Transplantation of Mesenchymal Stem Cells Derived from Old Rats Improves Healing and Biomechanical Properties of Vaginal Tissue Following Surgical Incision in Aged Rats
Abstract
1. Introduction
2. Results
2.1. Homing and Survival of Transplanted Old-Donor-Derived MSCs Following Full-Thickness Vaginal Incision
2.2. The Effect of Old-Donor-Derived MSC Transplantation on the Inflammatory Response at the Vaginal Surgical Site
2.3. The Effect of Old-Donor-Derived MSC Transplantation on Acute and Chronic MMP9 Expression Following Injury
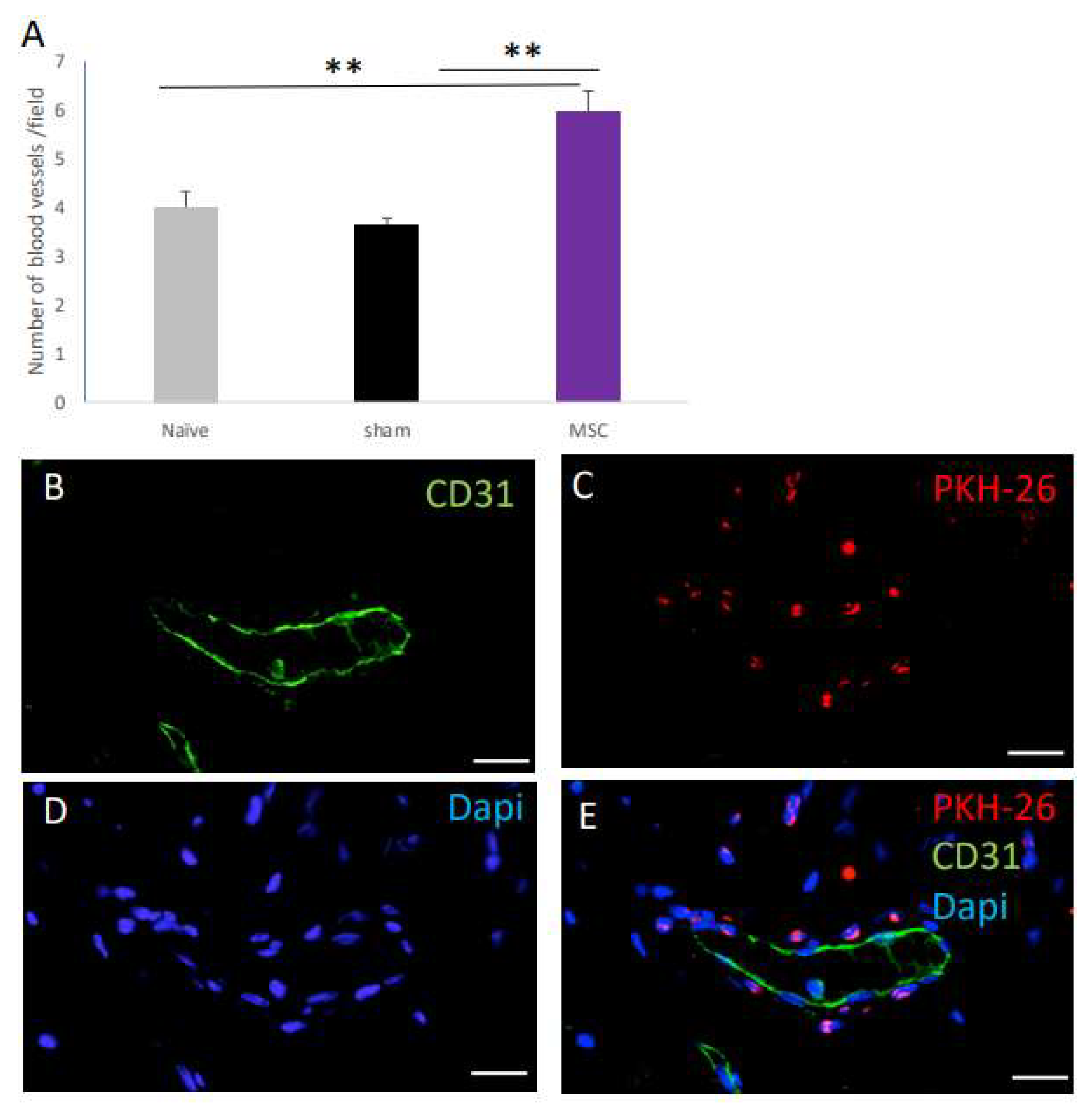
2.4. The Effect of MSC Transplantation on Blood Vessel Number on Day 30 Post Surgery
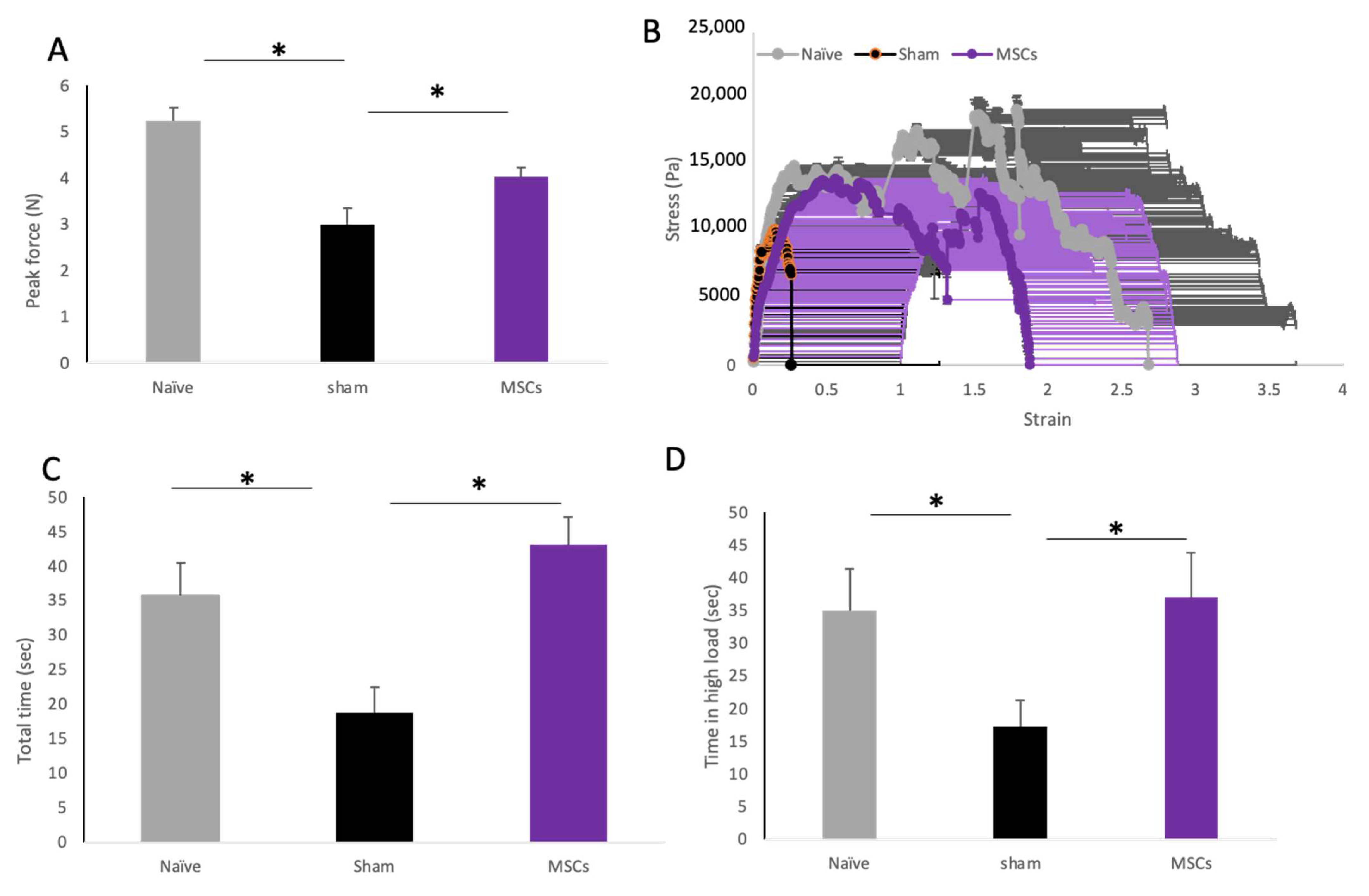
2.5. The Effect of MSC Transplantation on Biomechanical Properties of the Vaginal Tissue
3. Discussion
4. Materials and Methods
4.1. Animal Model and Procedure
4.2. Dissection of Vaginal Tissue and Histological Evaluation
4.3. Biomechanical Testing
4.4. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ben Menachem-Zidon, O.; Gropp, M.; Reubinoff, B.; Shveiky, D. Mesenchymal stem cell transplantation improves biomechanical properties of vaginal tissue following full-thickness incision in aged rats. Stem Cell Rep. 2022, 17, 2565–2578. [Google Scholar] [CrossRef] [PubMed]
- Glenn, J.D.; Whartenby, K.A. Mesenchymal stem cells: Emerging mechanisms of immunomodulation and therapy. World J. Stem Cells 2014, 6, 526–539. [Google Scholar] [CrossRef] [PubMed]
- Chavez-Munoz, C.; Nguyen, K.T.; Xu, W.; Hong, S.-J.; Mustoe, T.A.; Galiano, R.D. Transdifferentiation of adipose-derived stem cells into keratinocyte-like cells: Engineering a stratified epidermis. PLoS ONE 2013, 8, e80587. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Zhao, R.C.H.; Tredget, E.E. Concise review: Bone marrow-derived stem/progenitor cells in cutaneous repair and regeneration. Stem Cells 2010, 28, 905–915. [Google Scholar] [CrossRef] [PubMed]
- Katsara, O.; Mahaira, L.G.; Iliopoulou, E.G.; Moustaki, A.; Antsaklis, A.; Loutradis, D.; Stefanidis, K.; Baxevanis, C.N.; Papamichail, M.; Perez, S.A. Effects of donor age, gender, and in vitro cellular aging on the phenotypic, functional, and molecular characteristics of mouse bone marrow-derived mesenchymal stem cells. Stem Cells Dev. 2011, 20, 1549–1561. [Google Scholar] [CrossRef] [PubMed]
