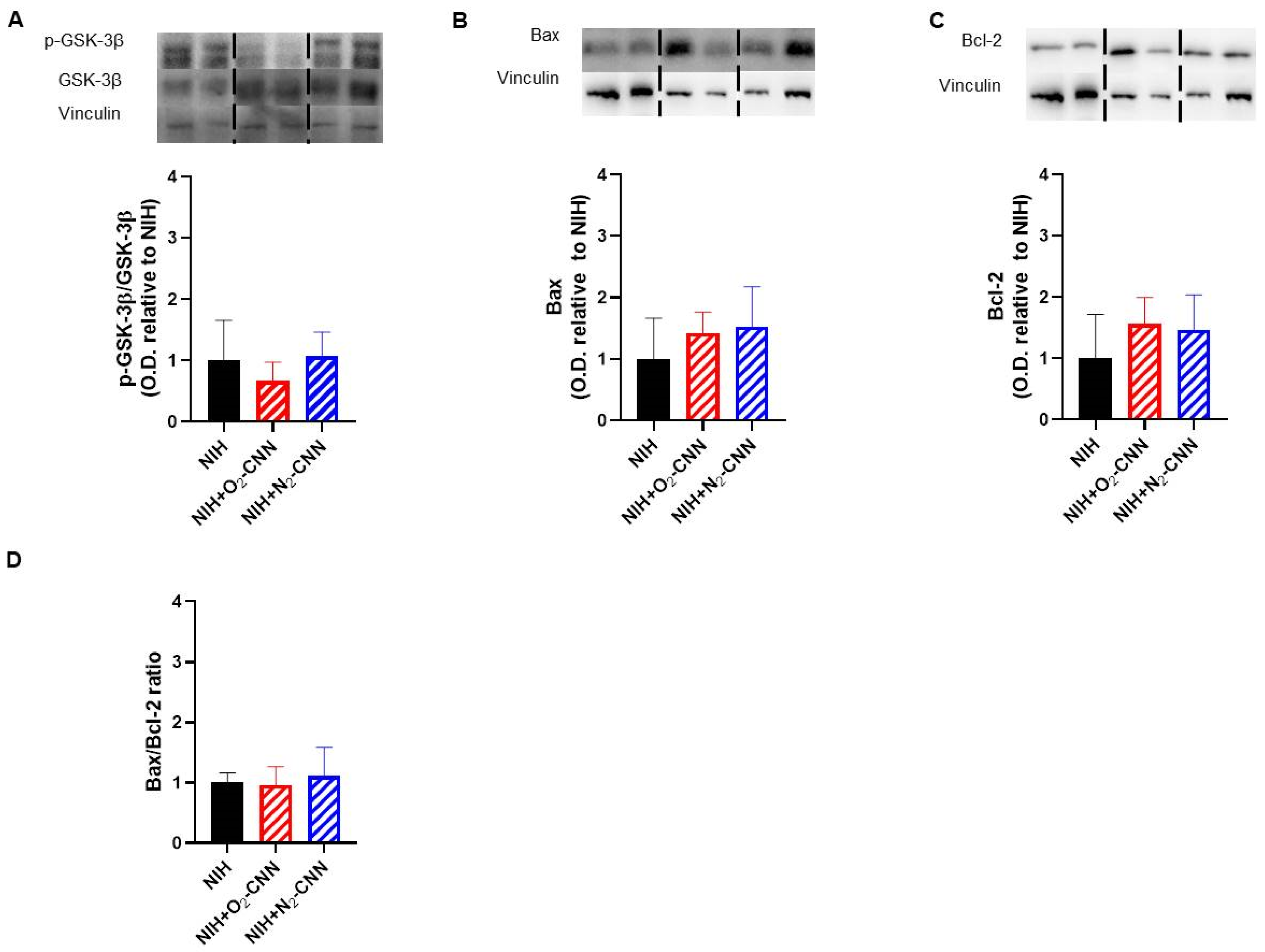
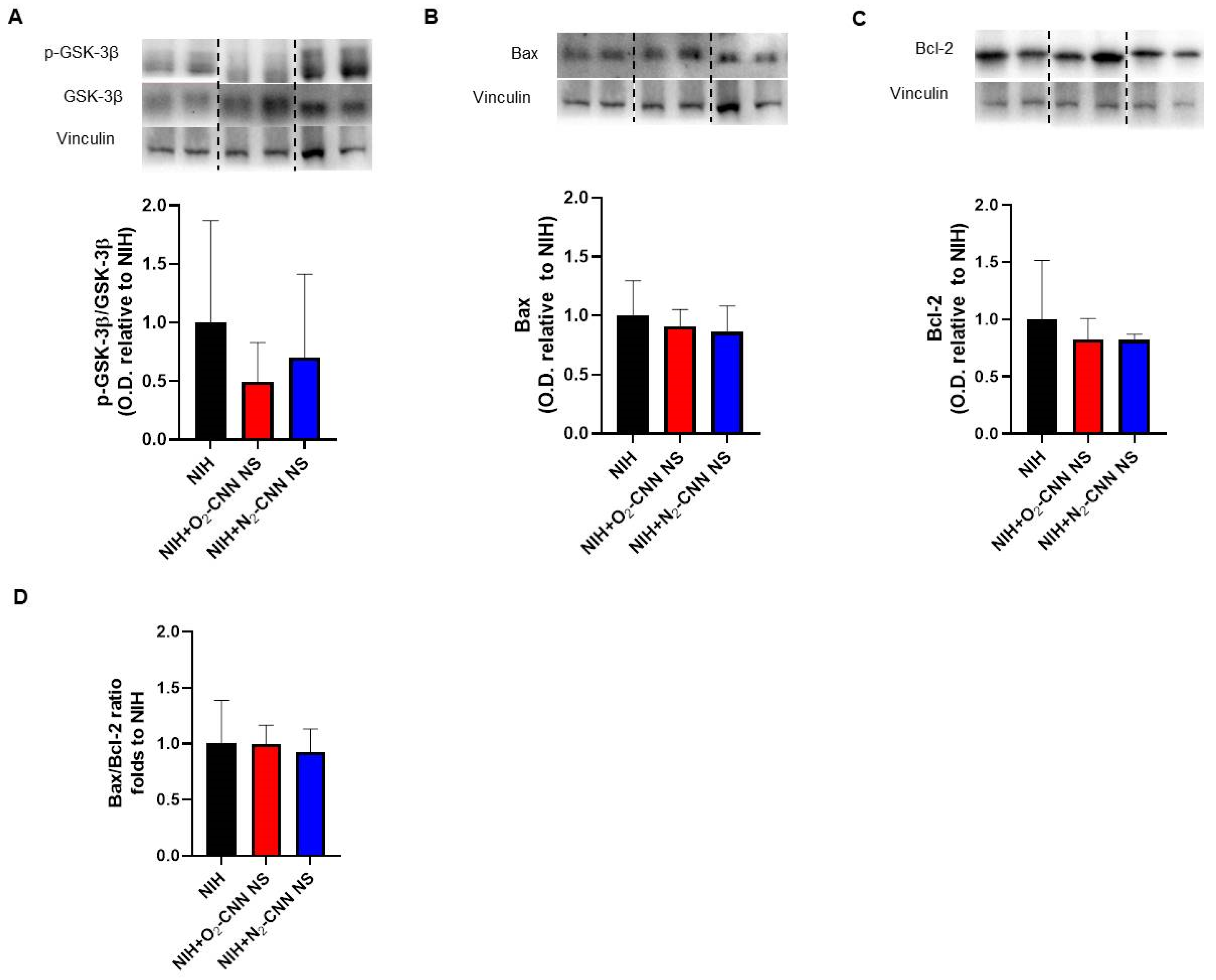
2.6. Levels of Apoptosis Indices in Hearts Treated with Different Nanocarriers
2.7. Levels of Autophagy and Oxidative Stress Indices in Hearts Treated with Different Nanocarriers
2.8. Catalase Activity in Hearts Stored with NIH and CNN
3. Discussion
3.1. Nanomonomers Transiently Increase Heart Performance Recovery
3.2. Nanosponges Do Not Improve Heart Performance Recovery
3.3. Gas-Loaded Nanocarriers: Influence on Redox Stress, Apoptosis, and Autophagy
3.4. Methodological Considerations
4. Materials and Methods
4.1. Preparation of Nanomonomers and Nanosponges
4.2. Animals and Ethics Statement
4.3. Heart Perfusion
- NIH only (NIH, n = 5);
- NIH plus CNN carrying O2 (NIH + O2-CNN, n = 4);
- NIH plus CNN-NS carrying O2 (NIH + O2-CNN-NS, n = 5);
- NIH plus CNN carrying N2 (NIH + N2-CNN, n = 4);
- NIH plus CNN-NS carrying N2 (NIH + O2-CNN-NS, n = 4).