- Scruggs, B.A.; Semon, J.A.; Zhang, X.; Zhang, S.; Bowles, A.C.; Pandey, A.C.; Imhof, K.M.P.; Kalueff, A.V.; Gimble, J.M.; Bunnell, B.A. Age of the donor reduces the ability of human adipose-derived stem cells to alleviate symptoms in the experimental autoimmune encephalomyelitis mouse model. Stem Cells Transl. Med. 2013, 2, 797–807. [Google Scholar] [CrossRef] [PubMed]
- Selle, M.; Koch, J.D.; Ongsiek, A.; Ulbrich, L.; Ye, W.; Jiang, Z.; Krettek, C.; Neunaber, C.; Noack, S. Influence of age on stem cells depends on the sex of the bone marrow donor. J. Cell. Mol. Med. 2022, 26, 1594–1605. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.W.; Wang, Y.-L.; Christensen, J.M.; Khalifian, S.; Schneeberger, S.; Raimondi, G.; Cooney, D.S.; Lee, W.P.A.; Brandacher, G. Donor age negatively affects the immunoregulatory properties of both adipose and bone marrow derived mesenchymal stem cells. Transpl. Immunol. 2014, 30, 122–127. [Google Scholar] [CrossRef] [PubMed]
- Turinetto, V.; Vitale, E.; Giachino, C. Senescence in Human Mesenchymal Stem Cells: Functional Changes and Implications in Stem Cell-Based Therapy. Int. J. Mol. Sci. 2016, 17, 1164. [Google Scholar] [CrossRef] [PubMed]
- Siegel, G.; Kluba, T.; Hermanutz-Klein, U.; Bieback, K.; Northoff, H.; Schäfer, R. Phenotype, donor age and gender affect function of human bone marrow-derived mesenchymal stromal cells. BMC Med. 2013, 11, 146. [Google Scholar] [CrossRef]
- Baker, N.; Boyette, L.B.; Tuan, R.S. Characterization of bone marrow-derived mesenchymal stem cells in aging. Bone 2015, 70, 37–47. [Google Scholar] [CrossRef] [PubMed]
- Sethe, S.; Scutt, A.; Stolzing, A. Aging of mesenchymal stem cells. Ageing Res. Rev. 2006, 5, 91–116. [Google Scholar] [CrossRef] [PubMed]
- Vallant, N.; Sandhu, B.; Hamaoui, K.; Prendecki, M.; Pusey, C.; Papalois, V. Immunomodulatory Properties of Mesenchymal Stromal Cells Can Vary in Genetically Modified Rats. Int. J. Mol. Sci. 2021, 22, 1181. [Google Scholar] [CrossRef] [PubMed]
- Choudhery, M.S.; Khan, M.; Mahmood, R.; Mehmood, A.; Khan, S.N.; Riazuddin, S. Bone marrow derived mesenchymal stem cells from aged mice have reduced wound healing, angiogenesis, proliferation and anti-apoptosis capabilities. Cell Biol. Int. 2012, 36, 747–753. [Google Scholar] [CrossRef]