4.4. Western Blots
4.5. Catalase Activity Assay
4.6. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Westerdahl, D.E.; Kobashigawa, J.A. Heart Transplantation for Advanced Heart Failure. Card. Intensive Care 2019, 504–524. [Google Scholar]
- Martins, R.P.; Hamel-Bougault, M.; Bessière, F.; Pozzi, M.; Extramiana, F.; Brouk, Z.; Guenancia, C.; Sagnard, A.; Ninni, S.; Goemine, C.; et al. Heart Transplantation as a Rescue Strategy for Patients with Refractory Electrical Storm. Eur. Heart J. Acute Cardiovasc. Care 2023, 12, 571–581. [Google Scholar] [CrossRef]
- Kounatidis, D.; Brozou, V.; Anagnostopoulos, D.; Pantos, C.; Lourbopoulos, A.; Mourouzis, I. Donor Heart Preservation: Current Knowledge and the New Era of Machine Perfusion. Int. J. Mol. Sci. 2023, 24, 16693. [Google Scholar] [CrossRef]
- Nakamura, Y.; Saito, S.; Miyagawa, S.; Yoshikawa, Y.; Hata, H.; Yoshioka, D.; Toda, K.; Sawa, Y. Perioperative Ischaemic Reperfusion Injury and Allograft Function in the Early Post-Transplantation Period. Interact. Cardiovasc. Thorac. Surg. 2019, 29, 230–236. [Google Scholar] [CrossRef]
- Femminò, S.; Penna, C.; Bessone, F.; Caldera, F.; Dhakar, N.; Cau, D.; Pagliaro, P.; Cavalli, R.; Trotta, F. α-Cyclodextrin and α-Cyclodextrin Polymers as Oxygen Nanocarriers to Limit Hypoxia/Reoxygenation Injury: Implications from an in Vitro Model. Polymers 2018, 10, 211. [Google Scholar] [CrossRef]
- Penna, C.; Femminò, S.; Caldera, F.; Rubin Pedrazzo, A.; Cecone, C.; Alfì, E.; Comità, S.; Higashiyama, T.; Trotta, F.; Pagliaro, P.; et al. Cyclic Nigerosyl-Nigerose as Oxygen Nanocarrier to Protect Cellular Models from Hypoxia/Reoxygenation Injury: Implications from an In Vitro Model. Int. J. Mol. Sci. 2021, 22, 4208. [Google Scholar] [CrossRef]
- Opoku-Damoah, Y.; Zhang, R.; Ta, H.T.; Xu, Z.P. Therapeutic Gas-Releasing Nanomedicines with Controlled Release: Advances and Perspectives. Exploration 2022, 2, 20210181. [Google Scholar] [CrossRef]
- Khan, A.R.; Bin Abdulhak, A.; Luni, F.K.; Farid, T.A.; Riaz, H.; Ruzieh, M.; Pham, L.; Hirsch, G.; Bolli, R. Oxygen Administration Does Not Influence the Prognosis of Acute Myocardial Infarction: A Meta-Analysis. Am. J. Ther. 2019, 26, 151–160. [Google Scholar] [CrossRef]
- Lucia Appleton, S.; Navarro-Orcajada, S.; Martínez-Navarro, F.J.; Caldera, F.; López-Nicolás, J.M.; Trotta, F.; Matencio, A. Cyclodextrins as Anti-Inflammatory Agents: Basis, Drugs and Perspectives. Biomolecules 2021, 11, 1384. [Google Scholar] [CrossRef]
- Tannous, M.; Hoti, G.; Trotta, F.; Cavalli, R.; Higashiyama, T.; Pagliaro, P.; Penna, C. Oxygen Nanocarriers for Improving Cardioplegic Solution Performance: Physico-Chemical Characterization. Int. J. Mol. Sci. 2023, 24, 10073. [Google Scholar] [CrossRef]
- Sakamoto, S.; Bochimoto, H.; Shibata, K.; Zin, N.K.M.; Fukai, M.; Nakamura, K.; Ishikawa, T.; Fujiyoshi, M.; Shimamura, T.; Taketomi, A. Exploration of Optimal PH in Hypothermic Machine Perfusion for Rat Liver Grafts Retrieved after Circulatory Death. J. Clin. Med. 2023, 12, 3845. [Google Scholar] [CrossRef]