- Andrzejewska, A.; Catar, R.; Schoon, J.; Qazi, T.H.; Sass, F.A.; Jacobi, D.; Blankenstein, A.; Reinke, S.; Krüger, D.; Streitz, M.; et al. Multi-Parameter Analysis of Biobanked Human Bone Marrow Stromal Cells Shows Little Influence for Donor Age and Mild Comorbidities on Phenotypic and Functional Properties. Front. Immunol. 2019, 10, 2474. [Google Scholar] [CrossRef]
- Carvalho, M.S.; Alves, L.; Bogalho, I.; Cabral, J.M.S.; da Silva, C.L. Impact of Donor Age on the Osteogenic Supportive Capacity of Mesenchymal Stromal Cell-Derived Extracellular Matrix. Front. Cell Dev. Biol. 2021, 9, 747521. [Google Scholar] [CrossRef]
- Kim, M.; Kim, C.; Choi, Y.S.; Kim, M.; Park, C.; Suh, Y. Age-related alterations in mesenchymal stem cells related to shift in differentiation from osteogenic to adipogenic potential: Implication to age-associated bone diseases and defects. Mech. Ageing Dev. 2012, 133, 215–225. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Xia, X.; Li, B. Mesenchymal stem cell aging: Mechanisms and influences on skeletal and non-skeletal tissues. Exp. Biol. Med. 2015, 240, 1099–1106. [Google Scholar] [CrossRef]
- Stolzing, A.; Jones, E.; McGonagle, D.; Scutt, A. Age-related changes in human bone marrow-derived mesenchymal stem cells: Consequences for cell therapies. Mech. Ageing Dev. 2008, 129, 163–173. [Google Scholar] [CrossRef]
- Allameh, A.; Jazayeri, M.; Adelipour, M. In Vivo Vascularization of Endothelial Cells Derived from Bone Marrow Mesenchymal Stem Cells in SCID Mouse Model. Cell J. 2016, 18, 179–188. [Google Scholar] [CrossRef]
- Pacini, S.; Petrini, I. Are MSCs angiogenic cells? New insights on human nestin-positive bone marrow-derived multipotent cells. Front. Cell Dev. Biol. 2014, 2, 20. [Google Scholar] [CrossRef] [PubMed]
- Silva, G.V.; Litovsky, S.; Assad, J.A.R.; Sousa, A.L.S.; Martin, B.J.; Vela, D.; Coulter, S.C.; Lin, J.; Ober, J.; Vaughn, W.K.; et al. Mesenchymal Stem Cells Differentiate into an Endothelial Phenotype, Enhance Vascular Density, and Improve Heart Function in a Canine Chronic Ischemia Model. Circulation 2005, 111, 150–156. [Google Scholar] [CrossRef] [PubMed]
- Yue, W.-M.; Liu, W.; Bi, Y.-W.; He, X.-P.; Sun, W.-Y.; Pang, X.-Y.; Gu, X.-H.; Wang, X.-P. Mesenchymal stem cells differentiate into an endothelial phenotype, reduce neointimal formation, and enhance endothelial function in a rat vein grafting model. Stem Cells Dev. 2008, 17, 785–793. [Google Scholar] [CrossRef] [PubMed]
- Reiss, M.; Han, Y.; Garcia, E.; Goldberg, M.; Hong, Y.; Garner, W. Matrix Metalloproteinase-9 Delays Wound Healing in a Murine Wound Model. Surgery 2010, 147, 295. [Google Scholar] [CrossRef]
- Kandhwal, M.; Behl, T.; Singh, S.; Sharma, N.; Arora, S.; Bhatia, S.; Al-Harrasi, A.; Sachdeva, M.; Bungau, S. Role of matrix metalloproteinase in wound healing. Am. J. Transl. Res. 2022, 14, 4391–4405. [Google Scholar] [PubMed]
- Widgerow, A.D. Chronic wound fluid--thinking outside the box. Wound Repair Regen. 2011, 19, 287–291. [Google Scholar] [CrossRef]
- Caley, M.P.; Martins, V.L.C.; O’Toole, E.A. Metalloproteinases and Wound Healing. Adv. Wound Care 2015, 4, 225–234. [Google Scholar] [CrossRef] [PubMed]
- Cho, H.; Balaji, S.; Hone, N.L.; Moles, C.M.; Sheikh, A.Q.; Crombleholme, T.M.; Keswani, S.G.; Narmoneva, D.A. Diabetic wound healing in a MMP9-/- mouse model. Wound Repair Regen. 2016, 24, 829–840. [Google Scholar] [CrossRef] [PubMed]
- Mohan, R.; Chintala, S.K.; Jung, J.C.; Villar, W.V.L.; McCabe, F.; Russo, L.A.; Lee, Y.; McCarthy, B.E.; Wollenberg, K.R.; Jester, J.V.; et al. Matrix metalloproteinase gelatinase B (MMP-9) coordinates and effects epithelial regeneration. J. Biol. Chem. 2002, 277, 2065–2072. [Google Scholar] [CrossRef]
- Musiał-Wysocka, A.; Kot, M.; Majka, M. The Pros and Cons of Mesenchymal Stem Cell-Based Therapies. Cell Transplant. 2019, 28, 801–812. [Google Scholar] [CrossRef]
- Ben Menachem-Zidon, O.; Gropp, M.; Ben Shushan, E.; Reubinoff, B.; Shveiky, D. Systemically transplanted mesenchymal stem cells induce vascular-like structure formation in a rat model of vaginal injury. PLoS ONE 2019, 14, e0218081. [Google Scholar] [CrossRef] [PubMed]
- Dai, M.; Xu, P.; Hou, M.; Teng, Y.; Wu, Q. In vivo imaging of adipose-derived mesenchymal stem cells in female nude mice after simulated childbirth injury. Exp. Ther. Med. 2015, 9, 372–376. [Google Scholar] [CrossRef] [PubMed]