- Baicu, S.C.; Taylor, M.J. Acid-Base Buffering in Organ Preservation Solutions as a Function of Temperature: New Parameters for Comparing Buffer Capacity and Efficiency. Cryobiology 2002, 45, 33–48. [Google Scholar] [CrossRef]
- Rosenthial, T.B. The Effect of Temperature on the PH of Blood and Plasma in Vitro. J. Biol. Chem. 1948, 173, 25–30. [Google Scholar] [CrossRef]
- Gelpi, R.J.; Morales, C.; Cohen, M.V.; Downey, J.M. Xanthine Oxidase Contributes to Preconditioning’s Preservation of Left Ventricular Developed Pressure in Isolated Rat Heart: Developed Pressure May Not Be an Appropriate End-Point for Studies of Preconditioning. Basic Res. Cardiol. 2002, 97, 40–46. [Google Scholar] [CrossRef]
- García-Dorado, D.; Rodríguez-Sinovas, A.; Ruiz-Meana, M. Gap Junction-Mediated Spread of Cell Injury and Death during Myocardial Ischemia-Reperfusion. Cardiovasc. Res. 2004, 61, 386–401. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, T.; He, Q. Strategies for Engineering Advanced Nanomedicines for Gas Therapy of Cancer. Natl. Sci. Rev. 2020, 7, 1485–1512. [Google Scholar] [CrossRef]
- Penna, C.; Trotta, F.; Cavalli, R.; Pagliaro, P. Nanocarriers Loaded with Oxygen to Improve the Protection of the Heart to Be Transplanted. Curr. Pharm. Des. 2022, 28, 468–470. [Google Scholar] [CrossRef] [PubMed]
- Djordjevic, T.; Pogrebniak, A.; BelAiba, R.S.; Bonello, S.; Wotzlaw, C.; Acker, H.; Hess, J.; Görlach, A. The Expression of the NADPH Oxidase Subunit P22phox Is Regulated by a Redox-Sensitive Pathway in Endothelial Cells. Free. Radic. Biol. Med. 2005, 38, 616–630. [Google Scholar] [CrossRef]
- Hamacher-Brady, A.; Brady, N.R.; Gottlieb, R.A. The Interplay between Pro-Death and pro-Survival Signaling Pathways in Myocardial Ischemia/Reperfusion Injury: Apoptosis Meets Autophagy. Cardiovasc. Drugs Ther. 2006, 20, 445–462. [Google Scholar] [CrossRef]
- Chen-Scarabelli, C.; Agrawal, P.R.; Saravolatz, L.; Abuniat, C.; Scarabelli, G.; Stephanou, A.; Loomba, L.; Narula, J.; Scarabelli, T.M.; Knight, R. The Role and Modulation of Autophagy in Experimental Models of Myocardial Ischemia-Reperfusion Injury. J. Geriatr. Cardiol. 2014, 11, 338–348. [Google Scholar]
- Li, G.; Chen, Y.; Saari, J.T.; Kang, Y.J. Catalase-Overexpressing Transgenic Mouse Heart Is Resistant to Ischemia-Reperfusion Injury. Am. J. Physiol. 1997, 273, 1090–1095. [Google Scholar] [CrossRef]
- Neethling, E.; Moreno Garijo, J.; Mangalam, T.K.; Badiwala, M.V.; Billia, P.; Wasowicz, M.; Van Rensburg, A.; Slinger, P. Intraoperative and Early Postoperative Management of Heart Transplantation: Anesthetic Implications. J. Cardiothorac. Vasc. Anesth. 2020, 34, 2189–2206. [Google Scholar] [CrossRef] [PubMed]
- Freundt, M.; Lavanga, E.; Brehm, C. Optimal, Early Postoperative Management of Cardiac Transplant and Durable Left Ventricular Assist Recipients. Curr. Cardiol. Rep. 2022, 24, 2023–2029. [Google Scholar] [CrossRef] [PubMed]