- Kasap, B.; Kasap, Ş.; Vatansever, S.; Kendirci, R.; Yılmaz, O.; Çalışır, M.; Edgünlü, T.; Akın, M.N. Effects of adipose and bone marrow-derived mesenchymal stem cells on vaginal atrophy in a rat menopause model. Gene 2019, 711, 143937. [Google Scholar] [CrossRef] [PubMed]
- Mori da Cunha, M.G.M.C.; Mackova, K.; Hympanova, L.H.; Bortolini, M.A.T.; Deprest, J. Animal models for pelvic organ prolapse: Systematic review. Int. Urogynecol. J. 2021, 32, 1331–1344. [Google Scholar] [CrossRef]
- Ripperda, C.M.; Maldonado, P.A.; Acevedo, J.F.; Keller, P.W.; Akgul, Y.; Shelton, J.M.; Word, R.A. Vaginal estrogen: A dual-edged sword in postoperative healing of the vaginal wall. Menopause 2017, 24, 838–849. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Chan, C. Isolation and enrichment of rat mesenchymal stem cells (MSCs) and separation of single-colony derived MSCs. J. Vis. Exp. 2010, 37, 1852. [Google Scholar] [CrossRef]
- Abercrombie, M. Estimation of nuclear population from microtome sections. Anat. Rec. 1946, 94, 239–247. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ben Menachem-Zidon, O.; Reubinoff, B.; Shveiky, D. Transplantation of Mesenchymal Stem Cells Derived from Old Rats Improves Healing and Biomechanical Properties of Vaginal Tissue Following Surgical Incision in Aged Rats. Int. J. Mol. Sci. 2024, 25, 5714. https://doi.org/10.3390/ijms25115714
Ben Menachem-Zidon O, Reubinoff B, Shveiky D. Transplantation of Mesenchymal Stem Cells Derived from Old Rats Improves Healing and Biomechanical Properties of Vaginal Tissue Following Surgical Incision in Aged Rats. International Journal of Molecular Sciences. 2024; 25(11):5714. https://doi.org/10.3390/ijms25115714
Chicago/Turabian StyleBen Menachem-Zidon, Ofra, Benjamin Reubinoff, and David Shveiky. 2024. "Transplantation of Mesenchymal Stem Cells Derived from Old Rats Improves Healing and Biomechanical Properties of Vaginal Tissue Following Surgical Incision in Aged Rats" International Journal of Molecular Sciences 25, no. 11: 5714. https://doi.org/10.3390/ijms25115714
APA StyleBen Menachem-Zidon, O., Reubinoff, B., & Shveiky, D. (2024). Transplantation of Mesenchymal Stem Cells Derived from Old Rats Improves Healing and Biomechanical Properties of Vaginal Tissue Following Surgical Incision in Aged Rats. International Journal of Molecular Sciences, 25(11), 5714. https://doi.org/10.3390/ijms25115714