- Inoue, N. Vascular C-Reactive Protein in the Pathogenesis of Coronary Artery Disease: Role of Vascular Inflammation and Oxidative Stress. Cardiovasc. Hematol. Disord. Drug Targets 2006, 6, 227–231. [Google Scholar] [CrossRef] [PubMed]
- Qiao, S.-G.; Sun, Y.; Sun, B.; Wang, A.; Qiu, J.; Hong, L.; An, J.-Z.; Wang, C.; Zhang, H.-L. Sevoflurane Postconditioning Protects against Myocardial Ischemia/Reperfusion Injury by Restoring Autophagic Flux via an NO-Dependent Mechanism. Acta Pharmacol. Sin. 2019, 40, 35–45. [Google Scholar] [CrossRef] [PubMed]
- Wittenberg, J.B.; Wittenberg, B.A. Myoglobin Function Reassessed. J. Exp. Biol. 2003, 206, 2011–2020. [Google Scholar] [CrossRef] [PubMed]
- Bøtker, H.E.; Hausenloy, D.; Andreadou, I.; Antonucci, S.; Boengler, K.; Davidson, S.M.; Deshwal, S.; Devaux, Y.; Di Lisa, F.; Di Sante, M.; et al. Practical Guidelines for Rigor and Reproducibility in Preclinical and Clinical Studies on Cardioprotection. Basic Res. Cardiol. 2018, 113, 39. [Google Scholar] [CrossRef] [PubMed]
- Caldera, F.; Argenziano, M.; Trotta, F.; Dianzani, C.; Gigliotti, L.; Tannous, M.; Pastero, L.; Aquilano, D.; Nishimoto, T.; Higashiyama, T.; et al. Cyclic Nigerosyl-1,6-Nigerose-Based Nanosponges: An Innovative PH and Time-Controlled Nanocarrier for Improving Cancer Treatment. Carbohydr. Polym. 2018, 194, 111–121. [Google Scholar] [CrossRef]
- Quagliariello, V.; Vecchione, R.; Coppola, C.; Di Cicco, C.; De Capua, A.; Piscopo, G.; Paciello, R.; Narciso, V.; Formisano, C.; Taglialatela-Scafati, O.; et al. Cardioprotective Effects of Nanoemulsions Loaded with Anti-Inflammatory Nutraceuticals against Doxorubicin-Induced Cardiotoxicity. Nutrients 2018, 10, 1304. [Google Scholar] [CrossRef]



| pH ± SD | Viscosity ± SD (mpa) | |
|---|---|---|
| NIH | 7.93 ± 0.01 | 1.194 ± 0.001 |
| NIH + O2-CNN | 8.07 ± 0.04 | 1.204 ± 0.004 |
| NIH + O2-CNN-NS | 8.37 ± 0.01 | 1.190 ± 0.001 |
| NIH + N2-CNN | 8.20 ± 0.02 | 1.204 ± 0.04 |
| NIH + N2-CNN-NS | 8.42 ± 0.01 | 1.195 ± 0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rubeo, C.; Hoti, G.; Giordano, M.; Molinar, C.; Aragno, M.; Mantuano, B.; Comità, S.; Femminò, S.; Cavalli, R.; Trotta, F.; et al. Enhancing Heart Transplantation: Utilizing Gas-Loaded Nanocarriers to Mitigate Cold/Hypoxia Stress. Int. J. Mol. Sci. 2024, 25, 5685. https://doi.org/10.3390/ijms25115685
Rubeo C, Hoti G, Giordano M, Molinar C, Aragno M, Mantuano B, Comità S, Femminò S, Cavalli R, Trotta F, et al. Enhancing Heart Transplantation: Utilizing Gas-Loaded Nanocarriers to Mitigate Cold/Hypoxia Stress. International Journal of Molecular Sciences. 2024; 25(11):5685. https://doi.org/10.3390/ijms25115685
Chicago/Turabian StyleRubeo, Chiara, Gjylije Hoti, Magalì Giordano, Chiara Molinar, Manuela Aragno, Beatrice Mantuano, Stefano Comità, Saveria Femminò, Roberta Cavalli, Francesco Trotta, and et al. 2024. "Enhancing Heart Transplantation: Utilizing Gas-Loaded Nanocarriers to Mitigate Cold/Hypoxia Stress" International Journal of Molecular Sciences 25, no. 11: 5685. https://doi.org/10.3390/ijms25115685






